Abstract
Purpose:
To evaluate the diagnostic value of dynamic contrast-enhanced (DCE) magnetic resonance imaging (MRI) for patients with atypical ductal hyperplasia (ADH) in predicting malignant upgrade.
Materials and Methods:
3T DCE-MRI was performed for 17 patients with ADH (median age 52, range 42–76) proven by stereotactic biopsy (n = 15), and ultrasound-guided biopsy (n = 2) from January 2011 to April 2015. All patients underwent surgical excision after the MRI. Two radiologists prospectively reviewed the MRI to determine the presence or absence of suspicious findings at the site of biopsy, and evaluated the MR features of any lesion present according to the Breast Imaging Reporting and Data System (BI-RADS) lexicon. MRI findings and clinical information were correlated with the final surgical pathology by multivariate analysis.
Results:
Nine of 17 lesions were upgraded to malignancy. MRI demonstrated suspicious nonmass enhancement (NME) at the site of biopsy in all upgraded patients. The median size was 19.5 mm (range, 9–44mm). In the eight patients without upgrade, no enhancement (n = 2), linear enhancement along the biopsy track (n = 4), thin rim enhancement around hematoma (n = 1), and a focal NME (n = 1) were seen. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of MRI findings were 100, 87.5, 90, and 100%, respectively. Multivariate analysis revealed that the presence of suspicious enhancement on MRI was the most significant predictor of upgrade to malignancy (P = 0.0006)
Conclusion:
Our study revealed a high NPV of DCE-MRI for patients with ADH in terms of malignant upgrade at subsequent surgery. This suggests that patients with ADH without suspicious enhancement on DCE-MRI might be followed with DCE-MRI rather than undergoing surgical excision.
The widespread use of image-guided percutaneous core needle biopsy (CNB) targeting breast lesions has caused an increase in the detection of high-risk lesions on pathological results.1 High-risk lesions include atypical ductal hyperplasia (ADH), lobular carcinoma in situ (LCIS), atypical lobular hyperplasia (ALH), radial scar, papillary lesions, and flat epithelial atypia (FEA). Due to the possibility of their upgrade to malignancy at final pathology from surgical excision, when these lesions are found on CNB they are often managed with surgery.
ADH is reported to demonstrate a various upgrade rate (7–54%).2–10 In the pathology literature, ADH is defined as a lesion that has some but not all of the histologic and cytologic features of low-grade ductal carcinoma in situ (DCIS), a lesion that has all of the features of low-grade DCIS but involves one duct, or a lesion that has all of the features of low-grade DCIS but measures less than 2 mm in diameter.11–13 Because a main difference between ADH and low-grade DCIS is the extent of the lesion, a definite differentiation between these two histologies is not always easy on core biopsy because the limited sample size creates the possibility of undersampling a lesion. Hence, surgical excision is the standard management of ADH found on CNB.14 However, it is debatable whether all patients with ADH need surgical excision, as only a minority of cases are upgraded to DCIS on final surgical pathology.
Although several investigators studied the utility of dynamic contrast-enhanced (DCE) magnetic resonance imaging (MRI) for predicting subsequent upgrade to malignancy in high-risk lesions proven on CNB, many of these studies clumped them together in one entity, and did not investigate each type of high-risk lesions individually.15–18 We believe that it would be reasonable to analyze each high-risk lesion individually because the likelihood of upgrade is different in each type of lesion.
In this study, we conducted a prospective multivariate analysis of breast MRI for predicting an upgrade to malignancy in patients with biopsy proven ADH.
Materials and Methods
Institutional Review Board (IRB) approval was obtained for this prospective study. All patients provided written informed consent to participate in this study.
Patients
The study subjects were patients who had undergone stereotactic or ultrasound-guided CNB of a breast lesion, with the pathologic diagnosis of ADH from biopsy. Patients with any malignant lesions proven in the specimen were excluded from the study. The study protocol required patients to undergo DCE MRI after the biopsy. Although a surgical excision of the lesion after the MRI was an option for the patients, only the patients who underwent a surgical excision were included in this study. Between January 2011 and April 2015, 22 patients were prospectively registered and provided informed consent. Five patients were excluded; four patients declined surgical excision and one patient had a study deviation in performing DCE MRI (a 1.5T MR machine, which was out of the study protocol, was used). In total, 17 patients were included in our prospective study. MRI was performed 9–126 days (mean 38 days) after the biopsy, and the surgery was performed 2–50 days (mean 17 days) after the MRI. The interval between the biopsy and the surgery was 16–132 days (mean 55 days). The patients’ menopausal status and personal and family history of breast cancer were recorded using the institutional electronic medical record.
Interpretation of Conventional Images
Interpretation of mammography and ultrasound images was performed according to the Breast Imaging Reporting and Data System (BI-RADS) lexicon for lesion’s characteristics,19 in consensus by two radiologists (K.T., H.A.) with at least 4 years of experience in the interpretation of mammography, ultrasound images, and breast MR images. Overall BI-RADS assessment was determined using both mammography and ultrasound images.
Procedure
Percutaneous CNB was performed under stereotactic or ultrasound guidance. Stereotactic biopsy was performed in 15 patients (88%) using 8-, 9-, 11-, and 12-gauge core needle with a vacuum-assisted device. The number of cores samples obtained varied in these patients (median: 7, range: 3–12). Ultrasound-guided biopsy was performed in the remaining two patients (12%) with 14-gauge core needle, and three core samples were obtained in each case. A marking clip was placed at the biopsy site in all patients. In patients with mammographically detected microcalcifications, a specimen radiograph was performed to confirm the presence of calcifications.
MR Technique
MRI examination was performed using a 3T system (Achieva, Philips Healthcare, Best, Netherlands) with a 16-channel breast coil and the patients were set in the prone position. The DCE study was performed using one pre- and five postcontrast fat-saturated axial T1-weighted images with a temporal resolution of 75 seconds. Subtraction images were made by postcontrast T1-weighted images minus precontrast T1-weighted images. Imaging parameters were as follows: repetition time / echo time (TR/TE), 4.92/2.46; flip angle, 10°; matrix size, 448 × 448; field of view (FOV), 360 × 360; slice thickness, 1.6 mm. Gadobutrol (Gadavist, Bayer, Germany) was injected IV at a dose of 0.1 mM/kg followed by a 20-ml saline flush at a rate of 2 ml/s for the dynamic contrast study. Subtraction images and kinetic analysis including color-coding map were processed by Dynacad (v. 2.1.7.113583, Philips Healthcare). Subtraction images were utilized in assessing the lesions.
MR Interpretation
MR images were interpreted independently by the same two radiologists who interpreted conventional images. Discordance between two radiologists’ assessment was solved by consensus. There was a washout period of 1 month between the two readings and the radiologists were blinded to other imaging studies. The readers evaluated both nonsubtraction and subtraction DCE MR images together with multiplanar reconstructions and maximum intensity projections on Dynacad workstation. The presence or absence of enhancement at the site of needle biopsy was noted. An enhancement along the biopsy track and a thin rim enhancement around hematoma/seroma were considered benign (BI-RADS 2) or probably benign (BI-RADS 3). When there was no enhancement or only benign enhancement, it was considered a “nonsuspicious finding.” On the other hand, any contrast enhancement with measurable extent was considered a “suspicious finding.” When there was a suspicious finding (BI-RADS 4 or 5), lesion type (mass, nonmass enhancement [NME] or focus), morphologic features (shape, margin, and internal enhancement characteristics for mass; distribution and internal enhancement patterns for nonmass enhancement) and kinetics (initial phase, delayed phase) were assessed according to the BI-RADS lexicon. The maximum diameter of the evaluated lesion was recorded as the size of lesion.
Pathologic Assessment
All biopsy specimens and surgically excised specimens were stained with hematoxylin and eosin (H&E) and assessed as per clinical standard of care. All pathological results of image guided biopsy and surgery were obtained from the electronic medical record.
Statistical Analysis
Final surgical histology was considered the reference standard for comparison with the MR findings. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of breast MRI in predicting the presence of malignancy was calculated with 95% confidence intervals (CIs). Lesions correctly classified as suspicious on MR images were categorized as true-positive findings, whereas lesions incorrectly classified as suspicious were deemed false-positive findings. Likewise, lesions correctly classified as nonsuspicious on MR images were considered true-negative findings, whereas those incorrectly classified as nonsuspicious were deemed false-negative findings.
For the multivariate analysis, we selected patient age, menopausal status (postmenopausal vs. others), family history of breast cancer in a first- or second-degree relative (presence vs. absence), personal history of breast cancer (presence vs. absence), the number of core samples, gauge of biopsy needle (10G or larger vs. others), BI-RADS assessment on conventional imaging (4A vs. 4B or higher), residual calcifications on mammography after biopsy (presence vs. absence) and lesions that were 0.7 cm or larger vs. smaller than 0.7cm on conventional images as confounding factors with MR findings (suspicious enhancement on MRI vs. nonsuspicious enhancement on MRI). We adopted 0.7 cm as a cutoff for lesion size because a previous study20 suggested that the upgrade rate was higher when the high-risk lesion was larger than 0.7 cm regardless of the type of pathology. To assess the association between the documented variables and results of final histology (benign or malignant) in univariate analysis, categorical variables were compared by Fisher’s exact tests and continuous variables were assessed using paired Wilcoxon and Mann-Whitney U-test. Variables were selected for inclusion in the multivariate models based on univariate associations when P < 0.2. The logistic regression model was used for multivariate analysis. P ≤ 0.05 was considered statistically significant at multivariate analysis. We also performed a subgroup analysis for the cases with ADH diagnosed by stereotactic biopsy using the same uni- and multivariate analysis method. Data analyses was performed with commercially available software (JMP 11, SAS Institute, Cary, NC).
Results
Patient Characteristics
Among 17 patients (median age 52, range 42–76), 8 were postmenopausal (47%) and nine were premenopausal (53%). There was one patient (14%) who has a history of contralateral breast cancer and there were six patients (35%) with family history of breast cancer within second-degree.
Lesion’s Characteristics on Mammography or Ultrasound
Fourteen lesions (82%) were evident only mammographically and manifested as calcifications. Their patterns are: grouped amorphous calcifications (n = 9), grouped coarse heterogeneous calcifications (n = 4), and segmental amorphous calcifications (n = 1). Three lesions (18%) were detected both on mammography and on ultrasound as grouped coarse heterogeneous calcifications / hypoechoic mass, focal asymmetry / hypoechoic mass, and segmental amorphous calcifications / hypoechoic mass with calcifications (mammographic/ultrasound findings).
Surgical Pathological Finding
Final surgical pathology revealed malignancy in 9 of 17 patients (upgrade rate in CNB: 53%). Among them, ductal carcinoma in situ (DCIS) was found in seven patients (n = 2, low grade; n = 5, intermediate grade) and grade 1 invasive ductal carcinoma (IDC) was found in two patients. Among patients without malignant pathology results (47%), high-risk lesions were found in seven patients (41%) and benign histologic finding in one patient (6%). For these patients, high-risk lesions were ADH in five patients, ALH in one patient and FEA in one patient, and one benign finding was usual ductal hyperplasia.
MR Findings
The lesion characteristics of all imaging and pathological findings are summarized in Table 1, and the biopsy information is shown on Table 2. All of the nine upgraded lesions showed suspicious enhancement (true-positive finding; Fig. 1). All of these findings were NME. The distribution of NME types were focal (n = 3), linear (n = 5), and segmental (n = 1); internal enhancement patterns were clumped (n = 6), heterogeneous (n = 2), and homogeneous (n = 1); kinetics were rapid-plateau (n = 5), medium-plateau (n = 1), medium-washout (n = 1), and slow-persistent (n = 2); the mean size was 21.6 mm (range, 9–48 mm). Among the eight patients without upgrade, seven showed no suspicious enhancement (n = 2, no enhancement; n = 4, enhancement along biopsy track; n = 1, thin rim enhancement around hematoma) (true-negative finding; Fig. 2). One of the eight patients without upgrade showed 12 mm of focal homogeneous NME with rapid-washout kinetic at the site of biopsy, hence it was the only false-positive case (false-positive finding; Fig. 3). Surgical pathology of this case was ADH with intraductal papilloma. Sensitivity, specificity, PPV, and NPV with the exact 95% CIs of MRI findings were 100 (80.7–100), 87.5 (65.7–87.5), 90 (72.6–90.0) and 100% (75.1–100) respectively.
TABLE 1.
Imaging Characteristics and Histological Results of 17 Lesions
| Conventional Imaging Findings | MRI Findings | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non mass Enhancement | Signal Intensity Curve |
|||||||||
| 1 | grouped amorphous calcs | NA | 4a | over | linear | clumped | rapid | plateau | 4 | DCIS grade 2 |
| 2 | grouped amorphous calcs | NA | 4b | over | linear | clumped | medium | plateau | 4 | DCIS grade 1 |
| 3 | grouped amorphous calcs | occult | 4b | under | NA | NA | NA | NA | 1 | UDH |
| 4 | grouped heterogeneous calcs | occult | 4b | under | NA | NA | NA | NA | 2 | ADH |
| 5 | grouped amorphous calcs | occult | 4b | over | focal | heterogeneous | rapid | plateau | 4 | DCIS grade 1 |
| 6 | grouped amorphous calcs | NA | 4a | under | NA | NA | NA | NA | 2 | ADH |
| 7 | grouped coarse heterogeneous calcs | NA | 4c | under | NA | NA | NA | NA | 2 | ALH |
| 8 | grouped amorphous calcs | NA | 4c | over | linear | clumped | slow | persistent | 4 | IDC grade 1 |
| 9 | grouped coarse heterogeneous calcs | NA | 4c | under | focal | heterogeneous | rapid | plateau | 4 | DCIS grade 2 |
| 10 | grouped coarse heterogeneous calcs | hypaechoic mass | 4b | over | linear | clumped | rapid | plateau | 4 | DCIS grade 2 |
| 11 | grouped amorphous calcs | NA | 4a | under | NA | NA | NA | NA | 1 | ADH |
| 12 | grouped amorphous calcs | NA | 4c | under | NA | NA | NA | NA | 2 | ADH |
| 13 | grouped coarse heterogeneous calcs | occuit | 4b | over | NA | NA | NA | NA | 2 | FEA |
| 14 | segmental amorphous calcs | NA | 4b | over | linear | clumped | slow | persistent | 4 | DCIS grade 2 |
| 15 | focal asymmetry | hypoechoic mass | 4b | over | focal | homogeneous | rapid | washout | 4 | ADH, papilloma |
| 16 | segmental amorphous calcs | hypoechoic mass | 4b | over | segmental | clumped | rapid | plateau | 4 | IDC grade 1 |
| 17 | grouped amorphous calcs | NA | 4a | over | focal | homogeneous | medium | washout | 4 | DCIS grade 1 |
calcs: calcifications, NA: not available, UDH: usual ductal hyperplasia, ADH: atypical ductal hyperplasia, ALH: atypical lobular hyperplasia, FEA: flat epithelial atypia, DCIS: ductal carcinoma in situ, IDC: invasive ductal carcinoma
TABLE 2.
Biopsy Information of 17 Lesions
| 1 | stereotactic | 9 | 12 | + |
| 2 | stereotactic | 9 | 12 | − |
| 3 | stereotactic | 9 | 6 | − |
| 4 | stereotactic | 9 | 12 | + |
| 5 | stereotactic | 9 | 8 | + |
| 6 | stereotactic | 9 | 7 | − |
| 7 | stereotactic | 12 | 6 | + |
| 8 | stereotactic | 12 | 12 | + |
| 9 | stereotactic | 9 | 7 | + |
| 10 | ultrasound-guided | 14 | 3 | + |
| 11 | stereotactic | 9 | 6 | − |
| 12 | stereotactic | 9 | 6 | + |
| 13 | stereotactic | 9 | 10 | − |
| 14 | stereotactic | 9 | 12 | + |
| 15 | ultrasound-guided | 14 | 3 | − |
| 16 | stereotactic | 3 | 6 | + |
| 17 | stereotactic | 12 | 12 | + |
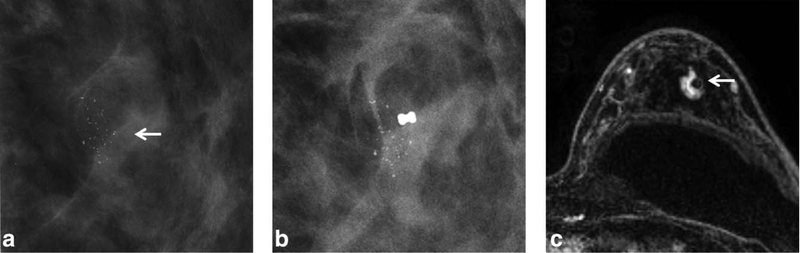
FIGURE 1:
a: A 48-year-old woman (Table 1, No. 9) with screen mammography detected grouped coarse heterogeneous and linear calcifications (BIRADS 4c) in the left upper outer quadrant (arrow). b: The stereotactic biopsy with 9G core needle was performed for the calcifications and seven core samples were obtained. The pathologic result was a single focus of atypical ductal hyperplasia (ADH). Postbiopsy mammogram showed multiple residual calcifications besides biopsy clip. c: Axial T1-weighted contrast-enhanced subtracted MR image 48 days after biopsy showed clip artifact within 11 mm focal heterogeneous nonmass enhancement (NME) with rapid plateau kinetics. The retro-pectoral implant was also observed (arrow). Surgical pathology showed scattered foci of ductal carcinoma in situ (DCIS) intermediate grade. The MRI finding is true-positive.
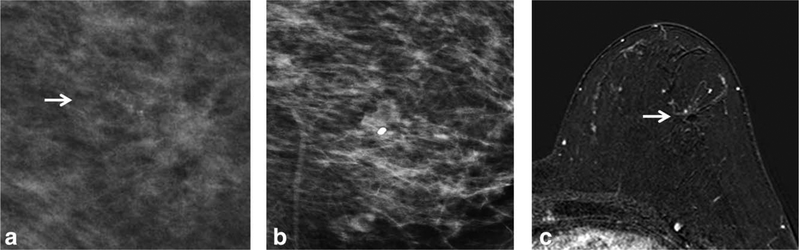
FIGURE 2:
a: A 44-year-old woman (Table 1, No. 11) with screening mammogram detected grouped amorphous calcification (BIR-ADS 4a) in the left upper outer quadrant (arrow). b: The stereotactic biopsy with 9G core needle was performed for calcifications and six core samples were obtained. The pathologic result was a single focus of ADH. Postbiopsy mammogram showed biopsy clip with no residual calcifications. c: Axial T1-weighted contrast-enhanced subtracted MR image 43 days after biopsy showed clip artifact with no suspicious enhancement (arrow). Surgical pathology showed rare foci of ADH with flat epithelial atypia (FEA). The MRI finding is a true-negative.
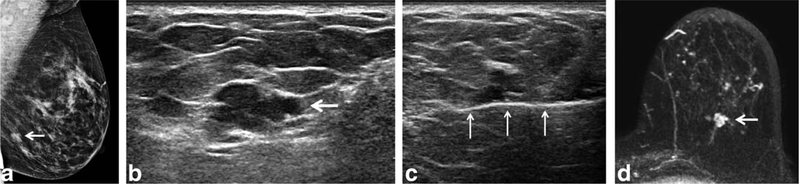
FIGURE 3:
a: A 52-year-old woman (Table 1, No. 15) with new focal asymmetry (BIRADS 4b) in the left lower outer quadrant at screening mammography (arrow). b: A targeted ultrasound showed an irregular hypoechoic mass with indistinct margins measuring 12 × 7 × 6 mm with minimal vascularity at the 6 o’clock position (arrow). c: The ultrasound-guided biopsy with 14G core needle (arrows) was performed for the lesion and three core samples were obtained. The pathology result was a single focus of ADH. d: Axial T1-weighted contrast-enhanced subtracted MR image 16 days after biopsy showed clip artifact within 12 mm focal homogeneous NME with rapid washout kinetics (arrow). Surgical pathology showed focal ADH with intraductal papilloma. The MRI finding is a false-positive.
Univariate and Multivariate Analysis
On comparing the clinical characteristics and the imaging features between patients with benign results and those with malignancies by univariate analysis, the following variables did not fulfill the inclusion criterion (P < 0.2) for the multivariate analysis; patient age (P = 0.335), menopausal status (P = 0.637), the presence or absence of personal history of breast cancer (P = 1.000), or family history of breast cancer within second degree (P = 0.620), the gauge of biopsy needle (over 10G or under 10G) (P = 0.620), BI-RADS assessment on conventional imaging (4A vs. 4B or higher) (P = 1.000). On the other hand, the following variables fulfilled the inclusion criterion (P < 0.2) for multivariate analysis: residual calcifications on mammography after biopsy (presence vs. absence) (P = 0.0498), lesions that were 0.7 cm or larger vs. smaller than 0.7 cm on conventional images (P = 0.0152), the presence or absence of suspicious enhancement (P = 0.0004), and the number of core samples (P = 0.110). Multivariate analysis using these four factors revealed that the presence or absence of suspicious enhancement on MR was the only significant predictor of upgrade to malignancy (P = 0.0006). P values for other three factors were: the number of core samples (P = 0.0959), lesions that were 0.7cm or larger vs. smaller than 0.7cm on conventional images (P = 0.9993), residual calcifications on mammography after biopsy (presence vs. absence) (P = 0.0959) (Table 3).
TABLE 3.
Correlation of Clinico-Pathologic Findings and Imaging Features
| All Patients (n=17) | Patients with Stereotactic Biopsy (n=15) | |||||||
|---|---|---|---|---|---|---|---|---|
| Surgical Histopathological Findings | Surgical Histopathological Findings | |||||||
| Age | ||||||||
| mean±SD (range) | 56.1 + 12.7 (42–76) | 48 ± 3.8 (43–54) | 0.335 | 54 ± 11.8 (42–76) | 47 ± 3.7 (43–54) | 0.4867 | ||
| Menopause (n[%]) | ||||||||
| yes | 5 (62.5) | 3 (37.5) | 0.637 | 4 (66.7) | 2(33.3) | 0.6084 | ||
| no | 4 (44.4) | 5 (55.6) | 4 (44.4) | 5 (55.6) | ||||
| Past History (n[%]) | ||||||||
| yes | 1 | 0 | 1.000 | 0 | 0 | 1.000 | ||
| no | 8 | 8 | 8 | 7 | ||||
| Family History (n[%]) | ||||||||
| yes | 4 (66.7) | 2 (33.3) | 0.620 | 4 (66.7) | 2 (33.3) | 0.6084 | ||
| no | 5 (45.5) | 6 (54.5) | 4 (44.4) | 5 (55.6) | ||||
| Number of samples | ||||||||
| mean±SD (range) | 9.3 ± 3.4 (3–12) | 7 ± 2.8 (3–12) | 0.110 | 0.0959 | 10.1 + 2.6 (6–12) | 7.6 + 2.4 (6–12) | 0.0587 | 1.000 |
| Biopsy Gauge (n[%]) | ||||||||
| 10G≤ | 4 (66.7) | 2 (33.3) | 0.620 | 3 (75.0) | 1 (25.0) | 0.5692 | ||
| 10G≥ | 5 (45.5) | 6 (54.5) | 5 (45.5) | 6 (54.5) | ||||
| 4b ≤ on conventional imsges (n[%]) | ||||||||
| yes | 7 (53.8) | 6 (46.2) | 1.000 | 6 (54.5) | 5 (45.5) | 1.000 | ||
| no | 2 (50.0) | 2 (50.0) | 2 (50.0) | 2 (50.0) | ||||
| 0.7 cm ≤ on conventional images (n[%I) | ||||||||
| yes | 8 (80.0) | 2 (20.0) | 0.0152 | 0.9993 | 7 (87.5) | 1 (12.5) | 0.0101 | 1.000 |
| no | 1 (14.3) | 6 (85.7) | 1 (14.3) | 6 (85.7) | ||||
| Residual calcification [n[%]) | ||||||||
| yes | 8 (72.7) | 3 (27.3) | 0.0498 | 0.0959 | 7 (70.0) | 3 (30.0) | 0.1189 | 1.000 |
| no | 1 (16.7) | 5 (83.3) | 1 (20.0) | 4 (80.0) | ||||
| MR enhancement (n[%]) | ||||||||
| suspicious | 9 (90.0) | 1 (10.0) | 0.0004 | 0.0006 | 8 (100) | 0 (0) | 0.0002 | 0.0071 |
| non-suspicious | 0 (0) | 7 (100) | 0 (0) | 7 (100) | ||||
As for the subgroup analysis of patients with ADH proven by stereotactic biopsy, the following four factors fulfilled the inclusion criterion (P < 0.2) for multivariate analysis: the number of core samples (P = 0.0587), lesions that were 0.7 cm or larger vs. smaller than 0.7 cm on conventional images (P = 0.0101), residual calcifications on mammography after biopsy (presence vs. absence) (P = 0.1189), the presence or absence of suspicious enhancement on MR (P = 0.0002). Just as with the whole group analysis, multivariate analysis using these four factors revealed that the presence or absence of suspicious enhancement on MR was the only significant predictor of upgrade to malignancy (P = 0.0071) (Table 3).
Discussion
Our study revealed that the upgrade rate for biopsy proven ADH on final surgical pathology was 53%, which is relatively high, but still within the range of previously reported results (7–54%).2–10 The previous four studies which examined the utility of MRI for predicting subsequent upgrade to malignancy at final pathology in high-risk lesions enrolled 6, 13, 16, and 23 patients with ADH, respectively.15–18 We studied 17 ADH patients, which is a relatively high number collected from a single institution. Among these four prior studies, two studies16,17 were prospective, and their decision criteria of MRI findings using BI-RADS assessment are the same as ours. The other two studies were retrospective. Linda et al15 used the Fischer score, which is known to correlate well with BI-RADS assessment,21 defining I-III as nonsuspicious and IV-V as suspicious. Londero et al18 used a simple assessment of the presence or absence of enhancement. Linda et al15 and Pediconi et al16 reported overall PPV (33.3% and 91%) and NPV (98.2% and 96%) of DCE-MRI in predicting upgrade of all high-risk lesions as a whole, but they did not provide those for ADH specifically. The later study of Linda et al17 and Londero et al18 reported PPV (50% and 44%) and NPV (90% and 100%) in predicting upgrade of ADH with DCE-MRI. So when the studies are reviewed together, PPV varies greatly (33.3–91%) while the NPV ranges narrow (90–100%). Thus, our study is in line with other literature showing that DCE-MRI can predict non-upgrade of ADH (NPV: 100%).
The high NPV of DCE-MRI in predicting the lack of malignant upgrade of ADH might be explained when the histopathology of ADH is considered. In its pathological definition, ADH lacks the histological features of low-grade DCIS or is histologically the same as low-grade DCIS but is localized to one duct or less than 2 mm in diameter.11–13 It is known that among patients with low-grade DCIS, there are 20–60% in which MRI does not show enhancement.22–24 This is probably because low-grade DCIS is associated with a lower angiogenic potential than intermediate-high-grade DCIS.25–27 By this principle, it would be expected that ADH would enhance even less, if it is a kind of incomplete or tiny low-grade DCIS.
In the studies described above15–18 and reported here-in, there were no false-negative patients of invasive cancer or high-grade DCIS at final pathology, but the majority of false-negative cases were low-grade DCIS. Sanders et al reported that low-grade DCIS had a capability to evolve into invasive carcinoma in 35–50% of cases, but the time course might span >40 years.28 Sagara et al reported that there was no statistically significant survival benefit for performing breast surgery for patients with low-grade DCIS in their large population-based cohort study, and they recommended a strategy of nonoperative management with active surveillance for those patients with low-grade DCIS.29 They also reported that patients with intermediate- and high-grade DCIS had a statically significant survival benefit by undergoing breast surgery. Since we know that the DCE-MRI is the most sensitive imaging modality for detecting breast cancers, especially high-grade DCIS and invasive car-cinoma,22,30 patients with low-grade DCIS might undergo a watchful waiting strategy, and be followed up with DCE-MRI. Any significant change on MRI would indicate the need for surgery. For those with ADH proven at CNB, if their DCE-MRI does not demonstrate any suspicious finding, surgical excision may be viewed as overtreatment in view of the high NPV of DCE-MRI in discriminating ADH with and without upgrade to malignancy. Further, any false-negative MRI is likely to be low-grade DCIS, a lesion that may not always be clinically significant. However, for patients with highly suspicious findings on mammography or ultrasound (BI-RADS 5) or pathologic results which are discordant, surgery should not be deferred and a treatment decision should be made based on the referring physician’s discretion.
A differentiating feature of our study is that multivariate analysis was performed. DCE-MR was the only effective factor for predicting upgrade to malignancy in the multivariate analysis of both whole group and subgroup of patients. We believe that the result of the subgroup analysis strengthens the efficacy of DCE-MRI because the patients in this group are less variable in terms of biopsy method and number of core samples.
We did not set a specific rule for the time interval between the initial biopsy and the breast MRI. The actual time interval varied from 9–126 days (mean 38 days), and there was no detrimental effect from the biopsy on the MR images in this study. Based on our review, researchers have reported mixed results regarding the optimal time interval needed to minimize excisional biopsy effects on MRI. Some reported that a breast MRI should be delayed a few weeks after the biopsy, such as 28 days31 or 14 days,32 but others reported that it should be performed within 28 days,33 or within 7 days.34 Thus, there is no established optimal interval between an excisional biopsy and a breast MRI. A percutaneous needle biopsy is less invasive than an excisional biopsy. Therefore, the magnitude of biopsy changes is typically smaller, and the effect of time interval may not be as important. In any case, further work may help determine the optimal timing between percutaneous biopsy and breast MRI.
This study has several limitations. One limitation is the small number of patients. Although this study was assessed prospectively and the results were consistent with those of previous studies, which also reported high NPV, a further prospective study with a large cohort would be needed to confirm the value of MRI for predicting subsequent upgrade of ADH lesions. The second limitation was that we did not include patients with ADH proven by MRI-guided biopsy. MR-detected ADH lesions had been detected for their contrast-enhancement on MRI; therefore, those lesions were likely to be enhancing again on MRI after biopsy. Thus, we considered that it might mislead to a biased distribution of subjects. The third limitation was that we only used 3T MRI for this study. Because 1.5T MR is more widely accessible, further studies including images obtained on 1.5T MR could be beneficial. The fourth limitation to consider is selection bias. Although surgery was recommended to all patients with ADH, 4 of the 22 patients (18%) declined surgery. Because the decision whether to receive surgery was made based on a discussion between the patients and a breast surgeon, there could be selection bias in our study. To minimize selection bias, the study protocol could mandate that patients have surgery. However, patients’ right to decline surgery at any time during the study would still need to be respected. Therefore, some drop-offs would always be inevitable, and we believe our drop-off rate to be acceptable. The fifth limitation to our study was that the biopsy needles and the number of core samples were not standardized. While standardization of these variables would be ideal, making such a study protocol would be quite challenging because we need to include the initial biopsy in the study protocol. All the subjects need to have received a percutaneous biopsy as part of routine clinical care to be eligible for this study. Presuming we include patients’ initial clinical biopsy in the protocol, we will need a large number of participants because the eligible patients for this study (patients with ADH) would be a minority of all the participants. Therefore, making such a protocol is not realistic in a single institution, outside of a much larger research study including all breast biopsies, regardless of predicted pathology results.
In conclusion, our prospective study revealed a high NPV of DCE-MRI for patients with ADH in predicting the lack of upgrade to malignancy. This suggests that patients with ADH without suspicious enhancement on DCE-MRI might be followed with DCE-MRI rather than performing surgical excision.
Acknowledgments
Part of this work was supported by Bayer Healthcare.
References
- 1.Berg WA. Image-guided breast biopsy and management of high-risk lesions. Radiol Clin North Am 2004;42:935–946. [DOI] [PubMed] [Google Scholar]
- 2.Menes TS, Rosenberg R, Balch S, Jaffer S, Kerlikowske K, Miglioretti DL. Upgrade of high-risk breast lesions detected on mammography in the Breast Cancer Surveillance Consortium. Am J Surg 2014;207:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polat AK, Kanbour-Shakir A, Andacoglu O, et al. Atypical hyperplasia on core biopsy: is further surgery needed? Am J Med Sci 2012;344: 28–31. [DOI] [PubMed] [Google Scholar]
- 4.Winchester DJ, Bernstein JR, Jeske JM, et al. Upstaging of atypical ductal hyperplasia after vacuum-assisted 11-gauge stereotactic core needle biopsy. Arch Surg 2003;138:619–623. [DOI] [PubMed] [Google Scholar]
- 5.Kohr JR, Eby PR, Allison KH, et al. Risk of upgrade of atypical ductal hyperplasia after stereotactic breast biopsy: effects of number of foci and complete removal of calcifications. Radiology 2010;255:723–730. [DOI] [PubMed] [Google Scholar]
- 6.Ely KA, Carter BA, Jensen RA, Simpson JF, Page DL. Core biopsy of the breast with atypical ductal hyperplasia: a probabilistic approach to reporting. Am J Surg Pathol 2001;25:1017–1021. [DOI] [PubMed] [Google Scholar]
- 7.Ingegnoli A, d′ Aloia C, Frattaruolo A, et al. Flat epithelial atypia and atypical ductal hyperplasia: carcinoma underestimation rate. Breast J 2010;16:55–59. [DOI] [PubMed] [Google Scholar]
- 8.Darvishian F, Singh B, Simsir A, Ye W, Cangiarella JF. Atypia on breast core needle biopsies: reproducibility and significance. Ann Clin Lab Sci 2009;39:270–276. [PubMed] [Google Scholar]
- 9.Sneige N, Lim SC, Whitman GJ, et al. Atypical ductal hyperplasia diagnosis by directional vacuum-assisted stereotactic biopsy of breast microcalcifications: considerations for surgical excision. Am J Clin Pathol 2003;119:248–253. [DOI] [PubMed] [Google Scholar]
- 10.Fuhrman GM, Cederbom GJ, Bolton JS, et al. Image-guided core-needle breast biopsy is an accurate technique to evaluate patients with nonpalpable imaging abnormalities. Ann Surg 1998;227:932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tavassoli FA, Norris HJ. A comparison of the results of long-term follow-up for atypical lntraductal hyperplasia and lntraductal hyperplasia of the breast. Cancer 1990;65:518–529. [DOI] [PubMed] [Google Scholar]
- 12.Page DL, Dupont WD, Rogers LW, Rados MS. Atypical hyperplastic lesions of the female breast a long-term follow-up study. Cancer 1985;55:2698–2708. [DOI] [PubMed] [Google Scholar]
- 13.Page DL, Rogers LW. Combined histologic and cytologic criteria for the diagnosis of mammary atypical ductal hyperplasia. Hum Pathol 1992;23:1095–1097. [DOI] [PubMed] [Google Scholar]
- 14.Degnim AC, King TA. Surgical management of high-risk breast lesions. Surg Clin North Am 2013;93:329–340. [DOI] [PubMed] [Google Scholar]
- 15.Linda A, Zuiani C, Bazzocchi M, Furlan A, Londero V. Borderline breast lesions diagnosed at core needle biopsy: Can magnetic resonance mammography rule out associated malignancy? Preliminary results based on 79 surgically excised lesions. Breast 2008;17:125–131. [DOI] [PubMed] [Google Scholar]
- 16.Pediconi F, Padula S, Dominelli V, et al. Role of breast MR imaging for predicting malignancy of histologically borderline lesions diagnosed at core needle biopsy: prospective evaluation. Radiology 2010; 257:653–661. [DOI] [PubMed] [Google Scholar]
- 17.Linda A, Zuiani C, Furlan A, et al. Nonsurgical management of high-risk lesions diagnosed at core needle biopsy: Can malignancy be ruled out safely with breast MRI? AJR Am J Roentgenol 2012;198: 272–280. [DOI] [PubMed] [Google Scholar]
- 18.Londero V, Zuiani C, Linda A, Girometti R, Bazzocchi M, Sardanelli F. High-risk breast lesions at imaging-guided needle biopsy: usefulness of MRI for treatment decision. AJR Am J Roentgenol 2012;199:W240–250. [DOI] [PubMed] [Google Scholar]
- 19.Morris EA, Comstock CE, Lee CH, et al. ACR BI-RADS® magnetic resonance imaging. In: ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- 20.Gumus H, Mills P, Gumus M, et al. Factors that impact the upgrading of atypical ductal hyperplasia. Diagn Interv Radiol 2013;19:91–96. [DOI] [PubMed] [Google Scholar]
- 21.Al-Khawari H, Athyal R, Kovacs A, Al-Saleh M, Madda JP. Accuracy of the Fischer scoring system and the Breast Imaging Reporting and Data System in identification of malignant breast lesions. Ann Saudi Med 2009;29:280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuhl CK, Schrading S, Bieling HB, et al. MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study. Lancet 2007;370:485–492. [DOI] [PubMed] [Google Scholar]
- 23.Neubauer H, Li M, Kuehne-Heid R, Schneider A, Kaiser WA. High grade and non-high grade ductal carcinoma in situ on dynamic MR mammography: characteristic findings for signal increase and morphological pattern of enhancement. Br J Radiol 2003;76:3–12. [DOI] [PubMed] [Google Scholar]
- 24.Rosen EL, Smith-Foley SA, DeMartini WB, Eby PR, Peacock S, Lehman CD. BI-RADS MRI enhancement characteristics of ductal carcinoma in situ. Breast J 2007;13:545–550. [DOI] [PubMed] [Google Scholar]
- 25.Guidi AJ, Schnitt SJ, Fischer L, et al. Vascular permeability factor (vascular endothelial growth factor) expression and angiogenesis in patients with ductal carcinoma in situ of the breast. Cancer 1997;80: 1945–1953. [DOI] [PubMed] [Google Scholar]
- 26.Brown LF, Guidi AJ, Schnitt SJ, et al. Vascular stroma formation in carcinoma in situ, invasive carcinoma, and metastatic carcinoma of the breast. Clin Cancer Res 1999;5:1041–1056. [PubMed] [Google Scholar]
- 27.Ottinetti A, Sapino A. Morphometric evaluation of microvessels surrounding hyperplastic and neoplastic mammary lesions. Breast Cancer Res Treat 1988;11:241–248. [DOI] [PubMed] [Google Scholar]
- 28.Sanders ME, Schuyler PA, Simpson JF, Page DL, Dupont WD. Continued observation of the natural history of low-grade ductal carcinoma in situ reaffirms proclivity for local recurrence even after more than 30 years of follow-up. Mod Pathol 2015;28:662–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sagara Y, Mallory MA, Wong S, et al. Survival benefit of breast surgery for low-grade ductal carcinoma in situ: a population-based cohort study. JAMA Surg 2015;150:739. [DOI] [PubMed] [Google Scholar]
- 30.Berg WA, Gutierrez L, NessAiver MS, et al. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology 2004;233:830–849. [DOI] [PubMed] [Google Scholar]
- 31.Frei KA, Kinkel K, Bonel HM, Lu Y, Esserman LJ, Hylton NM. MR imaging of the breast in patients with positive margins after lumpectomy: influence of the time interval between lumpectomy and MR imaging. AJR Am J Roentgenol 2000;175:1577–1584. [DOI] [PubMed] [Google Scholar]
- 32.Yang H, Liu H, Peng W, Hua Y. Magnetic resonance imaging of the breast in evaluating residual diseases at lumpectomy site soon after excisional biopsy. J Comput Assist Tomogr 2012;36:196–199. [DOI] [PubMed] [Google Scholar]
- 33.Stucky C-CH, McLaughlin SA, Dueck AC, et al. Does magnetic resonance imaging accurately predict residual disease in breast cancer? Am J Surg 2009;198:547–552. [DOI] [PubMed] [Google Scholar]
- 34.Chae EY, Cha JH, Kim HH, et al. Evaluation of residual disease using breast MRI after excisional biopsy for breast cancer. AJR Am J Roentgenol 2013;200:1167–1173. [DOI] [PubMed] [Google Scholar]