Abstract
Background
Preoperative predictors for the need of prophylactic lymph node dissection in the lateral neck have been studied in patients with medullary thyroid carcinoma (MTC).
Objectives
To evaluate the ability of serum calcitonin to predict the extent of surgery needed in the lateral neck.
Methods
This retrospective population-based cohort study includes data from 94 of 139 patients with MTC surgically treated in Norway from 2003 to 2016. Patients were identified in the 4 regional centers treating MTC and by the Cancer Registry of Norway, and grouped according to calcitonin levels. In 58 patients without distant metastases or disease progression to the next tumor level (NPNL), data were compared in prognostic groups (N0-NPNL), (N1a-NPNL), and (N1b-NPNL).
Results
At calcitonin levels ≤500, 501–1,000, and >1,000 pmol/L, metastatic lymph nodes in the lateral neck were found in 16, 50, and 71% of the patients, respectively. In the prognostic groups, 19% of N0-NPNL patients had calcitonin >500 pmol/L and 17% of N1b-NPNL patients had calcitonin ≤500 pmol/L. In multivariate analysis, factors predicting biochemical cure and calcitonin level ≤500 pmol/L were no metastatic lymph nodes in the lateral neck (p = 0.030) and tumor diameter ≤20 mm (p < 0.001), respectively. Factors related to metastatic lymph nodes in the lateral neck were extrathyroidal extension (p = 0.007) and no biochemical cure (p = 0.028).
Conclusions
Basal calcitonin cannot predict the need for prophylactic lateral lymph node dissection in patients with MTC. Further prospective, randomized studies are warranted.
Keywords: Calcitonin, Prophylactic lymph node dissection, Medullary thyroid carcinoma, Diagnostics, Surgery, Pathology, Outcome
Introduction
Medullary thyroid carcinoma (MTC) accounts for 1–10% of thyroid malignancies [1, 2, 3, 4]. MTC occurs sporadically in 75% of the patients; hereditary MTC was noted in 25% as part of multiple endocrine neoplasia type 2 [1, 2, 5, 6].
The prospect of curation and survival from MTC are inferior to that of follicle-derived thyroid carcinomas, with 5- and 10-year disease-specific survival reported from 79 to 99% and from 70 to 87%, respectively [1, 7, 8, 9, 10, 11].
If performed to a sufficient extent, surgery is the only means of potential cure for MTC [2, 3, 12, 13]. Total thyroidectomy and central lymph node dissection in the neck is standard treatment, as is therapeutic lateral lymph node dissection at clinical disease in the lateral neck [3, 14].
High-resolution ultrasound is important in disease staging, enabling the detection of even very small metastatic lymph nodes but not micrometastases. Calcitonin is a good tumor marker in MTC. As a rule, it is proportional to tumor load, making the hormone a good marker for pretherapeutic staging and surgical strategy planning [12, 15, 16]. Major studies have analyzed diagnostic predictors for the necessity of prophylactic lymph node dissection in the lateral neck considering tumor features at ultrasound [17], calcitonin level [12, 16], or metastatic lymph node load in the central neck [14, 18].
The aim of this study is to evaluate the relation between preoperative basal serum calcitonin levels and tumor stage, and the ability of the calcitonin level to predict the extent of surgery needed in the lateral neck in patients with MTC.
Materials and Methods
Study Population
This retrospective cohort study is based on the Norwegian MTC project database covering all patients with MTC in Norway from 1994 to 2016, with clinical, genetic, and histopathological data recorded by the regional centers dealing with MTC. In addition, the Cancer Registry of Norway provided information on all patients registered with MTC. Data collection was done by reviewing patient files. The censoring date for follow-up data was March 1, 2017.
Due to more sensitive calcitonin analysis from 2003, this study is limited to patients with MTC treated after 2003. Figure 1 presents a flowchart of the study cohort.
Fig. 1.
Study cohort with inclusion and exclusion flowchart.
Ninety-four patients treated 2003–2016 were included. The patients were grouped according to the preoperative calcitonin level. To minimize bias related to stage migration during follow-up, the data were compared in prognostic groups according to lymph node and distant metastatic status after completed primary surgery and during follow-up. Patients without distant metastasis (M0) and no lymph node metastasis (pN0), metastatic lymph nodes in the central neck (pN1a), or metastatic lymph nodes in the lateral neck (pN1b) at primary surgery, and with no disease progression to the next tumor level during follow-up (NPNL) were grouped as (N0-NPNL), (N1a-NPNL), and (N1b-NPNL), respectively, counting 58 patients in all.
The study patients have been included in previous studies with other objectives [4, 19, 20].
Surgical Treatment and Follow-Up
Standard surgical treatment comprised total thyroidectomy and lymph node dissection in the central neck. Lymph node dissection in the lateral neck was performed preoperatively for localized metastatic lymph nodes. Lobectomy alone and prophylactic lateral lymph node dissection were performed occasionally. Completed primary surgery was defined as one or more surgical procedures within the first year.
Follow-up was generally performed early postoperatively, every 3–6 months in the first postoperative year, and then once a year. However, the follow-up frequencies have varied depending on the patients' biochemical and clinical status. Regular follow-up included basal serum calcitonin analysis and ultrasound of the neck. Other radiological and nuclear medical examinations were performed when indicated.
Postoperative biochemical cure was evaluated after completed primary surgery. The lowest measured calcitonin value after primary surgery and before known disease progression was used to evaluate postoperative biochemical cure. At the latest MTC-specific follow-up, clinical and biochemical status were evaluated. The outcome was evaluated by basal serum calcitonin in 93 (99%) patients, including 2 patients with calcitonin analysis earlier than the latest follow-up year. Neck ultrasound was performed in 76 (81%) patients, including 12 patients with neck ultrasound performed earlier than the latest follow-up year due to biochemical cure, parallel radiological or nuclear medical examinations, or unknown reason (8, 3, and 1 patients, respectively). In 18 (19%) patients, neck ultrasound was not performed due to biochemical cure, parallel radiological or nuclear medical examinations, short postoperative follow-up, or other serious illness (5, 7, 4, and 2 patients, respectively).
Biochemical and Histopathological Analysis
Calcitonin was analyzed by routine immunoassay in 2 laboratories: the Oslo University Hospital (OUH) and the Haukeland University Hospital (HUH), Bergen. Calcitonin was measured with Immulite® Siemens with varying detection limits (from 2003: from <1.5 to <1.0 pmol/L and from 2007 <0.6 pmol/L). From 2015, calcitonin was analyzed using Roche COBAS® Modul E at OUH (detection limit <0.3 pmol/L or <1.0 pg/mL). Reference values were <2.1 and <3.1 pmol/L at OUH and <1.6 and <2.2 pmol/L at HUH in females and males, respectively. In the present study, 3.0 pmol/L was used as the cutoff value for both sexes. After total thyroidectomy, biochemical cure was defined as no calcitonin detected with the assay actually used, and after lobectomy as calcitonin <3.0 pmol/L. The conversion factor to pg/mL is 0.2926. Stimulated calcitonin analyses were performed in only 11 (12%) study patients and are not reported.
MTC stage was classified according to the 7th edition of the American Joint Committee of Cancer (AJCC) tumor-node-metastasis (TNM) classification (UICC 2010), the current classification during data collection [21]. At primary surgery, pTNM was defined as the total pTNM after primary surgery. When indicating the MTC stage, clinically unknown preoperative metastatic status (Mx) and no lymph node dissection (Nx) were classified as no metastasis (M0) and no metastatic lymph nodes (pN0) in the patients who achieved biochemical cure after primary surgery.
Statistical Analysis
Data were analyzed using SPSS software (SPSS for Windows, version 25). For continuous variables not following normal distribution, a nonparametric test for independent samples (Mann-Whitney U test) was used. Group differences and associations between categorical variables were analyzed using the Pearson χ2 2-sided test. Factors predicting postoperative biochemical cure, preoperative calcitonin level, and disease in the lateral neck were explored in multivariate analysis using logistic regression. Statistical significance was set at p < 0.05.
Results
Preoperative Calcitonin Levels Related to Clinical and Histopathological Data
Table 1 presents clinical and histopathological results related to preoperative calcitonin levels in 94 study patients. Extent of surgery, number of surgical procedures at primary surgery, tumor load, and tumor stage increased significantly with raising calcitonin levels (p < 0.001, p = 0.007, and p = 0.002). Ultrasound of the neck was performed before primary surgery in all study patients. At preoperative basal calcitonin levels ≤500, 501–1,000, 1,001–3,000, and >3,000 pmol/L, metastatic lymph nodes in the lateral neck were found in 16, 50, 58, and 100% of the patients, respectively. Of all the patients with metastatic lymph nodes in the lateral neck, >50% had preoperative calcitonin >1,000 pmol/L. Furthermore, 50% of the patients with clinical distant metastases had calcitonin level ≤3,000 pmol/L, and of the patients with calcitonin level >3,000 pmol/L, 50% were without distant metastases. In the 50 patients with calcitonin ≤500 pmol/L, metastatic lymph nodes in the lateral neck were found in 8 (16%) and distant metastases in 2 (4%) of the patients. Five (10%) patients died of MTC.
Table 1.
Clinical and histopathological data related to preoperative calcitonin in 94 MTC patients1 surgically treated in 2003–2016
| Total | Serum calcitonin before surgery (median) [range], pmol/L |
P value | |||||
|---|---|---|---|---|---|---|---|
| ≤20 (4.7) [1.1–20] | 21–500 (152) [23–474] | 501–1,000 (790) [528–1,000] | 1,001-3,000 (1,650) [1,020–2,450] | >3,000 (4,878) [3,010–9,890] | |||
| Patients (total),n | 13 | 37 | 16 | 19 | 9 | ||
| Gender | |||||||
| Female | 8 (62%) | 22 (59%) | 10 (63%) | 11 (58%) | 2 (22%) | 0.307 | |
| Male | 5 (38%) | 15 (41%) | 6 (37%) | 8 (42%) | 7 (78%) | ||
| Median age at thyroid surgery, years | 50 | 58 | 56 | 62 | 53 | 0.317 | |
| Range | 6–72 | 13–82 | 28–79 | 32–83 | 16–81 | ||
| Ultrasound of the neck performed | |||||||
| before primary surgery | 13 (100%) | 37 (100%) | 16 (100%) | 19 (100%) | 9 (100%) | N/A | |
| Primary surgery | |||||||
| Lobectomy ± ipsilateral CND/LND2 | 1 (8%) | 4 (11%) | 2 (12.5%) | 2 (10.5%) | 1 (11%) | ||
| TT | 2 (15%) | 0 | 0 | 0 | 0 | <0.001 | |
| TT + CND | 9 (69%) | 23 (62%) | 4 (15%) | 6 (31.5%) | 0 | ||
| TT + CND + LND | 1 (8%) | 10 (27%) | 10 (62.5%) | 11 (58%) | 8 (89%) | ||
| Surgical procedures | |||||||
| 1 | 12 (92%) | 35 (95%) | 11 (69%) | 12 (63%) | 5 (56%) | 0.007 | |
| ≥2 | 1 (8%) | 2 (5%) | 5 (31%) | 7 (37%) | 4 (44%) | ||
| Tumor diameter, mm | |||||||
| Available data,n | 90 | 12 | 36 | 15 | 18 | 9 | |
| Median [range] | 2.8 [1–33] | 17 [3–78] | 23 [4–58] | 35 [5–80] | 50 [23–125] | <0.001 | |
| Extrathyroidal extension | |||||||
| Available data,n | 91 | 12 | 37 | 15 | 18 | 9 | |
| Not present | 11 (92%) | 28 (76%) | 8 (53%) | 9 (50%) | 1 (11%) | ||
| Minimal/muscle/soft tissue | 1 (8%) | 8 (22.5%) | 7 (47%) | 6 (33%) | 4 (44.5%) | <0.001 | |
| Esophagus/larynx/vessel/nerve | 0 | 1 (2.5%) | 0 | 3 (17%) | 4 (44.5%) | ||
| Nodal status at primary surgery | |||||||
| pN0 | 10 (77%)3 | 24 (25%) | 3 (19%) | 4 (21%) | 0 | ||
| pN1a | 2 (15%) | 6 (16%) | 5 (31%) | 4 (21%) | 0 | <0.001 | |
| pN1b ipsilateral | 1 (8%) | 5 (13.5%) | 5 (31%) | 9 (47%) | 5 (66%) | ||
| pN1b bilateral | 0 | 2 (5.5%) | 3 (19%) | 2 (11%) | 4 (44%) | ||
| Metastatic lymph nodes | |||||||
| Available data,n | 88 | 10 | 37 | 14 | 18 | 9 | |
| Median [range] | 0 [0–13] | 0 [0–21] | 4 [0–36] | 8 [0–34] | 22 [9–43] | <0.001 | |
| Metastatic status at primary surgery | |||||||
| Available data,n | 82 | 13 | 32 | 13 | 16 | 8 | |
| M0 | 13 (100%) | 30 (94%) | 12 (92%) | 15 (94%) | 4 (50%) | 0.002 | |
| M1 | 0 | 2 (6%) | 1 (8%) | 1 (6%) | 4 (50%) | ||
| Stage at primary surgery4 | |||||||
| Available data,n | 87 | 13 | 32 | 15 | 18 | 9 | |
| I | 10 (77%) | 17 (53%) | 0 | 1 (5.5%) | 0 | ||
| II | 0 | 4 (13%) | 3 (20%) | 3 (17%) | 0 | <0.001 | |
| III | 2 (15%) | 3 (9%) | 4 (27%) | 2 (11%) | 0 | ||
| IV | 1 (8%) | 8 (25%) | 8 (53%) | 12 (66.5%) | 9 (100%) | ||
| Biochemical cure7 | |||||||
| Postoperatively | 12 (92%) | 22 (61%)5 | 5 (33%) | 5 (26%) | 1 (11%) | <0.001 | |
| Latest MTC-specific follow-up | 10 (77%) | 20 (56%)5 | 3 (20%) | 5 (26%)6 | 0 | <0.001 | |
| Died of MTC | 1 (8%) | 4 (11%) | 0 | 1 (5%) | 3 (33%) | 0.088 | |
| MTC-specific follow-up, months | |||||||
| Median [range]7 | 74 [2–142] | 38 [1–119] | 50 [3–158] | 20 [2–134] | 29 [5–65] | 0.155 | |
TT, total thyroidectomy; CND, lymph node dissection in the central neck; LND, lymph node dissection in the lateral neck; N/A, not applicable.
Only patients with available data were included in the analysis.
CND ipsilateral in 9 patients and LND ipsilateral in 2 patients.
Three patients with no lymph node dissection (Nx) achieved biochemical cure after primary surgery (classified as pN0).
Unknown preoperative metastatic status (Mx) and no lymph node dissection (Nx) were classified as clinically no metastasis (M0) and no metastatic lymph nodes (pN0) in the patients who achieved biochemical cure after completed primary surgery.
Information about biochemical cure was not available in 1 patient.
One patient had calcitonin 0.4 pmol/L at the latest follow-up and included as biochemically cured.
Available data: n = 93.
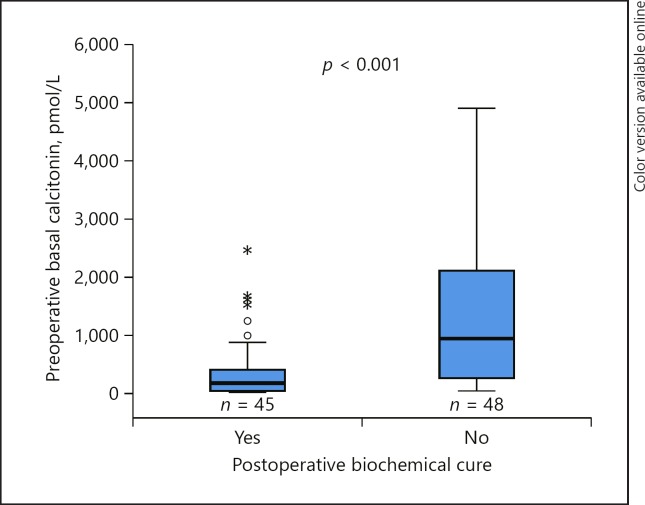
Less than 50% of the patients experienced postoperative biochemical cure at preoperative calcitonin >500 pmol/L. In the patients with calcitonin level 21–500 pmol/L, 22 (61%) of 36 patients were biochemically cured. The median preoperative calcitonin level in this group was 162 pmol/L (range 23–384) and 106 pmol/L (range 25–474) in the cured versus noncured patients, and the difference was not statistically significant (p = 0.490). Figure 2 illustrates the significant difference between preoperative calcitonin and postoperative biochemical cure in the study population (p < 0.001).
Fig. 2.
Postoperative biochemical cure related to preoperative basal calcitonin in 93 patients with available data (p < 0.001). One and 3 patients had calcitonin >6,000 pmol/L in the cured and noncured patients: 8,630 and 6,900, 7,130, and 9,890 pmol/L, respectively (not marked).
Prophylactic lymph node dissection in the lateral neck was performed in 6 patients with no evidence of metastatic lymph nodes in the lateral neck: 4 in ipsilateral and 2 in the ipsi-and contralateral lateral neck.
Prognostic Groups
Table 2 presents clinical- and histopathological results related to prognostic groups in 58 patients. Due to the small number of patients, pN1b was not differentiated into ipsilateral and ipsi- and contralateral lateral neck. Ten of 12 patients (83%) with pN1b status had preoperative calcitonin >500 pmol/L. However, preoperative calcitonin >500 pmol/L was found in 7 of 37 patients (19%) with no metastatic lymph nodes at primary surgery or clinical recurrence during follow-up.
Table 2.
Clinical and histopathological data related to prognostic groups in 58 patients with M01
| Variables | N0-NPNL | N1a-NPNL | N1b-NPNL2 | p value |
|---|---|---|---|---|
| Patients,n (%) | 37 (63) | 9 (16) | 12 (20) | |
| Gender,n (%) | ||||
| Female | 24 (65) | 5 (56) | 6 (50) | 0.625 |
| Male | 13 (35) | 4 (44) | 6 (50) | |
| Age at thyroid surgery, years | ||||
| Median [range] | 53 [6–80] | 59 [29–77] | 62.5 [13–82] | 0.218 |
| Preoperative serum calcitonin | ||||
| Median [range], pmol/L | 146 [1.1–2,450] | 554 [4.0-2,100] | 1,355 [271–8,630] | <0.001 |
| Patients,n (%) | ||||
| ≤20 pmol/L | 10 (27) | 2 (22) | 0 | |
| 21–500 pmol/L | 20 (54) | 2 (22) | 2 (16.5) | |
| 501–1,000 pmol/L | 3 (8) | 4 (34) | 2 (16.5) | 0.001 |
| 1,001–3,000 pmol/L | 4 (11) | 2 (22) | 5 (42) | |
| >3,000 pmol/L | 0 | 0 | 3 (25) | |
| Tumor diameter, mm | ||||
| Median [range] | 17 [1–45] | 23 [6–58] | 27.5 [8–80] | 0.002 |
| Patients,n (%) | ||||
| ≤20 mm | 28 (76) | 4 (44) | 3 (25) | 0.004 |
| >20 mm | 9 (34) | 5 (56) | 9 (75) | |
| Extrathyroidal extension, n (%) | ||||
| Not present | 35 (97) | 9 (100) | 3 (25) | <0.001 |
| Present | 1 (3) | 0 | 9 (75) | |
| Available data (n =57) | 36 | 9 | 12 | |
| Number of metastatic lymph nodes | ||||
| Median [range] | 0 | 1 [1–6] | 12 [3–33] | <0.001 |
| Biochemical cure3, n (%) | 35 (95) | 6 (67) | 2 (17) | <0.001 |
| Available data (n =57) | 36 | 9 | 12 | |
Univariate analysis. NPNL, no clinical progression to next tumor level.
Patients with Mx or M1 at primary surgery or progression to the next clinical tumor level were excluded.
Ipsi- and contralateral pN1b in 1 patient and ipsilateral pN1b in 11 patients.
After completed primary surgery.
Multivariate logistic regression analyses were performed to evaluate correlations between postoperative biochemical cure, preoperative calcitonin levels, metastatic lymph nodes in the lateral neck, tumor diameter, and extrathyroidal extension (Table 3). Lack of metastatic lymph nodes in the lateral neck was the only independent predictive factor for biochemical cure (p = 0.030; odds ratio, OR, 17.9; 95% confidence interval, CI, 1.33–241.4). Using calcitonin as a dependent variable showed that tumor diameter ≤20 mm was independently predictive for calcitonin level ≤500 pmol/L (p < 0.001; OR 48.9; 95% CI 7.4–322.9). Finally, factors related to metastatic lymph nodes in the lateral neck were extrathyroidal extension (p = 0.007; OR 97.2; 95% CI 3.49–2,705.3) and no biochemical cure (p = 0.028; OR 18.7; 95% CI 1.38–252.4).
Table 3.
Correlations between postoperative biochemical cure, preoperative levels of calcitonin, metastatic lymph nodes in the lateral neck, tumor diameter, and extrathyroidal extension in 56 (97%) of 58 patients with M01
| Variables Dependant and Independent | Patients, n | OR (95% CI) | p value | |
|---|---|---|---|---|
| Biochemical cure2 Preoperative calcitonin level | yes vs. no ≤500 vs. >500 pmol/L | 42 vs. 14 34 vs. 22 | 3.64 (0.24–56.3) | 0.355 |
| Tumor diameter | ≤20 vs. >20 mm | 33 vs. 23 | 1.098 (0.074–16.3) | 0.946 |
| Extrathyroidal extension | no vs. yes | 46 vs. 10 | 2.21 (0.12–41.5) | 0.595 |
| Prognostic groups | N0/N1a-NPNL vs. N1b-NPNL3 | 44 vs. 12 | 17.9 (1.33–241.4) | 0.030 |
| Preoperative calcitonin level | ≤500 vs. >500 | 34 vs. 22 | ||
| Prognostic groups | pmol/L N0/N1a-NPNL vs. N1b-NPNL3 | 44 vs. 12 | 2.46 (0.095–63.7) | 0.587 |
| Tumor diameter | ≤20 vs. >20 mm | 33 vs. 23 | 48.9 (7.4–322.9) | <0.001 |
| Extrathyroidal extension | no vs. yes | 46 vs. 10 | 3.53 (0.11–111.5) | 0.474 |
| Biochemical cure2 | yes vs. no | 42 vs. 14 | 4.15 (0.32–53.8) | 0.277 |
| Prognostic groups | N0/N1a-NPNL vs. N1b-NPNL3 | 44 vs. 12 | ||
| Preoperative calcitonin level | <500 vs. >500 pmol/L | 34 vs. 22 | 3.37 (0.019–606.5) | 0.647 |
| Tumor diameter | ≤20 vs. >20 mm | 33 vs. 23 | 1.37 (0.007–252.8) | 0.907 |
| Extrathyroidal extension | no vs. yes | 46 vs. 10 | 97.2 (3.49–2,705.3) | 0.007 |
| Biochemical cure2 | yes vs. no | 42 vs. 14 | 18.7 (1.38–252.4) | 0.028 |
Logistic regression, in 3 analysis, with biochemical cure, preoperative levels of calcitonin, and prognostic groups as dependent variables. CI, confidence interval; NPNL, no clinical progression to next tumor level.
Patients with Mx or M1 at primary surgery or progression to next clinical tumor level were excluded.
After completed primary surgery.
Ipsi- and contralateral pN1b in 1 patient and ipsilateral pN1b in 11 patients.
Discussion
Preoperative calcitonin levels are significantly related to the extent of disease, and metastatic lymph nodes in the lateral neck have negative prognostic impact. However, the present study does not predict a clear cutoff calcitonin value forecasting the extent of surgery needed in the lateral neck. Hence, in patients with MTC and without distant metastases, other factors influence the calcitonin level in addition to the extent of disease in the neck.
Major studies have been carried out in order to identify the calcitonin cutoff level that could predict prophylactic lymph node dissection in the lateral neck [12, 22]. The American Thyroid Association guidelines (2015) recommend consideration of prophylactic lymph node dissection in the lateral neck based on serum calcitonin levels in patients without distant metastases, but the Task Force did not achieve consensus regarding this recommendation [3].
Guidelines from the United Kingdom recommend ipsilateral prophylactic lateral lymph node dissection based on the presence of metastatic lymph nodes in the central neck [14]. Machens et al. [18] found that the presence of 1–3 metastatic lymph nodes in the central neck compartment, or >4 metastatic lymph nodes, predicted the risk of metastatic lymph nodes in the ipsilateral lateral neck in 77 and 98%, respectively. In the present study, the median number of metastatic lymph nodes in the N1a-NPNL group was 1 (range 1–6) (Table 2).
The present study found that extrathyroidal tumor extension was predictive of metastatic lymph nodes in the lateral neck. A study by Oh et al. [17] evaluated ultrasound features in primary tumors at preoperative neck ultrasound. Patients with larger tumors of irregular shape with spiculated margins and a subcapsular location were more likely to have metastatic lymph nodes in the lateral neck. These features can represent extrathyroidal extension.
Preoperative ultrasound of the neck in the evaluation of thyroid nodules was performed in all patients. In experienced hands, high-resolution ultrasonography is the most valuable examination for detecting metastatic lymph nodes in the lateral neck, in both papillary and medullary thyroid carcinoma [23, 24, 25]. However, a negative examination does not rule out a possible presence of micrometastases in the lymph nodes. Kocharyan et al. [25] found that preoperative ultrasonography of the lateral neck has a 85.4% positive predictive value in detecting metastatic lymph nodes in the lateral neck.
As patients with calcitonin ≤500 pmol/L had metastases in the lateral neck, whereas other patients with calcitonin >500 pmol/L, or even >1,000 pmol/L, had not (Tables 1, 2), the study could not offer strict guidance for when to perform lateral neck lymph node dissection. Lateral lymph node dissection includes the risk of surgical complications, with nerve injuries as the most severe among these [26, 27]. The dilemma then is the possible presence of undetectable micrometastases versus the risk of morbidity due to prophylactic lateral lymph node dissection. Hence, in patients without any sign of distant metastases, prophylactic lateral lymph node dissection should be considered as a second operation if biochemical cure is not achieved after primary surgery. The extension of surgical resection has to be planned according to the preoperative lymph node status in the central and lateral compartment [28]. Furthermore, postponed surgery of the lateral compartment is a valid option in order to limit unnecessary complications without affecting adequate lymph node clearance. However, the level of preoperative calcitonin together with the assessment of nodal involvement in the central compartment compensate the diagnostic limits of ultrasound [28].
The present study found a significant independent relation between tumor diameter <20 mm and preoperative calcitonin <500 pmol/L. Furthermore, 14 (30%) of the 46 patients in the prognostic groups without metastatic lymph nodes in the lateral neck had tumor diameter >20 mm. Desmoplastic stromal reaction (DSR) is today not a part of regular evaluation of the histological specimens. However, studies have evaluated DSR as a possible predictor for disease aggressiveness and lymph node metastases in the lateral neck [29, 30]. A speculation might be if larger tumors without DSR by itself have high calcitonin values, and the calcitonin level is not reflecting the extent of the disease in the lateral neck but the tumor diameter in these patients.
Advanced MTC with low preoperative calcitonin was found in the present study population. Dedifferentiation in advanced MTC with loss of ability to produce calcitonin has been reported [31].
More than 50% of the patients with preoperative calcitonin level 21–500 pmol/L, median 152 pmol/L, achieved postoperative biochemical cure, with no significant difference between the cured and noncured patients in this group. Machens et al. [16] showed that preoperative basal calcitonin levels >500 pg/mL (146 pmol/L) best predicted the failure to achieve biochemical remission.
Clinical data collection combined with data from the Cancer Registry of Norway made this study possible and complete with minimal to absent selection bias. This represents a significant strength of the present study. However, there are also certain limitations, such as incomplete records. Further, as there was no preoperative calcitonin analysis in 28% (39/139) of the surgically treated patients, these patients were excluded from the study. Hence, the possibility of reduced power and diagnostic selection bias exist, as patients with MTC randomly discovered might have less severe disease. However, the variation in correlations between preoperative calcitonin and extent of disease in the present and included study population, remains valid. Furthermore, in the multivariate analysis, there are low numbers in some variables, and statistically type two error might occur.
In conclusion, the present study found that the preoperative calcitonin variation did not consistently relate to tumor stage and metastatic lymph nodes in the lateral neck. Calcitonin is therefore not an optimal marker in predicting the need for prophylactic lateral lymph node dissection. However, prophylactic lateral lymph node dissection should be considered in a second séance when biochemical cure is not achieved after primary surgery in patients without systemic disease. Further, prospective randomized studies are needed to find appropriate predictors for the need of prophylactic lymph node dissection in the lateral neck.
Statement of Ethics
The Regional Committee for Medical and Health Research Ethics (REC) of Western Norway approved the study (case No. 2013/1499). All of the included patients or their parents, if the patients were children, gave written, informed consent. Furthermore, REC granted permission to include deceased patients.
Disclosure Statement
This work was not funded by any organization and was not supported by any grants. There are no conflicts of interest and no competing financial interest exists for any of the authors.
Acknowledgment
We acknowledge the Norwegian Radium Hospital's Endowments for Research at the Oslo University Hospital for providing research grants to cover travel expenses related to the data collection.
References
- 1.Kebebew E, Ituarte PH, Siperstein AE, Duh QY, Clark OH. Medullary thyroid carcinoma: clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer. 2000 Mar;88((5)):1139–48. doi: 10.1002/(sici)1097-0142(20000301)88:5<1139::aid-cncr26>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 2.Pelizzo MR, Boschin IM, Bernante P, Toniato A, Piotto A, Pagetta C, et al. Natural history, diagnosis, treatment and outcome of medullary thyroid cancer: 37 years experience on 157 patients. Eur J Surg Oncol. 2007 May;33((4)):493–7. doi: 10.1016/j.ejso.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 3.Wells SA, Jr, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, et al. American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015 Jun;25((6)):567–610. doi: 10.1089/thy.2014.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Opsahl EM, Akslen LA, Schlichting E, Aas T, Brauckhoff K, Hagen AI, et al. Trends in Diagnostics, Surgical Treatment, and Prognostic Factors for Outcomes in Medullary Thyroid Carcinoma in Norway: A Nationwide Population-Based Study. Eur Thyroid J. 2019 Jan;8((1)):31–40. doi: 10.1159/000493977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donis-Keller H, Dou S, Chi D, Carlson KM, Toshima K, Lairmore TC, et al. Mutations in the RET proto-oncogene are associated with MEN 2A and FMTC. Hum Mol Genet. 1993 Jul;2((7)):851–6. doi: 10.1093/hmg/2.7.851. [DOI] [PubMed] [Google Scholar]
- 6.Eng C, Smith DP, Mulligan LM, Nagai MA, Healey CS, Ponder MA, et al. Point mutation within the tyrosine kinase domain of the RET proto-oncogene in multiple endocrine neoplasia type 2B and related sporadic tumours. Hum Mol Genet. 1994 Feb;3((2)):237–41. doi: 10.1093/hmg/3.2.237. [DOI] [PubMed] [Google Scholar]
- 7.de Groot JW, Plukker JT, Wolffenbuttel BH, Wiggers T, Sluiter WJ, Links TP. Determinants of life expectancy in medullary thyroid cancer: age does not matter. Clin Endocrinol (Oxf) 2006 Dec;65((6)):729–36. doi: 10.1111/j.1365-2265.2006.02659.x. [DOI] [PubMed] [Google Scholar]
- 8.Gatta G, Ciccolallo L, Kunkler I, Capocaccia R, Berrino F, Coleman MP, et al. EUROCARE Working Group Survival from rare cancer in adults: a population-based study. Lancet Oncol. 2006 Feb;7((2)):132–40. doi: 10.1016/S1470-2045(05)70471-X. [DOI] [PubMed] [Google Scholar]
- 9.Modigliani E, Cohen R, Campos JM, Conte-Devolx B, Maes B, Boneu A, et al. Prognostic factors for survival and for biochemical cure in medullary thyroid carcinoma: results in 899 patients. The GETC Study Group. Groupe d'étude des tumeurs à calcitonine. Clin Endocrinol (Oxf) 1998 Mar;48((3)):265–73. doi: 10.1046/j.1365-2265.1998.00392.x. [DOI] [PubMed] [Google Scholar]
- 10.Torresan F, Cavedon E, Mian C, Iacobone M. Long-Term Outcome After Surgery for Medullary Thyroid Carcinoma: A Single-Center Experience. World J Surg. 2018 Feb;42((2)):367–75. doi: 10.1007/s00268-017-4321-z. [DOI] [PubMed] [Google Scholar]
- 11.Randle RW, Balentine CJ, Leverson GE, Havlena JA, Sippel RS, Schneider DF, et al. Trends in the presentation, treatment, and survival of patients with medullary thyroid cancer over the past 30 years. Surgery. 2017 Jan;161((1)):137–46. doi: 10.1016/j.surg.2016.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Machens A, Dralle H. Biomarker-based risk stratification for previously untreated medullary thyroid cancer. J Clin Endocrinol Metab. 2010 Jun;95((6)):2655–63. doi: 10.1210/jc.2009-2368. [DOI] [PubMed] [Google Scholar]
- 13.Dralle H, Machens A. Surgical management of the lateral neck compartment for metastatic thyroid cancer. Curr Opin Oncol. 2013 Jan;25((1)):20–6. doi: 10.1097/CCO.0b013e328359ff1f. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell AL, Gandhi A, Scott-Coombes D, Perros P. Management of thyroid cancer: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016 May;130(S2):S150–60. doi: 10.1017/S0022215116000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elisei R, Bottici V, Luchetti F, Di Coscio G, Romei C, Grasso L, et al. Impact of routine measurement of serum calcitonin on the diagnosis and outcome of medullary thyroid cancer: experience in 10,864 patients with nodular thyroid disorders. J Clin Endocrinol Metab. 2004 Jan;89((1)):163–8. doi: 10.1210/jc.2003-030550. [DOI] [PubMed] [Google Scholar]
- 16.Machens A, Schneyer U, Holzhausen HJ, Dralle H. Prospects of remission in medullary thyroid carcinoma according to basal calcitonin level. J Clin Endocrinol Metab. 2005 Apr;90((4)):2029–34. doi: 10.1210/jc.2004-1836. [DOI] [PubMed] [Google Scholar]
- 17.Oh HS, Kwon H, Song E, Jeon MJ, Song DE, Kim TY, et al. Preoperative Clinical and Sonographic Predictors for Lateral Cervical Lymph Node Metastases in Sporadic Medullary Thyroid Carcinoma. Thyroid. 2018 Mar;28((3)):362–8. doi: 10.1089/thy.2017.0514. [DOI] [PubMed] [Google Scholar]
- 18.Machens A, Hauptmann S, Dralle H. Prediction of lateral lymph node metastases in medullary thyroid cancer. Br J Surg. 2008 May;95((5)):586–91. doi: 10.1002/bjs.6075. [DOI] [PubMed] [Google Scholar]
- 19.Brauckhoff M, Machens A, Lorenz K, Bjøro T, Varhaug JE, Dralle H. Surgical curability of medullary thyroid cancer in multiple endocrine neoplasia 2B: a changing perspective. Ann Surg. 2014 Apr;259((4)):800–6. doi: 10.1097/SLA.0b013e3182a6f43a. [DOI] [PubMed] [Google Scholar]
- 20.Opsahl EM, Brauckhoff M, Schlichting E, Helset K, Svartberg J, Brauckhoff K, et al. A Nationwide Study of Multiple Endocrine Neoplasia Type 2A in Norway: Predictive and Prognostic Factors for the Clinical Course of Medullary Thyroid Carcinoma. Thyroid. 2016 Sep;26((9)):1225–38. doi: 10.1089/thy.2015.0673. [DOI] [PubMed] [Google Scholar]
- 21.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. 7th ed. New York (NY): Springer-Verlag; 2010. AJCC Cancer Staging Manual. [Google Scholar]
- 22.Chandeze MM, Noullet S, Faron M, Trésallet C, Godiris-Petit G, Tissier F, et al. Can We Predict the Lateral Compartment Lymph Node Involvement in RET-Negative Patients with Medullary Thyroid Carcinoma? Ann Surg Oncol. 2016 Oct;23((11)):3653–9. doi: 10.1245/s10434-016-5292-2. [DOI] [PubMed] [Google Scholar]
- 23.Leenhardt L, Erdogan MF, Hegedus L, Mandel SJ, Paschke R, Rago T, et al. 2013 European thyroid association guidelines for cervical ultrasound scan and ultrasound-guided techniques in the postoperative management of patients with thyroid cancer. Eur Thyroid J. 2013 Sep;2((3)):147–59. doi: 10.1159/000354537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stulak JM, Grant CS, Farley DR, Thompson GB, van Heerden JA, Hay ID, et al. Value of preoperative ultrasonography in the surgical management of initial and reoperative papillary thyroid cancer. Arch Surg. 2006 May;141((5)):489–94. doi: 10.1001/archsurg.141.5.489. [DOI] [PubMed] [Google Scholar]
- 25.Kocharyan D, Schwenter F, Bélair M, Nassif E. The relevance of preoperative ultrasound cervical mapping in patients with thyroid cancer. Can J Surg. 2016 Apr;59((2)):113–7. doi: 10.1503/cjs.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polistena A, Di Lorenzo P, Sanguinetti A, Buccelli C, Conzo G, Conti A, Niola M, Avenia N. Medicolegal implications of surgical errors and complications in neck surgery: A review based on the Italian current legislation. Open medicine (Warsaw, Poland) 2016;11:298–306. doi: 10.1515/med-2016-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polistena A, Monacelli M, Lucchini R, Triola R, Conti C, Avenia S, et al. Surgical morbidity of cervical lymphadenectomy for thyroid cancer: A retrospective cohort study over 25 years. Int J Surg. 2015 Sep;21:128–34. doi: 10.1016/j.ijsu.2015.07.698. [DOI] [PubMed] [Google Scholar]
- 28.Polistena A, Sanguinetti A, Lucchini R, Galasse S, Monacelli M, Avenia S, et al. Timing and extension of lymphadenectomy in medullary thyroid carcinoma: A case series from a single institution. Int J Surg. 2017 May;41(Suppl 1):S70–4. doi: 10.1016/j.ijsu.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 29.Koperek O, Bergner O, Pichlhöfer B, Oberndorfer F, Hainfellner JA, Kaserer K, et al. Expression of hypoxia-associated proteins in sporadic medullary thyroid cancer is associated with desmoplastic stroma reaction and lymph node metastasis and may indicate somatic mutations in the VHL gene. J Pathol. 2011 Sep;225((1)):63–72. doi: 10.1002/path.2926. [DOI] [PubMed] [Google Scholar]
- 30.Scheuba C, Kaserer K, Kaczirek K, Asari R, Niederle B. Desmoplastic stromal reaction in medullary thyroid cancer-an intraoperative “marker” for lymph node metastases. World J Surg. 2006 May;30((5)):853–9. doi: 10.1007/s00268-005-0391-4. [DOI] [PubMed] [Google Scholar]
- 31.Brutsaert EF, Gersten AJ, Tassler AB, Surks MI. Medullary thyroid cancer with undetectable serum calcitonin. J Clin Endocrinol Metab. 2015 Feb;100((2)):337–41. doi: 10.1210/jc.2014-3095. [DOI] [PubMed] [Google Scholar]