Abstract
Background
Patients with undiagnosed hypothyroidism are not treated for the disease and are at high risk of developing serious complications, with major impact on public health. There is a need to systematically review the available evidence on this topic.
Objective
To identify the prevalence of undiagnosed hypothyroidism in Europe.
Methods
A systematic review of the literature (Medline, EMBASE, and Cochrane Central) was performed to identify epidemiological studies on the prevalence of undiagnosed hypothyroidism among European populations published between January 2008 and April 2018. The Newcastle-Ottawa Scale was used to assess the methodological quality of the included studies. Random-effects meta-analyses were performed to pool estimates of proportions (with 95% confidence intervals [CIs]) of undiagnosed (1) subclinical, (2) overt, and (3) total hypothyroidism.
Results
The search returned 15,565 citations (4,526 duplicates). Twenty papers were included in the study. Fourteen and 6 studies were of good and moderate methodological quality, respectively. The results of the meta-analyses were as follows for the prevalence of undiagnosed hypothyroidism: subclinical, 4.11% (95% CI 3.05–5.31%, I<sup>2</sup> = 99.32%); overt, 0.65% (95% CI 0.38–0.99%, I<sup>2</sup> = 96.67%); and total, 4.70% (95% CI 2.98–6.79%, I<sup>2</sup> = 99.53%). According to the sensitivity analysis, the prevalence of hypothyroidism tends to be higher in female patients, in those aged ≥65 years, among studies with lower sample sizes, in those with thyroid-stimulating hormone levels <4.5 mIU/L, and in Eastern and Southern Europe.
Conclusions
The current evidence suggests that a considerable proportion of the European population has hypothyroidism, particularly subclinical hypothyroidism, which is undiagnosed. This issue deserves further investigation because of possible deleterious consequences for public health.
Keywords: Undiagnosed hypothyroidism, Subclinical hypothyroidism, Prevalence, Systematic review, Meta-analysis
Introduction
Hypothyroidism is a condition of thyroid hormone deficiency which is essentially defined based on biochemical parameters. Overt hypothyroidism is the combination of an elevated level of serum thyroid-stimulating hormone (TSH) with a decreased level of serum free thyroxin (fT4) as compared to the reference ranges in the general population. Subclinical hypothyroidism is defined as an elevated serum TSH level in combination with a normal serum fT4 level [1, 2]. The reference ranges for TSH and fT4 currently used to define thyroid dysfunction are subject of discussion because of the arbitrary nature of the cutoffs. This issue is of clinical importance for diagnosis and treatment decision purposes [1, 3].
The prevalence of hypothyroidism varies considerably across the general population. There is a number of factors that can influence the prevalence of this condition. For example, the occurrence of hypothyroidism is affected by differences in the iodine status between populations, with higher prevalence among those with high iodine intake and in severely iodine-deficient populations [4]. The prevalence of hypothyroidism is also higher in women, in senior populations, and in Caucasian individuals [5, 6, 7, 8, 9]. Furthermore, there is an increased risk of hypothyroidism in patients with autoimmune diseases, including diabetes mellitus, rheumatoid arthritis, or systemic lupus erythematosus, and also in patients with other conditions, such as HIV infection [10, 11, 12, 13, 14].
Several studies have been conducted to estimate the prevalence of hypothyroidism. The estimates can vary between 0.1 and 12.5%, depending on the definition used [15, 16]. For example, some studies evaluated the prevalence of undiagnosed and untreated subclinical and/or overt hypothyroidism [17, 18, 19], while others addressed previously diagnosed and treated hypothyroidism [20, 21]. Patients with undiagnosed hypothyroidism are not treated for the disease and therefore might be at higher risk of developing long-term complications, such as serious and even fatal cardiovascular diseases, diabetes, or others [22, 23, 24, 25]. This situation may result in important implications for public health. Although a systematic review with a meta-analysis on the European prevalence and incidence of (undiagnosed and diagnosed) thyroid dysfunction has been published, that work considered only studies that reported simultaneously on both hypo- and hyperthyroidism. This approach led to the exclusion of studies that exclusively addressed hypothyroidism. Furthermore, several studies were concluded after the publication of that meta-analysis [26]. Therefore, there is rationale to perform a systematic review and meta-analysis aiming to determine the prevalence of undiagnosed hypothyroidism in Europe.
Methods
This study conforms to standard guidelines and was written according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [27]. Online supplementary Table 1 (for all online suppl. material, see www.karger.com/doi/10.1159/000499751) presents the PRISMA checklist.
Search Strategy
The Medline, EMBASE, and Cochrane Central databases were searched for articles published between January 2008 and April 2018. Bibliographic reference lists of all relevant studies, meta-analyses, and systematic reviews were hand searched to identify additional eligible articles. The electronic database search strategy is available in online supplementary Tables 2 and 3.
Study Selection
The titles and abstracts of all retrieved citations were screened by two independent reviewers (D.M. and C.A.) to identify potentially relevant publications. Full texts were retrieved for relevant citations. Discrepancies were resolved by majority decision (two of three) involving a third investigator (F.B.M.).
Studies were included if they met the following criteria: (1) based on a European population, (2) included participants from the general population (without age or sex restrictions) without previously known thyroid disease, (3) provided epidemiological data on the prevalence of subclinical, overt, and/or total hypothyroidism, and (4) provided definitions for subclinical and overt hypothyroidism according to laboratory measurements, namely TSH and fT4 levels. Studies involving only participants with underlying diseases (e.g., diabetes) or conditions (e.g., pregnancy) were excluded. Reviews, case reports, abstracts, and conference proceedings were excluded. No further exclusion criteria were applied.
Data Extraction
Data were extracted by two independent reviewers (D.M. and C.A.). The data retrieved from each study included first author's name, bibliographic reference, year of publication, country/region, study design, demographic characteristics (mean age, proportion of females, number of subjects included), prevalence of hypothyroidism, reference ranges for thyroid function tests and thyroid disorder categories, as well as assays used to measure thyroid function in each study.
Evaluation of Methodological Quality
The Newcastle-Ottawa Scale was used to assess the methodological quality of the included studies. This scale considers the following: selection of the study groups, comparability of the groups, and ascertainment of either the exposure (for case-control studies) or outcome of interest (for cohort studies) [28]. A maximum of 1 point for each item within the “selection” and “exposure/outcome” categories could be awarded. For the “comparability” category, a maximum of 2 points could be awarded. The summary score equals the number of points earned by each study, totaling a maximum of 9 (maximum of 8 points for cross-sectional analysis). An adapted form of the Newcastle-Ottawa Scale was used to assess cross-sectional studies [29]. Studies scoring ≥7 points were considered to be of good quality, those scoring <7 and ≥5 points were considered to be of moderate quality, and those scoring <5 points were considered to be of poor quality.
Statistical Analysis
The co-primary outcomes were the proportion of people with undiagnosed (1) subclinical, (2) overt, and (3) total hypothyroidism (i.e., the sum of subclinical and overt cases). The classification of cases as subclinical or overt hypothyroidism was the one provided by the authors of the studies included in this systematic review.
Pooled estimates of proportions with corresponding 95% confidence intervals (CIs) were calculated using the “exact” method within a random-effects model [30, 31]. Between-study heterogeneity was assessed using the χ2 test and the I2 measure of inconsistency [32]. An I2 estimate >50% was considered indicative of substantial heterogeneity. Publication bias was evaluated by Egger's regression asymmetry test and visually examined by a funnel plot [33]. All reported p values are two-sided with significance being set as <0.05. A sensitivity analysis was carried out to test the influence of sex (male vs. female), age (mean age <65 years vs. ≥65 years), TSH reference values for determining hypothyroidism (<4.5 mIU/L vs. ≥4.5 mIU/L), the sample size of the studies (number of patients <1,000 vs. 1,000–10,000 vs. >10,000), and geographic region (Northern, Southern, Western, and Eastern Europe) on the estimates of prevalence. All statistical analyses were performed in STATA version 13 (StataCorp, 2013).
Results
Included Studies
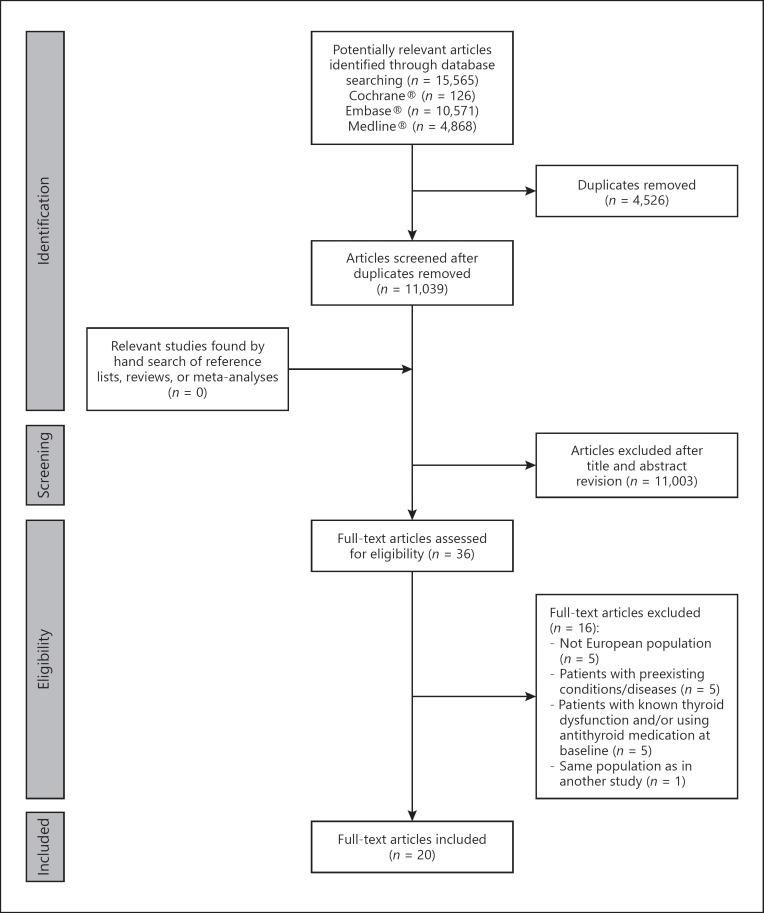
Figure 1 presents the search strategy flowchart. The search returned 15,565 citations. After excluding 4,526 duplicates and 11,003 studies based on revision of titles and abstracts, 36 full-text articles were assessed for eligibility. Of those, 20 articles were selected. One article reported results for two populations [15].
Fig. 1.
Flowchart of study selection in the systematic review.
Study Characteristics
Table 1 presents the main characteristics of the studies selected for systematic review. The sample size of selected studies varied between 307 [34] and 80,490 [35]. The studies included sample populations from Austria [35], Belgium [36], Bulgaria [37], Denmark [38], Germany [39, 40], Italy [16, 18, 41], Norway [15, 42], Spain [17, 19, 34, 43], The Netherlands [44, 45], the UK [46, 47], and Turkey [48].
Table 1.
Main characteristics of studies selected for systematic review
| Study (first author) | Location | Period | Design | Characteristics of the source population | Participants, n | Participants' age, years | Definition of hypothyroidism | Assays |
|---|---|---|---|---|---|---|---|---|
| Heeringa, 2008 [44]1 | Rotterdam, The Netherlands | 1990 | cross-sectional analysis within a prospective cohort study (The Rotterdam Study) | all residents of the Rotterdam suburb of Ommoord, The Netherlands, aged ≥55 years | T: 1,607; M: 632 (39.3%); F: 975 (60.7%) | median (range): 68 (55–93) | SH: TSH >4.5 and <19.9 mlU/L, fT 4 in the reference range1 | TSH: Lumitest, Henning, Berlin, Germany; fT4 Vitros ECi Immunodiagnostic System, Ortho-Clinical Diagnostics, Amersham, UK |
| Hogervorst, 2008 [46] | UK (England and Wales ) | 1998 | cross-sectional analysis within a prospective cohort study (MRC-CFAS) | subset of respondents originally recruited for the MRC-CFAS aged ≥65 years | T: 1,047; M: 513 (49.0%); F: 534 (51.0%) | mean ± SD: 73.6±6.2 | OH: TSH >4.8 mlU/L, fT4 <13 pmol/L; SH: TSH >4.8 IU/L, fT4 = 13–23 pmol/L | TSH: enzyme-amplified immunometric assay kit (Novobiolabs Ltd., Cambridge, UK); fT 4: analogue radioimmunoassay kit (Amerlex-M, Kodak, Amersham, UK) |
| Ceresini, 2009 [41]2 | Italy | 1998 | cross-sectional analysis | community-dwelling adults aged ≥65 years living in Tuscany, Italy | T: 1,171; M: 519 (44.3%); F: 652 (55.7%) | mean ± SD: 75.3±7.3 (800 euthyroid participants) | OH: TSH >4.68 mlU/L, fT4 <10.04 pmol/L; SH: TSH >4.68 mIU/L, fT4 = 9.91–28.19 pmol/L | TSH/fT4: chemiluminescent immunoassay (Vitros Reagent, Ortho-Clinical Diagnostics, Johnson and Johnson Medical Section, Milan, Italy) |
| Ittermann, 2010 [39]1 | Pomerania, Germany | 1997–2001 | cross-sectional analysis within a prospective cohort study | adults aged 20–79 years living in West Pomerania, Germany | T: 2,339; M: 1,148 (49.1%); F: 1,191 (50.9%) | median (range): 45 (20–85) | SH: TSH >4.5 and <19.9 mIU/L, fT 4 in the reference range1 | TSH/fT4: immunochemiluminescent procedures (LIA-mat, Byk Sangtec Diagnostica GmbH, Frankfurt, Germany) |
| Lucas,2010 [17]3 | Catalonia, Spain | 2001 | cross-sectional study within the Health Survey of Catalonia (based on interviewer-directed questionnaires with 165 items) | nonhospitalized adults aged 18–74 years living in Catalonia, Spain | T: 1,124; M: 500 (44.5%); F: 624 (55.5%) | mean ± SD: 44.8±15.2 | OH: TSH >4 mIU/L, fT4 <10.30 pmol/L; SH: TSH >4 mIU/L, fT4 = 10.30–24.46 pmol/L | TSH: Immulite Third Generation TSH Chemiluminescent Enzyme Immunoassay; fT4: Immulite fT4 Chemiluminescent Enzyme Immunoassay; all from DPC, Los Angeles, CA, USA |
| Asvold, 2011 [42] (HUNT 1) | Nord-Trøndelag, Norway | 1995–1997 | cross-sectional analysis within a survey (comprehensive questionnaires, clinical examination, blood sampling, and thyroid function measurements) | all adults living in Nord-Trøndelag county in Norway | T: 29,480; M: 9,769 (33.1%); F: 19,711 (66.9%) | median (range): 57 (41–98) | OH: TSH >4.0 mIU/L, fT4 <8.0 pmol/L; SH: TSH = 3.6–4.0 mIU/L, or TSH >4.0 mIU/L and fT4 ≥8.0 pmol/L | TSH: DELFIA hTSH Ultra Kit; fT4: DELFIA fT4 Kit; all from Wallac Oy, Turku, Finland |
| Schultz, 2011 [38] | Copenhagen, Denmark | 1998–2000 | cross-sectional analysis within a survey (comprehensive questionnaires, clinical examination, blood sampling, and thyroid function measurements) | participants aged 50–91 years recruited from the general population inFrederiksberg,Copenhagen, Denmark | T: 605; M: 253 (41.8%); F: 352 (58.2%) | mean ± SD: 68.0±10.7 | SH: TSH >4.0 mIU/L, fT4 in the reference range | TSH: immunoradiometric method (RIA-GNOST,Behringwerke AB, Marburg, Germany); fT4: solid-phase, chemiluminescent, competitive immunoassay (Immulite 2500) |
| de Jongh, 2011 [45] | The Netherlands | 1992–1993 | cross-sectional analysis within a prospective cohort study | individuals aged 55–85 years included in LASA | T: 1,219; M: 603 (49.5%); F: 616 (50.5%) | mean ± SD: 75.5±6.6 | SH: TSH >4.5 mIU/L, fT4 = 11–22 pmol/L, fT3 = 3.5–6.5 pmol/L | TSH: RIA (Centaur, BayerDiagnostics); fT4: competitive immunoassay (Centaur,BayerDiagnostics) |
| Dişel, 2012 [48]3 | Adana, Turkey | 2008–2009 | cross-sectional analysis within a case-control study (cancer patients vs. healthy people) | healthy people (age- and sexmatched control group for cancer patients) | T: 373; M: 198 (53.1%); F: 175 (46.9%) | mean (95% CI) = 55.6 (54.2–57.0) | OH: TSH >5.5 mIU/L, fT4 <9.52 pmol/L, fT 3 <2.96 pmol/L; SH: TSH >5.5 mIU/L, fT4 = 9.52–19.56 pmol/L, fT3 = 2.96–5.40 pmol/L | TSH/fT4: third-generationchemiluminescent immunoassay (ADVIA Centaur, Siemens Diagnostic) |
| Díez, 2012 [43] | Segovia, Spain | 2004–2010 | cross-sectional analysis within a case-control study (diabetic patients vs. healthy people) | control group in the study, patients without diabetes nor known thyroid diseases | T: 911; M: 332 (36.4%); F: 579 (63.6%) | mean ± SD: 57.4±16.1 | OH: TSH >5 mIU/L, fT4 <9 pmol/L; SH: TSH >5 mIU/L, normal fT4 | TSH/fT4: immunochemiluminescent assay (Immulite, Diagnostic Products, Los Angeles, CA, USA) |
| Resta, 2012 [16]3 | Bari, Italy | 2008–2010 | cross-sectional analysis | patients aged 64–86 years attending a geriatric service of the University of Bari | T: 337; M: 177 (52.5%); F: 160 (47.5%) | mean ± SD: 74.3±5.8 | OH: TSH >3.6 mIU/L, fT4 <10.30 pmol/L; SH: TSH >3.6 mIU/L, fT4 = 10.30–21.88 pmol/L | TSH/fT4: commercial kits (DiaSorin S.p.A., Saluggia, Italy) |
| Asvold, 2013 [15] (HUNT 2)2 | Nord-Trøndelag, Norway | 1995– 1997 | cross-sectional analysis within a survey (comprehensive questionnaires, clinical examination, blood sampling, and thyroid function measurements) | adult residents in the Nord-Trøndelag county | T: 33,917; M: 10,643 (31.4%); F: 23,274 (68.6%) | median (range): 49 (36–64) | OH: TSH >4.5 mIU/L, fT4 <8 pmol/L; SH: TSH >4.5 mIU/L, fT4 = 8–20 pmol/L | TSH: DELFIA hTSH Ultra Kit; fT4:DELFIA fT4 Kit; all from Wallac Oy, Turku, Finland |
| Asvold, 2013 [15] (HUNT 3)2 | Nord-Trøndelag, Norway | 2006–2008 | cross-sectional analysis within a survey (comprehensive questionnaires, clinical examination, blood sampling, and thyroid function measurements) | adult residents in the Nord-Trøndelag county | T: 49,180; M: 22,358 (45.5%); F: 26,822 (54.5%) | median (range): 53 (40–64) | OH: TSH >4.5 mlU/L, fT4 <8 pmol/L; SH: TSH>4.5 mIU/L, fT4 = 9–19 pmol/L | TSH: DELFIA hTSH Ultra Kit; fT4: DELFIA fT Kit; all from Wallac Oy, Turku, Finland |
| Delitala, 2014 [18]3 | Sardinia, Italy | 2001 | cross-sectional analysis within the Sardinian survey | participants in the Sardinian survey | T: 6,252; M: 2,826 (45.2%); F: 3,426 (54.8%) | median (range): 41.7 (28.8–57) | OH: TSH >4.0 mlU/L, fT4 <11.46 pmol/L; SH: TSH >4.0 mIU/L, fT4 = 11.46–22.65 pmol/L | TSH/fT4: automated chemiluminescence assay system (Immulite 2000, Erlangen, Germany) |
| Formiga, 2013 [34] | Barcelona, Spain | 2009 | cross-sectional analysis within a prospective cohort study | subjects from the follow-up prospective cohort study of participants from the OCTABAIX study | T: 307; M: 123 (45.4%); F: 184 (54.6%) | N/A | OH: TSH >5 mIU/L, fT4 <10 pmol/L; SH: TSH >5 mIU/L, fT4 = 10–26 pmol/L | TSH/fT4: electrochemiluminescence immunoassay based on the sandwich principle with MABs (Roche Diagnostics) |
| Kovar, 2015 [35]3 | Vienna, Austria | 1993–2004 | cross-sectional analysis within a retrospective cohort study | individuals aged ≥18 years admitted to the Institute of Medical and Chemical Laboratory Diagnostics of the Medical University of Vienna, Austria; fT4values within normal range | T: 80,490; M: 31,577 (39.2%); F: 48,913 (60.8%) | median (range): 48 (34–61) | SH: TSH >4.5 and >20.0 mIU/L, fT4= 9.01–21.88 pmol/L | TSH/fT4: electrochemiluminescence immunoassays “ECLIA” (Elecsys 2010 and Modular Analytics E170, respectively, Roche Diagnostics, Mannheim, Germany) |
| Ludwig, 2015 [40] | Leutkirch, Germany | 2002 | population-based cross-sectional study based on a standardized questionnaire and documentation of physical, biochemical, and ultrasonographic findings | individuals aged ≥18 years living in Leutkirch, Germany who participated in the EMIL study | T: 1,276; M: 674 (52.8%); F: 602 (47.2%) | mean ± SD: 40.7±12.7 | OH: TSH ≥3.4 mlU/L, fT4 <12.8 pmol/L; SH: TSH ≥3.4 mIU/L, fT4 = 12.8–20.4 pmol/L, fT3 = 3.92–6.74 pmol/L | TSH/fT4: Elecsys® 2010 Disk and Rack Analyzers (Roche Diagnostics, Indianapolis, IN, USA) |
| Pfister,2015 [47]1 | Norfolk, UK | 1993– 1997 | cross-sectional analysis within a prospective cohort study | adults aged 40–79 years living in Norfolk, England | T: 11,642; M: 5,461 (46.9%); F: 6,181 (53.1%) | median (range): 58 (39–78) | SH: TSH >4.5 and <19.9 mIU/L, fT 4 in the reference range1 | N/A |
| Valdés, 2017 [19] | Spain | 2009–2010 | cross-sectional, population-based survey | adults aged >18 years living in Spain | T: 4,554; M: 1,932 (42.4%); F: 2,622 (57.6%) | median (range): 50 (18–93) | OH: TSH >5.0 mlU/L, fT4 <11.0 pmol/L; SH: TSH > 5.0 mIU/L, fT4≥11.0 pmol/L | TSH/fT4: electrochemiluminescence immunoassay (Modular Analytics E170, cobas e 602; Roche Diagnostics, Basel, Switzerland) |
| Veltri, 2017 [36] | Brussels, Belgium | 2015 | cross-sectional analysis within a retrospective cohort study | individuals aged≥20years living in Brussels | T: 676; M: 151 (22.3%; F: 525 (77.7%) | mean ± SD: 44.2±15.3 | SH: TSH >4.0 mlU/L, fT4in the reference range | TSH/fT4: chemiluminescence Centaur XP Siemens immunoanalyzer |
| Elenkova, 2017 [37] | Sofia, Bulgaria | 2009–2012 | cross-sectional analysis within a case-control study | control group in a case-control study: healthy women aged ≥18 years | T: 106; M: 0 (0.0%); F: 106 (100%) | mean ± SD: 35.5±8.46 | OH: TSH >4.0 mlU/L, fT4 <10.0 pmol/L; SH: TSH > 4.0 mIU/L, fT4≥10.0 pmol/L | TSH/fT4: commercial kits produced by BRAHMS GmbH, Germany |
CI, confidence interval; EMIL, EchinococcusMultilocularis Infection and other medical disorders in Leutkirch; F, female; LASA, Longitudinal Aging Study Amsterdam; M, male; MRC-CFAS, Medical Research Council Cognitive Function and Ageing Study; N/A, not available; OH, overt hypothyroidism; SD, standard deviation; SH, subclinical hypothyroidism; T, total; TSH, thyroid-stimulating hormone.
According to Baumgartneret al.[50].
Patients aged >65 years.
Units for reference ranges for fT4(ng/dL) and/or fT3(pg/mL) originally presented in the study were converted to SI units (pmol/L).
The report of selected articles results from cross-sectional analysis of 8 population-based surveys [15, 16, 17, 18, 40, 41, 42, 49], 7 prospective cohort studies [34, 38, 39, 44, 45, 46, 47], 2 retrospective cohort studies [35, 36], and 3 case-control studies [37, 43, 48].
The conceptual definition of subclinical hypothyroidism (elevated TSH and normal free thyroid hormone) and overt hypothyroidism (elevated TSH and low free thyroid hormone) was the same across the selected studies, but the reference ranges for TSH and free thyroid hormones (i.e., fT4 and/or fT3) were not. The highest limit of the reference range for TSH varied between 3.4 mIU/L [40] and 5.5 mIU/L [48]. The lowest limits of the reference ranges varied between 8.0 pmol/L [42] and 12.8 pmol/L [40] for fT4 and between 2.96 pmol/L [48] and 3.92 pmol/L [40] for fT3.
Risk of Bias in Selected Studies
The studies' methodological quality scores are available in online supplementary Tables 4–6. All cohort studies (n = 9), 2 case-control studies, and 3 cross-sectional studies were considered to have good methodological quality; 1 case-control study and 5 cross-sectional studies were considered to have moderate quality.
Prevalence of Undiagnosed Hypothyroidism
The prevalence of undiagnosed hypothyroidism in European countries is presented in Table 2.
Table 2.
Prevalence of undiagnosed hypothyroidism in European countries
| Study | Location | Period | Sex | Source population | Undiagnosed hypothyroidism |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| subclinical |
overt |
total |
||||||||||
| n | % | n | % | n | % | n | % | |||||
| Heeringa, 2008 [44]1 | Rotterdam, The Netherlands | 1990 | M | 632 | 39.3 | − | − | − | ||||
| F | 975 | 60.7 | − | − | − | |||||||
| T | 1,607 | 100 | 91 | 5.7 | − | − | ||||||
| Hogervorst, 2008 [46] | UK (England and Wales) | 1998 | M | 513 | 49.0 | − | − | − | ||||
| F | 534 | 51.0 | − | − | − | |||||||
| T | 1,047 | 100 | 33 | 3.2 | 34 | 3.2 | 67 | 6.4 | ||||
| Ceresini, 2009 [41] | Italy | 1998 | M | 519 | 44.3 | − | − | − | ||||
| F | 652 | 55.7 | − | − | − | |||||||
| T | 1,171 | 100 | 26 | 2.2 | 6 | 0.5 | 32 | 2.7 | ||||
| Ittermann, 2010 [39]1 | Pomerania,Germany | 1997– | M | 1,148 | 49.1 | − | − | − | ||||
| 2001 | F | 1,191 | 50.9 | − | − | − | ||||||
| T | 2,339 | 100 | 12 | 0.5 | − | − | ||||||
| Lucas, 2010 [17] | Catalonia, Spain | 2001 | M | 500 | 44.5 | 18 | 3.6 | 0 | 0.0 | 18 | 3.6 | |
| F | 624 | 55.5 | 22 | 3.5 | 3 | 0.5 | 25 | 4.0 | ||||
| T | 1,124 | 100 | 40 | 3.5 | 3 | 0.2 | 43 | 3.8 | ||||
| Asvold, 2011 [42] | Nord-Trøndelag, Norway | 1995– | M | 9,769 | 33.1 | − | − | − | ||||
| 1997 | F | 19,711 | 66.9 | − | − | − | ||||||
| (HUNT 1) | T | 29,480 | 100 | 2,024 | 6.9 | 172 | 0.6 | 2,196 | 7.4 | |||
| Schultz, 2011 [38] | Copenhagen, Denmark | 1998– | M | 253 | 41.8 | 5 | 2.0 | − | − | |||
| 2000 | F | 352 | 58.2 | 26 | 7.4 | − | − | |||||
| T | 605 | 100 | 31 | 5.1 | − | − | ||||||
| de Jongh, 2011 [45] | The Netherlands | 1992– | M | 603 | 49.5 | − | − | − | ||||
| 1993 | F | 616 | 50.5 | − | − | − | ||||||
| T | 1,219 | 100 | 64 | 5.3 | − | − | ||||||
| Dişel, 2012 [48] | Adana, Turkey | 2008– | M | 198 | 53.1 | − | − | − | ||||
| 2009 | F | 175 | 46.9 | − | − | − | ||||||
| T | 373 | 100 | 20 | 5.4 | 4 | 1.1 | 24 | 6.4 | ||||
| Díez, 2012 [43] | Segovia, Spain | 2004– | M | 332 | 36.4 | 3 | 0.9 | 1 | 0.3 | 4 | 1.2 | |
| 2010 | F | 579 | 63.6 | 17 | 2.9 | 5 | 0.9 | 22 | 3.8 | |||
| T | 911 | 100 | 20 | 2.2 | 6 | 0.7 | 26 | 2.9 | ||||
| Resta, 2012 [16] | Bari, Italy | 2008– | M | 177 | 52.5 | 15 | 8.5 | 0 | 0.0 | 15 | 8.5 | |
| 2010 | F | 160 | 47.5 | 27 | 16.9 | 1 | 0.6 | 28 | 17.5 | |||
| T | 337 | 100 | 42 | 12.5 | 1 | 0.3 | 43 | 12.8 | ||||
| Asvold, 2013 [15] | Nord-Trøndelag, Norway | 1995– | M | 10,643 | 31.4 | 224 | 2.1 | 21 | 0.2 | 245 | 2.3 | |
| 1997 | F | 23,274 | 68.6 | 698 | 3.0 | 186 | 0.8 | 884 | 3.8 | |||
| (HUNT 2)2,3 | T | 33,917 | 100 | 922 | 2.7 | 207 | 0.6 | 1,129 | 3.3 | |||
| Asvold, 2013 [15] | Nord-Trøndelag, Norway | 2006– | M | 22,358 | 45.5 | 224 | 1.0 | 22 | 0.1 | 246 | 1.1 | |
| 2008 | F | 26,822 | 54.5 | 295 | 1.1 | 27 | 0.1 | 322 | 1.2 | |||
| (HUNT 3)2,3 | T | 49,180 | 100 | 519 | 1.1 | 49 | 0.1 | 568 | 1.2 | |||
| Delitala, 2014 [18] | Sardinia, Italy | 2001 | M | 2,826 | 45.2 | − | − | − | ||||
| F | 3,426 | 54.8 | − | − | − | |||||||
| T | 6,252 | 100 | 293 | 4.7 | 42 | 0.7 | 335 | 5.4 | ||||
| Formiga, 2013 [34] | Barcelona, Spain | 2009 | M | 123 | 45.4 | 9 | 7.3 | − | − | |||
| F | 184 | 54.6 | 11 | 6.0 | − | − | ||||||
| T | 307 | 100 | 20 | 6.5 | − | − | ||||||
| Kovar, 2015 [35] | Vienna, Austria | 1993– 2004 | M | 31,577 | 39.2 | 868 | 2.7 | − | − | |||
| F | 48,913 | 60.8 | 3,066 | 6.3 | − | − | ||||||
| T | 80,490 | 100 | 3,934 | 3.7 | − | − | ||||||
| Ludwig, 2015 [40] | Leutkirch, Germany | 2002 | M | 674 | 52.8 | − | − | − | ||||
| F | 602 | 47.2 | − | − | − | |||||||
| T | 1,276 | 100 | 34 | 2.7 | 18 | 1.4 | 52 | 4.1 | ||||
| Pfister, 2015 [47]1 | Norfolk, UK | 1993– 1997 | M | 5,461 | 46.9 | − | − | − | ||||
| F | 6,181 | 53.1 | − | − | − | |||||||
| T | 11,642 | 100 | 607 | 5.2 | − | − | ||||||
| Valdés,2017 [19] | Spain | 2009–2010 | M | 1,932 | 42.4 | 65 | 3.4 | 1 | 0.1 | 66 | 3.4 | |
| F | 2,622 | 57.6 | 145 | 5.5 | 16 | 0.6 | 161 | 6.1 | ||||
| T | 4,554 | 100 | 210 | 4.6 | 17 | 0.3 | 227 | 5.0 | ||||
| Veltri, 2017 [36] | Brussels, Belgium | 2015 | M | 151 | 22.3 | − | − | − | ||||
| F | 525 | 77.7 | − | − | − | |||||||
| T | 676 | 100 | 47 | 6.9 | − | − | ||||||
| Elenkova, 2017 [37] | Sofia, Bulgaria | 2009–2012 | M | 0 | 0.0 | − | − | − | ||||
| F | 106 | 100 | 3 | 2.8 | − | − | ||||||
| T | 106 | 100 | 3 | 2.8 | − | − | ||||||
M, male; F, female; T, total.
Data obtained from Baumgartner et al. [50].
Table presents data from Asvold et al. [15]. Fleiner et al. [81] reported results for untreated total hypothyroidism in patients without diabetes from HUNT 2 (men, 2.2%; women, 3.7%; both sexes, 3.7%) and HUNT 3 (men, 1.2%; women, 1.3%; both sexes, 1.2%).
Absolute values were calculated based on percentages.
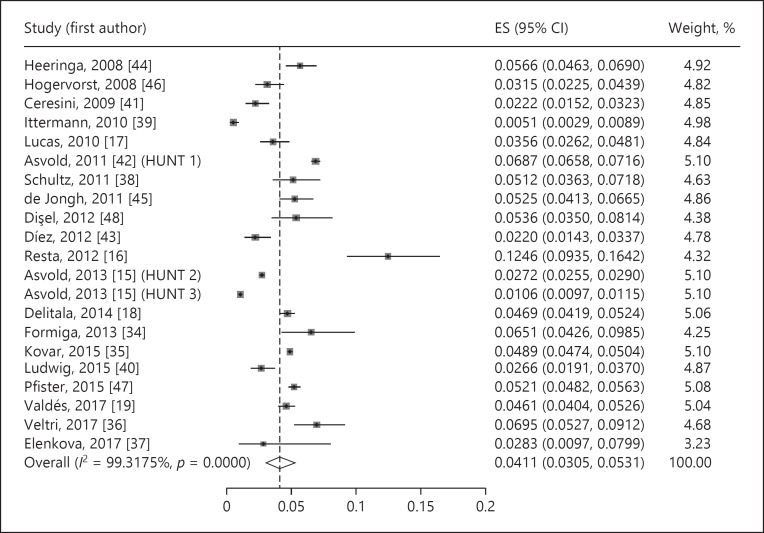
With the exception of one study [15], all studies reported the prevalence of undiagnosed subclinical hypothyroidism, which varied between 0.5% in Germany [39, 50] and 12.5% in Italy [16].
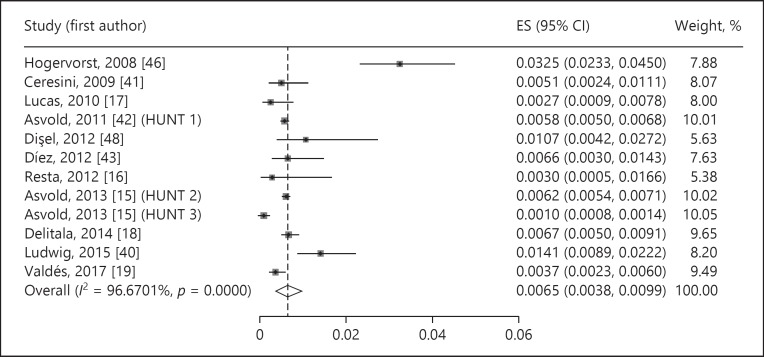
The prevalence of undiagnosed overt hypothyroidism was reported in 11 studies [15, 16, 17, 18, 19, 40, 41, 42, 43, 46, 48] and varied between 0.1% in Norway [15] and 3.2% in Germany [46].
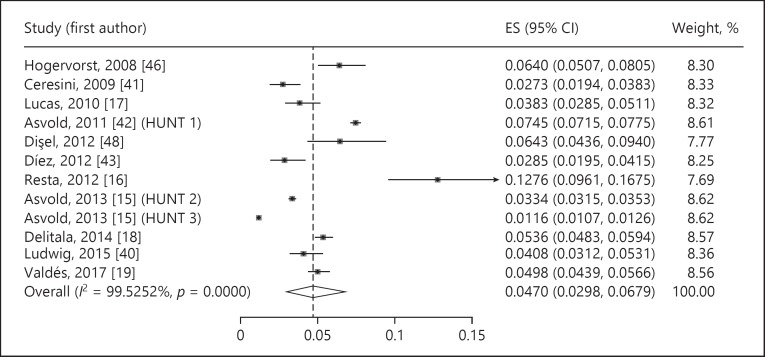
The prevalence of undiagnosed total (subclinical plus overt) hypothyroidism was ascertained in 11 studies [15, 16, 17, 18, 19, 40, 41, 42, 43, 46, 48], with estimates ranging between 2.7% [41] and 12.8% [16]. The prevalence of hypothyroidism was lower in men than in women for subclinical (men: min. 0.9%, max. 8.5%; women: min. 2.8%, max. 16.9%), overt (men: min. 0.0%, max. 0.3%; women: min. 0.5%, max. 0.9%), and total hypothyroidism (men: min. 1.2%, max. 8.5%; women: min. 3.8%, max. 17.5%).
Meta-Analysis
The estimated prevalence of undiagnosed subclinical hypothyroidism was 4.11% (95% CI 3.05–5.31%, p < 0.001, I2 = 99.32%) (Fig. 2). The prevalence of undiagnosed overt hypothyroidism was 0.65% (95% CI 0.38–0.99%, p < 0.001, I2 = 96.67%) (Fig. 3). Undiagnosed total hypothyroidism was estimated with a prevalence of 4.70% (95% CI 2.98–6.79%, p < 0.001, I2 = 99.53%) (Fig. 4).
Fig. 2.
Prevalence of undiagnosed subclinical hypothyroidism. ES, estimate of proportions; CI, confidence interval.
Fig. 3.
Prevalence of undiagnosed overt hypothyroidism. ES, estimate of proportions; CI, confidence interval.
Fig. 4.
Prevalence of undiagnosed total hypothyroidism. ES, estimate of proportions; CI, confidence interval.
The funnel plots (for studies studying subclinical, overt, and total hypothyroidism) appear asymmetric, with smaller studies tending to report larger proportions of hypothyroidism, which may suggest publication bias (online suppl. Fig. 1–3). Egger's regression asymmetry test detected publication bias among the studies included to assess the proportion of overt hypothyroidism, but not among the subclinical or total hypothyroidism analysis (subclinical hypothyroidism: coefficient 6.06, standard error 3.56, p = 0.105; overt hypothyroidism: coefficient 4.00, standard error 1.46, p = 0.021; total hypothyroidism: coefficient 9.43, standard error 4.45, p = 0.06). The test provides weak evidence for the presence of small-study effects. However, there is evidence of substantial heterogeneity among the results of the three proportion estimates, which makes the publication bias analysis difficult. Moreover, there are studies reporting a reduced number of events, particularly in the estimation of overt hypothyroidism, which can lead to false-positive publication bias test results.
Sensitivity Analysis
Table 3 presents the results of the sensitivity analysis. In general, the prevalence of hypothyroidism tends to be higher in female patients, those aged ≥65 years, among studies with lower sample sizes, in case of TSH reference levels <4.5 mIU/L, and in Eastern and Southern Europe.
Table 3.
Prevalence of hypothyroidism according to the results obtained in the sensitivity analysis
| Variable | Subclinical hypothyroidism | Overt hypothyroidism | Total hypothyroidism |
|---|---|---|---|
| Sex | |||
| Men | 2.70% (1.81–3.76%) | 0.06% (0.01–0.12%) | 2.67% (1.62–3.96%) |
| I2 = 97.15%* | I2 = 30.37%*** | I2 = 96.08%* | |
| Women | 4.80% (2.85–7.20%) | 0.49% (0.11–1.09%) | 4.83% (2.74–7.46%) |
| I2 = 99.45%* | I2 = 97.13%*** | I2 = 99.06%* | |
| Age1 | |||
| <65 years | 3.58% (2.39–4.98%) | 0.54% (0.29–0.86%) | 4.16% (2.34–6.48%) |
| I2 = 99.54%* | I2 = 96.67%*** | I2 = 99.54%* | |
| >65 years | 5.12% (3.30–7.30%) | 1.10% (0.03–3.42%) | 6.60% (2.54–12.35%) |
| I2 = 91.67%* | I2 = 97.00%*** | I2 = NR* | |
| Sample size (number of patients) | |||
| <1,000 | 5.61% (3.40–8.32%) | 0.64% (0.28–1.11%) | 6.72% (2.11–13.60%) |
| I2 = 88.65%* | I2 = NR* | I2 = NR* | |
| 1,000–10,000 | 3.37% (2.20–4.77%) | 0.87% (0.09–1.52%) | 4.54% (3.73–5.42%) |
| I2 = 95.92%* | I2 = 91.72%* | I2 = 81.41%* | |
| >10,000 | 3.85% (1.92–6.39%) | 0.39% (0.01–0.88%) | 3.54% (0.87–7.93%) |
| I2 = 99.85%* | <SMALLCAPS><ITAL>I</ITAL><UPPER>2</UPPER> = NR*** </SMALLCAPS> | I2 = NR*** | |
| TSH reference values | |||
| <4.5 mlU/L | 5.34% (3.98–6.88%) | 0.62% (0.40–0.87%) | 6.11% (4.48–7.97%) |
| I2 = 94.37%* | I2 = 67.94%* | I2 = 95.70%* | |
| >4.5 mlU/L | 3.46% (2.30–4.85%) | 0.70% (0.30–1.25%) | 3.68% (2.22–5.49%) |
| I2 = 99.43%* | I2 = 97.65%* | I2 = 99.10%* | |
| European geographic region | |||
| North | 3.75% (1.74–6.47%) | 0.76% (0.31–1.41%) | 4.16% (1.48–8.11%) |
| I2 = 99.78%* | I2 = 98.97%* | I2 = 99.86%* | |
| West | 3.90% (2.12–6.20%) | 1.41% (0.89–2.22%) | 4.08% (3.12–5.31%) |
| I2 = 97.82%* | I2 = NR* | I2 = NR* | |
| East | 4.67% (2.91–6.80%) | 1.07% (0.42–2.72%) | 6.43% (4.36–9.40%) |
| I2 = NR* | I2 = NR*** | I2 = NR* | |
| South | 4.50% (3.28–5.91%) | 0.47% (0.33–0.63%) | 4.78% (3.52–6.23%) |
| I2 = 91.18%* | I2 = 21.68%* | I2 = 91.38%* | |
NR, not reported; TSH, thyroid-stimulating hormone.
The mean age of patients included in each study was considered.
p < 0.0001
p < 0.001
p < 0.05.
Discussion
This systematic review and meta-analysis aimed to identify the prevalence of undiagnosed hypothyroidism (subclinical, overt, and total) in the general population of Europe. As we were interested in studying current figures, the time horizon of the search strategy was limited to articles published over the last 10 years. Several studies were dedicated to characterizing the epidemiology of thyroid dysfunctions, but only few addressed the problem of underdiagnosing such diseases [26]. For example, some studies applied drug utilization approaches or electronic health record analysis based on prescriptions data, pharmacy claims, or surveys to identify consumption of antithyroid medication and estimate the prevalence of treated hypothyroidism [21, 51, 52, 53, 54]. Furthermore, many studies investigated the prevalence of this disorder or its role as a risk factor for other diseases in specific populations, for example in pregnant women, patients with diabetes, patients with cardiovascular diseases, or immunocompromised individuals [12, 24, 55, 56, 57, 58, 59]. Therefore, there is room and need for systematically review of the evidence on the prevalence of undiagnosed hypothyroidism in the general population. As such, patients with known thyroid dysfunction and/or using antithyroid medication at baseline were not considered in this review, as defined in the inclusion criteria.
The present results point out that the current prevalence of undiagnosed subclinical hypothyroidism (4.11%) is higher than the prevalence of undiagnosed overt hypothyroidism (0.65%). This is an unsurprising finding taking into account the conclusions of other studies. A previous meta-analysis on thyroid dysfunctions estimated the mean prevalence of undiagnosed hypothyroidism in Europe at 4.94%, with a clear predominance of the subclinical form of the disease: subclinical hypothyroidism 4.59%, overt hypothyroidism 0.62% [26]. The higher prevalence of subclinical hypothyroidism may be explained by the fact that many patients are asymptomatic or report fewer and milder symptoms than patients with overt hypothyroidism [2]. Furthermore, 75% of patients with subclinical hypothyroidism have a serum TSH level <10 mIU/L (i.e., mild subclinical hypothyroidism) [60], being less prone to hypothyroid symptoms and, for example, cardiovascular events than patients with severe subclinical hypothyroidism [2, 22, 23, 24]. Moreover, the TSH concentration usually normalizes within 2 years for 46% of patients with subclinical hypothyroidism if the TSH level is <7 mIU/L in a single measurement [61, 62, 63]. Yet, the risk of progression to overt hypothyroidism among patients with severe forms of subclinical hypothyroidism is estimated at 2–6% per year [51, 61, 62].
The results of this meta-analysis should be interpreted with caution given its high heterogeneity (I2 > 96%). There are considerable differences between the selected studies, including study design (e.g., prospective and retrospective cohort studies, population-based surveys), studied population (e.g., inclusion/exclusion criteria, sex, age), sample size (i.e., range from hundreds to tens of thousands), laboratory tests performed (e.g., TSH, fT4, fT3, antibodies), laboratory techniques and material, and reference ranges of circulating thyroid hormones used to diagnose the several forms of hypothyroidism. Despite the limitations of the present meta-analysis, a quantitative synthesis that matches the research question is possibly preferred over qualitative interpretations of results or unclear quasi-quantitative analyses [64]. In addition to the production of overall estimates, meta-analysis provides the advantage of assessing the consistency of findings and improving the understanding of moderator variables, boundary conditions, and generalizability [64, 65, 66].
The prevalence of hypothyroidism (both subclinical and overt) was higher in women than in men, which is also in line with previous findings [26]. Monitoring the thyroid status is particularly important in the group of pregnant women or women of childbearing potential since correction of overt hypothyroidism reduces the risk of fetal loss and preterm birth [67, 68]. Treatment of overt hypothyroidism is therefore recommended during pregnancy [69]. Pregnant women with subclinical hypothyroidism before 20 weeks of gestation are at a higher risk of miscarriage [70]. Treatment may reduce miscarriage in thyroid autoantibodies (TPOAb)-positive pregnant women, and therefore women with TSH concentrations >2.5 mIU/L should be evaluated for TPOAb status. Furthermore, TPOAb-positive women with TSH levels greater than the pregnancy-specific ranges as well as women with TPOAb-negative status and TSH levels >10 mIU/L should be treated with levothyroxine. Other subgroups of pregnant women may be considered for treatment depending on TSH levels [69]. Nevertheless, universal thyroid screening in pregnancy is still a matter of debate [71]. The results of randomized controlled trials pointed out that treating pregnant women with subclinical hypothyroidism with levothyroxine provided no benefit on the IQ of the offspring or obstetric outcomes [72, 73]. However, levothyroxine therapy was initiated after the critical phase of fetal brain development, i.e., from the end of the first trimester of pregnancy.
The results of our sensitivity analysis also indicated that the prevalence of any form of hypothyroidism is higher among patients aged ≥65 years (subclinical 5.12%, overt 1.10%) as compared to younger ones (subclinical 3.58%, overt 0.54%). The study by Lucas et al. [17] also illustrates the influence of age on estimates of prevalence: when the authors restricted the analysis to subjects >60 years, the prevalence of subclinical and overt hypothyroidism was estimated at 6.2 and 0.44%, respectively (vs. 3.5 and 0.2% in the general population).
With regards to the reference ranges of serum thyroid hormones, the variation of the highest limit of the reference range for TSH concentration in the studies included in this meta-analysis is considerable (between 3.4 and 5.5 mIU/L) [40, 48]. According to the sensitivity analysis, a higher prevalence of subclinical and total hypothyroidism was found for studies using lower TSH reference values. The most commonly cited reference range for TSH concentration in the clinical literature set the highest limit at 4.0 mIU/L (and the lowest limit at 0.4 mIU/L), while the reference range for fT4 depends on the type of assay and the population in question [1]. Nevertheless, the reference level cutoffs used to determine overt and subclinical hypothyroidism have changed over the years [46]. Thus, in the context of the present meta-analysis, the lack of consensus on reference ranges for levels of thyroid hormones used to establish diagnoses may result in inaccurate comparisons because of nonoverlapping definitions of disease across the included studies.
Moreover, the sensitivity analysis also detected differences in the prevalence of hypothyroidism depending on the sample sizes of included studies – higher estimates of prevalence among those with lower number of patients – and on the European region – the prevalence of hypothyroidism tended to be higher in Eastern and Southern Europe.
There is great variation in the clinical manifestations of hypothyroidism, ranging from the absence of signs or symptoms to life-threatening conditions, such as myxedema coma [1]. The most common symptoms are nonspecific and vary depending on different individual factors, such as the age or sex of patients. Furthermore, autoimmune hypothyroidism is asymptomatic or associated with only one symptom in nearly 15% of patients with the condition, while 70% of euthyroid individuals have at least one symptom that is usually related with hypothyroidism [74]. These issues may contribute to preclude or at least delay the diagnosis of hypothyroidism. In addition, with the exception of some populations, such as patients with type 1 diabetes mellitus [75, 76], there is no consensus on TSH screening in the general population [1].
The long-term consequences of hypothyroidism are clinically important and adversely affect the health of patients. Those consequences have been mainly studied in patients with subclinical hypothyroidism, given that overt hypothyroidism is usually treated. The body of evidence is particularly robust with regards to the association between hypothyroidism and adverse cardiovascular outcomes. A meta-analysis of individual data on >2,500 elderly participants found that the risk of coronary heart disease and mortality is increased in subjects with higher serum TSH levels [22]. Conversely, patients with TSH concentrations >10 mIU/L are at higher risk of heart failure [22, 23]. Hence, timely diagnosis and treatment initiation are potentially important for preventing adverse consequences of hypothyroidism in patients with the condition. However, the results of a double-blind, randomized, placebo-controlled, parallel-group trial pointed out that giving levothyroxine to senior patients (>65 years) with subclinical hypothyroidism (TSH level 4.60–19.99 mIU/L, fT4 level within the reference range) provided no significant beneficial effects on thyroid-related quality of life symptoms or cardiovascular events [77]. Other illnesses potentially associated with hypothyroidism include nonalcoholic fatty liver disease, cancer mortality, arthritis, renal failure, and diabetes [25, 78, 79, 80].
In conclusion, the current evidence suggests that a considerable proportion of the European population has hypothyroidism, particularly subclinical hypothyroidism, which is not diagnosed. This issue deserves further investigation because of possible deleterious consequences for public health.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
This work was financially supported by Merck S.A., Portugal.
Supplementary Material
Supplementary data
References
- 1.Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet. 2017 Sep;390((10101)):1550–62. doi: 10.1016/S0140-6736(17)30703-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peeters RP. Subclinical Hypothyroidism. N Engl J Med. 2017 Oct;377((14)):1404–1404. doi: 10.1056/NEJMc1709853. [DOI] [PubMed] [Google Scholar]
- 3.LeFevre ML, U.S. Preventive Services Task Force Screening for thyroid dysfunction: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015 May;162((9)):641–50. doi: 10.7326/M15-0483. [DOI] [PubMed] [Google Scholar]
- 4.Laurberg P, Cerqueira C, Ovesen L, Rasmussen LB, Perrild H, Andersen S, et al. Iodine intake as a determinant of thyroid disorders in populations. Best Pract Res Clin Endocrinol Metab. 2010 Feb;24((1)):13–27. doi: 10.1016/j.beem.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007 Dec;92((12)):4575–82. doi: 10.1210/jc.2007-1499. [DOI] [PubMed] [Google Scholar]
- 6.Boucai L, Surks MI. Reference limits of serum TSH and free T4 are significantly influenced by race and age in an urban outpatient medical practice. Clin Endocrinol (Oxf) 2009 May;70((5)):788–93. doi: 10.1111/j.1365-2265.2008.03390.x. [DOI] [PubMed] [Google Scholar]
- 7.Vadiveloo T, Donnan PT, Murphy MJ, Leese GP. Age- and gender-specific TSH reference intervals in people with no obvious thyroid disease in Tayside, Scotland: the Thyroid Epidemiology, Audit, and Research Study (TEARS) J Clin Endocrinol Metab. 2013 Mar;98((3)):1147–53. doi: 10.1210/jc.2012-3191. [DOI] [PubMed] [Google Scholar]
- 8.McLeod DS, Caturegli P, Cooper DS, Matos PG, Hutfless S. Variation in rates of autoimmune thyroid disease by race/ethnicity in US military personnel. JAMA. 2014 Apr;311((15)):1563–5. doi: 10.1001/jama.2013.285606. [DOI] [PubMed] [Google Scholar]
- 9.Mammen JS, McGready J, Ladenson PW, Simonsick EM. Unstable Thyroid Function in Older Adults Is Caused by Alterations in Both Thyroid and Pituitary Physiology and Is Associated with Increased Mortality. Thyroid. 2017 Nov;27((11)):1370–7. doi: 10.1089/thy.2017.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Journy NM, Bernier MO, Doody MM, Alexander BH, Linet MS, Kitahara CM. Hyperthyroidism, Hypothyroidism, and Cause-Specific Mortality in a Large Cohort of Women. Thyroid. 2017 Aug;27((8)):1001–10. doi: 10.1089/thy.2017.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emamifar A, Hangaard J, Jensen Hansen IM. Thyroid disorders in patients with newly diagnosed rheumatoid arthritis is associated with poor initial treatment response evaluated by disease activity score in 28 joints-C-reactive protein (DAS28-CRP): an observational cohort study. Medicine (Baltimore) 2017 Oct;96((43)):e8357. doi: 10.1097/MD.0000000000008357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domingues SL, Gonçalves FT, Jorge ML, Limongi JE, Ranza R, Jorge PT. HIGH PREVALENCE OF HYPOTHYROIDISM IN SYSTEMIC LUPUS ERYTHEMATOSUS PATIENTS WITHOUT AN INCREASE IN CIRCULATING ANTI-THYROID ANTIBODIES. Endocr Pract. 2017 Nov;23((11)):1304–10. doi: 10.4158/EP161664.OR. [DOI] [PubMed] [Google Scholar]
- 13.Beltran S, Lescure FX, Desailloud R, Douadi Y, Smail A, El Esper I, et al. Thyroid and VIH Group Increased prevalence of hypothyroidism among human immunodeficiency virus-infected patients: a need for screening. Clin Infect Dis. 2003 Aug;37((4)):579–83. doi: 10.1086/376626. [DOI] [PubMed] [Google Scholar]
- 14.Ji S, Jin C, Höxtermann S, Fuchs W, Xie T, Lu X, et al. Prevalence and Influencing Factors of Thyroid Dysfunction in HIV-Infected Patients. BioMed Res Int. 2016;2016:3874257. doi: 10.1155/2016/3874257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asvold BO, Vatten LJ, Bjøro T. Changes in the prevalence of hypothyroidism: the HUNT Study in Norway. Eur J Endocrinol. 2013 Oct;169((5)):613–20. doi: 10.1530/EJE-13-0459. [DOI] [PubMed] [Google Scholar]
- 16.Resta F, Triggiani V, Barile G, Benigno M, Suppressa P, Giagulli VA, et al. Subclinical hypothyroidism and cognitive dysfunction in the elderly. Endocr Metab Immune Disord Drug Targets. 2012 Sep;12((3)):260–7. doi: 10.2174/187153012802002875. [DOI] [PubMed] [Google Scholar]
- 17.Lucas A, Julián MT, Cantón A, Castell C, Casamitjana R, Martínez-Cáceres EM, et al. Undiagnosed thyroid dysfunction, thyroid antibodies, and iodine excretion in a Mediterranean population. Endocrine. 2010 Dec;38((3)):391–6. doi: 10.1007/s12020-010-9397-2. [DOI] [PubMed] [Google Scholar]
- 18.Delitala AP, Pilia MG, Ferreli L, Loi F, Curreli N, Balaci L, et al. Prevalence of unknown thyroid disorders in a Sardinian cohort. Eur J Endocrinol. 2014 Jul;171((1)):143–9. doi: 10.1530/EJE-14-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valdés S, Maldonado-Araque C, Lago-Sampedro A, Lillo JA, Garcia-Fuentes E, Perez-Valero V, et al. Population-Based National Prevalence of Thyroid Dysfunction in Spain and Associated Factors: Di@bet.es Study. Thyroid. 2017 Feb;27((2)):156–66. doi: 10.1089/thy.2016.0353. [DOI] [PubMed] [Google Scholar]
- 20.Hoogendoorn EH, Hermus AR, de Vegt F, Ross HA, Verbeek AL, Kiemeney LA, et al. Thyroid function and prevalence of anti-thyroperoxidase antibodies in a population with borderline sufficient iodine intake: influences of age and sex. Clin Chem. 2006 Jan;52((1)):104–11. doi: 10.1373/clinchem.2005.055194. [DOI] [PubMed] [Google Scholar]
- 21.Leese G. P., Flynn R. V., Jung R. T., MacDonald T. M., Murphy M. J., Morris A. D. “Increasing prevalence and incidence of thyroid disease in Tayside, Scotland: the Thyroid Epidemiology Audit and Research Study (TEARS),”. Clin. Endocrinol. (Oxf). 2007 Oct;vol. 0(no. 0):p. 071029015918001. doi: 10.1111/j.1365-2265.2007.03051.x. [DOI] [PubMed] [Google Scholar]
- 22.Rodondi N, den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh JP, et al. Thyroid Studies Collaboration Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010 Sep;304((12)):1365–74. doi: 10.1001/jama.2010.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gencer B, Collet TH, Virgini V, Bauer DC, Gussekloo J, Cappola AR, et al. Thyroid Studies Collaboration Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation. 2012 Aug;126((9)):1040–9. doi: 10.1161/CIRCULATIONAHA.112.096024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaker L, Baumgartner C, den Elzen WP, Ikram MA, Blum MR, Collet TH, et al. Thyroid Studies Collaboration Subclinical Hypothyroidism and the Risk of Stroke Events and Fatal Stroke: An Individual Participant Data Analysis. J Clin Endocrinol Metab. 2015 Jun;100((6)):2181–91. doi: 10.1210/jc.2015-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gronich N, Deftereos SN, Lavi I, Persidis AS, Abernethy DR, Rennert G. Hypothyroidism is a Risk Factor for New-Onset Diabetes: A Cohort Study. Diabetes Care. 2015 Sep;38((9)):1657–64. doi: 10.2337/dc14-2515. [DOI] [PubMed] [Google Scholar]
- 26.Garmendia Madariaga A, Santos Palacios S, Guillén-Grima F, Galofré JC. The incidence and prevalence of thyroid dysfunction in Europe: a meta-analysis. J Clin Endocrinol Metab. 2014 Mar;99((3)):923–31. doi: 10.1210/jc.2013-2409. [DOI] [PubMed] [Google Scholar]
- 27.Moher D., Liberati A., Tetzlaff J., D. G., Altman, and PRISMA Group “Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement.,”. Ann. Intern. Med. 2009 Aug;vol. 151(no. 4):pp. 264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. W64. [DOI] [PubMed] [Google Scholar]
- 28.Wells GA, et al. Ottawa Hosp. Res. Inst. no. 3. 2013. “The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses.,”; pp. pp 1–4. [Google Scholar]
- 29.Alshabanat A, Zafari Z, Albanyan O, Dairi M, FitzGerald JM. Asthma and COPD Overlap Syndrome (ACOS): A Systematic Review and Meta Analysis. PLoS One. 2015 Sep;10((9)):e0136065. doi: 10.1371/journal.pone.0136065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014 Nov;72((1)):39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986 Sep;7((3)):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 32.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003 Sep;327((7414)):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. “Publication bias,” in Introduction to meta-analysis. In: Rothstein MB, editor. Chichester: John Wiley & Sons, Ltd; 2009. pp. pp. 277–91. [Google Scholar]
- 34.Formiga F, Ferrer A, Padros G, Contra A, Corbella X, Pujol R, Octabaix Study Group Thyroid status and functional and cognitive status at baseline and survival after 3 years of follow-up: the OCTABAIX study. Eur J Endocrinol. 2013 Nov;170((1)):69–75. doi: 10.1530/EJE-13-0722. [DOI] [PubMed] [Google Scholar]
- 35.Kovar FM, Fang IF, Perkmann T, Haslacher H, Slavka G, Födinger M, et al. Subclinical hypothyroidism and mortality in a large Austrian cohort: a possible impact on treatment? Wien Klin Wochenschr. 2015 Dec;127((23-24)):924–30. doi: 10.1007/s00508-015-0846-z. [DOI] [PubMed] [Google Scholar]
- 36.Veltri F, Rocha FO, Willems D, Praet JP, Grabczan L, Kleynen P, et al. Prevalence of thyroid dysfunction and autoimmunity in the older population and implications of age-specific reference ranges. Clin Chim Acta. 2017 Feb;465:34–9. doi: 10.1016/j.cca.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 37.Elenkova A, Аtanasova I, Кirilov G, Natchev Е, Ivanova R, Кovatcheva R, et al. Autoimmune hypothyroidism is three times more frequent in female prolactinoma patients compared to healthy women: data from a cross-sectional case-control study. Endocrine. 2017 Sep;57((3)):486–93. doi: 10.1007/s12020-017-1372-8. [DOI] [PubMed] [Google Scholar]
- 38.Schultz M, Kistorp C, Raymond I, Dimsits J, Tuxen C, Hildebrandt P, et al. Cardiovascular events in thyroid disease: a population based, prospective study. Horm Metab Res. 2011 Aug;43((9)):653–9. doi: 10.1055/s-0031-1283162. [DOI] [PubMed] [Google Scholar]
- 39.Ittermann T, Haring R, Sauer S, Wallaschofski H, Dörr M, Nauck M, et al. Decreased serum TSH levels are not associated with mortality in the adult northeast German population. Eur J Endocrinol. 2010 Mar;162((3)):579–85. doi: 10.1530/EJE-09-0566. [DOI] [PubMed] [Google Scholar]
- 40.Ludwig U, Holzner D, Denzer C, Greinert A, Haenle MM, Oeztuerk S, et al. EMIL-Study Subclinical and clinical hypothyroidism and non-alcoholic fatty liver disease: a cross-sectional study of a random population sample aged 18 to 65 years. BMC Endocr Disord. 2015 Aug;15((1)):41. doi: 10.1186/s12902-015-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ceresini G, Lauretani F, Maggio M, Ceda GP, Morganti S, Usberti E, et al. Thyroid function abnormalities and cognitive impairment in elderly people: results of the Invecchiare in Chianti study. J Am Geriatr Soc. 2009 Jan;57((1)):89–93. doi: 10.1111/j.1532-5415.2008.02080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asvold BO, Bjøro T, Vatten LJ. Association of thyroid function with estimated glomerular filtration rate in a population-based study: the HUNT study. Eur J Endocrinol. 2011 Jan;164((1)):101–5. doi: 10.1530/EJE-10-0705. [DOI] [PubMed] [Google Scholar]
- 43.Díez JJ, Iglesias P. An analysis of the relative risk for hypothyroidism in patients with Type 2 diabetes. Diabet Med. 2012 Dec;29((12)):1510–4. doi: 10.1111/j.1464-5491.2012.03687.x. [DOI] [PubMed] [Google Scholar]
- 44.Heeringa J, Hoogendoorn EH, van der Deure WM, Hofman A, Peeters RP, Hop WC, et al. High-normal thyroid function and risk of atrial fibrillation: the Rotterdam study. Arch Intern Med. 2008 Nov;168((20)):2219–24. doi: 10.1001/archinte.168.20.2219. [DOI] [PubMed] [Google Scholar]
- 45.de Jongh RT, Lips P, van Schoor NM, Rijs KJ, Deeg DJ, Comijs HC, et al. Endogenous subclinical thyroid disorders, physical and cognitive function, depression, and mortality in older individuals. Eur J Endocrinol. 2011 Oct;165((4)):545–54. doi: 10.1530/EJE-11-0430. [DOI] [PubMed] [Google Scholar]
- 46.Hogervorst E, Huppert F, Matthews FE, Brayne C. Thyroid function and cognitive decline in the MRC Cognitive Function and Ageing Study. Psychoneuroendocrinology. 2008 Aug;33((7)):1013–22. doi: 10.1016/j.psyneuen.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 47.Pfister R, Brägelmann J, Michels G, Wareham NJ, Luben R, Khaw KT. Performance of the CHARGE-AF risk model for incident atrial fibrillation in the EPIC Norfolk cohort. Eur J Prev Cardiol. 2015 Jul;22((7)):932–9. doi: 10.1177/2047487314544045. [DOI] [PubMed] [Google Scholar]
- 48.Dişel U, Beşen A, Karadeniz C, Mertsoylu H, Sezer A, Köse F, et al. Prevalence of thyroid dysfunction in untreated cancer patients: a cross-sectional study. Med Oncol. 2012 Dec;29((5)):3608–13. doi: 10.1007/s12032-012-0254-4. [DOI] [PubMed] [Google Scholar]
- 49.Tanno LK, Calderon MA, Goldberg BJ, Gayraud J, Bircher AJ, Casale T, et al. Constructing a classification of hypersensitivity/allergic diseases for ICD-11 by crowdsourcing the allergist community. Allergy. 2015 Jun;70((6)):609–15. doi: 10.1111/all.12604. [DOI] [PubMed] [Google Scholar]
- 50.Baumgartner C, da Costa BR, Collet TH, Feller M, Floriani C, Bauer DC, et al. Thyroid Studies Collaboration Thyroid Function Within the Normal Range, Subclinical Hypothyroidism, and the Risk of Atrial Fibrillation. Circulation. 2017 Nov;136((22)):2100–16. doi: 10.1161/CIRCULATIONAHA.117.028753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf) 1995 Jul;43((1)):55–68. doi: 10.1111/j.1365-2265.1995.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 52.Giorda CB, Carnà P, Romeo F, Costa G, Tartaglino B, Gnavi R. Prevalence, incidence and associated comorbidities of treated hypothyroidism: an update from a European population. Eur J Endocrinol. 2017 May;176((5)):533–42. doi: 10.1530/EJE-16-0559. [DOI] [PubMed] [Google Scholar]
- 53.Rodriguez-Gutierrez R, Maraka S, Ospina NS, Montori VM, Brito JP. Levothyroxine overuse: time for an about face? Lancet Diabetes Endocrinol. 2017 Apr;5((4)):246–8. doi: 10.1016/S2213-8587(16)30276-5. [DOI] [PubMed] [Google Scholar]
- 54.Flynn RW, MacDonald TM, Morris AD, Jung RT, Leese GP. The thyroid epidemiology, audit, and research study: thyroid dysfunction in the general population. J Clin Endocrinol Metab. 2004 Aug;89((8)):3879–84. doi: 10.1210/jc.2003-032089. [DOI] [PubMed] [Google Scholar]
- 55.Diéguez M, Herrero A, Avello N, Suárez P, Delgado E, Menéndez E. Prevalence of thyroid dysfunction in women in early pregnancy: does it increase with maternal age? Clin Endocrinol (Oxf) 2016 Jan;84((1)):121–6. doi: 10.1111/cen.12693. [DOI] [PubMed] [Google Scholar]
- 56.Blumenthal NJ, Byth K, Eastman CJ. Prevalence of thyroid dysfunction and thyroid antibodies in a private obstetrical practice in Sydney. Aust N Z J Obstet Gynaecol. 2016 Jun;56((3)):307–11. doi: 10.1111/ajo.12462. [DOI] [PubMed] [Google Scholar]
- 57.Song F, Bao C, Deng M, Xu H, Fan M, Paillard-Borg S, et al. The prevalence and determinants of hypothyroidism in hospitalized patients with type 2 diabetes mellitus. Endocrine. 2017 Jan;55((1)):179–85. doi: 10.1007/s12020-016-1095-2. [DOI] [PubMed] [Google Scholar]
- 58.Jia F, Tian J, Deng F, Yang G, Long M, Cheng W, et al. Subclinical hypothyroidism and the associations with macrovascular complications and chronic kidney disease in patients with Type 2 diabetes. Diabet Med. 2015 Aug;32((8)):1097–103. doi: 10.1111/dme.12724. [DOI] [PubMed] [Google Scholar]
- 59.Blum MR, Wijsman LW, Virgini VS, Bauer DC, den Elzen WP, Jukema JW, et al. PROSPER study group Subclinical Thyroid Dysfunction and Depressive Symptoms among the Elderly: A Prospective Cohort Study. Neuroendocrinology. 2016;103((3-4)):291–9. doi: 10.1159/000437387. [DOI] [PubMed] [Google Scholar]
- 60.Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000 Feb;160((4)):526–34. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- 61.Huber G, Staub JJ, Meier C, Mitrache C, Guglielmetti M, Huber P, et al. Prospective study of the spontaneous course of subclinical hypothyroidism: prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. J Clin Endocrinol Metab. 2002 Jul;87((7)):3221–6. doi: 10.1210/jcem.87.7.8678. [DOI] [PubMed] [Google Scholar]
- 62.Somwaru LL, Rariy CM, Arnold AM, Cappola AR. The natural history of subclinical hypothyroidism in the elderly: the cardiovascular health study. J Clin Endocrinol Metab. 2012 Jun;97((6)):1962–9. doi: 10.1210/jc.2011-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meyerovitch J, Rotman-Pikielny P, Sherf M, Battat E, Levy Y, Surks MI. Serum thyrotropin measurements in the community: five-year follow-up in a large network of primary care physicians. Arch Intern Med. 2007 Jul;167((14)):1533–8. doi: 10.1001/archinte.167.14.1533. [DOI] [PubMed] [Google Scholar]
- 64.Ioannidis JP, Patsopoulos NA, Rothstein HR. Reasons or excuses for avoiding meta-analysis in forest plots. BMJ. 2008 Jun;336((7658)):1413–5. doi: 10.1136/bmj.a117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith TC, Spiegelhalter DJ, Thomas A. Bayesian approaches to random-effects meta-analysis: a comparative study. Stat Med. 1995 Dec;14((24)):2685–99. doi: 10.1002/sim.4780142408. [DOI] [PubMed] [Google Scholar]
- 66.Berlin JA. Invited commentary: benefits of heterogeneity in meta-analysis of data from epidemiologic studies. Am J Epidemiol. 1995 Aug;142((4)):383–7. doi: 10.1093/oxfordjournals.aje.a117645. [DOI] [PubMed] [Google Scholar]
- 67.Krassas GE, Poppe K, Glinoer D. Thyroid function and human reproductive health. Endocr Rev. 2010 Oct;31((5)):702–55. doi: 10.1210/er.2009-0041. [DOI] [PubMed] [Google Scholar]
- 68.Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, et al. American Thyroid Association Taskforce on Thyroid Disease During Pregnancy and Postpartum Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011 Oct;21((10)):1081–125. doi: 10.1089/thy.2011.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid. 2017 Mar;27((3)):315–89. doi: 10.1089/thy.2016.0457. [DOI] [PubMed] [Google Scholar]
- 70.Pennington JA. A review of iodine toxicity reports. J Am Diet Assoc. 1990 Nov;90((11)):1571–81. [PubMed] [Google Scholar]
- 71.Taylor PN, Okosieme OE, Premawardhana L, Lazarus JH. Should all women be screened for thyroid dysfunction in pregnancy? Womens Health (Lond) 2015 Jun;11((3)):295–307. doi: 10.2217/whe.15.7. [DOI] [PubMed] [Google Scholar]
- 72.Lazarus JH, Bestwick JP, Channon S, Paradice R, Maina A, Rees R, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012 Feb;366((6)):493–501. doi: 10.1056/NEJMoa1106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Casey BM, Thom EA, Peaceman AM, Varner MW, Sorokin Y, Hirtz DG, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network Treatment of Subclinical Hypothyroidism or Hypothyroxinemia in Pregnancy. N Engl J Med. 2017 Mar;376((9)):815–25. doi: 10.1056/NEJMoa1606205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carlé A, Pedersen IB, Knudsen N, Perrild H, Ovesen L, Laurberg P. Hypothyroid symptoms and the likelihood of overt thyroid failure: a population-based case-control study. Eur J Endocrinol. 2014 Nov;171((5)):593–602. doi: 10.1530/EJE-14-0481. [DOI] [PubMed] [Google Scholar]
- 75.American Diabetes Association “Executive Summary: Standards of Medical Care in Diabetes—2014,” Diabetes Care, vol. 37, no. Supplement. 2014 Jan;1:S5–13. doi: 10.2337/dc14-S005. [DOI] [PubMed] [Google Scholar]
- 76.Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, et al. American Association Of Clinical Endocrinologists And American Thyroid Association Taskforce On Hypothyroidism In Adults Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012 Dec;22((12)):1200–35. doi: 10.1089/thy.2012.0205. [DOI] [PubMed] [Google Scholar]
- 77.Stott DJ, Rodondi N, Kearney PM, Ford I, Westendorp RG, Mooijaart SP, et al. TRUST Study Group Thyroid Hormone Therapy for Older Adults with Subclinical Hypothyroidism. N Engl J Med. 2017 Jun;376((26)):2534–44. doi: 10.1056/NEJMoa1603825. [DOI] [PubMed] [Google Scholar]
- 78.Tseng FY, Lin WY, Li CI, Li TC, Lin CC, Huang KC. Subclinical hypothyroidism is associated with increased risk for cancer mortality in adult Taiwanese-a 10 years population-based cohort. PLoS One. 2015 Apr;10((4)):e0122955. doi: 10.1371/journal.pone.0122955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bano A, Chaker L, Plompen EP, Hofman A, Dehghan A, Franco OH, et al. Thyroid Function and the Risk of Nonalcoholic Fatty Liver Disease: the Rotterdam Study. J Clin Endocrinol Metab. 2016 Aug;101((8)):3204–11. doi: 10.1210/jc.2016-1300. [DOI] [PubMed] [Google Scholar]
- 80.Zhang Y, Chang Y, Ryu S, Cho J, Lee WY, Rhee EJ, et al. Thyroid hormone levels and incident chronic kidney disease in euthyroid individuals: the Kangbuk Samsung Health Study. Int J Epidemiol. 2014 Oct;43((5)):1624–32. doi: 10.1093/ije/dyu126. [DOI] [PubMed] [Google Scholar]
- 81.Fleiner, et al. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data