Abstract
Background
In Europe IgA nephropathy (IgAN) is detected in 20% of children with glomerular diseases diagnosed by renal biopsy. The outcome during childhood is generally good, but progression in the long-term follow-up may occur in about 20% of children after 20 years.
Summary
In Europe, urine screening programs are not active, and there is variability in the policy to perform renal biopsies in oligo-symptomatic children. Hence, a suitable observational approach to pediatric IgAN is offered by the VALIGA study which included 174 children aged < 18 years from 13 European countries followed over a median of 4.4 (2.5–7.5) years. Renal pathology lesions were centrally scored according to the Oxford Classification of IgAN (mesangial hypercellularity, M; endocapillary hypercellularity, E; segmental glomerulosclerosis, S; tubular atrophy/interstitial fibrosis, T; crescents, C [MEST-C]). Children had renal biopsy mostly with normal estimated glomerular filtration rate (eGFR) and moderate proteinuria of a median of 0.84 g/day/1.73 m<sup>2</sup> (< 0.30 g/day/1.73 m<sup>2</sup> in 30% of the cases). Children showed M1 in 21.8%, E1 in 13.8%, S1 in 42.5%, T1–2 in 6.3%, and C1 in 14.9%. The survival at the combined endpoint of 50% eGFR decrease or end-stage renal disease at 15 years was 94%. The slow progression rate and the limited number of cases progressing to the combined endpoint (6.4%) did not allow the detection of a predictive value of the MEST-C score. Moreover, the predictive value of clinical and pathological features was likely blunted by the use of corticosteroid/immunosuppressive treatment (CS/IS) in 50% of the cases. The survival tree analysis also proved that children < 16 years old with IgAN without mesangial hypercellularity (M0) and well preserved eGFR (> 90 mL/min/1.73 m<sup>2</sup>) had a high probability of proteinuria remission during follow-up. Moreover, in this subgroup of children, the benefits of CS/IS therapy reached statistical significance. In Europe, the use of CS/IS treatment in IgAN is still a debated issue, but most children tend to be treated more commonly than adults with CS/IS. A recent uncontrolled study reports a favorable outcome in European children with IgAN and very active acute forms of IgAN with improvement in eGFR and reduction in proteinuria.
Key Messages
In Europe, children with IgAN have a favorable prognosis in the short term, and this may be due also to the frequently adopted CS/IS therapy, particularly with acute and active pathological features. The risk of progression over decades of follow-up remains an unsolved problem which needs to be addressed by controlling subtle chronic pathogenetic factors which work in children as well as in adult cases of IgAN.
Keywords: IgA nephropathy, Children, Risk factors for progression, Renal pathology, Treatment
Introduction and Interest for European Pediatric Nephrology
IgA nephropathy (IgAN) is a common glomerular disease in children and adolescents all over the world. Europe is the second continent with a frequency of this disease accounting for 20% of renal biopsies performed in pediatric age [1, 2, 3], while Asia has a 40% frequency [4, 5]. This different prevalence might be due to genetic or environmental factors, which play a greater role in Asia than in Europe, particularly favoring the early onset of IgAN [6]. However, most probably, the frequency increases where screening programs are active, like in Japan and Korea [5, 7]. In Europe, this policy has not been adopted, and IgAN is diagnosed in children undergoing renal biopsy because of persistent heavy isolated microscopic hematuria or hematuria associated with non-nephrotic proteinuria [1, 2, 3]. The prevalence of pediatric IgAN in Europe varies in different reports, due to the variable criteria for performing renal biopsy in young subjects shortly after the detection of urine anomalies or limiting renal biopsy to cases having developed proteinuria. It is likely that in Europe several cases of IgAN originating in the pediatric age are missed because most of them are asymptomatic. The presence of isolated microscopic hematuria or hematuria associated with minimal proteinuria had been considered a benign clinical feature in children by general pediatricians in Europe before the new millennium. However, the European Registry of patients in regular replacement therapy (ERA-EDTA Registry) reported in a focused study that most subjects with IgAN entering regular replacement therapy are young adults [8], and since the decline of renal function in these patients is slow, it becomes manifest that progressive IgAN in Europe frequently begins in childhood. The interest for pediatric IgAN increased in the last decades, since detecting IgAN at the beginning of its natural history in childhood may offer the possibility for early treatment and improvement of the natural history of the disease in adult age.
Clinical Features of Children and Young Subjects with IgAN in Europe
The different criteria to perform renal biopsies in European children with oligo-symptomatic urinary abnormalities account for the variability in the reports on the clinical presentation at renal biopsy and the natural course of pediatric IgAN in Europe [1, 2, 3]. For this reason, to gain a general overview of this glomerular disease in children, a suitable observational approach is offered by the cohort gathered by the collaborative study to validate the Oxford Classification of IgAN, VALIGA [9]. This study enrolled 1,147 patients from 13 European countries followed over a median of 4.7 years [10]. This cohort included 174 children aged < 18 years from 13 European countries, offering the possibility of presenting European data without the bias of selection due to different biopsy policies [11]. All renal biopsies were centrally reviewed and scored according to the Oxford Classification [9]: mesangial hypercellularity, M0/M1 (≤/> 50% of glomeruli with mesangial hypercellularity, defined as 4 or > 4 mesangial cells/mesangial area); endocapillary hypercellularity, E0/E1 (absent/present); segmental glomerulosclerosis, S0/S1 (absent/present); and tubular atrophy/interstitial fibrosis, T0/T1–2 (≤/> 25% of renal biopsy tissue); crescents were assessed as C0/C1 (absent/present) (MEST-C score).
The baseline clinical data and pathology features of the 174 children form 13 European countries are reported in Table 1. At renal biopsy, the mean age was 12.7 ± 3.6 years (only 6% were < 6 years old, 40% were < 12 years old), and the children were followed for a median of 4.6 (interquartile range 2.5–7.3) years. Males were more frequent than females. Renal biopsy was performed mostly with normal estimated glomerular filtration rate (eGFR). Proteinuria at renal biopsy was at a median of 0.84 g/day/1.73 m2, being < 0.30 g/day/1.73 m2 in 25% of the cases, while nephrotic range proteinuria was very rare. Hypertension (mean arterial blood pressure [MAP] > 95th percentile for age) was found in 20% of children who were hypertensive and/or were receiving antihypertensive medications.
Table 1.
Demographic and clinical data at renal biopsy and over the follow-up in 174 children <18 years old enrolled in the VALIGA cohort
| Clinical data at biopsy | |
| Female gender | 49 (28.16) |
| Age, years | 12.72±3.63 |
| eGFR, mL/min/1.73 m2 | 117.02 (96.17–120) |
| Proteinuria, g/day/1.73 m2 | 0.84 (0.34–2.18) |
| MAP, mm Hg | 87.53±11.35 |
| Biopsy features | |
| M1 | 38 (21.84) |
| E1 | 24 (13.79) |
| S1 | 74 (42.53) |
| T1–2 | 11 (6.32) |
| Follow-up data | |
| Duration of follow-up, years | 4.63 (2.48–7.35) |
| TA MAP, mm Hg | 86.64±8.54 |
| TA proteinuria, g/day/1.73 m2 | 0.56 (0.27–1.02) |
| RASB treatment | 116 (66.67) |
| CsA/IS treatment | 88 (50.57) |
| ΔeGFR, mL/min/1.73 m2 | 2.01±15.38 |
| ΔMAP, mm Hg | −1.29±11.75 |
| ΔProteinuria, g/day/1.73 m2 | −1.31±3.99 |
| Clinical outcomes | |
| Rate of eGFR loss, mL/min/1.73 m2/year | 0 (−1.72 to 0.76) |
| 15-year survival free from combined event | 163 (93.68) |
| TA proteinuria ≤0.5 in patients with baseline proteinuria >0.5 g/day/1.73 | m2 4/53 (7.54) |
Values are n (%), means ± standard deviations, or medians (interquartile ranges). eGFR, estimated glomerular filtration rate; MAP, mean arterial pressure; M1, mesangial hypercellularity (>50 of glomeruli with mesangial hypercellularity); E1, presence of endocapillary hypercellularity; S1, presence of segmental glomerular sclerosis; T1–2, tubular atrophy/interstitial fibrosis in >25% of renal biopsy tissues; TA, time-average; RASB, renin-angiotensin blockade; CsA, cyclosporine A; IS, steroid/immunosuppressive drugs.
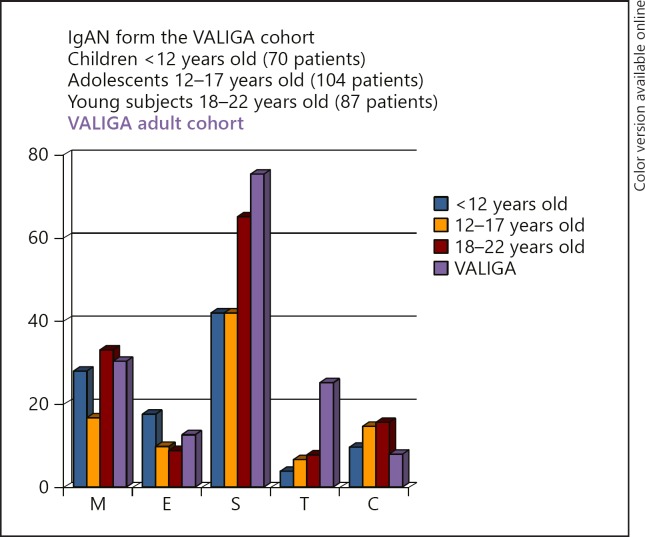
According to the Oxford Classification criteria, children showed M1 in 21.8%, E1 in 13.8%, S1 in 42.5%, T1–2 in 6.3%, and C1 in 14.9%. In comparison to the adult VALIGA cohort of 973 subjects, children showed a lower frequency of S1 and T1 lesions and a higher frequency of crescentic lesions (Fig. 1).
Fig. 1.
Frequency of renal pathology lesions in European children (174 subjects) and adults (973 subjects) enrolled in the VALIGA study [10], classified according to the Oxford Classification. IgAN, IgA nepropathy; M, mesangial hypercellularity (> 50% of glomeruli with 4 or more mesangial cells/area); E, endocapillary hypercellularity (present/absent); S, segmental glomerulosclerosis (present/absent); T, tubular atrophy/interstitial fibrosis (> 25%); C, crescents (present/absent).
During the follow-up, renin-angiotensin system blockers (RASBs) were adopted in 66.6% of the cases, and 50% of the children received corticosteroid/immunosuppressive treatment (CS/IS).
Two sub-cohorts of children were considered: 70 cases aged < 12 years and 104 cases aged 12–17 years. No significant differences in mean or median values of clinical data at renal biopsy were observed, while some differences were found for the MEST score, in agreement with the observation of the original Oxford study on a much lower number of children [12]. Mesangial hypercellularity was more frequent in children < 12 years of age. An opposite trend was found for tubular-interstitial damage; T1–2 was detected in only 4.3% of children < 12 years old but in 7.7% of those between 12 and 17 years of age (Fig. 1).
In the whole group of 174 children aged < 18 years, the rate of renal function decline was at a median of 0 mL/min/1.73 m2/year, with stabilization or improvement in 50% of children and decrease by less than −1.8 mL/min/1.73 m2/year in 25%. The combined endpoint of end-stage renal disease (ESRD) and/or 50% reduction in eGFR was reached in 11/174 (6.3%) children: 7 (4.0%) reached ESRD, 8 (4.6%) had a 50% loss of initial eGFR, and 4 (2.3%) had both.
The Kaplan-Meier survival curve of children showed a survival from the combined endpoint at 15 years of 94%, but a remission in time-averaged (TA) proteinuria to values < 0.5 g/day/1.73 m2 was found in only 7.5% of the cases who had initial proteinuria > 0.5 g/day/1.73 m2. During the follow-up, TA proteinuria was 0.56 (0.27–1.02) g/day/1.73 m2.
On univariate analysis, age was significantly associated (p < 0.01) with the rate of decline in eGFR. Mean MAP was also associated with eGFR slope, while no association was found with eGFR or baseline proteinuria. On multiple linear regression analysis, no data at renal biopsy were predictive of the rate of functional decline, while the value of proteinuria and blood pressure over the follow-up (TA proteinuria and TA MAP) predicted eGFR slope (p < 0.0001).
No MEST-C score, by univariate analysis, was predictive of progression (eGFR slope or the combined endpoint). Similarly, there was no association between MEST-C scores and the combined endpoint by CS/IS treatment.
The children enrolled in VALIGA included mild cases, with minimal proteinuria and acute glomerular lesions, but also active cases. Renal function decline was in the median absent, due to improvement in half of the cases, and the combined outcome of 50% reduction in eGFR or ESRD was attained in 6.3% of the cases only. This suggests that in children, a large part of the damage is potentially reversible possibly after CS/IS drugs, a treatment given in more than half of the European children.
In the VALIGA cohort, a relationship was found between age at renal biopsy and log-hazard of the combined endpoint, which increased in both treated and untreated young subjects with age until a plateau was reached at 23 years [11]. Some age-related protective effect was supposed, which seemed to be lost after the age of 23 years. The analysis of this expanded cohort of 216 subjects < 23 years of age, who did not differ in baseline clinical and pathological features from the 174 children aged < 18 years, proved the validation of the MST features as predictive of the combined event. Performing a survival tree multivariate analysis, young subjects presenting with M1 or proteinuria > 0.4 g/day/1.73 m2 were found to be at higher risk for IgAN progression. The tree analysis also provided an interesting observation in children < 16 years old with IgAN without mesangial hypercellularity (M0) and well-preserved eGFR (> 90 mL/min/1.73 m2), who had a high probability of proteinuria remission during follow-up. Moreover, in this subgroup of children, the benefits of CS/IS therapy reached statistical significance.
The long-term outcome of pediatric IgAN in the VALIGA cohort was investigated in a recent study updating the database and prolonging the follow-up from 4.7 to 7.0 years [13]. At 20 years from renal biopsy, 20% of the 174 children developed 50% eGFR loss or ESRD. The fact that a persistent follow-up proteinuria in the pediatric cases was at a median of > 0.5 g/day/1.73 m2 suggests the persistence of a significant risk factor for progression. Interestingly, over this long follow-up, the prognostic value of M1, S1, and T lesions was confirmed decades after renal biopsy, and it was independent of age, i.e., valid in children as well as in adults. The annual loss of eGFR was independently predicted in this long-term follow-up study by T lesions, but in untreated cases also by crescents. Again, this was independent of age and valid for children as well as for adults.
Data from Selected European Cohorts
In Europe, Sweden had a long-lasting interest in pediatric IgAN (Tommy Linné and Ulla Berg as major representatives), and Swedish studies reported pioneer data on the progression of this disease in children [14]. These pediatric nephrologists recently reported on their cohort of 99 children with IgAN aiming at validating the predictive value of the Oxford scores [15]. Their cohort included 25% of children with chronic kidney disease 2–4. Proteinuria was nephrotic in 15% of the cases. MEST scores were similar to those of the pediatric VALIGA cohort as far as M and E lesions were concerned, with a lower frequency of S1 and higher tubular-interstitial damage (M1, 31%; E1, 10%; S1, 23%; T1–2, 23%; C1, 17%). ESRD was reached by 15% of the cases and the combined endpoint by 22% of children. At univariate analysis, clinical baseline data (low eGFR, hypertension, and proteinuria) predicted outcome, and M1, E1, T1–2, and C were associated with combined outcome. However, on multivariate analysis, only in models constructed combining 1 histologic lesion with proteinuria at renal biopsy or at 1-year follow-up reached statistical significance. If clinical variables were not added, only models including 2 histological variables provided significant prediction by multivariate Cox analysis. A treatment was deserved by severe cases (34% of children). CS/IS drugs were most frequently used in children with E1 and C1.
Pediatric nephrology in Paris has a long and glorious history since the discovery of IgAN by Jean Berger in 1968 [16] and relevant clinicopathologic studies by Patrick Niaudet and Renée Habib as major representatives [17, 18]. Severe cases of crescentic pediatric IgAN referred to the Necker Hospital were described and successfully treated with pulse steroid therapy [18]. A recent report from the Necker and Debré Hospitals in Paris [19] has reported on the results of CS/IS therapy in a cohort of pediatric IgAN with rather unusual severe clinical and histological presentation. This cohort of 82 French children with IgAN presented with an age and gender distribution similar to those of to the VALIGA multi-country cohort, but the history of gross hematuria was more frequent (33%) as well as the presence of acute kidney failure (25%) or nephrotic range proteinuria (7% of the cases) at the time of biopsy. The MEST-C distribution was much more severe than in previously reported national or pan-European cohorts (M1, 80%; E1, 71%; S1, 61%; C, 46%), while tubular-interstitial lesions were exceptional (T1–2, 1%). The reason for this difference in MEST-C lesions is likely due to the short time elapsed between clinical onset and renal biopsy, since the median time from onset to biopsy was shorter than 2 months. The prompt indication to perform renal biopsy may favor the detection of acute or active renal lesions, while the prolongation of waiting time before renal biopsy is correlated with a reduction in M and E lesions but with an increase in tubulointerstitial damage [20]. The enrolment of early active cases without chronic lesions allowed a comparison, though not balanced, between 2 groups of children with different treatments as detailed below.
Treatment of Pediatric IgAN in Europe
In Europe as well as in Asia, one of the first treatments considered for IgAN, particularly in children with recurrent gross hematuria, has been tonsillectomy. However, the results gathered in Europe (though never confirmed by randomized controlled trials [RCTs]) failed to show significant benefits in adults, which did not encourage the choice of tonsillectomy in children with IgAN [21]. In Europe, tonsillectomy is indicated when tonsils are a true infectious focus, in case of recurrent tonsillitis (> 3 per year). In children with IgAN, this choice is usually made when there is an infective focus associated with recurrent gross hematuria.
The use of RASBs has a strong rationale in IgAN, not only because these drugs improve 2 principal progression factors (hypertension and proteinuria), but because they can inhibit the long series of potentially negative effects caused by angiotensin II on mesangial cells, particularly in the presence of mesangial immune deposits [22]. We performed the first European multicenter RCT including children and young subjects with a constant level of proteinuria (> 1 and < 3.5 g/day/1.73 m2 over the 3 months before enrolment) and normal or moderately reduced renal function [23]. Patients randomized to receive benazepril 0.2 mg/kg/day for 42 months showed a significant protection against a 30% decline in eGFR and/or worsening of proteinuria to the nephrotic range in comparison to the placebo group. A stable remission of proteinuria was observed in 56% of patients on treatment versus 8% of those on placebo. The multivariate analysis showed that treatment with RASB was an independent predictor of prognosis. This RCT was considered by the KDIGO to suggest RASB treatment for children with IgAN and persistent proteinuria > 1 g/day (suggesting also to expand the indication to children with proteinuria > 0.5 g/1.73 m2/day) [24]. Only in cases with persistent proteinuria after 3–6 months on RASB, the KDIGO suggested 6 months of CS/IS therapy.
However, in children, at variance with adults, it is not common to detect slowly progressive cases of IgAN, which can wait for 6 months on supportive care with RASB alone. Pediatric nephrologists have always been worried about a subtle progression not completely blocked by RASB and needing a more aggressive anti-inflammatory treatment with CS/IS drugs. In Japan, a RCT obtained good results treating children with severely proliferative IgAN over 2 years with CS/IS drugs in combination with antiplatelets and anticoagulants [25]. The results lasted for a decade after the end of treatment [26]; however, the side effects of this long-term heavy therapy were of some relevance. In Europe, much of the interest was focused on treating acute cases presenting with crescents and compromised renal function, with a few months of treatment obtaining encouraging results without side effects in some case series [18, 27, 28].
A great present debate in Europe as well as all over the word is about the use of steroids in adult patients with IgAN [29], and this is reflected in children as well. Differences in treatment exist among countries, but in general, in Europe CS are more used in pediatric than in adult patients, with the rationale of treating early acute disease before the development of irreversible changes.
A recent report from 2 hospitals in Paris, gathering 82 cases of pediatric IgAN (described above), suggests benefits of an early aggressive CS/IS therapy in children with IgAN and severe clinical and pathological features [19]. Children were divided retrospectively into 2 groups, one treated with CS (some in association with cyclophosphamide) and supportive care (RASB) and the other treated by RASB alone. The children in the 2 groups were very different for proteinuria (median baseline values 1.6 vs. 0.3 g/g protein/creatinine; p < 0.001). Moreover, MES-C scores were significantly different between the 2 groups. Hence, the choice of treating the children was dictated by a medical decision based on the severity and possible theoretical benefit of CS/IS because of the activity of the disease in individual cases. A great benefit of CS/IS therapy was reported, as eGFR in the CS/IS group was significantly improved after 6 months of treatment from 90 to 110 mL/min/1.73 m2 (p < 0.001). Proteinuria significantly decreased from 1.6 to 0.3 g/g (p < 0.001). In the supportive care group, eGFR and proteinuria remained stable. Podocytopathic features (tip lesions, found in 9 patients treated and in 3 in the control group) suggested a negative predictive value, though the limited number of cases renders this observation rather preliminary. MEST-C scores were not predictive of outcome, but this is likely to be due to the favorable evolution of most cases, which blunted the risk factor value. In conclusion, this study indicates in a rather large cohort of European children with IgAN and acute and active onset, who received renal biopsy within a few weeks from onset, that there is an improvement associated with CS/IS treatment, and no relevant adverse effects were reported. Though these results have been produced by a retrospective noncontrolled study, the excellent outcome of severe cases has to be considered. The use of CS in children with IgAN and active clinical and histological features is rather common in Europe. The severity and frequency of side effects is not reported to be severe, probably because prolonged treatment is avoided. The Japanese reports on an increase in T lesions in children in whom the decision for renal biopsy was delayed or when CS were not used [25, 26] have further stressed the attitude for Europe to treat progressive IgAN in children with aggressive therapy [18, 27, 28]. In Europe, the results of the long-term follow-up of the VALIGA study [13] clearly indicated that when T lesions develop, the disease enters a relentless phase of progression, and that M1, S1, and also C lesions in untreated cases can have an impact on disease outcome decades later in children as well as in adults.
These considerations tend to suggest adopting a rather aggressive therapy for children with IgAN, considering the long life expectancy and the limited side effects of short-term CS/IS therapy [30]. Anyway, future RCTs are needed to estimate the lowest dose of CS/IS which produces benefits and to carefully report the side effects also in the long-term follow-up [31].
Children with IgAN may present acute onset more frequently than adults, with acute nephritic syndrome or heavy proteinuria and sometimes with acute kidney injury, which is likely due to a peculiar response of the immune system to a triggering event. Remissions, either spontaneous or induced by treatment, are common [32], but the initial immune system dysregulation likely persists a lifetime in most cases, and a not negligible percentage of children with IgAN are exposed to progressive relentless loss of renal function. Treatments given for a long time, with minimal side effects, are the next hope for these children, and Europe and the whole nephrology community are awaiting new results, hoping to find less toxic and more efficient drugs, which are particularly needed in children with IgAN [33].
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.Coppo R, Gianoglio B, Porcellini MG, Maringhini S, Group of Renal Immunopathology of the Italian Society of Pediatric Nephrology and Group of Renal Immunopathology of the Italian Society of Nephrology Frequency of renal diseases and clinical indications for renal biopsy in children (report of the Italian National Registry of Renal Biopsies in Children) Nephrol Dial Transplant. 1998 Feb;13((2)):293–7. doi: 10.1093/oxfordjournals.ndt.a027821. [DOI] [PubMed] [Google Scholar]
- 2.Verde E, Quiroga B, Rivera F, López-Gómez JM. Renal biopsy in very elderly patients: data from the Spanish Registry of Glomerulonephritis. Am J Nephrol. 2012;35((3)):230–7. doi: 10.1159/000336307. [DOI] [PubMed] [Google Scholar]
- 3.Mizerska-Wasiak M, Turczyn A, Such A, Cichoń-Kawa K, Małdyk J, Miklaszewska M, et al. IgA Nephropathy in Children: A Multicenter Study in Poland. Adv Exp Med Biol. 2016;952:75–84. doi: 10.1007/5584_2016_65. [DOI] [PubMed] [Google Scholar]
- 4.Cho BS, Hahn WH, Cheong HI, Lim I, Ko CW, Kim SY, et al. A nationwide study of mass urine screening tests on Korean school children and implications for chronic kidney disease management. Clin Exp Nephrol. 2013 Apr;17((2)):205–10. doi: 10.1007/s10157-012-0672-9. [DOI] [PubMed] [Google Scholar]
- 5.Shibano T, Takagi N, Maekawa K, Mae H, Hattori M, Takeshima Y, et al. Epidemiological survey and clinical investigation of pediatric IgA nephropathy. Clin Exp Nephrol. 2016 Feb;20((1)):111–7. doi: 10.1007/s10157-015-1129-8. [DOI] [PubMed] [Google Scholar]
- 6.Gharavi AG, Kiryluk K, Choi M, Li Y, Hou P, Xie J, et al. Genome-wide association study identifies susceptibility loci for IgA nephropathy. Nat Genet. 2011 Mar;43((4)):321–7. doi: 10.1038/ng.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamagata K, Takahashi H, Tomida C, Yamagata Y, Koyama A. Prognosis of asymptomatic hematuria and/or proteinuria in men. High prevalence of IgA nephropathy among proteinuric patients found in mass screening. Nephron. 2002 May;91((1)):34–42. doi: 10.1159/000057602. [DOI] [PubMed] [Google Scholar]
- 8.Brunner FP, Fassbinder W, Broyer M, Oulès R, Brynger H, Rizzoni G, et al. Survival on renal replacement therapy: data from the EDTA Registry. Nephrol Dial Transplant. 1988;3((2)):109–22. [PubMed] [Google Scholar]
- 9.Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, et al. Working Group of the International IgA Nephropathy Network and the Renal Pathology Society The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009 Sep;76((5)):534–45. doi: 10.1038/ki.2009.243. [DOI] [PubMed] [Google Scholar]
- 10.Coppo R, Troyanov S, Bellur S, Cattran D, Cook HT, Feehally J, et al. VALIGA study of the ERA-EDTA Immunonephrology Working Group Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int. 2014 Oct;86((4)):828–36. doi: 10.1038/ki.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coppo R, Lofaro D, Camilla RR, Bellur S, Cattran D, Cook HT, et al. Risk factors for progression in children and young adults with IgA nephropathy: an analysis of 261 cases from the VALIGA European cohort. Pediatr Nephrol. 2017 Jan;32((1)):139–50. doi: 10.1007/s00467-016-3469-3. [DOI] [PubMed] [Google Scholar]
- 12.Coppo R, Troyanov S, Camilla R, Hogg RJ, Cattran DC, Cook HT, et al. Working Group of the International IgA Nephropathy Network and the Renal Pathology Society The Oxford IgA nephropathy clinicopathological classification is valid for children as well as adults. Kidney Int. 2010 May;77((10)):921–7. doi: 10.1038/ki.2010.43. [DOI] [PubMed] [Google Scholar]
- 13.Coppo R, D'Arrigo G, Tripepi G, Russo ML, Roberts IS, Bellur S, et al. Is there long-term value of pathology scoring in IgA nephropathy? A VALIGA update. Nephrol Dial Transplant Forthcoming. 2018 doi: 10.1093/ndt/gfy302. [DOI] [PubMed] [Google Scholar]
- 14.Linné T, Berg U, Bohman SO, Sigström L. Course and long-term outcome of idiopathic IgA nephropathy in children. Pediatr Nephrol. 1991 Jul;5((4)):383–6. doi: 10.1007/BF01453658. [DOI] [PubMed] [Google Scholar]
- 15.Edström Halling S, Söderberg MP, Berg UB. Predictors of outcome in paediatric IgA nephropathy with regard to clinical and histopathological variables (Oxford classification) Nephrol Dial Transplant. 2012 Feb;27((2)):715–22. doi: 10.1093/ndt/gfr339. [DOI] [PubMed] [Google Scholar]
- 16.Berger J, Hinglais N. [Intercapillary deposits of IgA-IgG] J Urol Nephrol (Paris) 1968 Sep;74((9)):694–5. [PubMed] [Google Scholar]
- 17.Habib R, Murcia I, Beaufils H, Niaudet P. Primary IgA nephropathies in children. Biomed Pharmacother. 1990;44((3)):159–62. doi: 10.1016/0753-3322(90)90003-r. [DOI] [PubMed] [Google Scholar]
- 18.Niaudet P, Murcia I, Beaufils H, Broyer M, Habib R. Primary IgA nephropathies in children: prognosis and treatment. Adv Nephrol Necker Hosp. 1993;22:121–40. [PubMed] [Google Scholar]
- 19.Cambier A, Rabant M, Peuchmaur M, Hertig A, Deschenes G, Couchoud C, et al. Immunosuppressive Treatment in Children With IgA Nephropathy and the Clinical Value of Podocytopathic Features. Kidney Int Rep. 2018 Mar;3((4)):916–25. doi: 10.1016/j.ekir.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shima Y, Nakanishi K, Hama T, Sato M, Mukaiyama H, Togawa H, et al. Biopsy timing and Oxford classification variables in childhood/adolescent IgA nephropathy. Pediatr Nephrol. 2015 Feb;30((2)):293–9. doi: 10.1007/s00467-014-2862-z. [DOI] [PubMed] [Google Scholar]
- 21.Feehally J, Coppo R, Troyanov S, Bellur SS, Cattran D, Cook T, et al. VALIGA study of ERA-EDTA Immunonephrology Working Group Tonsillectomy in a European Cohort of 1,147 Patients with IgA Nephropathy. Nephron. 2016;132((1)):15–24. doi: 10.1159/000441852. [DOI] [PubMed] [Google Scholar]
- 22.Takamatsu M, Urushihara M, Kondo S, Shimizu M, Morioka T, Oite T, et al. Glomerular angiotensinogen protein is enhanced in pediatric IgA nephropathy. Pediatr Nephrol. 2008 Aug;23((8)):1257–67. doi: 10.1007/s00467-008-0801-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coppo R, Peruzzi L, Amore A, Piccoli A, Cochat P, Stone R, et al. IgACE: a placebo-controlled, randomized trial of angiotensin-converting enzyme inhibitors in children and young people with IgA nephropathy and moderate proteinuria. J Am Soc Nephrol. 2007 Jun;18((6)):1880–8. doi: 10.1681/ASN.2006040347. [DOI] [PubMed] [Google Scholar]
- 24.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group KDIGO Clinical Practice Guideline for Glomerulonephritis. Immunoglobulin A nephropathy. Kidney Int Suppl. 2012;2:139–274. [Google Scholar]
- 25.Yoshikawa N, Ito H, Sakai T, Takekoshi Y, Honda M, Awazu M, et al. A controlled trial of combined therapy for newly diagnosed severe childhood IgA nephropathy. The Japanese Pediatric IgA Nephropathy Treatment Study Group. J Am Soc Nephrol. 1999 Jan;10((1)):101–9. doi: 10.1681/ASN.V101101. [DOI] [PubMed] [Google Scholar]
- 26.Kamei K, Nakanishi K, Ito S, Saito M, Sako M, Ishikura K, et al. Japanese Pediatric IgA Nephropathy Treatment Study Group Long-term results of a randomized controlled trial in childhood IgA nephropathy. Clin J Am Soc Nephrol. 2011 Jun;6((6)):1301–7. doi: 10.2215/CJN.08630910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coppo R, Basolo B, Giachino O, Roccatello D, Lajolo D, Mazzucco G, et al. Plasmapheresis in a patient with rapidly progressive idiopathic IgA nephropathy: removal of IgA-containing circulating immune complexes and clinical recovery. Nephron. 1985;40((4)):488–90. doi: 10.1159/000183527. [DOI] [PubMed] [Google Scholar]
- 28.Roccatello D, Ferro M, Coppo R, Mazzucco G, Quattrocchio G, Piccoli G. Treatment of rapidly progressive IgA nephropathy. Contrib Nephrol. 1995;111:177–82. doi: 10.1159/000423894. [DOI] [PubMed] [Google Scholar]
- 29.Coppo R. Corticosteroids in IgA Nephropathy: Lessons from Recent Studies. J Am Soc Nephrol. 2017 Jan;28((1)):25–33. doi: 10.1681/ASN.2016060647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarcina C, Tinelli C, Ferrario F, Pani A, De Silvestri A, Scaini P, et al. Changes in Proteinuria and Side Effects of Corticosteroids Alone or in Combination with Azathioprine at Different Stages of IgA Nephropathy. Clin J Am Soc Nephrol. 2016 Jun;11((6)):973–81. doi: 10.2215/CJN.02300215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai Q, Xie X, Wang J, Shi S, Liu L, Chen Y, et al. Severe Adverse Effects Associated With Corticosteroid Treatment in Patients With IgA Nephropathy. Kidney Int Rep. 2017 Feb;2((4)):603–9. doi: 10.1016/j.ekir.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shima Y, Nakanishi K, Hama T, Mukaiyama H, Togawa H, Sako M, et al. Spontaneous remission in children with IgA nephropathy. Pediatr Nephrol. 2013 Jan;28((1)):71–6. doi: 10.1007/s00467-012-2294-6. [DOI] [PubMed] [Google Scholar]
- 33.Yeo SC, Liew A, Barratt J. Emerging therapies in immunoglobulin A nephropathy. Nephrology (Carlton) 2015 Nov;20((11)):788–800. doi: 10.1111/nep.12527. [DOI] [PubMed] [Google Scholar]