Abstract
Objective
To study if the follicle-stimulating hormone receptor (FSHR) variant asparagine/serine in amino acid 680 (N680S) can predict hypersensitivity to gonadotropins in women undergoing assisted reproduction.
Patients and methods
In this retrospective study, 586 women undergoing their first in-vitro fertilisation treatment were enroled, and their FSHR N680S genetic variant was analysed. The main outcome measures were number of retrieved oocytes and any grade of ovarian hyperstimulation syndrome (OHSS). Experimental studies were performed on FSHR variants transfected into eukaryotic cells treated with 1–90 IU recombinant follicle-stimulating hormone. The receptors’ ability to induce a second messenger 3′,5′-cyclic AMP was measured.
Results
The proportion of women who developed OHSS was 6% (n=36). None of the women who developed this condition had the homozygous serine variant. The N680S polymorphism in the FSHR was associated with the condition, Ptrend (genotype)=0.004 and Pallelic (alleles)=0.04. Mean oocyte number was 11±6 in women without OHSS and 16±8 in women who developed OHSS (P=0.001), despite exposure to lower total hormonal dose in the latter group. The odds ratio for developing OHSS in carriers of the asparagine allele was 1.7 (95% confidence interval: 1.025–2.839, P=0.04). A higher receptor activity in cells expressing asparagine compared with the serine was also evident at all concentrations of recombinant follicle-stimulating hormone used (P<0.05 for all).
Conclusion
This study confirms previous findings regarding higher hormonal sensitivity in carriers of asparagine in the N680S position. These women are at higher risk for OHSS during in-vitro fertilisation. Genetic testing could identify those at highest risk to develop this adverse effect.
Keywords: controlled ovarian stimulation, follicle-stimulating hormone receptor, follicle-stimulating hormone, ovarian hyperstimulation syndrome, ovarian hyperstimulation, polymorphism
Introduction
In western societies, an increasing number of women postpone childbearing, which in turn is leading to a growing need of assisted reproductive technology (ART) 1,2. In Europe, in 2012, 0.2–6.1% of all children were born as a result of powerful ART. The most widely used ARTs are in-vitro fertilisation (IVF), in which sperms are allowed to fertilise oocytes in a laboratory dish, or intracytoplasmic sperm injection (ICSI), in which one sperm is injected into an oocyte and the resulting embryo is transferred into the uterus 3. In the US, this proportion of children is 1.7% in total. Moreover, in Asia, the tendency towards ART is increasing, and the number of treatments related to childlessness has grown every year during the past decades 4.
During assisted reproduction treatment, high doses of follicle-stimulating hormone (FSH) is used to stimulate the ovaries to obtain a high number of follicles. Subsequently, human chorionic gonadotropin (hCG) is administered for triggering maturation of the oocytes produced up to that time. Following fertilisation and embryo development, the best embryo is selected for transfer. There are marked individual differences in the hormonal response, ranging from lack of increased ovulation to hyperstimulation and more than 15 follicles. Although low responses are bothersome, too high responses are feared by all fertility specialists, as this can trigger ovarian hyperstimulation syndrome (OHSS), which can be a life-threatening condition.
This most unwanted adverse effect develops after hCG treatment, or later, when pregnancy is established and the endogenous hCG production has begun. Nowadays, OHSS can to some extent be avoided, as triggering final follicular maturation by gonadotropin-releasing-hormone (GnRH) agonist instead of hCG in antagonist protocols is commonly used. Nevertheless, some women still are hyperresponding 5.
Milder forms of hormonal sensibility were at the time of enrolment to the study affecting up to 30% of all IVF patients worldwide, whereas 0.5–5% developed clinically significant OHSS. Mild forms cause only some discomfort that resolves within some days, whereas OHSS is characterised by multifollicular ovaries and subsequently increased ovarian size, abdominal pain, increased vascular permeability and outflow of intracellular fluid to extracellular room with hemoconcentration and increased risk for thrombosis 6. These women need medical intervention with parenteral fluids, evacuation of ascites and pleural fluid, thrombose prophylaxis and eventually treatment of deep thrombosis 7,8. When a high risk for OHSS is present, cycles are cancelled before ovum pickup, or a freeze-all embryos approach is chosen. In subsequent IVF cycles, the FSH dose is adjusted to avoid this adverse effect. The borderline between less severe and clinically significant OHSS is not sharp and therefore numbers regarding incident cases in the literature varies. However, mortality owing to thrombosis and dysfunction of multiple organs caused by OHSS is very rare 9.
The pathophysiology is not completely understood, but known risk factors for developing OHSS are polycystic ovarian syndrome, low weight, young age or high serum concentration of anti-Müllerian hormone 10–12. However, there are also cases of familial gestational spontaneous OHSS reported 13–17. In all cases, heterozygosity for follicle-stimulating hormone receptor (FSHR) mutations was identified 16–19. All mutant FSHR variants were located in the transmembrane part of the receptor, which is involved in signalling into the cell and not in the hormone-binding domain (Fig. 1). Nevertheless, these mutated receptors displayed reduction of ligand specificity, allowing activation by hCG during pregnancy, indicating that high level of hCG is capable of stimulating mutated FSHRs even if the mutation is in the membrane binding part of the receptor. An intracellular FSHR mutation has also been reported in a young woman with recurrent spontaneous OHSS events, despite any pregnancy, finally resulting in ovarian torsion 17,20.
Fig. 1.
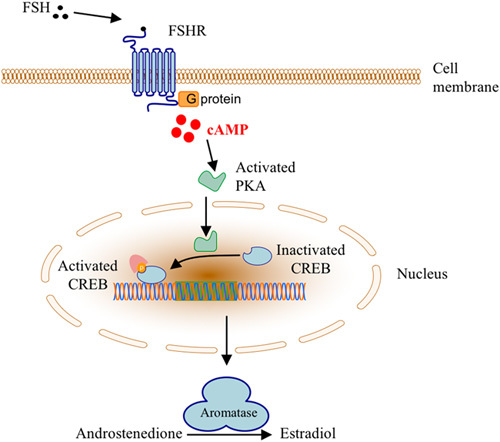
Cellular action of FSH and FSHR through the classical cyclic AMP/protein kinase A signalling pathway. FSH, follicle-stimulating hormone; FSHR, follicle-stimulating hormone receptor; CREB, 3′,5′-cyclic AMP (cAMP) response element-binding (protein); PKA, protein kinase A.
In iatrogenic cases of OHSS, mutations in the FSHR are absent 21, or at least rare, but a genetic component may still be operating. The FSHR gene encompasses two common single nucleotide polymorphisms (SNPs) T307A (rs6165) and N680S (rs6166), which are in high linkage disequilibrium. The AAT to AGT change, substituting asparagine with serine in codon 680, is located in the intracellular part of the receptor. Homozygous carriers of asparagine have in clinical studies on women undergoing IVF been associated with requirement of lower total dose of exogenous FSH for ovulation than those with NS or SS in the same position 22–24. This phenomenon has been interpreted as increased hormone sensibility for carriers of asparagine. If correct, these individuals should be more at risk for developing grades of OHSS than those with serine in the same position. The asparagine-variant should also be capable of inducing higher amounts of the second messenger 3′,5′-cyclic AMP (cAMP, Fig. 1) in cell-based experiments in such case.
The objective of this study was therefore to investigate the effect of the N680S polymorphism in women undergoing assisted reproduction and to also analyse the genetic variants in a cell-based setting.
Patients and methods
Patients
Data for this study were collected from 586 women. Details of the cohort have been described previously 25. In brief, all attended the Reproductive Medicine Centre, Skåne University Hospital, Malmö, Sweden, for their first cycle of IVF/ICSI treatment during the period 2007–2016. Inclusion criteria for all participants were regular menstruation cycle of 21–35 days, bilateral ovaries, BMI less than 30 kg/m2, age less than 40 years and non-smoking. Exclusion criteria were PCOS, amenorrhoea or unilateral ovarium. Cases with PCOS were excluded, as this category of patients has a high risk for OHSS; they follow another stimulation protocol where special caution is taken, such as lower starting dose of hormone, more frequent ultrasound investigations to monitor follicle development, and use of a shorter treatment protocol.
A venous blood sample was drawn before initiation of IVF/ICSI treatment for DNA extraction and subsequent genotyping of the rs6166 polymorphism N680S in the FSHR.
Patients underwent either a short antagonist protocol (43% of the cohort), using the GnRH antagonist Ganirelix (Orgalutran, Organon Ltd, Swords, Dublin, Ireland) or a long agonist protocol (57% of the cohort), with the GnRH agonist Nafarelin (Synarela; Pfizer AB, Sollentuna, Sweden) or Buserelin (Suprecur; Sanofi AB, Stockholm, Sweden).
Ovarian hyperstimulation was performed using individualised flexible doses of either Follitrophin alpha (GONAL-f; Merck-Serono, Darmstad, Germany), Follitropin beta (Puregon; Organon Ltd, Ireland), Urofollitropin (Fostimon; Institut BIochimique SA, Lugano, Switzerland) or Menotropin (Menopur; Ferring GmbH, Kiel, Germany). Follicle development was monitored by vaginal ultrasound on stimulation days 6–8, and if needed, doses were adjusted. When three or more follicles reached 17 mm, hCG (Ovitrelle; Merck-Serono) was administrated, and 35–36 h later, transvaginal oocyte retrieval was performed. Triggering with GnRH agonist in antagonist protocol, which is nowadays common in hyperresponders, was not routine at the time of the inclusion of the patients. At that time, to reduce the risk of OHSS, total freezing of oocytes was used when the hyperresponse was a fact.
Ovarian hyperstimulation syndrome was defined according to the criteria suggested by Humaidan et al. 5. In short, in addition to classical symptoms of OHSS (fatigue, nausea, vomiting, abdominal bloating, shortness of breath and weight gain) at least one positive finding at further screening was necessary to diagnose OHSS, that is, ultrasound-confirmed ascites, elevated liver enzymes, hemoconcentration, elevated creatinine or electrolyte imbalance. Data on clinical status and blood tests were retrieved from medical records.
All women participated with informed consent. The study was approved by the Regional Ethical Committee, Lund University, Lund, Sweden.
Genotyping of the follicle-stimulating hormone receptor
Genomic DNA was extracted from peripheral leucocytes using standard procedures. The SNP at amino acid position 680 (rs6166) in the FSHR was analysed by allele-specific PCR as previously described 26. The PCR results were confirmed by direct sequencing of 20 samples on an eight-capillary Applied Biosystems sequencing gear (Applied Biosystems, Stockholm, Sweden).
Site-directed mutagenesis
The FSHR cDNA (OriGene Technologies Inc., Rockville, Maryland, USA) was cloned into the pCMV6-XL5 vector (OriGene Technologies Inc.), by EcoRI restriction in the 5′ end and Sal I restriction in the 3′ end of the insert. Amino acid 680 was mutated from AAT (asparagine) to AGT (serine) by site-directed mutagenesis using the QuickChange II-E Site-Directed Mutagenesis Kit (Strategene, La Jolla, California, USA) according to the manufacturer’s instructions. For mutagenesis, primers with the following sequences were used: forward 5′-CAGCTCCCAGAGTCACCAGTGGTTCCACTTACATACTTG-3′ and reverse 5′-CAAGTATGTAAGTGGAACCACTGGTGACTCTGGGAGCTG-3′. The mutation was confirmed by direct sequencing on a 16-capillary Applied Biosystems 3130 sequencing gear (Applied Biosystems).
Transactivation studies
For transactivation, 1 µg of the plasmids containing the genetic variants was transiently transfected using JetPEI (PolyPlus Transfection, Illkirch, France) according to the manufacturer’s instructions, into ∼150 000 COS-1 cells (ECACC, Salisbury, UK), seeded into 12-well plates in Dulbecco’s modified Eagle’s medium (DMEM; Gibco Invitrogen, Carlsbad, California, USA), supplemented with 10% foetal bovine serum (FBS; Biological Industries, Beit HaEmek, Israel) and 1% penicillin–streptomycin (5000 Upenicillin and 5 mg/ml streptomycin; Sigma-Aldrich, Stockholm, Sweden). An empty vector was used as a transfection and background control. Twenty-four hours after transfection, cells were washed twice with Dulbecco’s PBS (Gibco Invitrogen) and incubated for 1 h at 37°C, 5% CO2, in phenol red-free and serum-free DMEM (LifeTechnologies, Stockholm, Sweden). Cells were stimulated with 0, 1, 10, or 90 IU of Follitropin alpha (GONAL-f; Merk-Serono) and incubated for 1 h at 37°C, 5% CO2, in phenol red-free and serum-free DMEM. Cell culture medium was aspirated and centrifuged for 20 min, 1000g at RT. Endogenous phosphodiesterases in the medium were inactivated by incubation for 5 min at 95°C. Cells were washed once with PBS and lysed with RIPA buffer (LifeTechnologies).
The capacity of FSHR variants to induce cAMP was measured in the cell culture medium using a cAMP enzyme-linked immunosorbent assay kit (ENZO Life Sciences, Lausen, Switzerland) and adjusted for total protein concentrations in the cell lysates, measured by use of bicinchoninic acid protein assay reagent (Thermo Fisher Scientific Inc., Waltham, Massachusetts, USA). All experiments were performed in duplicates and repeated three times.
For measurement of intracellular cAMP, COS-1 cells stably transfected with the pGloSensor-22 cAMP plasmid (Promega, Madison, Wisconsin, USA) were seeded into 6-cm dishes (106 cells/dish) and next day transfected with pCMV6-XL5 vector (OriGene Technologies Inc., Rockville, Maryland, USA) expressing FSHR N680, FSHR S680 or empty mock pCMV4 vector (EV). After 24 h, the cells from the dishes were trypsinized and seeded into the inner part of a Costar white flat bottom 96-well plate at a density of 40 000 cells/well. Next day, the medium was replaced with 100 µl of equilibration medium (88% CO2-independent medium+10% FBS+2% GloSensor cAMP reagent stock solution), and cells were pre-equilibrated for 2 h at RT before the addition of the tested compounds. Pre-read measurement was performed for 10 min, and results were used to normalise the data. Recombinant follicle-stimulating hormone (rFSH) (GONAL-f; Merck-Serono) at the final concentration of 10 IU/ml, forskolin (10 µmol/l) or PBS (negative control) was added (the final volume in each well was 110 µl), and data were collected every 30 s with integration time of 1000 ms for 50 min. Luminescence was measured at 25°C by using Infinite 200 plate reader and Magellan software (Tecan, Grödig, Austria). The experiment was performed in triplicates and repeated three times.
For comparing the transfection efficiency between the FSHR variants, the following green fluorescent protein (GFP) tagged receptors were used: OHu22510C_G2039A_pcDNA3.1(+)-C-eGFP (the N680 variant) and OHu22510C_pcDNA3.1(+)-C-eGFP (the S680 variant) (GenScript, Leiden, The Netherlands). In brief, ∼200 000 cells were seeded in six-well plates, and 24 h later, they were transfected with 1.5 µg plasmid DNA using jetPei (Polyplus Transfection) according to manufacturer’s instructions. As a positive control, a plasmid encoding the human luteinizing hormone receptor conjugated with GFP was used, and nontransfected cells served as negative control. After transfection, the cells were incubated for 24 h, trypsinized, and harvested. They were then centrifuged at 300g for 5 min and then washed in PBS supplemented with 10% FBS (Biological Industries, Beit HaEmek, Israel) twice before the proportion of GFP positive cells was measured in a CytoFLEX Flow Cytometer (Beckman Coulter, Brea, California, USA). The experiment was repeated twice and run in duplicate wells. Cells were gated for GFP signals based on the background signal from the nontransfected cells. The proportion of positively stained cells out of 10 000 counts was used for comparison of the transfection efficiency. Data acquisition and analysis was carried out by the CytExpert Software for the CytoFLEX platform (Beckman Coulter).
Statistical analysis
The SNP was studied for association with OHSS by using the χ2 for linearity trend test or Fisher’s exact test where appropriate. The odds ratio and associated 95% confidence interval were computed when analysing the allele frequencies. Differences in age, BMI, total FSH dose and number of oocytes were calculated with the independent samples t-test.
When calculating differences between genetic variants in means of cAMP production in vitro and transfection efficiency the two sample assuming equal variance t-test was used.
Data were analysed using SPSS software version 23 (SPSS, Inc., Chicago, Illinois, USA). All statistical calculations were two tailed, and a P value less than 0.05 was considered statistically significant.
Results
Odds ratio for ovarian hyperstimulation syndrome
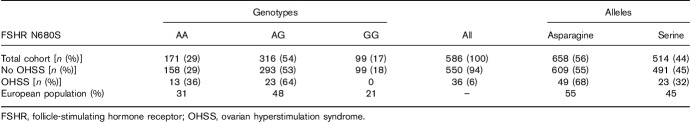
In the total cohort of 586 women, the genotype distribution for the FSHR was AA 29%, AG 54% and GG 17%. The frequencies of genotypes in the total cohort did not differ from general European population (http://www.ensembl.org; Table 1). The OHSS incidence was 6% (36 cases): 13 with the AA and 23 with the AG and no cases with the GG genotype. The expected number was six.
Table 1.
Genotype and allele distribution in the study cohort and the general European population (http://www.ensembl.org)
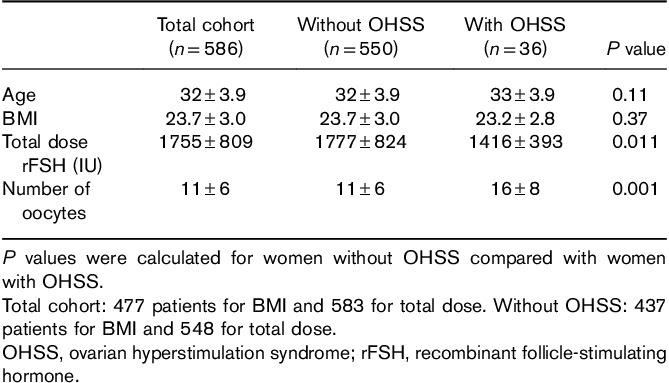
No difference in age or BMI was found when comparing women who developed OHSS with those who did not (Table 2). Most patients (76%) in the study were treated with GONAL-f (Merck-Serono). The total treatment dose was significantly lower in women with OHSS compared with those who did not develop OHSS (1416 IU for OHSS vs. 1777 IU for no OHSS, P=0.011). These women also produced significantly more oocytes compared to women without OHSS (16±8 vs. 11±6, P=0.001).
Table 2.
Characteristics of women included in the study presented as mean±SD value
The N680S polymorphism was associated with OHSS (Ptrend=0.004 and Pallele=0.038), with carriers of asparagine having an odds ratio for OHSS of 1.7, 95% confidence interval: 1.0–2.8, P=0.04, in comparison with carriers of serine.
Transactivation studies
In COS cells transfected with hFSHR, the homozygous asparagine variant displayed higher extracellular cAMP production per milligram total protein compared with the serine variant at all concentrations of rFSH tested (1 IU 54 vs. 25 pmol/mg, P<0.000014; 10 IU 58 vs. 38 pmol/mg, P=0.0043; and 90 IU 101 vs. 61 pmol/mg, P=0.0019; Fig. 2).
Fig. 2.
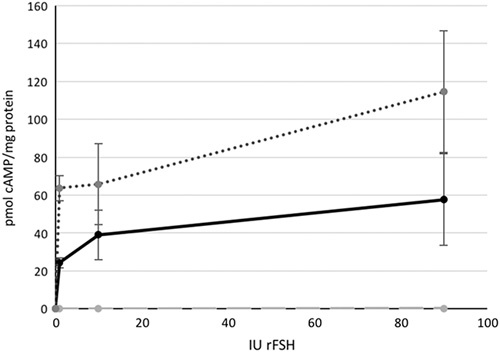
The FSHR activity measured as cyclic AMP (cAMP) adjusted for total protein amount, in response to 0, 1, 10 and 90 IU rFSH. Dotted line homozygous asparagine, solid line homozygous serine and dashed line negative control. FSHR, follicle-stimulating hormone receptor; rFSH, recombinant follicle-stimulating hormone.
The intracellular cAMP production was significantly higher in the homozygous asparagine variant of the FSHR compared with the serine variant (Fig. 3). Statistical calculations were done at three time points: 15 min (P=0.003), 40 min (P=0.001) and 50 min (P=0.001).
Fig. 3.
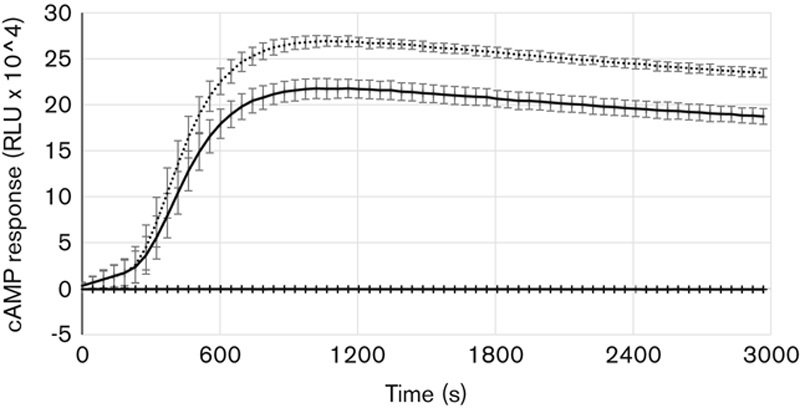
The FSHR activity measured as intracellular cyclic AMP (cAMP) adjusted for background luminescence, in response to 10 IU/ml rFSH. Dotted line homozygous asparagine, solid line homozygous serine, and grey line untreated negative control. FSHR, follicle-stimulating hormone receptor; rFSH, recombinant follicle-stimulating hormone.
The transfection efficiency analysis by FACS showed that the average proportion of transfected cells for the N680 variant was 25% (range: 22.8–26.6%) and for the S680 was 26% (range: 24.4–27.2%) (P=0.563) (Fig. 4a and b).
Fig. 4.
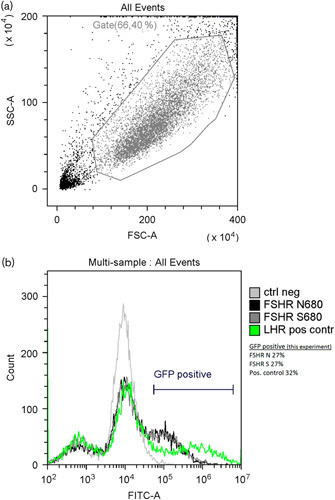
(a) Representative sample of gating of cells during FACS analysis. SSC-A side scatter, FSC-A forward scatter. (b) Representative histogram of analysed cells from one experiment, fluorescent intensity (FITC-A) versus number of events (count). Light grey line – negative control cells (background), black line – FSHR asparagine variant, dark grey line – FSHR serine variant, green line – positive control. The green fluorescent protein-positive bar indicates the cells with a fluorescence above background; the percentage of positive cells for the experiment is indicated. FSHR, follicle-stimulating hormone receptor.
Discussion
The main result of this work was that the N680S variant in the FSHR gene was associated with considerably increased risk for OHSS, almost doubled, in carriers of the asparagine variant, despite the fact that these women were treated with on average 20% lower hormonal dose for ovarian stimulation. This finding is in accordance with many previous clinical studies showing that women who are homozygous asparagine in amino acid 680 can be treated with lower doses of FSH when undergoing IVF 22–24.
In this study, none of the women who developed OHSS were homozygous serine, although the expected number according to the frequency in the study cohort would be six.
However, this finding is contradicting a previous report, showing lack of association between FSHR genotype and OHSS, although the asparagine variant was more common among severe OHSS cases 27. When the data from Daelemans et al. 27 were combined with the data on Brazilian women 28, there was still no association between FSHR and OHSS, but the combined data indicated that women with severe OHSS more often were homozygous serine in amino acid 680 in the FSHR. This discrepancy could probably be due to differences in the definition of OHSS. In the cohort used for the present study, no case had severe OHSS or needed hospitalisation. Nevertheless, this study did not show that women with the SS genotype would never develop OHSS but that the risk of developing OHSS is lower for them.
Present finding is also in contrast with a previous meta-analysis including 16 studies 29, this could be because that in the meta-analysis only two studies were included that reported the OHSS incidence, one from India 30 and one from Europe 31. The first was a very small study containing only 50 patients in total, and of those 15 had OHSS, and the other one including only seven cases who developed OHSS. Moreover, NN was compared with NS and SS combined, which could be a strategy that missed the possibility to show other differences than poor response. The analysis of combined genotypes disregards the previously mentioned fact that the SS variant has been linked to lower number of oocytes and higher FSH dose required to ovulate 23,24,32, indicating a lower FSH sensibility in those patients. This was also concluded in a meta-analysis including 4020 women showing that women homozygous for SS had a higher risk of poor response compared with the NS or NN 33.
In this study, the clinical finding was confirmed in cell-based assays, showing that the asparagine variant had a higher activity compared with the serine variant at all concentrations of rFSH tested. The fact that the two methods used for extracellular and intracellular cAMP measurements gave similar results, and that no difference in transfection efficiency between the genotypes was found, further strengthens this conclusion. In previous in-vitro studies on granulosa cells 34, on COS-7 cells 35 and 293T cells 24, no statistically significant differences between the genotypes in induction of cAMP was found. An explanation for the differences between results could be that the previous studies used hormone concentrations that were much lower. Moreover, the granulosa cells used came from women already treated with rFSH 34; these cells may well therefore have been refractory to further gonadotropin stimulation, and consequently, no differences in cAMP response could be noted.
A mechanistic explanation is not obvious. The fact that OHSS occurs at the time when hCG is administered would rather be linked to the luteinizing hormone receptor than to the FSHR. One could speculate that the asparagine variant of the FSHR allows too high follicular proliferation in response to rFSH and that the subsequent extensive hCG administration to induce luteinization of these follicles, is leading to loss of ligand specificity, activation of downstream signals, and subsequently a hyperreaction in terms of vascular permeability triggering this syndrome in women with genetic predisposition. This has previously been discussed regarding activating mutations and spontaneous OHSS where mutated FSHR responds to hCG and in some cases also thyroid-stimulating hormone 14,17,19,36.
Conclusion
Women with asparagine in the FSHR N680S position are hyperresponsive to FSH and consequently are at increased risk for OHSS when undergoing IVF treatment. Genetic testing may be beneficial to add to already known predictors to identify these women.
Acknowledgements
This work was supported by Interreg IV A, EU (grant 167158) and ALF governments grant (F2014/354). Merck-Serono (Darmstadt, Germany) supported the enrolment of the patients.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Luke B. Pregnancy and birth outcomes in couples with infertility with and without assisted reproductive technology: with an emphasis on US population-based studies. Am J Obstet Gynecol 2017; 217:270–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prag P, Mills MC. Cultural determinants influence assisted reproduction usage in Europe more than economic and demographic factors. Hum Reprod 2017; 32:2305–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European IVF-Monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE), Calhaz-Jorge C, de Geyter C, Kupka MS, de Mouzon J, Erb K, Mocanu E, et al. Assisted reproductive technology in Europe, 2012: results generated from European registers by ESHRE. Hum Reprod 2016; 31:1638–1652.27496943 [Google Scholar]
- 4.Dyer S, Chambers GM, de Mouzon J, Nygren KG, Zegers-Hochschild F, Mansour R, et al. International Committee for Monitoring Assisted Reproductive Technologies world report: Assisted Reproductive Technology 2008, 2009 and 2010. Hum Reprod 2016; 31:1588–1609. [DOI] [PubMed] [Google Scholar]
- 5.Humaidan P, Nelson SM, Devroey P, Coddington CC, Schwartz LB, Gordon K, et al. Ovarian hyperstimulation syndrome: review and new classification criteria for reporting in clinical trials. Hum Reprod 2016; 31:1997–2004. [DOI] [PubMed] [Google Scholar]
- 6.Kaiser UB. The pathogenesis of the ovarian hyperstimulation syndrome. N Engl J Med 2003; 349:729–732. [DOI] [PubMed] [Google Scholar]
- 7.Delvigne A, Rozenberg S. Epidemiology and prevention of ovarian hyperstimulation syndrome (OHSS): a review. Hum Reprod Update 2002; 8:559–577. [DOI] [PubMed] [Google Scholar]
- 8.Schenker JG, Ezra Y. Complications of assisted reproductive techniques. Fertil Steril 1994; 61:411–422. [DOI] [PubMed] [Google Scholar]
- 9.Venn A, Hemminki E, Watson L, Bruinsma F, Healy D. Mortality in a cohort of IVF patients. Hum Reprod 2001; 16:2691–2696. [DOI] [PubMed] [Google Scholar]
- 10.Alper MM, Smith LP, Sills ES. Ovarian hyperstimulation syndrome: current views on pathophysiology, risk factors, prevention, and management. J Exp Clin Assist Reprod 2009; 6:3. [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar P, Sait SF, Sharma A, Kumar M. Ovarian hyperstimulation syndrome. J Hum Reprod Sci 2011; 4:70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Practice Committe of the American Society for Reproductive M. Ovarian hyperstimulation syndrome. Fertil Steril 2003; 80:1309–1314. [DOI] [PubMed] [Google Scholar]
- 13.Cepni I, Erkan S, Ocal P, Ozturk E. Spontaneous ovarian hyperstimulation syndrome presenting with acute abdomen. J Postgrad Med 2006; 52:154–155. [PubMed] [Google Scholar]
- 14.Montanelli L, Delbaere A, Di Carlo C, Nappi C, Smits G, Vassart G, et al. A mutation in the follicle-stimulating hormone receptor as a cause of familial spontaneous ovarian hyperstimulation syndrome. J Clin Endocrinol Metab 2004; 89:1255–1258. [PubMed] [Google Scholar]
- 15.Olatunbosun OA, Gilliland B, Brydon LA, Chizen DR, Pierson RA. Spontaneous ovarian hyperstimulation syndrome in four consecutive pregnancies. Clin Exp Obstet Gynecol 1996; 23:127–132. [PubMed] [Google Scholar]
- 16.Uchida S, Uchida H, Maruyama T, Kajitani T, Oda H, Miyazaki K, et al. Molecular analysis of a mutated FSH receptor detected in a patient with spontaneous ovarian hyperstimulation syndrome. PLoS One 2013; 8:e75478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vasseur C, Rodien P, Beau I, Desroches A, Gerard C, de Poncheville L, et al. A chorionic gonadotropin-sensitive mutation in the follicle-stimulating hormone receptor as a cause of familial gestational spontaneous ovarian hyperstimulation syndrome. N Engl J Med 2003; 349:753–759. [DOI] [PubMed] [Google Scholar]
- 18.De Leener A, Caltabiano G, Erkan S, Idil M, Vassart G, Pardo L, et al. Identification of the first germline mutation in the extracellular domain of the follitropin receptor responsible for spontaneous ovarian hyperstimulation syndrome. Hum Mutat 2008; 29:91–98. [DOI] [PubMed] [Google Scholar]
- 19.Smits G, Olatunbosun O, Delbaere A, Pierson R, Vassart G, Costagliola S. Ovarian hyperstimulation syndrome due to a mutation in the follicle-stimulating hormone receptor. N Engl J Med 2003; 349:760–766. [DOI] [PubMed] [Google Scholar]
- 20.Hugon-Rodin J, Sonigo C, Gompel A, Dode C, Grynberg M, Binart N, et al. First mutation in the FSHR cytoplasmic tail identified in a non-pregnant woman with spontaneous ovarian hyperstimulation syndrome. BMC Med Genet 2017; 18:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerkela E, Skottman H, Friden B, Bjuresten K, Kere J, Hovatta O. Exclusion of coding-region mutations in luteinizing hormone and follicle-stimulating hormone receptor genes as the cause of ovarian hyperstimulation syndrome. Fertil Steril 2007; 87:603–606. [DOI] [PubMed] [Google Scholar]
- 22.Lledo B, Dapena P, Ortiz JA, Morales R, Llacer J, Bernabeu R. Clinical efficacy of recombinant versus highly purified follicle-stimulating hormone according to follicle-stimulating hormone receptor genotype. Pharmacogenet Genomics 2016; 26:288–293. [DOI] [PubMed] [Google Scholar]
- 23.Perez Mayorga M, Gromoll J, Behre HM, Gassner C, Nieschlag E, Simoni M. Ovarian response to follicle-stimulating hormone (FSH) stimulation depends on the FSH receptor genotype. J Clin Endocrinol Metab 2000; 85:3365–3369. [DOI] [PubMed] [Google Scholar]
- 24.Sudo S, Kudo M, Wada S, Sato O, Hsueh AJ, Fujimoto S. Genetic and functional analyses of polymorphisms in the human FSH receptor gene. Mol Hum Reprod 2002; 8:893–899. [DOI] [PubMed] [Google Scholar]
- 25.Lindgren I, Baath M, Uvebrant K, Dejmek A, Kjaer L, Henic E, et al. Combined assessment of polymorphisms in the LHCGR and FSHR genes predict chance of pregnancy after in vitro fertilization. Hum Reprod 2016; 31:672–683. [DOI] [PubMed] [Google Scholar]
- 26.Lindgren I, Giwercman A, Axelsson J, Lundberg Giwercman Y. Association between follicle-stimulating hormone receptor polymorphisms and reproductive parameters in young men from the general population. Pharmacogenet Genomics 2012; 22:667–672. [DOI] [PubMed] [Google Scholar]
- 27.Daelemans C, Smits G, de Maertelaer V, Costagliola S, Englert Y, Vassart G, et al. Prediction of severity of symptoms in iatrogenic ovarian hyperstimulation syndrome by follicle-stimulating hormone receptor Ser680Asn polymorphism. J Clin Endocrinol Metab 2004; 89:6310–6315. [DOI] [PubMed] [Google Scholar]
- 28.d’Alva CB, Serafini P, Motta E, Latronico AC, Mendonca BB. Letter re: FSH receptor polymorphisms and iatrogenic ovarian hyperstimulation. J Clin Endocrinol Metab 2005; 90:4978–4982. [DOI] [PubMed] [Google Scholar]
- 29.Tang H, Yan Y, Wang T, Zhang T, Shi W, Fan R, et al. Effect of follicle-stimulating hormone receptor Asn680Ser polymorphism on the outcomes of controlled ovarian hyperstimulation: an updated meta-analysis of 16 cohort studies. J Assist Reprod Genet 2015; 32:1801–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Achrekar SK, Modi DN, Desai SK, Mangoli VS, Mangoli RV, Mahale SD. Follicle-stimulating hormone receptor polymorphism (Thr307Ala) is associated with variable ovarian response and ovarian hyperstimulation syndrome in Indian women. Fertil Steril 2009; 91:432–439. [DOI] [PubMed] [Google Scholar]
- 31.Mohiyiddeen L, Newman WG, Cerra C, McBurney H, Mulugeta B, Roberts SA, et al. A common Asn680Ser polymorphism in the follicle-stimulating hormone receptor gene is not associated with ovarian response to gonadotropin stimulation in patients undergoing in vitro fertilization. Fertil Steril 2013; 99:149–155. [DOI] [PubMed] [Google Scholar]
- 32.Behre HM, Greb RR, Mempel A, Sonntag B, Kiesel L, Kaltwasser P, et al. Significance of a common single nucleotide polymorphism in exon 10 of the follicle-stimulating hormone (FSH) receptor gene for the ovarian response to FSH: a pharmacogenetic approach to controlled ovarian hyperstimulation. Pharmacogenet Genomics 2005; 15:451–456. [DOI] [PubMed] [Google Scholar]
- 33.Pabalan N, Trevisan CM, Peluso C, Jarjanazi H, Christofolini DM, Barbosa CP, et al. Evaluating influence of the genotypes in the follicle-stimulating hormone receptor (FSHR) Ser680Asn (rs6166) polymorphism on poor and hyper-responders to ovarian stimulation: a meta-analysis. J Ovarian Res 2014; 7:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nordhoff V, Sonntag B, von Tils D, Gotte M, Schuring AN, Gromoll J, et al. Effects of the FSH receptor gene polymorphism p.N680S on cAMP and steroid production in cultured primary human granulosa cells. Reprod Biomed Online 2011; 23:196–203. [DOI] [PubMed] [Google Scholar]
- 35.Simoni M, Gromoll J, Hoppner W, Kamischke A, Krafft T, Stahle D, et al. Mutational analysis of the follicle-stimulating hormone (FSH) receptor in normal and infertile men: identification and characterization of two discrete FSH receptor isoforms. J Clin Endocrinol Metab 1999; 84:751–755. [DOI] [PubMed] [Google Scholar]
- 36.Delbaere A, Smits G, Olatunbosun O, Pierson R, Vassart G, Costagliola S. New insights into the pathophysiology of ovarian hyperstimulation syndrome. What makes the difference between spontaneous and iatrogenic syndrome? Hum Reprod 2004; 19:486–489. [DOI] [PMC free article] [PubMed] [Google Scholar]