Abstract
The effect of chemotherapy on programmed cell death-ligand 1 (PD-L1) expression has been previously studied in lung cancer, while the results remain controversial. The aim of this study was to investigate the variation of PD-L1 expression after neoadjuvant chemotherapy and explore the association between chemotherapy response, prognosis and the variation of PD-L1 expression in lung cancer patients. A total of 63 lung cancer patients who received platinum-based neoadjuvant chemotherapy and subsequently underwent surgical resection were selected. PD-L1 expression on tumor cells (TC) and tumor-infiltrating immune cells (IC) was assessed by immunohistochemistry using 22C3 monoclonal antibody in these 63 matched lung cancer specimens before and after neoadjuvant chemotherapy. The positivity of PD-L1 on TC changed from 17.5% to 39.7% after neoadjuvant chemotherapy and the positivity of PD-L1 on IC changed from 19.0% to 71.4% after neoadjuvant chemotherapy. The elevation of PD-L1 expression on TC after neoadjuvant chemotherapy was more frequently observed in patients achieving stable disease or progressive disease than in patients achieving partial response (P=0.026). Patients with elevated PD-L1 expression on TC after neoadjuvant chemotherapy showed a trend to have a shorter progression-free survival than patients without elevated PD-L1 expression on TC, although the difference was not statistically significant in multivariate analysis (hazard ratio=2.38, 95% confidence interval=0.99–5.73, P=0.053). PD-L1 expression can be elevated by chemotherapy in lung cancer. Furthermore, elevation of PD-L1 expression on TC after neoadjuvant chemotherapy was associated with reduced chemotherapy response and inferior progression-free survival in patients with lung cancer.
Key Words: programmed death ligand 1 (PD-L1), chemotherapy, lung cancer, survival
Lung cancer remains the leading cause of cancer-related mortality worldwide.1 In the past decades, molecular targeted therapy had led to a great shift in the treatment landscape of advanced lung cancer patients harboring genetic aberrations, such as EGFR mutation and ALK translocation.2–8 For patients without genetic aberrations, platinum-based chemotherapy is the most commonly used therapeutic modality in the treatment of advanced lung cancer and the prognosis remains poor. Currently, blockade of programmed cell death-1 (PD-1)/programmed cell death-ligand 1 (PD-L1) pathway with immune checkpoint inhibitors has shown feasibility and efficacy in immunotherapy for advanced lung cancer and immunohistochemical (IHC) analysis of PD-L1 expression on tumor cells (TC) has been demonstrated to be a predictive biomarker in selecting appropriate patients for immunotherapy.9–13
Today, immunotherapy can be used as first-line treatment for advanced or metastatic patients without gene abnormality or with progression from prior targeted therapy, especially for non–small cell lung cancer patients with PD-L1 expression ≥50%.13 For patients with low PD-L1 expression, chemotherapy combined with immunotherapy also achieved better therapeutic effect compared with chemotherapy alone.14 Recently, several previous studies have shown that chemotherapy is associated with variation of PD-L1 expression, but the results remain inconsistent and inconclusive. For example, Song et al15 and Sun et al16 reported that neoadjuvant chemotherapy upregulated the expression of PD-L1 on TC in lung cancer patients, whereas Sheng et al17 and Moldvay et al18 found that chemotherapy decreased PD-L1 expression on TC in lung cancer patients.
We conducted this study using Dako 22C3 assay to explore the role of neoadjuvant chemotherapy in variation of PD-L1 expression on both TC and tumor-infiltrating immune cells (IC) evaluated by IHC staining, and the association between chemotherapy response, prognosis and the variation of PD-L1 expression in lung cancer patients.
MATERIALS AND METHODS
Patients and Samples
A total of 63 lung cancer patients who received platinum-based neoadjuvant chemotherapy and subsequently underwent surgical resection between September 2011 and March 2018 were enrolled in our study. The eligibility criteria for patients included in this study were as follows: (1) had a pathologic diagnosis of primary lung cancer by biopsy before neoadjuvant chemotherapy; (2) received 1–4 cycles of neoadjuvant chemotherapy before surgery without other preoperative treatments; (3) with sufficient tumor specimens for assessment of PD-L1 expression by IHC. Formalin-fixed paraffin-embedded paired lung cancer specimens obtained before and after neoadjuvant chemotherapy of these 63 patients were collected from the archive of the Department of Pathology, Cancer Hospital, Chinese Academy of Medical Sciences (Beijing, China). This study was approved by The Ethics Committee of the Cancer Hospital, Chinese Academy of Medical Sciences.
IHC Analysis of PD-L1 Expression
IHC analysis of PD-L1 expression was performed as previously reported.19 In brief, tissue sections (4 mm thick) were cut from formalin-fixed, paraffin-embedded blocks containing representative tumors and processed for PD-L1 IHC. The presence of at least 100 viable TC was required for the specimen to be considered adequate for quantification of PD-L1 expression. The expression of PD-L1 was evaluated by IHC staining using Dako 22C3 monoclonal antibody. PD-L1 IHC 22C3 pharmDx was performed on the DAKO Autostainer Link 48. TC showing either partial or complete cell membrane staining for PD-L1 were evaluated as positive cells. Tumor proportion score (TPS) was used to evaluate PD-L1 expression on TC, which was the percentage of PD-L1-positive TC showing partial or complete membrane staining in the overall tumor sections. We classified PD-L1 expression on TC into 4 levels: PD-L1 TPS <1%, PD-L1 TPS 1%–49%, and PD-L1 TPS ≥50%. PD-L1 expression on IC was assessed as the proportion of tumor area occupied by PD-L1-positive immune cells of any intensity. For 22C3 assay, there are no recommended cutoffs which are appropriate for assessment of PD-L1 expression on IC. Consistent with our previous study,19 PD-L1 expression on IC was classified into 2 levels: PD-L1 on IC <1% and PD-L1 on IC ≥1%. All slides were assessed by 2 experienced pathologists. In cases of disagreement, the slides were reviewed by all 2 observers together to achieve consensus.
Statistical Analysis
The Fisher exact test was used for evaluating the relationship between clinical characteristics and variation of PD-L1 expression. The variation of PD-L1 positivity status was analyzed by Wilcoxon signed-rank test. Overall survival (OS) was defined as the time from first diagnosis of lung cancer to death or the last follow-up. Disease-free survival (DFS) was calculated from the date of surgery to recurrence or the last follow-up. The survival curves were calculated by the Kaplan-Meier method and differences in survival were tested by the log-rank test. Univariate and multivariate analysis were performed with a Cox proportional hazard model. Statistics were performed using SPSS 20.0 software (Chicago, IL) and the significance level was set at P-value (2-sided) <0.05.
RESULTS
Patient Characteristics
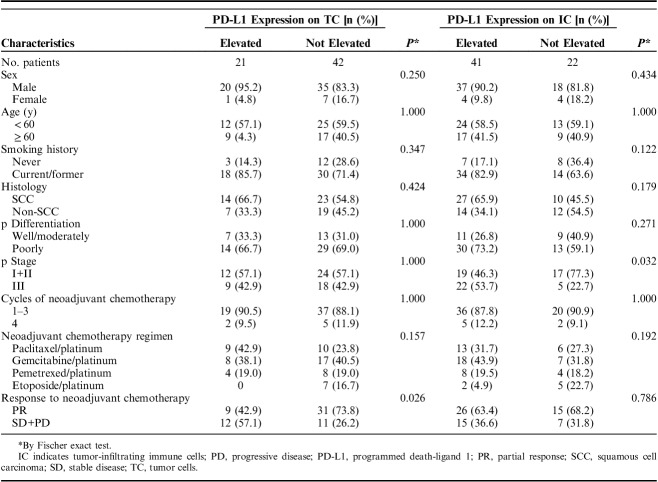
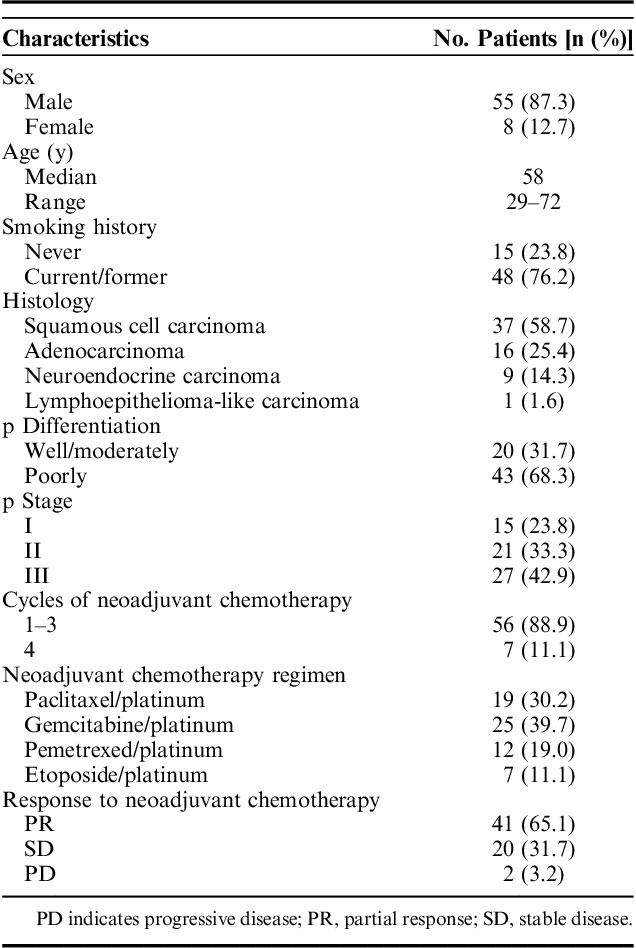
A total of 63 patients who received neoadjuvant chemotherapy, followed by curative surgery, were enrolled in this study and their clinicopathologic characteristics were shown in Table 1. Among them, the majority of patients were male (87.3%) and smokers (76.2%). More than half of them were diagnosed with lung squamous cell carcinoma (58.7%). All patients received neoadjuvant platinum-based chemotherapy regimens. Pemetrexed/platinum were used in nonsquamous histologies and paclitaxel/platinum were mainly used in squamous histologies. Regimens included gemcitabine/platinum (n=25, 39.7%), paclitaxel/platinum (n=19, 30.2%), pemetrexed/platinum (n=12, 19.0%) and etoposide/platinum (n=7, 11.1%). Considering the increased difficulty of operation and occurrence of surgical complications caused by more cycles of chemotherapy before surgery, more than half of patients (58.7%) in our case series received only 2 cycles of neoadjuvant chemotherapy and the remaining 2 cycles were administrated after surgery. Seven patients received all 4 cycles before surgery. Forty-one patient (65.1%) showed a partial response, 20 (31.7%) a stable disease and 2 (3.2%) a progressive disease after neoadjuvant chemotherapy.
TABLE 1.
Patient Characteristics (n=63)
Variation of PD-L1 Expression on TC and IC Between Paired Prechemotherapy and Postchemotherapy Specimens
Among the 63 patients, 11 patients (17.5%) had a positive expression of PD-L1 on TC in the prechemotherapy specimens, while 25 patients (39.7%) had a positive expression of PD-L1 in the surgical specimens (Fig. 1A). Twenty-one patients (33.3%) showed elevation, 2 patients (3.2%) showed reduction, and 40 patients (63.5%) showed no variation in the expression of PD-L1 on TC after neoadjuvant chemotherapy. The positive expression rate of PD-L1 on TC significantly increased between the prechemotherapy and postchemotherapy specimens (P<0.001).
FIGURE 1.
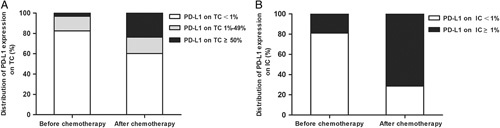
The distribution of programmed cell death-ligand 1 (PD-L1) expression. A, The distribution of PD-L1 expression on tumor cells (TC) before and after chemotherapy. B, The distribution of PD-L1 expression on tumor-infiltrating immune cells (IC) before and after chemotherapy.
In the whole cohort, 12 patients (19.0%) had a positive expression of PD-L1 on IC in the prechemotherapy specimens, while 45 patients (71.4%) had a positive expression of PD-L1 in the surgical specimens (Fig. 1B). Forty-one patients (65.1%) showed elevation, 1 patient (1.6%) showed reduction, and 21 patients (33.3%) showed no variation in the expression of PD-L1 on IC after neoadjuvant chemotherapy. The positive expression rate of PD-L1 on IC significantly increased between the prechemotherapy and postchemotherapy specimens (P<0.001).
Correlation Between Clinicopathologic Characteristics, Chemotherapy Response, and Variation of PD-L1 Expression
The correlation between clinicopathologic characteristics, chemotherapy response and variation of PD-L1 expression was analyzed by Fisher exact test (Table 2). The elevation of PD-L1 expression on TC after neoadjuvant chemotherapy was more frequently observed in patients achieving stable disease or progressive disease than in patients achieving partial response (P=0.026). In addition, the elevation of PD-L1 expression on IC was more common in advanced-stage patients compared with early-stage patients (P=0.032).
TABLE 2.
Association Between Clinicopathologic Characteristics and Alteration of PD-L1 Expression on TC and IC
Survival Analysis
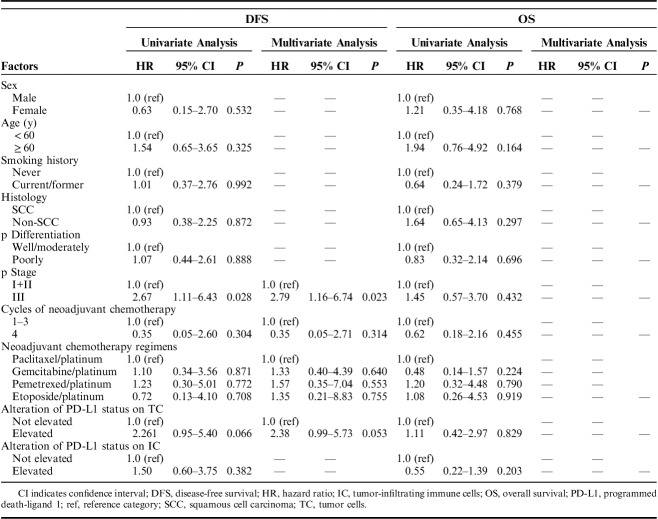
We investigated the association between clinicopathologic characteristics, variation of PD-L1 expression and survival time (Table 3). The median progression-free survival (PFS) and OS were 15 and 20 months, respectively. The survival times according to the variation of PD-L1 expression on TC and IC were assessed (Fig. 2). Multivariate Cox regression analysis revealed that a significantly shorter PFS was observed in patients with advanced stage [hazard atio (HR)=2.79, 95% confidence interval (CI)=1.16-6.74, P=0.023]. Patients with elevated PD-L1 expression on TC after neoadjuvant chemotherapy showed a trend to have a shorter PFS than patients without elevated PD-L1 expression on TC, although the difference was not statistically significant in multivariate analysis (HR=2.38, 95% CI=0.99-5.73, P=0.053). Elevated PD-L1 expression on IC after neoadjuvant chemotherapy has no significant association with PFS (HR=1.50, 95% CI=0.60-3.75, P=0.382). Cycles of neoadjuvant chemotherapy and regimens were also included into the multivariate analysis, whereas these 2 factors showed no significant association with PFS. All clinicopathologic characteristics and variation of PD-L1 expression had no significant correlation with OS.
TABLE 3.
Univariate and Multivariate Analysis of DFS and OS in All Patients
FIGURE 2.
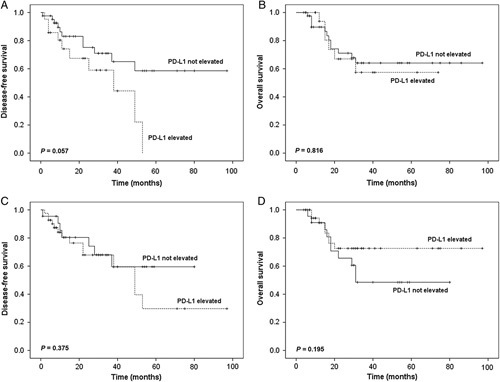
Kaplan-Meier curves for disease-free survival (DFS) and overall survival (OS). Comparison of patient DFS (A) and OS (B) according to variation of programmed cell death-ligand 1 (PD-L1) expression on tumor cells (TC) after chemotherapy. Comparison of patient DFS (C) and OS (D) according to variation of PD-L1 expression on tumor-infiltrating immune cells (IC) after chemotherapy.
DISCUSSION
In the present study, we assessed the variation of PD-L1 expression of TC and IC after platinum-based neoadjuvant chemotherapy and its association with clinicopathologic characteristics and outcome in a cohort of patients with lung cancer. Our results showed that chemotherapy might play a role in upregulating PD-L1 expression on TC and IC and the elevation of PD-L1 expression on TC was associated with reduced response to chemotherapy and inferior PFS.
The effect of chemotherapy on PD-L1 expression has been previously investigated in lung cancer, while the results remain conflicting and controversial. Sheng et al17 reported that the positivity of PD-L1 on TC changed from 75% to 37.5% after neoadjuvant chemotherapy in non–small cell lung cancer, while no significant changes were observed on IC. Rojkó et al20 revealed that PD-L1 expression showed no significant changes after neoadjuvant chemotherapy in patients with lung cancer. Song et al15 demonstrated that the expression of PD-L1 could be upregulated by neoadjuvant chemotherapy in lung squamous cell carcinoma patients. In our case series, PD-L1 expression significantly increased on both TC and IC after neoadjuvant chemotherapy. The mechanism underlying the role of chemotherapy in variation of PD-L1 expression has not been fully elucidated in lung cancer, but it is already known that several prior studies demonstrated that chemotherapy could induce PD-L1 expression in ovarian cancer and breast cancer. In the study by Peng et al,21 chemotherapy could upregulate PD-L1 expression through the nuclear factor-κB to induce local immune suppression in ovarian cancer. Zhang et al22 revealed that paclitaxel, etoposide and 5-fluorouracil were able to potentiate IFN-γ-induced PD-L1 expression in human breast cancer cells and increase PD-L1-mediated T-cell apoptosis. For patients receiving different chemotherapy regimens in this study, PD-L1 expression on TC elevated in 9 of 19 patients who received paclitaxel/cisplatin chemotherapy, but none of the 7 patients who received etoposide/cisplatin chemotherapy showed elevation of PD-L1 expression on TC, which is not consistent with Zhang et al’s study.22 The discordance may be attributable to the heterogeneity among various cancers and further studies are warranted to validate the impact of chemotherapy on PD-L1 expression in lung cancer.
The prognostic role of PD-L1 expression has been extensively investigated among various cancers. Several previous studies reported that PD-L1-positive expression on TC was associated with poor prognosis in lung cancers,23–26 but few studies focused on the relationship between chemotherapy response, prognosis and the variation of PD-L1 expression after chemotherapy. The findings of Zhang et al’s27 study suggested that change of PD-L1 expression was significantly associated with chemotherapy response, and the upregulation of PD-L1 promoted a resistance response in lung cancer cells. In line with this prior study, the results of our study confirmed that the elevation of PD-L1 expression on TC was linked to inferior response to neoadjuvant chemotherapy and shorter PFS. With regard to the underlying mechanism of elevated PD-L1 expression increases the chemoresistance, the results of a previous research28 indicated that the biological interaction between PD-L1 and chemoresistance occurred through the microRNA regulatory cascade. MicroRNA-197 is downregulated in lung cancer tissues in patients resistant to platinum-based chemotherapy and the downregulation of microRNA-197 enhances PD-L1 expression. MicroRNA-197 regulates lung cancer drug resistance and tumor progression by directly targeting the cyclin-dependent kinase CKS1B as well as by indirectly targeting the transcription factor STAT3. PD-L1 expression on ICs has been strongly correlated with the density of CD4 or CD8+ tumor-infiltrating lymphocytes.29 High expression of PD-L1 on ICs may predict effective host immune responses in the presence of a favorable immune microenvironment infiltrated with increased CD4 and CD8+ T cells, which may restrain tumor growth.30 In our study, although 65.1% of patients showed elevation in the expression of PD-L1 on IC after neoadjuvant chemotherapy, no significant association was found between PD-L1 expression changes on IC and outcomes. Relatively small samples may contribute to this result and further studies are still needed to investigate the association between PD-L1 expression changes on IC and outcomes as well as its underlying mechanisms.
Presently, non–small cell lung cancer patients with low PD-L1 expression may receive immunotherapy after chemotherapy. Because of the variation of PD-L1 expression before and after chemotherapy, some patients who have elevated PD-L1 expression after chemotherapy may achieve better therapeutic effect from immunotherapy. Therefore, tumor tissue rebiopsy and PD-L1 expression reassessment should be performed if patients want to receive immunotherapy after chemotherapy.
The current study had several limitations. First, it was a retrospective, single institutional study and the sample size was relatively small. Second, only one PD-L1 antibody, 22C3, was used in this study. Third, the PD-L1 positivity in the surgical specimens was higher than that in the biopsy specimens after chemotherapy, and the difference in specimens might be a confounding factor when comparing PD-L1 expression prechemotherapy and postchemotherapy. Hence, future prospective studies are needed to validate our results. Notwithstanding the limitations as shown above, this study provided evidence of elevated PD-L1 expression after chemotherapy and underline the necessity of tumor tissue rebiopsy and PD-L1 expression reassessment after treatment.
In conclusion, chemotherapy significantly increased the PD-L1 expression in lung cancer. Furthermore, elevation of PD-L1 expression on TC after neoadjuvant chemotherapy correlated with reduced response to chemotherapy and inferior PFS in patients with lung cancer.
CONFLICTS OF INTEREST/FINANCIAL DISCLOSURES
Supported by the grant 2016YFC0905400 from the National Key R&D Program of China, Beijing Municipal Science & Technology Commission No. LC2015A13 and CAMS Initiative for Innovative Medicine (CAMS-2017-I2M-4-002).
All authors have declared that there are no financial conflicts of interest with regard to this work.
Footnotes
L.G. and P.S. contributed equally to this work.
J.Y and S.G. are co-corresponding authors.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Lee CK, Wu YL, Ding PN, et al. Impact of specific epidermal growth factor receptor (EGFR) mutations and clinical characteristics on outcomes after treatment with EGFR tyrosine kinase inhibitors versus chemotherapy in EGFR-mutant lung cancer: a meta-analysis. J Clin Oncol. 2015;33:1958–1965. [DOI] [PubMed] [Google Scholar]
- 3.Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30:1122–1128. [DOI] [PubMed] [Google Scholar]
- 4.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. [DOI] [PubMed] [Google Scholar]
- 5.Mitsudomi T, Morita S, Yatabe Y, et al. West Japan Oncology Group Gefitinib versus cisplatin plus docetaxel inpatients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. [DOI] [PubMed] [Google Scholar]
- 6.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. [DOI] [PubMed] [Google Scholar]
- 7.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–2394. [DOI] [PubMed] [Google Scholar]
- 8.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. [DOI] [PubMed] [Google Scholar]
- 9.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. [DOI] [PubMed] [Google Scholar]
- 12.Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–1846. [DOI] [PubMed] [Google Scholar]
- 13.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. [DOI] [PubMed] [Google Scholar]
- 14.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. [DOI] [PubMed] [Google Scholar]
- 15.Song Z, Yu X, Zhang Y. Altered expression of programmed death-ligand 1 after neo-adjuvant chemotherapy in patients with lung squamous cell carcinoma. Lung Cancer. 2016;99:166–171. [DOI] [PubMed] [Google Scholar]
- 16.Sun W, Ma K, Wang X, et al. Altered expression of programmed death-1 receptor (PD-1) and its ligand PD-L1, PD-L2 after neo-adjuvant chemotherapy in lung cancer. J Thorac Oncol. 2017;12:S1997. [Google Scholar]
- 17.Sheng J, Fang W, Yu J, et al. Expression of programmed death ligand-1 on tumor cells varies pre and post chemotherapy in non-small cell lung cancer. Sci Rep. 2016;6:20090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moldvay J, Rojkó L, Téglási V, et al. Platinum-based chemotherapy is associated with altered PD-L1 expression in lung cancer. J Thorac Oncol. 2018;13:S733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song P, Guo L, Li W, et al. Clinicopathologic correlation with expression of PD-L1 on both tumor cells and tumor-infiltrating immune cells in patients with non-small cell lung cancer. J Immunother. 2019;42:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rojkó L, Reiniger L, Téglási V, et al. Chemotherapy treatment is associated with altered PD-L1 expression in lung cancer patients. J Cancer Res Clin Oncol. 2018;144:1219–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng J, Hamanishi J, Matsumura N, et al. Chemotherapy induces programmed cell death-ligand 1 overexpression via the nuclear factor-kappaB to foster an immunosuppressive tumor microenvironment in ovarian cancer. Cancer Res. 2015;75:5034–5045. [DOI] [PubMed] [Google Scholar]
- 22.Zhang P, Su DM, Liang M, et al. Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1) surface expression in breast cancer cells and promote PDL1-mediated T cell apoptosis. Mol Immunol. 2008;45:1470–1476. [DOI] [PubMed] [Google Scholar]
- 23.Cao L, Wang X, Li S, et al. PD-L1 is a prognostic biomarker in resected NSCLC patients with moderate/high smoking history and elevated serum SCCA level. J Cancer. 2017;8:3251–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azuma K, Ota K, Kawahara A, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol. 2014;25:1935–1940. [DOI] [PubMed] [Google Scholar]
- 25.Takada K, Okamoto T, Shoji F, et al. Clinical significance of PD-L1 protein expression in surgically resected primary lung adenocarcinoma. J Thorac Oncol. 2016;11:1879–1890. [DOI] [PubMed] [Google Scholar]
- 26.Igawa S, Sato Y, Ryuge S, et al. Impact of PD-L1 expression in patients with surgically resected non-small-cell lung cancer. Oncology. 2017;92:283–290. [DOI] [PubMed] [Google Scholar]
- 27.Zhang P, Ma Y, Lv C, et al. Upregulation of programmed cell death ligand 1 promotes resistance response in non-small-cell lung cancer patients treated with neo-adjuvant chemotherapy. Cancer Sci. 2016;107:1563–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujita Y, Yagishita S, Hagiwara K, et al. The clinical relevance of the miR-197/CKS1B/STAT3-mediated PD-L1 network in chemoresistant non-small-cell lung cancer. Mol Ther. 2015;23:717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim HR, Ha SJ, Hong MH, et al. PD-L1 expression on immune cells, but not on tumor cells, is a favorable prognostic factor for head and neck cancer patients. Sci Rep. 2016;6:36956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang CY, Lin MW, Chang YL, et al. Programmed cell death-ligand 1 expression is associated with a favourable immune microenvironment and better overall survival in stage I pulmonary squamous cell carcinoma. Eur J Cancer. 2016;57:91–103. [DOI] [PubMed] [Google Scholar]