Supplemental Digital Content is available in the text.
Keywords: angiotensin II, blood pressure, hypertension, mice, microbiota
Abstract
Gut microbial metabolites have been implicated in contributing to blood pressure regulation; however, only a few microbial metabolites have been examined to date. In this study, we hypothesized that an unbiased screen for changes in gut microbial metabolites in a chronic Ang II (angiotensin II) infusion model would identify novel microbial metabolites associated with blood pressure regulation. To accomplish this, we used both conventional and germ-free mice, which had been implanted with minipumps to infuse either saline or Ang II. Our aim was to identify metabolites that were altered with Ang II treatment in conventional mice, but not in germ-free mice, indicating that they are dependent on the gut microbiota. Both plasma and feces samples were processed and analyzed using liquid chromatography-tandem mass spectroscopy. In plasma, we identified 4 metabolites that were significantly upregulated and 8 metabolites that were significantly downregulated with Ang II treatment in conventional mice; none of these metabolites changed in germ-free mice. Similarly, in feces, we identified 25 metabolites that were significantly upregulated and 71 metabolites that were significantly downregulated with Ang II treatment in conventional mice; none of these metabolites changed in germ-free mice. Finally, fecal 16S sequencing revealed significant shifts in the microbiome of conventional mice with Ang II treatment, including sex-specific changes. These data demonstrate that the metabolites that are differentially regulated with Ang II are dependent on the gut microbiome.
Hypertension is a global health problem, and multiple pathways are known to play important roles in the development of hypertension, including the renin-angiotensin-aldosterone system,1 the immune system,2 and endothelial dysfunction.3,4 In recent years, emerging evidence in both humans and animal models has indicated that the gut microbiota also plays a role in the development of hypertension. Dysbiosis of the gut microbiota corresponds with hypertension in mice, rats, and humans,5–8 and correcting this dysbiosis using an antibiotic has been associated with a lowering of BP.6 In support of these concepts, a high-salt diet was shown to deplete a strain of Lactobacillus, and treating mice with this strain attenuated salt-sensitive hypertension.9 In addition, fecal transplants from hypertensive donors into germ-free (GF) mice (mice with no native microbiota) can transfer the hypertensive phenotype,10 implying that the microbiota may be not merely responding to a hypertensive phenotype but may be able to modulate the phenotype itself.
A primary way that the gut microbiota can influence the host is via the production of microbial metabolites. To date, studies have focused on a limited number of microbial metabolites as potential players in blood pressure regulation.8,11–14 In this study, we reasoned that a thorough and unbiased analysis of gut microbial metabolites altered in an Ang II (angiotensin II) infusion model may reveal novel candidates for future study. Thus, the aim of this study was to identify plasma or fecal metabolites which are altered in hypertension in a gut microbiota–dependent manner. To achieve this, we used a metabolomics approach to identify metabolites which are differentially regulated with Ang II treatment in conventional mice (which have gut microbiota) but not in GF mice (which lack gut microbiota).
Methods
Metabolomics data have been made publicly available at the Metabolomics Workbench and can be accessed at study ID ST001158 and ST001157 and project DOI 10.21228/M8CH5C. 16S data have been made publicly available at the National Center for Biotechnology Information and can be accessed at https://www.ncbi.nlm.nih.gov/Traces/study/?acc=PRJNA514044.
Animals and Housing
All animal protocols were approved by the Johns Hopkins University Animal Care and Use Committee. Conventional C57BL/6 and GF mice used in this study were bred at Johns Hopkins. GF mice were screened on a monthly basis to ensure their GF status; importantly, for the studies herein, fecal pellets were also analyzed from GF experimental mice near the time of sacrifice to ensure that the mice remained GF throughout the study. Detailed screening procedures are outlined in the online-only Data Supplement. This study used 4 groups of 6-week-old C57BL/6 mice: conventional (n=6) and GF (n=6) mice were infused with Ang II for 4 weeks (400 ng·kg−1·min−1; Alzet 1004), and in parallel control groups, conventional (n=6) and GF (n=6) mice instead received saline (0.9% w/v; Nova Tech, Inc) via minipumps (saline). Each group had equal numbers of males and females. GF mice (males and females) were from a total of 2 litters, and conventional mice (males and females) were from a total of 3 litters. Conventional mice were randomly allocated to saline or Ang II groups, whereas 1 litter of GF mice was used for saline and the other for Ang II. All mice were housed individually (to avoid cage effects due to coprophagia)15 on a 14/10 light/dark cycle, were fed the same diet (Purina Conventional Mouse Diet, LabDiet JL Rat/Mouse and Auto 6F No. 5K67), and had free access to water during the experimental period.
Minipump Implantation
For both conventional and GF mice, minipumps (Alzet) were implanted subcutaneously after intraperitoneal anesthesia (ketamine hydrochloride, 50 mg/kg; xylazine hydrochloride, 10 mg/kg). A 2- to 4-mm subcutaneous incision was made to insert the minipump on the dorsal flank. After surgery, mice received a single subcutaneous dose (4 mg/kg) of ostilox (meloxicam; Norbrook) to relieve postoperative pain and meloxicam in the drinking water (0.2 mg/kg) for next 24 hours.
Blood and Feces Collection
At the end of the study, blood and feces were collected from live mice. Blood was collected in heparin-coated tubes (BD Microtainer) by puncturing the submandibular vein located in the cheek pouch. Blood samples were kept on ice and were then centrifuged at 4500g for 15 minutes to separate plasma. Fecal pellets were collected directly into 1.5-mL sterile Eppendorf tube at time of defecation. Plasma and feces samples were flash frozen in liquid nitrogen and then stored at −80°C until analysis.
16S rRNA Microbiome Analysis
Fecal DNA extraction was performed on samples from conventional mice (saline and Ang II groups). Each sample comprised of 1 to 3 fecal pellets, and DNA was isolated using the Qiagen Fast Stool Isolation kit (cat No. 51604). 16s rRNA V3 through V4 region was amplified using primers 319F (CTCCTACGGGAGGCAGCAGT) and 806R (GGACTACHVGGGTWTCTAAT) following the method by Caporaso et al.16 The library was sequenced on MiSeq for 2×300 base pairs. The library preparation and sequencing were performed by the Johns Hopkins Transcriptomics and Deep Sequencing Core. Subsequently, they were analyzed by Resphera Biosciences as described in Methods in the online-only Data Supplement. Differential abundance analysis of α-diversity analyzed differences between groups (nonparametric difference test, Mann-Whitney U test, and t test). Multiple hypothesis testing was corrected using the false discovery rate.17 Generalized linear modeling was performed using R. 16S data have been deposited to the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/Traces/study/?acc=PRJNA514044).
Metabolomics on Blood and Feces Samples
Metabolomics were performed by Metabolon, as in the Methods in the online-only Data Supplement. Raw data were normalized in terms of raw area counts (the peak of the metabolite, quantified as area under the curve). The median for each individual metabolite was set to 1, with undetected metabolites being input as the minimum value. Values were normalized by the sample volume used for extraction. The y axis of all box plots represents the scaled intensity (an arbitrary unit, relative to the overall median of 1 for that metabolite). Of note, the metabolomics platform we chose was the broadest platform available to us but does not include short-chain fatty acids. Although short-chain fatty acids have been tied to blood pressure regulation,11 multiple groups have previously examined short-chain fatty acids in hypertension models.5,18–21 Thus, we reasoned that using this broad platform, which does not detect short-chain fatty acids, was an acceptable trade-off given that the aim of our study was to detect novel metabolites which may be altered in response to Ang II. Metabolomics data have been deposited to Metabolomics Workbench for both feces and plasma under study ID ST001158 and ST001157 and project DOI 10.21228/M8CH5C.
Results
To identify novel microbial metabolites which are altered with Ang II infusion, we implanted both conventional and GF mice with osmotic minipumps. In each group, half of the mice were infused with Ang II (400 ng·kg−1·min−1 for 4 weeks) and the other half with saline. After 4 weeks of infusion, plasma and feces were collected and metabolomics were performed. We detected a total of 822 metabolites in plasma and 944 in feces. Principal component analysis of the plasma (Figure 1A) and feces (Figure 1B) metabolomics data indicates that the conventional and GF groups generally segregate separately from one another and that (especially for feces) GF status has a larger influence than treatment status. Because a sex difference was seen on the principal component analysis for the plasma samples, plasma data were analyzed by sex and treatment.
Figure 1.

Principal component analysis of plasma and fecal metabolites reveals a strong role for germ free status. Principal component analysis of metabolites in plasma (A) and feces (B) of conventional (Con) and germ-free (GF) mice treated with Ang II (angiotensin II) or saline (Sal). For both plasma and fecal metabolites, GF status played a stronger role than treatment status. For the plasma metabolites, differences were observed both in GF status and in sex.
Plasma Metabolites Altered With Ang II
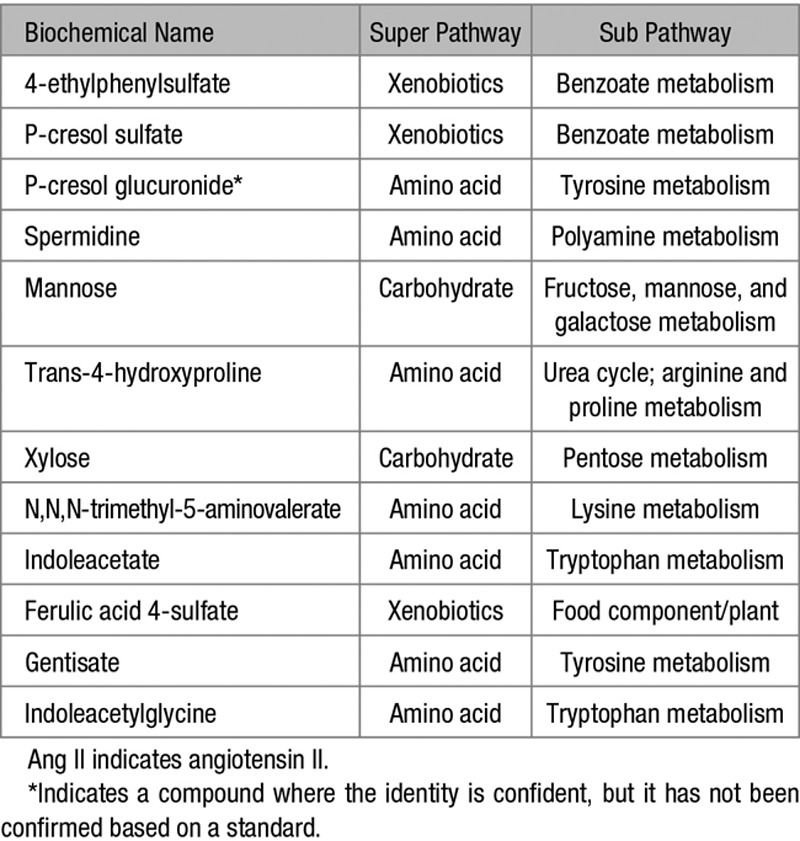
Our primary objective was to identify metabolites that were significantly altered with Ang II treatment in conventional but not GF mice. Metabolites shown in the Table are plasma metabolites which were differentially regulated by Ang II treatment in conventional mice. The Table lists the biochemical name of each differentially regulated metabolite and the superpathway and subpathway to which they belong.
Table.
Plasma Metabolites That Were Significantly Upregulated or Downregulated by Ang II Treatment in Conventional Mice: the Superpathway and Subpathway for Each Metabolite
In Figure 2, red indicates a statistically significant upregulation, whereas green indicates a statistically significant downregulation. The numbers in the figure reflect the fold change (ie, Ang II/saline) for each group. In plasma of conventional mice, we detected 4 significantly upregulated and 8 significantly downregulated metabolites (P<0.05, Q<0.2 for conventional Ang II versus conventional saline by ANOVA; Table S1 in the online-only Data Supplement shows the P and Q values for each metabolite in column 2 of Figure 2). Remarkably, none of the metabolites that were significantly altered with Ang II in conventional mice (column 2) were similarly changed in GF mice (column 3). Consistent with this finding, several of the metabolites differentially regulated in conventional mice with Ang II were either undetected or found at low levels in GF mice, as can be seen by comparing relative amounts in GF/conventional (columns 4 and 5). In these columns, the green color indicates that the compound was detected at a significantly lower level in GF mice versus conventional mice.
Figure 2.
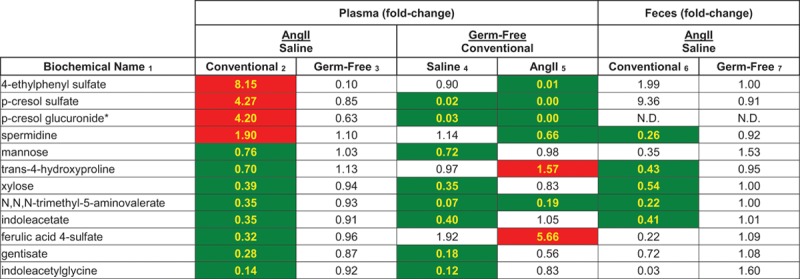
Plasma metabolites that were significantly upregulated (red) or significantly downregulated (green) by Ang II (angiotensin II) treatment in conventional mice are shown (P<0.05, Q<0.2, by ANOVA). Columns 2 and 3 show the fold change in metabolite abundance (expressed as Ang II/saline) for both conventional (column 2) and germ-free (GF; column 3) mice. Metabolites that are red in these columns were upregulated with Ang II; green indicates that the metabolite was downregulated with Ang II. In columns 4 and 5, the fold change in metabolite abundance for GF/conventional is shown for both saline and Ang II; in these columns, green indicates that the metabolite is found at a significantly lower abundance in GF animals. Finally, columns 6 and 7 show the fold change in abundance of these metabolites in the feces with Ang II treatment. N.D. indicates not detected. *Indicates a compound where the identity is confident, but it has not been confirmed based on a standard.
Finally, in columns 6 and 7, we analyzed the fold changes in fecal abundances for the metabolites in this figure. Whereas a subset of the conventional fecal samples showed changes that reflected the changes seen in plasma, in the GF fecal samples there were no significant changes in any of these metabolites.
Plasma Metabolites Upregulated With Ang II
The metabolites that were upregulated by Ang II in conventional mice are shown as box-and-whiskers plots in Figure 3A. Notably, 4-ethylphenylsulfate, p-cresol sulfate, and p-cresol glucuronide were nearly undetected under GF conditions, indicating that these metabolites are dependent on the microbiome for their production. In contrast, spermidine is still present in the GF animals but fails to upregulate with Ang II treatment. This indicates that the production of this compound is likely contributed to both by the microbiome and the host (or, host diet).22
Figure 3.
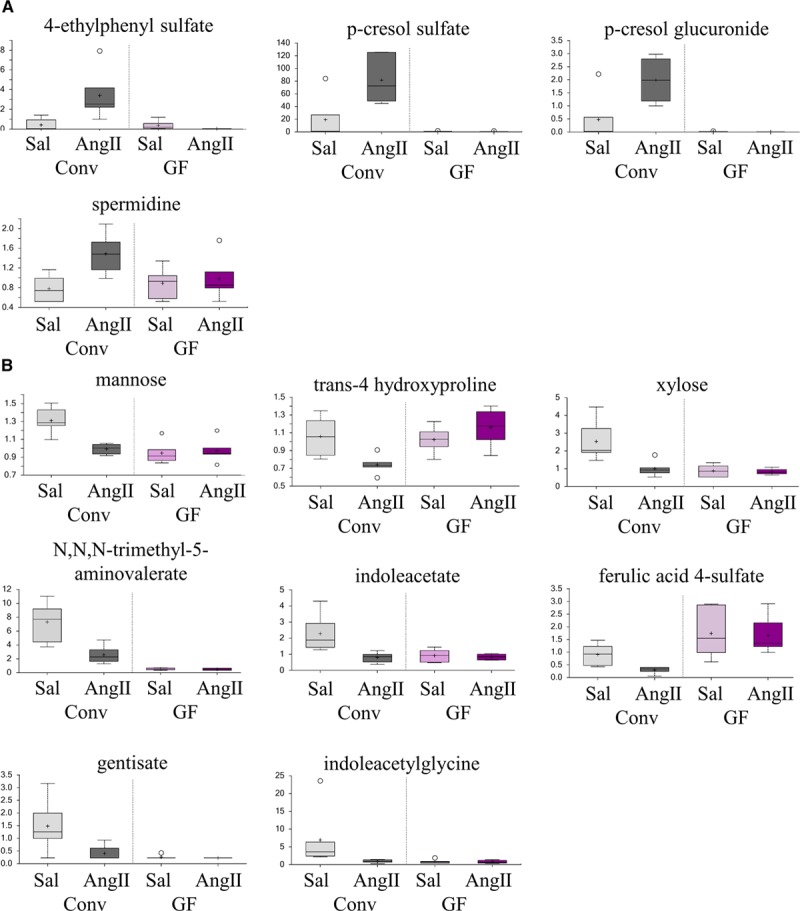
Plasma metabolites significantly upregulated or downregulated with angiotensin II (Ang II) in conventional mice are shown here. Plasma metabolites that were significantly upregulated with Ang II treatment in conventional (Conv) mice are shown in (A). Plasma metabolites that were significantly downregulated with Ang II treatment in Conv mice are shown in (B). All metabolites are statistically significant (Conv saline [Sal] vs Conv Ang II; P<0.05, P<0.2 by ANOVA). Data are plotted as scaled intensity (an arbitrary unit relative to an overall median of 1); the box-and-whisker plot indicates the minimum and maximum distribution (whiskers) and the upper and lower quartile limits (box), with the median value shown as a line and the mean values as a +. Extreme data points are plotted as circles. GF indicates germ-free.
Plasma Metabolites Downregulated With Ang II
We identified 8 plasma metabolites, which were significantly downregulated with Ang II treatment in conventional mice (Figure 3B). Some of these compounds were nearly undetected in GF mice (N,N,N-trimethyl-5-aminovalerate, gentisate, and indoleacetylglycine) implying that they are dependent on the gut microbiota and that gut microbial production is downregulated with Ang II treatment. In contrast, others were expressed at variable levels in GF mice but were not differentially regulated by Ang II, indicating that both the host and the microbiota contribute to metabolite production.
Sex Differences in Metabolite Changes
As the principal component analysis (Figure 1) indicated sex differences in plasma metabolites, plasma metabolome data were further analyzed by sex. However, it should be noted that although our study included animals of both sexes, it was not properly powered to compare males versus females. Nevertheless, we noted that several of the metabolites that were altered in plasma reached significance in only 1 sex (Table S2; Figure S1). The direction of the change (upregulation versus downregulation) was always the same in both sexes; only the magnitude of the change appeared to differ. The majority of these metabolites are found at lower levels in GF mice, indicating that they may be wholly or partially microbial in origin.
Fecal Metabolites Altered With Ang II
All fecal metabolites that were significantly upregulated (n=25) with Ang II treatment are shown in Table S3 with box-and-whiskers plots for the top 8 metabolites shown in Figure 4A and the remainder included as Figure S2 (P and Q values for Table S3 column 2 are shown in Table S4). Of the 25 metabolites that were upregulated with Ang II in conventional mice, none were upregulated in GF mice.
Figure 4.
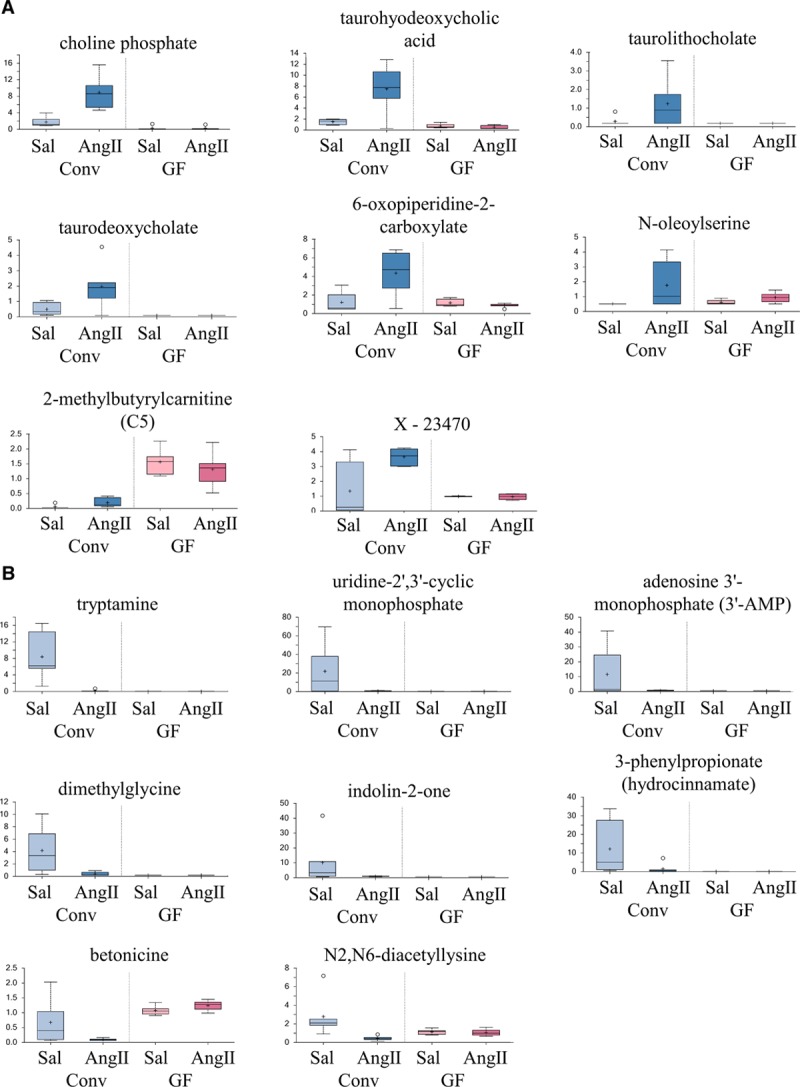
The top 8 fecal metabolites significantly upregulated or downregulated with angiotensin II (Ang II) in conventional mice are shown here. The top 8 upregulated fecal metabolites from Table S3B are shown in (A); the remaining upregulated fecal metabolites are plotted in Figure S2. Data are plotted as a box-and-whisker plot, as in Figure 3. The top 8 downregulated fecal metabolites from Table S5B are shown in (B); the remaining upregulated fecal metabolites are plotted in Figure S3. Data are plotted as a box-and-whisker plot, as in Figure 3. Ang II indicates Angiotensin II; Conv, conventional; GF, germ-free; and Sal, saline.
Fecal metabolites that were downregulated (n=71) with Ang II treatment are shown in Table S5A and S5B, with box-and-whiskers plots for the top 8 shown in Figure 4B and the remainder shown as Figure S3 (P and Q values for Table S5B column 2 are shown in Table S6). None of these 71 metabolites were also downregulated with Ang II in GF mice. Although many of the significantly altered fecal metabolites were found at lower abundances in GF mice (green color in columns 4 and 5 in Tables S3B and S5B), several metabolites were found at higher abundances (red color in Tables S3B and S5B). Metabolites found at higher abundances in the GF mice may be host-derived compounds, which normally are metabolized by the microbiota, and thus accumulate in the absence of the gut microbiota. Overall, the fact that none of the changes seen in the fecal metabolites in conventional mice are present in the GF mice (column 2 versus 3 in Tables S3B and S5B) demonstrates once again that the gut microbiota is required for the metabolomics changes seen with Ang II.
16S Microbial Sequencing
16S sequencing (V3 through V4) of fecal microbiota from conventional mice revealed taxonomic alterations in the microbial composition of saline-treated versus Ang II–treated mice. Although α-diversity was not different between saline and Ang II–treated groups (Shannon, Chao1), hierarchical clustering of taxonomic profiles at both the genus (top 50) and species (top 100) levels showed that animals were clustered by treatment modality (Figure 5A). This indicates that Ang II treatment significantly altered the microbiota. Analysis on the family level revealed 3 dominating phyla (firmicutes, bacteroidetes, and tenericutes; Figure 5B). In the Ang II–treated group, there was an expansion of Anaeroplasmataceae and a decrease in Lachnospiraceae.23 In agreement with an alteration in the microbiome induced by Ang II, principal component analysis of β-diversity (Figure 5C) showed that saline versus Ang II treatment mice harbored divergent microbial communities (P value: treatment, 0.029); however, we did not observe significant differences in β-diversity between males and females (P value: sex, 0.46). Of note, however, there were individual species/operational taxonomic units, which were changed in a sex-specific manner with Ang II treatment. For example, Clostridium celatum was nearly undetected in males treated with saline, males treated with Ang II, or in females treated with saline but was dramatically upregulated in females treated with Ang II (Figure 5D).
Figure 5.
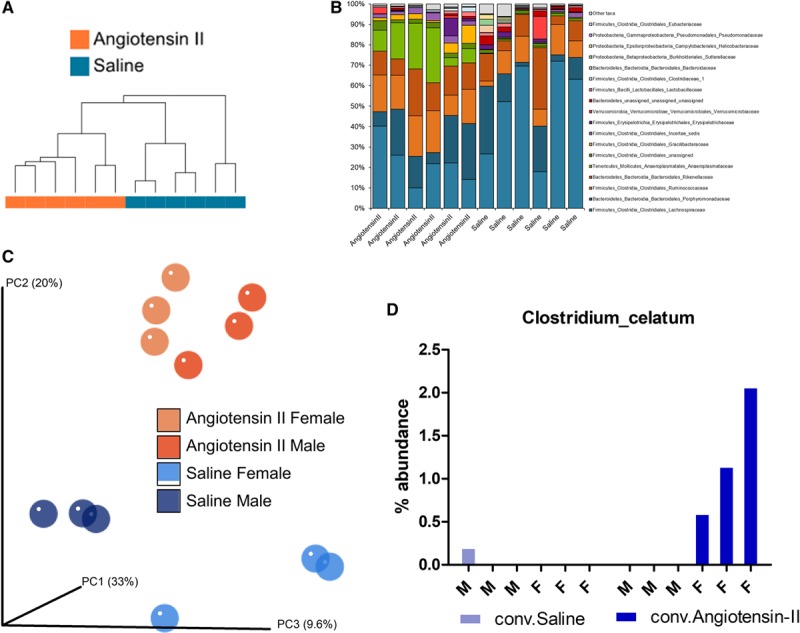
Microbial sequencing (16S) from feces of conventional (Conv) mice treated with either Ang II (angiotensin II) or with saline revealed significant alterations with Ang II treatment. Taxonomic clustering based on the top 100 species (A) demonstrates that the Ang II and saline samples are most similar to samples within the same group. Analysis on the family level revealed changes in phyla corresponding with Ang II treatment (B). Although differences were not seen between groups in α-diversity, analysis of β-diversity (C) revealed that the Ang II samples are most similar to each other, regardless of sex (PC, Principal Coordinate Axis). Permanova multivariate analysis of β-diversity associated with Ang II treatment was statistically significant (P=0.029). Although this analysis did not reveal sex differences in β-diversity (P=0.46), there were individual species/operational taxonomic units, which did change in a sex-specific manner, such as Clostridium celatum (D). F indicates female; and M, male.
Discussion
Although the causes of hypertension are multifaceted, recent studies have highlighted a novel player in hypertension: the gut microbiota.5,8,13,14,18 In this study, we demonstrate for the first time that the gut microbiota is the driving force behind the changes in plasma and fecal metabolomics in an Ang II infusion model. In total, we identified 12 plasma metabolites which are differentially regulated with Ang II infusion (400 ng·kg−1·min−1 for 4 weeks) in conventional mice; none of these molecules were similarly altered in GF animals. Similarly, we identified 96 fecal metabolites which were significantly altered with Ang II in conventional mice, none of which were similarly changed in GF animals. We also identified clear shifts in the gut microbiota of conventional mice with Ang II treatment, in support of the concept of a gut dysbiosis in hypertension which may be driving these metabolomics changes.6,24
Metabolites Upregulated With Ang II Treatment
The plasma metabolites that we identified as significantly upregulated in conventional mice with Ang II treatment include uremic toxins such as 4-ethylphenyl sulfate, p-cresol sulfate, and p-cresol glucuronide. It has been previously shown that the gut microbiota plays a substantial role in the production of these uremic toxins.25–30 Thus, our data demonstrate that at least some of the changes in the gut metabolome which occur in response to Ang II are likely detrimental. In contrast, however, the other metabolite upregulated with Ang II in our study (spermidine) has been reported to lower blood pressure.31–33 This raises the exciting and novel possibility that some of the so-called dysbiosis observed in the Ang II model may actually be protective; that is, the microbiota may be acting to help their host. Spermidine has been reported to lower blood pressure in dogs, and a 2016 study found that spermidine was cardioprotective in aged mice and in Dahl salt-sensitive rats.33 Further, this study found that higher levels of dietary spermidine in humans correlated with lowered blood pressure and a lower cardiovascular disease incidence. Although this study focused on dietary sources of spermidine, our data demonstrate that the gut microbiota is a significant source of plasma spermidine. Thus, we suggest that while some aspects of the gut dysbiosis associated with hypertension are likely harmful to the host, others may be beneficial.
The fecal metabolites that were upregulated with Ang II treatment include taurodeoxycholate and taurodeoxycholic acid. It is known that the host and the gut microbiota work together to achieve bile acid synthesis,34 and thus it is not surprising that the changes we observed in taurodeoxycholate and taurodeoxycholic acid did not occur in GF mice. Of note, taurodeoxycholate has been shown to lower blood pressure in rats.35
Metabolites Downregulated With Ang II Treatment
We also observed a decrease in 8 plasma and 71 fecal metabolites with Ang II treatment, none of which were altered in GF mice. Indoles are produced by the gut microbiota, and we observed several indoles that were downregulated in plasma with Ang II treatment (indoleacetylglycine and indoleacetate), which is interesting in light of a 2017 study, which reported a decrease in fecal indoles on a high-salt diet.9 We also observed a downregulation of the plasma metabolite N,N,N-trimethyl-5-aminovalerate with Ang II treatment; this compound was recently shown to be a predictor for progression to microalbuminuria in type 1 diabetics.36 This implies that a downregulation of this compound may be renoprotective. However, on the other hand, ferulic acid 4-sulfate has been reported to relax vessels and lower blood pressure in mice, implying that a decrease in this compound may exacerbate the effects of Ang II in conventional mice.37 These findings again lead us to speculate that the pleiotropic actions of the microbiota may be in some cases protective and in other cases detrimental.
Relating Changes in Fecal and Plasma Metabolites
None of the upregulated metabolites were similarly changed in both plasma and feces. However, there were 4 metabolites which were downregulated in both plasma and feces (trans-4-hydroxyproline, xylose, N,N,N-trimethyl-5-aminovalerate, and indoleacetate). Conversely, spermidine was significantly upregulated with Ang II treatment in conventional plasma but was downregulated in conventional feces. Of note, a change in the abundance of a metabolite in plasma may result from not only increased production but also may occur because of changes in the rate of uptake into the plasma or changes in the rate of clearance from the plasma. In addition, it is worth noting that fecal levels of metabolites may not reflect metabolite levels in the colon itself.
Relating Changes in 16S Sequencing and in Metabolites
Although we have both 16S and metabolomics data, at this time we cannot confidently assign individual metabolites to individual microbial species. This is especially difficult for metabolites which have been reported to be produced by multiple species, such as p-cresol metabolites.38 Although an increase in the firmicutes/bacteroidetes ratio has been used as an indicator of dysbiosis, we did not detect any difference in the firmicutes/bacteroidetes ratio between saline and Ang II groups in our study. Although some studies have reported an increase in the firmicutes/bacteroidetes ratio in the spontaneously hypertensive rat,6 our results are consistent with studies that found no changes in the firmicutes/bacteroidetes ratio in either the spontaneously hypertensive rat model or the Dahl salt-sensitive rat model of hypertension.5,20 Similarly, although a study by Wilck et al9 reported that Lactobacillus murinus is depleted by high-salt diet, we did not see depletion of L murinus with Ang II treatment. Of note, although we have focused on bacteria as potential sources of metabolites, microbiota-host interactions may also be responsible for metabolite generation (ie, a bacterial metabolite may act to upregulate the production of a host metabolite). Thus, the metabolite changes we see here are microbiota dependent but not necessarily microbiota derived. In addition, we cannot rule out other additional metabolite sources which may also differ between GF and conventional mice (ie, archaea, fungi). Finally, although our analyses focused on conventional mice, it is notable that GF mice treated with Ang II significantly upregulated 4 metabolites and downregulated 37 metabolites. Better understanding the roles of these metabolites in the host may also give us insights into microbe-host interactions.
Sex Differences
Hypertension has long been known to exhibit sex differences in humans and in animal models, with females being largely protected from hypertension premenopause. Sex hormones play a prominent role in driving these differences, as demonstrated by the fact that sex differences in the Ang II model are muted by gonadectomy.39 However, gonadectomy also profoundly changes the gut microbiota.40 Similarly, immune differences in males and females are known to be important in the Ang II model of hypertension,41 and gut microbiome is also known to play a role in immune system education and maintenance. Although the number of males and females in our study is too low to draw conclusions, it is intriguing to speculate that the microbiota may play a role in sex differences in blood pressure regulation. As precedent for this idea, the non-obese diabetic mouse model of type I diabetes mellitus has well-established sex differences, which are muted by gonadectomy. However, in 2013, it was shown that the sex differences in the NOD model are actually driven by sex differences in the microbiome; in fact, these sex differences disappeared in GF mice.42 The idea that hormonal differences and the microbiome intersect to explain sex differences in blood pressure regulation is also supported by the idea that uremic toxins (many of which are generated in large part via the microbiota) induce microvascular dysfunction and that estrogen may be protective against this mechanism.43 Future studies, better powered to examine sex differences, are needed in this area.
Response to Ang II in GF Mice
In this study, we took extreme care to confirm that the GF mice remained GF throughout the experiment. To achieve this, we minimized handling of the GF mice and euthanized the animals immediately on removing them from the GF isocages. Thus, although our study has yielded novel insights into the gut metabolome in response to Ang II, we were not able to measure blood pressure in this study and have not yet explored the pathophysiology of the response to Ang II in GF versus conventional mice. In addition, blood samples were prioritized for metabolomics studies, and thus we were not able to measure additional parameters in the plasma. However, our metabolomics data do contain compounds of interest, and these data show that neither conventional nor GF mice had significant changes in plasma creatinine, urea, glucose, cholesterol, or corticosterone when treated with Ang II versus saline.
Although we did not measure blood pressure in our study, a study by Karbach et al44 with a similar design (conventional versus GF mice, with and without Ang II infusion) did measure blood pressure. This same study also examined inflammatory markers and end organ damage in these 4 groups. It is important to note that the study by Karbach et al only used male mice, and the dose of Ang II in this study was higher (1 mg·kg−1·day−1), and the time of infusion was shorter (1 week) than in our study. Nevertheless, this dataset is a useful point of reference. Karbach et al found that Ang II treatment significantly increases blood pressure in GF mice; however, the increase in blood pressure in the GF mice was muted as compared with that seen in the conventional mice. In addition, this study found that Ang II–infused GF mice were protected from reactive oxygen species formation and exhibited less vascular inflammation, had lower numbers of infiltrating immune cells in the kidney, and decreased cardiac fibrosis. These data suggest that the microbiota is primarily detrimental in the setting of hypertension. However, our metabolomics data (increased spermidine, etc) suggest that although the microbiota have a negative effect on balance, some actions of the microbiota may serve to mitigate the effects of Ang II. It should be additionally noted here that we cannot rule out disparate effects of Ang II in GF versus conventional animals; nevertheless, differences in metabolites between GF and conventional mice still indicate a dependence of the metabolite effect on the microbiota.
Recently, a study by Mishima et al29 examined plasma metabolomics in a renal failure model using a similar study design to our own: conventional versus GF mice with and without renal failure (induced with an adenine-rich diet). Both our study and that by Mishima et al found an increase in p-cresol sulfate; however, no other metabolites that changed in the disease state were common between the 2 studies.
Perspectives
The gut microbiota produces a variety of metabolites which can enter the circulating blood and act as signaling molecules in the host; recent studies have shown a key role for the gut microbiome in the setting of hypertension. In this study, we show for the first time that Ang II–mediated changes in plasma and fecal metabolites are completely dependent on the gut microbiota. Gut-derived metabolites include both potentially beneficial and potentially harmful molecules, implying that the effects of the gut microbiota on host health may be pleotropic. We also found significant shifts in the gut microbiota themselves. In sum, this study reveals for the first time a central and primary role for the gut microbiota in altered metabolomics in response to Ang II; moving forward, it will be critical to validate these findings in additional cohorts and models.
Acknowledgments
We thank the members of the Pluznick Laboratory for helpful discussions.
Sources of Funding
This work was supported by the American Heart Association (16IRG27260265), National Institutes of Health (NIH) National Heart, Lung, and Blood Institute (R01HL-128512), and NIH National Institute of Diabetes and Digestive and Kidney Diseases (R01DK-107726).
Disclosures
None.
Supplementary Material
Footnotes
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.119.13155.
Novelty and Significance
What Is New?
We showed that all of the plasma and fecal metabolites which are altered with Ang II (angiotensin II), are derived from the gut microbiota.
While some of these metabolites are likely detrimental to the host, others may be beneficial in a setting of Ang II infusion.
What Is Relevant?
The gut microbiota has previously been implicated in changes in hypertension; however, to date, only a few gut microbial metabolites have been studied.
In this study, we used a discovery metabolomic approach to identify novel microbial metabolites which are altered in response to Ang II infusion.
We found that all of the plasma and fecal metabolites which are altered with Ang II infusion are dependent on the gut microbiota, indicating that the gut microbiome is the key player in metabolite changes in this model.
Summary
Metabolomic analyses revealed 12 significantly altered plasma metabolites, and 96 significantly altered fecal metabolites, in an Ang II infusion model in conventional mice. Intriguingly, none of these metabolites were altered in germ-free mice, indicating that gut microbiota plays the central role in metabolomic changes with Ang II infusion.
References
- 1.He WJ, Li C, Rao DC, Hixson JE, Huang J, Cao J, Rice TK, Shimmin LC, Gu D, Kelly TN. Associations of renin-angiotensin-aldosterone system genes with blood pressure changes and hypertension incidence. Am J Hypertens. 2015;28:1310–1315. doi: 10.1093/ajh/hpv033. doi: 10.1093/ajh/hpv033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurts C, Panzer U, Anders HJ, Rees AJ. The immune system and kidney disease: basic concepts and clinical implications. Nat Rev Immunol. 2013;13:738–753. doi: 10.1038/nri3523. doi: 10.1038/nri3523. [DOI] [PubMed] [Google Scholar]
- 3.Zhang HN, Xu QQ, Thakur A, Alfred MO, Chakraborty M, Ghosh A, Yu XB. Endothelial dysfunction in diabetes and hypertension: role of microRNAs and long non-coding RNAs. Life Sci. 2018;213:258–268. doi: 10.1016/j.lfs.2018.10.028. doi: 10.1016/j.lfs.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 4.Chopra S, Baby C, Jacob JJ. Neuro-endocrine regulation of blood pressure. Indian J Endocrinol Metab. 2011;15(suppl 4):S281–S288. doi: 10.4103/2230-8210.86860. doi: 10.4103/2230-8210.86860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, Haribabu B, Vijay-Kumar M, Pennathur S, Joe B. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics. 2015;47:187–197. doi: 10.1152/physiolgenomics.00136.2014. doi: 10.1152/physiolgenomics.00136.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, Sahay B, Pepine CJ, Raizada MK, Mohamadzadeh M. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durgan DJ, Ganesh BP, Cope JL, Ajami NJ, Phillips SC, Petrosino JF, Hollister EB, Bryan RM., Jr. Role of the gut microbiome in obstructive sleep apnea-induced hypertension. Hypertension. 2016;67:469–474. doi: 10.1161/HYPERTENSIONAHA.115.06672. doi: 10.1161/HYPERTENSIONAHA.115.06672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim S, Goel R, Kumar A, Qi Y, Lobaton G, Hosaka K, Mohammed M, Handberg EM, Richards EM, Pepine CJ, Raizada MK. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin Sci (Lond) 2018;132:701–718. doi: 10.1042/CS20180087. doi: 10.1042/CS20180087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilck N, Matus MG, Kearney SM, et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature. 2017;551:585–589. doi: 10.1038/nature24628. doi: 10.1038/nature24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Zhao F, Wang Y, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5:14. doi: 10.1186/s40168-016-0222-x. doi: 10.1186/s40168-016-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Natarajan N, Hori D, Flavahan S, Steppan J, Flavahan NA, Berkowitz DE, Pluznick JL. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol Genomics. 2016;48:826–834. doi: 10.1152/physiolgenomics.00089.2016. doi: 10.1152/physiolgenomics.00089.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pluznick JL, Protzko RJ, Gevorgyan H, et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA. 2013;110:4410–4415. doi: 10.1073/pnas.1215927110. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartolomaeus H, Balogh A, Yakoub M, et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. 2019;139:1407–1421. doi: 10.1161/CIRCULATIONAHA.118.036652. doi: 10.1161/CIRCULATIONAHA.118.036652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marques FZ, Nelson E, Chu PY, Horlock D, Fiedler A, Ziemann M, Tan JK, Kuruppu S, Rajapakse NW, El-Osta A, Mackay CR, Kaye DM. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135:964–977. doi: 10.1161/CIRCULATIONAHA.116.024545. doi: 10.1161/CIRCULATIONAHA.116.024545. [DOI] [PubMed] [Google Scholar]
- 15.Hildebrand F, Nguyen TL, Brinkman B, Yunta RG, Cauwe B, Vandenabeele P, Liston A, Raes J. Inflammation-associated enterotypes, host genotype, cage and inter-individual effects drive gut microbiota variation in common laboratory mice. Genome Biol. 2013;14:R4. doi: 10.1186/gb-2013-14-1-r4. doi: 10.1186/gb-2013-14-1-r4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 18.Adnan S, Nelson JW, Ajami NJ, Venna VR, Petrosino JF, Bryan RM, Jr, Durgan DJ. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics. 2017;49:96–104. doi: 10.1152/physiolgenomics.00081.2016. doi: 10.1152/physiolgenomics.00081.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bier A, et al. A high salt diet modulates the gut microbiota and short chain fatty acids production in a salt-sensitive hypertension rat model. Nutrients. 2018;10:e1154. doi: 10.3390/nu10091154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galla S, Chakraborty S, Cheng X, Yeo J, Mell B, Zhang H, Mathew AV, Vijay-Kumar M, Joe B. Disparate effects of antibiotics on hypertension. Physiol Genomics. 2018;50:837–845. doi: 10.1152/physiolgenomics.00073.2018. doi: 10.1152/physiolgenomics.00073.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganesh BP, Nelson JW, Eskew JR, Ganesan A, Ajami NJ, Petrosino JF, Bryan RM, Jr, Durgan DJ. Prebiotics, probiotics, and acetate supplementation prevent hypertension in a model of obstructive sleep apnea. Hypertension. 2018;72:1141–1150. doi: 10.1161/HYPERTENSIONAHA.118.11695. doi: 10.1161/HYPERTENSIONAHA.118.11695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto Y, et al. A metabolomic-based evaluation of the role of commensal microbiota throughout the gastrointestinal tract in mice. Microorganisms 20186pii: E101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vital M, Howe AC, Tiedje JM. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. MBio. 2014;5:e00889. doi: 10.1128/mBio.00889-14. doi: 10.1128/mBio.00889-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mishima E, Fukuda S, Kanemitsu Y, et al. Canagliflozin reduces plasma uremic toxins and alters the intestinal microbiota composition in a chronic kidney disease mouse model. Am J Physiol Renal Physiol. 2018;315:F824–F833. doi: 10.1152/ajprenal.00314.2017. doi: 10.1152/ajprenal.00314.2017. [DOI] [PubMed] [Google Scholar]
- 25.Wong J, Piceno YM, DeSantis TZ, Pahl M, Andersen GL, Vaziri ND. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol. 2014;39:230–237. doi: 10.1159/000360010. doi: 10.1159/000360010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanholder R, Schepers E, Pletinck A, Nagler EV, Glorieux G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review. J Am Soc Nephrol. 2014;25:1897–1907. doi: 10.1681/ASN.2013101062. doi: 10.1681/ASN.2013101062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, Patterson PH, Mazmanian SK. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koppe L, Alix PM, Croze ML, Chambert S, Vanholder R, Glorieux G, Fouque D, Soulage CO. p-Cresyl glucuronide is a major metabolite of p-cresol in mouse: in contrast to p-cresyl sulphate, p-cresyl glucuronide fails to promote insulin resistance. Nephrol Dial Transplant. 2017;32:2000–2009. doi: 10.1093/ndt/gfx089. doi: 10.1093/ndt/gfx089. [DOI] [PubMed] [Google Scholar]
- 29.Mishima E, Fukuda S, Mukawa C, et al. Evaluation of the impact of gut microbiota on uremic solute accumulation by a CE-TOFMS-based metabolomics approach. Kidney Int. 2017;92:634–645. doi: 10.1016/j.kint.2017.02.011. doi: 10.1016/j.kint.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 30.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marmo E, Berrino L, Cazzola M, Filippelli A, Cafaggi G, Persico N, Spadaro R, Nisticò G. Cardiovascular and respiratory effects of spermidine and spermine: an experimental study. Biomed Biochim Acta. 1984;43:509–515. [PubMed] [Google Scholar]
- 32.Eisenberg T, Abdellatif M, Zimmermann A, et al. Dietary spermidine for lowering high blood pressure. Autophagy. 2017;13:767–769. doi: 10.1080/15548627.2017.1280225. doi: 10.1080/15548627.2017.1280225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eisenberg T, Abdellatif M, Schroeder S, et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med. 2016;22:1428–1438. doi: 10.1038/nm.4222. doi: 10.1038/nm.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Rutishauser SC, Stone SL. Comparative effects of sodium taurodeoxycholate and sodium taurocholate on bile secretion in the rat, dog and rabbit. J Physiol. 1975;245:583–598. doi: 10.1113/jphysiol.1975.sp010863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haukka JK, Sandholm N, Forsblom C, Cobb JE, Groop PH, Ferrannini E. Metabolomic profile predicts development of microalbuminuria in individuals with type 1 diabetes. Sci Rep. 2018;8:13853. doi: 10.1038/s41598-018-32085-y. doi: 10.1038/s41598-018-32085-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Rymenant E, Van Camp J, Pauwels B, Boydens C, Vanden Daele L, Beerens K, Brouckaert P, Smagghe G, Kerimi A, Williamson G, Grootaert C, Van de Voorde J. Ferulic acid-4-O-sulfate rather than ferulic acid relaxes arteries and lowers blood pressure in mice. J Nutr Biochem. 2017;44:44–51. doi: 10.1016/j.jnutbio.2017.02.018. doi: 10.1016/j.jnutbio.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 38.Saito Y, et al. Identification of phenol- and p-cresol-producing intestinal bacteria by using media supplemented with tyrosine and its metabolites. FEMS Microbiol Ecol. 2018;94 doi: 10.1093/femsec/fiy125. doi: 10.1093/femsec/fiy125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xue B, Pamidimukkala J, Hay M. Sex differences in the development of angiotensin II-induced hypertension in conscious mice. Am J Physiol Heart Circ Physiol. 2005;288:H2177–H2184. doi: 10.1152/ajpheart.00969.2004. doi: 10.1152/ajpheart.00969.2004. [DOI] [PubMed] [Google Scholar]
- 40.Org E, Mehrabian M, Parks BW, Shipkova P, Liu X, Drake TA, Lusis AJ. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes. 2016;7:313–322. doi: 10.1080/19490976.2016.1203502. doi: 10.1080/19490976.2016.1203502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pollow DP, Uhrlaub J, Romero-Aleshire M, Sandberg K, Nikolich-Zugich J, Brooks HL, Hay M. Sex differences in T-lymphocyte tissue infiltration and development of angiotensin II hypertension. Hypertension. 2014;64:384–390. doi: 10.1161/HYPERTENSIONAHA.114.03581. doi: 10.1161/HYPERTENSIONAHA.114.03581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–1088. doi: 10.1126/science.1233521. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 43.Pei J, Harakalova M, den Ruijter H, Pasterkamp G, Duncker DJ, Verhaar MC, Asselbergs FW, Cheng C. Cardiorenal disease connection during post-menopause: the protective role of estrogen in uremic toxins induced microvascular dysfunction. Int J Cardiol. 2017;238:22–30. doi: 10.1016/j.ijcard.2017.03.050. doi: 10.1016/j.ijcard.2017.03.050. [DOI] [PubMed] [Google Scholar]
- 44.Karbach SH, et al. Gut microbiota promote angiotensin II-induced arterial hypertension and vascular dysfunction. J Am Heart Assoc. 2016;5:e003698. doi: 10.1161/JAHA.116.003698. [DOI] [PMC free article] [PubMed] [Google Scholar]


