Abstract
Background:
Although potential links between malaria parasitaemia and hypertension have been hypothesized, there is paucity of epidemiologic evidence on this link. We investigated in a population-based survey, the association between malaria parasitaemia and hypertension in Ivorian adults.
Methods:
We estimated the adjusted odds ratios (OR) and 95% confidence intervals (CI) of hypertension in relation to malaria parasitaemia using multinomial regression, in 997 randomly selected adults in the ‘Côte d’Ivoire Dual Burden of Disease Study’ (CoDuBu), in south-central Côte d’Ivoire. We defined malaria parasitaemia as a positive rapid diagnostic test or identification of Plasmodium spp. on microscopy. Using the mean of the last two of three blood pressure (BP) measurements and questionnaire data, we defined hypertension as SBP at least 140 mmHg or DBP at least 90 mmHg or clinician-diagnosed hypertension.
Results:
Prevalence of malaria parasitaemia and hypertension were 10 and 22%, respectively. Malaria parasitaemia was negatively associated with hypertension in participants with body temperature 36.5 °C or less [OR 0.23 (95% CI 0.06–0.84)]. Contrastingly, microscopic malaria parasitaemia showed positive associations with hypertension in participants with elevated body temperature [>36.5 °C; OR: 2.93 (95% CI 0.94–9.14)]. Participants having microscopic malaria parasitaemia with elevated body temperature had three-fold higher odds of hypertension [OR: 3.37 (95% CI 1.12–10.0)] than malaria parasitaemia-negatives with lower body temperature.
Conclusion:
Malaria parasitaemia and hypertension are prevalent and seemingly linked comorbidities in African settings. This link may depend on malaria parasitaemia symptomaticity/latency where individuals with more latent/asymptomatic malaria parasitaemia have lower risk of hypertension and those with more acute/symptomatic malaria parasitaemia have a tendency toward higher BP. The cross-sectional nature of the study limited the distinction of short-term BP elevation (interim pathophysiological stress) from hypertension development. Future longitudinal studies considering malaria/hypertension phenotypes and host molecular variations are needed to clarify involved biological mechanisms, toward comorbidity management.
Keywords: blood pressure, Côte d’Ivoire, hypertension, infectious disease, malaria, Plasmodium spp
INTRODUCTION
The prevalence of hypertension in low-income and middle-income countries (LMICs) is increasing [1,2], contributing to higher mortality rates because of cardiovascular diseases (CVD) [3]. Although this may be attributed to urbanization and westernization of lifestyle, they do not explain the high rates that have also been observed in rural areas [4]. Persons of African ethnic background are well known to have comparatively higher blood pressure (BP) and stroke risk than Caucasians [5]. At the same time, malaria parasitaemia remains prevalent and a significant contributor to mortality in LMICs [6]. Together with malaria parasitaemia, hypertension contributes to a state of double disease burden, which further strains individual health and poor health systems in LMICs.
A potential link between malaria parasitaemia and hypertension was hypothesized. Suggested pathways can be divided into causative malaria parasitaemia effects and shared origins [7,8]. Proposed direct effects of malaria parasitaemia include the promotion, in the long-term, of an atherosclerosis-promoting pro-inflammatory state because of repeated infections [7,9–11]. In-utero and early life effects of malaria parasitaemia on low birth weight, malnutrition and stunting, are established risk factors for CVD later in life [7,12–16]. In pregnant women, malaria parasitaemia effect on preeclampsia puts them at risk for hypertension [7]. In the short-term, malaria parasitaemia could induce BP fluctuations in either direction [11,17]. It is even conceivable that BP may first increase during the milder stages of malaria parasitaemia and subsequently falls as observed in severe malaria [18,19].
The shared origin hypothesis [7,8] is rooted in pathways common to malaria parasitaemia and hypertension, such as immunity, inflammation and body fluid regulation, and in the observation of higher rates of hypertension among individuals of African and Asian ancestry in malaria-free western settings [20]. It suggests selective pressure of genetic variants that protect against malaria or its pathogenesis, regardless of other potentially resulting negative systemic outcomes [8,21]. Malaria parasitaemia exerted the strongest selective pressure on the human genome in recent history, in part related to sickle cell trait [22]. But the latter was not related to 24-h BP measurements and arterial stiffness in African children from a malaria-free region [23]. Additional genes under selective pressure include those involved in oxidative stress, inflammation, immunity and endothelial function [22]. Genetic variants that promote angiotensin-converting enzyme (ACE) activity, and hypertension [24,25], have higher expression rates in African Americans compared with whites [26,27], and were associated with milder forms of malaria [28–30].
However, there is a paucity of population-based epidemiologic evidence on the association between malaria parasitaemia and hypertension. Malaria patients had lower SBP [31], and higher serum ACE activity [29] compared with controls in hospital-based studies. We investigated using a population-based approach, the association between malaria parasitaemia and hypertension in Ivorian adults from a malaria-endemic setting [32,33].
METHODS
Study population
This study was done within the Côte d’Ivoire Dual Burden of Disease Study (CoDuBu), which aims to investigate the co-occurrence of infectious diseases and noncommunicable diseases (NCDs), in the Taabo health and demographic surveillance system (HDSS) [34]. Details of CoDuBu have been described elsewhere [35]. Briefly, 1019 adults (50% men) were randomly recruited in 2017 from three Taabo HDSS communities (Amani-Ménou, Taabo-Cité and Tokohiri). Participants, in a fasting state, underwent health examinations, including anthropometry, BP, haemoglobin (Hb), malaria rapid diagnostic test (RDT), and thick and thin blood film microscopy. A biobank constituting blood, stool and urine was set up for future research on the molecular basis of infectious disease–NCD relationship. Participants also had detailed interviews covering their health status and lifestyle characteristics.
The CoDuBu study protocol was approved by the Côte d’Ivoire National Research Ethics Committee (ref. 032/IMSHP/CNER-kp; 24 March 2017) and the Ethics Committee of Northwest and Central Switzerland (ref. 2016–00143; 2 May 2016). All participants provided written informed consent before participating in the study, and consented to be re-contacted for a potential follow-up study.
Case definition of malaria parasitaemia
Venous blood from participants was applied toward RDT and microscopy for the identification of Plasmodium infection. RDT was done using Malaria Dual PF/PAN Antigen (Histidine-rich protein II (HRP2)/parasite lactate dehydrogenase (pLDH)) test (ICT Diagnostics, Cape Town, South Africa), which is an in-vitro immunochromatographic assay for the qualitative detection of Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale and Plasmodium malariae antigens in blood. RDTs were performed by experienced laboratory technicians, and only valid tests were recorded.
Thick and thin blood films were also prepared, air-dried, Giemsa-stained, and examined under a light microscope at high magnification, for Plasmodium spp. identification and quantification. Parasites were counted against 200 leukocytes (or 500 leukocytes if parasite count was less than 10), assuming a standard count of 8000 leukocytes/μl of blood [36]. For quality control, a random sample of 10% of the slides was re-read by a senior laboratory technician and inter-rater agreement was 100%.
We identified participants as malaria parasitaemia-positive, if they tested positive to either RDT or microscopy, regardless of Plasmodium spp. or density. As the RDT may detect low-density infections, and the HRP2 antigens of P. falciparum for a few weeks following parasite clearance [37], we defined two diagnosis-specific malaria parasitaemia, including RDT-only malaria parasitaemia (excluding those identified by microscopic malaria parasitaemia, representing more latent infection) and microscopic malaria parasitaemia (excluding RDT-only malaria parasitaemia, representing more active infection).
Case definition of prehypertension and hypertension
Participants had three BP measurements, 3 min apart, using Omron Oscillograph and appropriate-sized cuffs (OMRON Healthcare, Kyoto, Japan). The mean of the last two measurements was taken for further analyses. Prehypertension was defined as mean SBP of 120–139 mmHg or DBP of 80–89 mmHg, in the absence of clinician-diagnosed hypertension. Hypertension was defined as having mean SBP at least 140 mmHg or DBP at least 90 mmHg or clinician-diagnosed hypertension or antihypertensive medication use. All cases of hypertension underwent electrocardiography, had 1 month of free treatment, and were subsequently enrolled in a subsidized treatment and follow-up plan.
Measurement of potential confounders/modifiers
The following variables, which were measured using a questionnaire, were selected as covariates: age (years; continuous), sex (male/female), educational attainment (none/primary/secondary/tertiary), household wealth index derived as a principal component analysis of properties belonging to household of the participant [34] and study area. We further considered CVD risk factors including smoking status (never/former/current), intake of fruits and vegetables (never/monthly/weekly/several days per week), sedentariness (hours per week spent sitting or lying excluding sleep; continuous), family history of hypertension (yes/no) and general susceptibility to illness (in comparison to same sex and age group; lower/higher). Participants also reported fever (yes/no) before examination.
Weight (kg), height (m) and waist circumference (cm) were measured and BMI was calculated as the weight–height-squared ratio (kg/m2). Body temperature was measured using Omron auricular thermometer (Omron Healthcare, Kyoto, Japan) and was classified along the mean (≤36.5 °C/>36.5 °C; range: 34.6–37.9 °C) for analyses. Hb was measured with HemoCue Hb 301 (HemoCue AB, Ängelholm, Sweden), and anaemia was defined based on WHO recommendations [38].
Statistical analyses
We described the characteristics of participants using frequencies for categorical variables and means and SDs for continuous variables. We imputed age-adjusted, sex-adjusted, and area-adjusted mean of the missing covariate data. We stratified these characteristics by hypertension status and tested differences in proportions and means using χ2 and t-tests, respectively.
We performed multinomial logistic regression for associations between malaria parasitaemia and prehypertension and hypertension, in unadjusted and adjusted models. We performed covariate adjustment in a stepwise fashion and the covariates included age, sex, educational attainment, wealth index, area, family history of hypertension, smoking, intake of fruits and vegetables, BMI, waist circumference, sedentariness and anaemia. We performed sensitivity analyses by limiting malaria parasitaemia cases to (sensitivity model i) RDT malaria parasitaemia (regardless of microscopy); (sensitivity model ii) microscopic malaria parasitaemia (regardless of RDT); (sensitivity model iii) RDT-only malaria parasitaemia (excluding microscopy-positive cases) and (sensitivity model iv) microscopic malaria parasitaemia (excluding RDT-only positive cases).
Using the adjusted model, we tested effect modification by age, sex, self-reported fever, body temperature, family history of hypertension, and illness susceptibility. We also performed these effect modification analyses using the sensitivity models (iii) and (iv) mentioned above. Furthermore, we tested associations of prehypertension and hypertension (and of SBP and DBP) with combinations of elevated temperature and malaria parasitaemia diagnosis, in adjusted models. For these analyses, we classified participants into six exclusive groups, including (group i) malaria parasitaemia-negative without elevated body temperature (i.e. no self-reported fever and body temperature ≤36.5 ° C; reference group); (group ii) malaria parasitaemia-negative with elevated body temperature (i.e. self-reported fever or body temperature >36.5 °C); (group iii) RDT-only malaria parasitaemia-positive, without elevated body temperature; (group iv) RDT-only malaria parasitaemia-positive, with elevated body temperature; (group v) microscopic malaria parasitaemia-positive, without elevated body temperature and (group vi) microscopic malaria parasitaemia-positive, with elevated body temperature.
All results are reported as odds ratios (ORs) or beta coefficients (β) and their 95% confidence intervals (CIs). All analyses were performed with Stata version 14 (Stata Corporation, College Station, Texas, USA) and R Studio version 0.99.092 (R Foundation, Vienna, Austria).
RESULTS
We included 997 (98% inclusion rate) participants for analyses. Among the excluded were two participants who lacked BP data, and 20 pregnant women. Included participants were 51% men, aged 18–87 years, with 55% having at least primary education. Mean (standard deviation; SD) of SBP and DBP were 122 (18) mmHg and 79 (12) mmHg, respectively. Compared with nonhypertensive participants, hypertensive participants were older, more often women, had higher socioeconomic status, sedentariness, adiposity and family history of hypertension (Table 1).
TABLE 1.
Summary of participant characteristics
| All | No hypertension | Hypertension | P value | |
| Categorical variables | N (%) | N (%) | N (%) | χ2 |
| All | 997 (100) | 771 (77.3) | 226 (22.7) | |
| Sex | ||||
| Female | 485 (48.7) | 361 (46.8) | 124 (54.9) | 0.033 |
| Male | 512 (51.3) | 410 (53.2) | 102 (45.1) | |
| Formal education | ||||
| None | 448 (44.9) | 334 (43.2) | 114 (50.4) | 0.031 |
| Primary | 237 (23.8) | 199 (25.8) | 38 (16.8) | |
| Secondary | 235 (23.6) | 182 (23.6) | 53 (23.5) | |
| Tertiary | 77 (7.7) | 56 (7.3) | 21 (9.3) | |
| Wealth index tertiles | ||||
| T1 | 337 (33.8) | 279 (36.2) | 58 (25.7) | 0.002 |
| T2 | 330 (33.1) | 257 (33.3) | 73 (32.3) | |
| T3 | 330 (33.1) | 235 (30.5) | 95 (42.0) | |
| Smoking | ||||
| Never smokers | 827 (83.0) | 630 (81.7) | 197 (87.2) | 0.026 |
| Former smokers | 81 (8.1) | 62 (8.0) | 19 (8.4) | |
| Current smokers | 89 (8.9) | 79 (10.3) | 10 (4.4) | |
| Fruit intake | ||||
| Never | 5 (0.5) | 3 (0.4) | 2 (0.9) | 0.015 |
| Monthly | 271 (27.2) | 193 (25.0) | 78 (34.5) | |
| Weekly | 630 (63.2) | 498 (64.6) | 132 (58.4) | |
| Several days/week | 91 (9.1) | 77 (10.0) | 14 (6.2) | |
| Vegetable intake | ||||
| Never | 130 (13.0) | 104 (13.5) | 26 (11.5) | 0.494 |
| Monthly | 267 (26.8) | 205 (26.6) | 62 (27.4) | |
| Weekly | 460 (46.2) | 360 (46.7) | 100 (44.5) | |
| Several days/week | 140 (14.0) | 102 (13.2) | 38 (16.8) | |
| Study area | ||||
| Taabo-Cité | 500 (50.1) | 364 (47.2) | 136 (60.2) | 0.003 |
| Amani-Ménou | 244 (24.5) | 200 (25.9) | 44 (19.5) | |
| Tokohiri | 253 (25.4) | 207 (26.9) | 46 (20.3) | |
| Self-reported fever | 142 (14.2) | 100 (13.0) | 42 (18.6) | 0.034 |
| Anaemia | 207 (20.8) | 151 (19.6) | 56 (24.8) | 0.090 |
| General susceptibility to illness | 436 (43.7) | 321 (41.6) | 115 (50.9) | 0.014 |
| Malaria parasitaemia a | 103 (10.1) | 86 (11.5) | 14 (6.2) | 0.029 |
| RDT malaria parasitaemia-positive | 96 (9.6) | 84 (10.9) | 12 (5.3) | 0.012 |
| Microscopy malaria parasitaemia-positive | 54 (5.4) | 46 (5.9) | 8 (3.5) | 0.156 |
| Malaria medication | 55 (5.5) | 37 (4.8) | 18 (7.9) | 0.067 |
| Family history of hypertension | 277 (27.8) | 192 (24.9) | 85 (37.6) | <0.001 |
| Missing data, waist circumference | 4 (0.4) | 3 (0.4) | 1 (0.4) | 0.911 |
| Missing data, BMI | 3 (0.3) | 2 (0.3) | 1 (0.4) | 0.659 |
| Missing data, body temperature | 17 (1.7) | 12 (1.6) | 5 (2.2) | 0.503 |
| Continuous variables | Mean (SD) | Mean (SD) | Mean (SD) | t-test |
| Age (years) | 42 (13) | 40 (13) | 51 (12) | <0.001 |
| Waist circumference (cm) | 81 (11) | 79 (10) | 86 (13) | <0.001 |
| BMI (kg/m2) | 23.4 (4.2) | 22.9 (3.8) | 24.9 (4.8) | <0.001 |
| Body temperature (°C) | 36.5 (0.5) | 36.5 (0.5) | 36.5 (0.5) | 0.114 |
| SBP (mmHg) | 122 (18) | 115 (11) | 145 (20) | <0.001 |
| DBP (mmHg) | 79 (12) | 75 (7) | 94 (13) | <0.001 |
| Mean arterial pressure | 94 (13) | 88 (8) | 111 (14) | <0.001 |
| Haemoglobin level (g/dl) | 13.3 (1.6) | 13.4 (1.5) | 13.3 (1.6) | 0.437 |
| Sedentariness (h/week) | 33 (17) | 32 (16) | 36 (21) | 0.001 |
| Plasmodium density (per μl) | 558 (1750) | 602 (1873) | 263 (303) | 0.637 |
Hypertension was defined as SBP at least 140 mmHg or DBP at least 90 mmHg or clinician-diagnosed hypertension. Anaemia was indirectly measured using Hb level and categorized according to WHO guidelines [38]. Missing data represents the number of participants who had single imputations of age-adjusted, sex-adjusted and area-adjusted means of the respective variation. Wealth index was derived from a principal component analysis, including all assets owned by the household of participants. Sedentariness was defined as hours per week spent in a sitting or lying position, excluding sleeping hours. RDT, rapid diagnostic test.
aPositive malaria rapid diagnostic test or microscopy.
Prevalence of malaria parasitaemia was 10%. Prevalence of RDT-positive malaria parasitaemia was 9.6%, whereas microscopic malaria parasitaemia was 5.4%. Most (82%) of the malaria parasitaemia cases identified by microscopy or RDT were P. falciparum. Prevalence of self-reported fever was 14%. Agreement between body temperature and self-reported fever was 56.3%. Prevalence of prehypertension and hypertension was 33 and 22%, respectively. Prevalence of hypertension was higher among malaria parasitaemia-negative participants (24%) than malaria parasitaemia-positive participants (14%) but prehypertension was not different by malaria parasitaemia status (both 33%).
Associations between malaria parasitaemia and prehypertension and hypertension were generally negative. Unadjusted ORs of prehypertension and hypertension in relation to malaria parasitaemia were 0.81 (95% CI 0.51–1.29) and 0.48 (95% CI 0.26–0.89). Adjusting for age, sex and socioeconomic covariates attenuated the ORs of prehypertension and hypertension. Associations remained stable to further adjustment for family history of hypertension, smoking and nutritional factors, but were further attenuated on accounting for adiposity and sedentariness. Associations remained stable to further adjustment for anaemia. Thus, the fully adjusted ORs of prehypertension and hypertension in relation to malaria parasitaemia were 0.97 (95% CI 0.59–1.58) and 0.65 (95% CI 0.32–1.33), respectively (Table 2).
TABLE 2.
Association between malaria parasitaemia and hypertension phenotypes in the Côte d’Ivoire Dual Burden of Disease Study
| Normotension | Prehypertension | Hypertension | |
| Model | 441 | 330 | 226 |
| N | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Model 1: unadjusted | Reference | 0.81 (0.51–1.29) | 0.48 (0.26–0.89) |
| Model 2: model 1 combined with age and sex | Reference | 0.85 (0.53–1.36) | 0.56 (0.29–1.08) |
| Model 3: model 2 combined with education, wealth and area | Reference | 0.88 (0.54–1.41) | 0.65 (0.34–1.27) |
| Model 4: model 3 combined with family history of hypertension | Reference | 0.88 (0.55–1.41) | 0.65 (0.33–1.26) |
| Model 5: model 4 combined with smoking, fruits and vegetables | Reference | 0.89 (0.55–1.44) | 0.64 (0.33–1.25) |
| Model 6: model 5 combined with BMI, WC, and sedentariness | Reference | 0.95 (0.58–1.55) | 0.66 (0.33–1.33) |
| Model 7: model 6 combined with anaemiaa | Reference | 0.97 (0.59–1.58) | 0.65 (0.32–1.33) |
| Model 7, RDT positive regardless of microscopyb | Reference | 0.93 (0.57–1.53) | 0.55 (0.26–1.15) |
| Model 7, microscopy positive regardless of RDTc | Reference | 1.09 (0.57–2.11) | 1.05 (0.41–2.67) |
| Model 7, RDT-only positive (excluding microscopy positive)d | Reference | 0.81 (0.40–1.64) | 0.40 (0.14–1.14) |
| Model 7, microscopy positive excluding RDT-only positive casese | Reference | 1.08 (0.56–2.09) | 0.99 (0.39–2.51) |
All estimates were derived from multinomial logistic regression models comparing odds of prehypertension and hypertension in malaria-positive vs. malaria-negative participants. Age (years), BMI (kg/m2) and waist circumference (cm) were treated as continuous variables. Education was measured as formal education (yes/no); wealth index was derived from a principal component analysis, including all assets owned by the household of participants; smoking was categorized as never, former or current; fruit and vegetable intake was categorized into never/monthly/weekly/several days per week, respectively; waist circumference was measured at the level of the umbilicus at point of expiration; sedentariness was measured as hours per week spent in a sitting or lying position excluding night-time sleeping hours. Anaemia was indirectly measured using Hb level and categorized according to WHO guidelines [38]. CI, confidence interval; OR, odds ratio; RDT, rapid diagnostic test.
aN (parasitaemia) = 100.
bN (parasitaemia) = 96.
cN (parasitaemia) = 54.
dN (normotension) = 413; N (prehypertension) = 312; N (hypertension) = 218; N (parasitaemia) = 46.
eN (normotension) = 416; N (prehypertension) = 315; N (hypertension) = 220; N (parasitaemia) = 54.
Estimates of association between malaria parasitaemia and prehypertension and hypertension were robust to sensitivity analyses, especially when limiting malaria parasitaemia to RDT, with associations between RDT-only malaria parasitaemia and hypertension reaching borderline significance [OR 0.40 (95% CI 0.14–1.14). Associations between microscopic malaria parasitaemia and prehypertension and hypertension tended to be positive, with respective ORs of 1.09 (95% CI 0.57–2.11) and 1.05 (95% CI 0.41–2.67) (Table 2).
We observed differences by self-reported fever (Pinteraction = 0.04) and body temperature (Pinteraction = 0.04) on the association between malaria parasitaemia and hypertension in our study. We observed a negative association in participants without self-reported fever (OR 0.40; 95% CI 0.17–0.98), and a positive association in those reporting fever (OR 2.30; 95% CI 0.58–9.21). Similarly, we observed a negative association in participants with body temperature 36.5 °C or less (OR 0.23; 95% CI 0.06–0.84) and a positive association in those with body temperature greater than 36.5 °C (OR 1.21; 95% CI 0.50–2.92). This trend was similar for prehypertension (Fig. 1).
FIGURE 1.
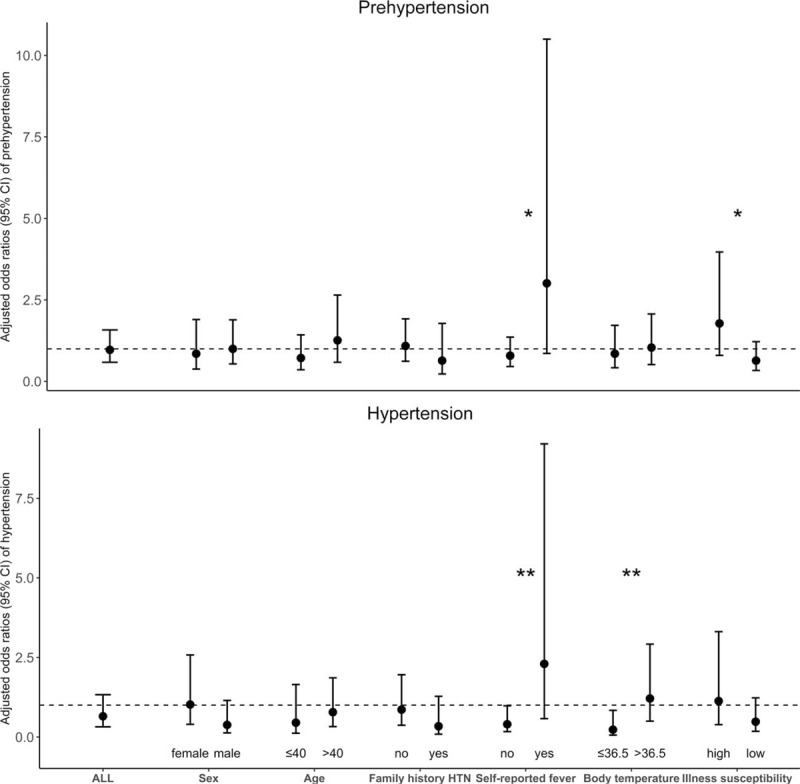
Adjusted odds ratios (circular points) and 95% confidence intervals (spikes) of the association between malaria parasitaemia (defined as positive microscopy or rapid diagnostic test) and hypertension phenotypes, stratified by potential modifiers. All estimates were derived from multivariable multinomial logistic regression models comparing odds of prehypertension and hypertension in malaria-positive vs. malaria-negative participants. All models were adjusted for age, sex, formal education, wealth index, area, family history of hypertension, smoking, fruit and vegetable intake, BMI, waist circumference, sedentariness and anaemia. Susceptibility was defined as a self-report of falling ill more frequently than people of the same sex and age group. CI, confidence interval; HTN, hypertension. ∗P value of interaction is 0.05; ∗∗P value of interaction is 0.04.
We observed a stronger magnitude of association in the participants with self-reported fever or having higher body temperature in models limited to microscopic malaria parasitaemia. Adjusted ORs of hypertension in relation to microscopic malaria parasitaemia was 4.89 (95% CI 0.90–26.90; P = 0.07) among those with self-reported fever (Pinteraction = 0.03), and 2.93 (95% CI 0.94–9.14; P = 0.06) among those with body temperature greater than 36.5 °C (Pinteraction = 0.02). We found differences by diagnostic test (Pinteraction(RDTvs. microscopy) = 0.03), in the modifying effect of body temperature on malaria parasitaemia–hypertension association. We also found similar test-specific trends with prehypertension (Table 3).
TABLE 3.
Association between malaria parasitaemia (rapid diagnostic test-diagnosed vs. microscopy-diagnosed) and hypertension in the Côte d’Ivoire Dual Burden of Disease Study, all participants and stratified by age, sex, the presence of elevated body temperature and self-reported susceptibility to illnesses
| Malaria diagnosis based on RDT only, excluding participants with malaria diagnosis based on microscopya | Malaria diagnosis based on microscopy, excluding participants with malaria diagnosis based only on RDTb | |||||
| Normotension | Prehypertension | Hypertension | Normotension | Prehypertension | Hypertension | |
| Subgroup | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| All participants | Reference | 0.81 (0.40–1.64) | 0.40 (0.14–1.14) | Reference | 1.08 (0.56–2.09) | 0.99 (0.39–2.51) |
| Self-reported fever | ||||||
| No | Reference | 0.73 (0.34–1.57) | 0.38 (0.12–1.19) | Reference | 0.81 (0.39–1.70) | 0.41 (0.11–1.61) |
| Yes | Reference | 1.61 (0.23–11.4) | 0.52 (0.05–5.98) | Reference | 4.60 (0.96–22.00) | 4.89 (0.90–26.90) |
| P value | 0.46 | 0.82 | 0.05 | 0.03 | ||
| Body temperaturec | ||||||
| 36.5 °C or less | Reference | 0.87 (0.31–2.46) | 0.30 (0.05–1.66) | Reference | 0.81 (0.32–2.03) | 0.15 (0.02–1.27) |
| >36.5 °C | Reference | 0.72 (0.27–1.92) | 0.47 (0.13–1.73) | Reference | 1.35 (0.53–3.46) | 2.93 (0.94–9.14) |
| P value | 0.79 | 0.68 | 0.44 | 0.02 | ||
| Illness susceptibility | ||||||
| Low | Reference | 0.57 (0.23–1.39) | 0.37 (0.10–1.38) | Reference | 0.71 (0.31–1.65) | 0.60 (0.16–2.23) |
| High | Reference | 1.39 (0.43–4.54) | 0.50 (0.09–2.82) | Reference | 2.04 (0.70–5.96) | 2.09 (0.54–8.12) |
| P value | 0.24 | 0.79 | 0.13 | 0.20 | ||
All estimates were derived from multivariable multinomial logistic regression models comparing odds of prehypertension and hypertension in malaria positive vs. negative participants. All models were adjusted for age, sex, formal education, wealth index, area, family history of hypertension, smoking, fruits and vegetables, BMI, waist circumference, sedentariness and anaemia. CI, confidence interval; OR, odds ratio; RDT, rapid diagnostic test. P values represent the significance level of the difference in estimates between comparative groups.
aN (normotension) = 413; N (prehypertension) = 312; N (hypertension) = 218; N (parasitaemia) = 46.
bN (normotension) = 416; N (prehypertension) = 315; N (hypertension) = 220; N (parasitaemia) = 54.
cP value of interaction (RDT vs. microscopy) for hypertension is 0.03.
Combinations of presence of malaria parasitaemia, elevated body temperature and diagnostic test showed positive associations between being microscopic malaria parasitaemia-positive and having elevated body temperature with hypertension [OR 3.37 (95% CI 1.12–10.10)] compared with being malaria parasitaemia-negative without elevated body temperature. This finding was also replicated with both SBP and DBP where their sample mean increased by 8.8 mmHg (95% CI 2.9–14.6 mmHg) and 4.1 mmHg (95% CI 0.3–7.9 mmHg), respectively (Table 4).
TABLE 4.
Association of hypertension and blood pressure with combinations of malaria parasitaemia, basis of malaria diagnosis and body temperature in the Côte d’Ivoire Dual Burden of Disease Study
| Normotension | Prehypertension | Hypertension | SBPa | DBPa | ||
| N | OR (95% CI) | OR (95% CI) | OR (95% CI) | β (95% CI) | β (95% CI) | |
| Malaria parasitaemia-negative without elevated body temperature | 433 | Reference | Reference | Reference | Reference | Reference |
| Malaria parasitaemia-negative with elevated body temperature | 464 | Reference | 1.17 (0.83–1.63) | 1.20 (0.80–1.80) | 1.89 (−0.29 to 4.06) | 0.81 (−0.6 to 2.22) |
| RDT-only malaria parasitaemia-positive, without elevated body temperature | 21 | Reference | 0.87 (0.31–2.46) | 0.30 (0.05–1.65) | −3.82 (−10.70 to 3.10) | −1.95 (−6.43 to 2.54) |
| RDT-only malaria parasitaemia-positive, with elevated body temperature | 25 | Reference | 0.94 (0.35–2.49) | 0.60 (0.16–2.22) | −0.38 (−6.78 to 6.01) | −2.47 (−6.61 to 1.67) |
| Microscopic malaria parasitaemia-positive, without elevated body temperature | 22 | Reference | 0.50 (0.17–1.48) | n.a | −6.21 (−13.00 to 0.59) | −6.69 (−11.10 to −2.29) |
| Microscopic malaria parasitaemia-positive, with elevated body temperature | 32 | Reference | 2.13 (0.88–5.16) | 3.37 (1.12–10.10) | 8.78 (2.92–14.60) | 4.08 (0.28–7.88) |
Estimates of prehypertension and hypertension were derived from multivariable multinomial logistic regression models whereas those of SBP and DBP were derived from multivariable linear regression models comparing malaria-positive vs. malaria-negative participants. Parasitaemia-negative implies a negative test to both RDT and microscopy. Elevated body temperature implies having self-reported fever or measured body temperature greater than 36.5 °C. All models were adjusted for age, sex, formal education, wealth index, area, family history of hypertension, smoking, fruit and vegetable intake, BMI, waist circumference, sedentariness and anaemia. n.a, not applicable because of lack of hypertension case in the group. β, beta-coefficient; CI, confidence interval; OR, odds ratio; RDT, rapid diagnostic test.
aModels additionally exclude 41 participants on antihypertensive medication.
DISCUSSION
We found that the cross-sectional distributions of BP and malaria parasitaemia were not independent. The associations depended on latency and symptomaticity of infection, and did not importantly depend on common hypertension risk factors. The differences in the direction of association observed between RDT malaria parasitaemia and microscopic malaria parasitaemia might be because of the long half-life of P. falciparum HRP-II antigens, which means that they can still be detected for several days following malaria treatment [39]. Thus, the RDTs may have picked up the more latent or asymptomatic cases, in comparison to microscopy, which may have identified more active malaria cases, thus preferred in practice for monitoring malaria treatment [37].
Among participants whose malaria parasitaemia was more likely latent and asymptomatic (RDT-diagnosed; no signs of elevated temperature), the risk of hypertension tended to be lower, compared with those with neither malaria parasitaemia nor elevated body temperature. Persons with this type of malaria parasitaemia have a higher likelihood of efficiently fighting infections, in particular, because self-reported low illness susceptibility further decreased the risk of hypertension. This result is consistent with a causal effect of malaria parasitaemia on hypertension, if the currently observed malaria parasitaemia circumstances reflect a more general and long-term propensity to efficiently fight a Plasmodium infection, which is more prevalent than among those not currently having malaria parasitaemia. In this case, the lower level of BP among the participants with more latent malaria may reflect that they possibly had fewer severe infections and pro-inflammatory episodes over the life course [7,9,12–14].
This result is also compatible with the shared origin hypothesis and the relation to selective pressure of genes protecting against malaria [40]. The observed association of latent malaria with lower hypertension risk suggests that genetic variants were selected, which protect humans against severe malaria and at the same time protect against high BP. This is also supported by the fact that the protective effect of latent malaria tended to be stronger with a positive family history of hypertension.
Candidate genes potentially relevant to the shared origin hypothesis exist. First, genetic variants of ACE (D allele of ACE I/D polymorphism), which were associated with higher ACE levels and increased conversion of angiotensin-I to angiotensin-II [28], were associated with hypertension [41], and milder forms of malaria in Indian population [28]. This is not in line with our finding of an inverse association between latent malaria and hypertension. But in line with our findings is a recent study from Nigeria where serum levels of ACE were higher in patients without malaria than in malaria cases, and ACE level was inversely correlated with malaria severity [29]. Second, genetic variants on chemokine receptor 1 (CR1) gene, which are related to atherosclerosis [42] also conferred protection from severe malaria [43,44].
Among participants with microscopic malaria, accompanied by elevated body temperature, whose infection is more likely acute and symptomatic, a tendency toward higher BP was observed compared with those without malaria parasitaemia and elevated body temperature. Several aspects may explain this positive association. First, elevated BP could be the acute outcome of disease and fever. Second, the current nonlatent disease could be representative of the person's more general reaction to infection. In this case, the participants may have experienced more severe infections and pro-inflammatory episodes in the past, mediating the effect on high BP. In-utero exposure to microscopic malaria was associated with BP in adolescents [12]. Fever, characteristic of symptomatic malaria results from inflammation and may lead to haemodynamic changes, which raise BP to improve perfusion and restore homeostasis [45,46]. Third, the observed positive association may again reflect shared genetics with individuals susceptible to severe malaria being at higher risk of hypertension. The mechanisms behind shared genetics are still unclear, but the strengthening of the endothelial–brain barrier via vasoconstriction is thought to be a pathway to reduce the occurrence of cerebral malaria, evidenced by experimental mouse models [30].
The strengths of our study include being the first population-based survey, to our knowledge, investigating the direct association of malaria parasitaemia and hypertension. We also investigated associations with prehypertension, a preclinical phenotype of hypertension. Our composite definition of hypertension using objectively measured BP as well as self-reported clinician diagnoses limited outcome misclassification. Likewise, the combination of RDT and microscopy for malaria parasitaemia diagnosis improved case detection, and allowed differentiating latent from nonlatent malaria as potential proxy measures for the efficiency of an organism in controlling infection. The availability of detailed information on risk factors enabled the control of potential confounding in our analyses.
Our study is limited by its cross-sectional nature, which precludes causal inference from our findings. We could, therefore, not distinguish between malaria parasitaemia effects on short-term BP elevation from malaria parasitaemia effects on risk of developing hypertension. We may have had some malaria parasitaemia exposure misclassification as we did not apply PCR test toward the identification of malaria parasitaemia cases. Although the combination of both microscopy and RDT has limited this misclassification, PCR might have identified additional malaria parasitaemia, and should be applied in future studies to more accurately capture the malaria parasitaemia profile of the population. A further limitation of the current and prior studies is the challenge in measuring the efficiency of human organisms in containing an infection over the life course. According immunological or other markers specific to this malaria-endemic setting do not exist to our knowledge. In addition, information on exposure to severe malaria infections in utero or early childhood was not obtainable. Despite our relatively low study sample, the prevalence of hypertension in our population corresponds to that (25.6%) of the 2005 subnational survey in Côte d’Ivoire [47], and the prevalence of hypertension in malaria in our study (14%) is also similar to that (20%) of another study [29].
In conclusion, the results of this study suggest that malaria and BP may influence each other or share a genetic background in a complex manner. The immediate implication of our findings is the recommendation to abstain from diagnosing a new hypertension in the setting of an acute malaria infection. From a public health perspective, it becomes obvious that burden of risk estimates for hypertension in LMICs should consider the dual disease burden. Well designed and highly powered longitudinal studies, considering malaria and hypertension phenotypes, are needed to test and differentiate between the hypothesized mechanisms mediating the malaria–hypertension association. Mendelian randomization approaches, as recently proposed on the basis of sickle cell trait variants, not associated with hypertension [23], would allow to specifically investigate the causality of malaria effect on hypertension.
ACKNOWLEDGEMENTS
We thank the CoDuBu field team for their efforts in data collection. The field team constituted staff from the Taabo health and demographic surveillance system, Centre Suisse de Recherches Scientifiques en Côte d’Ivoire, Université Félix Houphouët-Boigny, Institut National de Santé Publique and Ligue Ivoirienne contre l’Hypertension Artérielle et les Maladies Cardiovasculaires, Abidjan, Côte d’Ivoire. We thank the people of the Taabo district and the participants of this study for their long-term time and trust.
Funding: Unrestricted grant from Novartis Foundation (Project No. 2015–16).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Abbreviations: ACE, angiotensin-converting enzyme; CI, confidence interval; CoDuBu, Côte d’Ivoire Dual Burden of Disease Study; CR1, chemokine receptor 1; CVD, cardiovascular disease; Hb, haemoglobin; HDSS, health and demographic surveillance system; HRP2, histidine-rich protein 2; LMICs, low-income and middle-income countries; NCD, noncommunicable disease; OR, odds ratio; pLDH, parasite lactate dehydrogenase; RDT, rapid diagnostic test
REFERENCES
- 1.Guwatudde D, Nankya-Mutyoba J, Kalyesubula R, Laurence C, Adebamowo C, Ajayi I, et al. The burden of hypertension in sub-Saharan Africa: a four-country cross sectional study. BMC Public Health 2015; 15:1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cappuccio FP, Miller MA. Cardiovascular disease and hypertension in sub-Saharan Africa: burden, risk and interventions. Intern Emerg Medicine 2016; 11:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moran A, Forouzanfar M, Sampson U, Chugh S, Feigin V, Mensah G. The epidemiology of cardiovascular diseases in sub-Saharan Africa: the Global Burden of Diseases, Injuries and Risk Factors 2010 Study. Prog Cardiovasc Dis 2013; 56:234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koopman JJ, van Bodegom D, Jukema JW, Westendorp RG. Risk of cardiovascular disease in a traditional African population with a high infectious load: a population-based study. PLoS One 2012; 7:e46855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Opie LH, Seedat YK. Hypertension in sub-Saharan African populations. Circulation 2005; 112:3562–3568. [DOI] [PubMed] [Google Scholar]
- 6.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380:2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Etyang AO, Smeeth L, Cruickshank JK, Scott JA. The malaria-high blood pressure hypothesis. Circ Res 2016; 119:36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallego-Delgado J, Walther T, Rodriguez A. The high blood pressure-malaria protection hypothesis. Circ Res 2016; 119:1071–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moxon CA, Chisala NV, Wassmer SC, Taylor TE, Seydel KB, Molyneux ME, et al. Persistent endothelial activation and inflammation after Plasmodium falciparum Infection in Malawian children. J Infect Dis 2014; 209:610–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chuang SY, Hsu PF, Chang HY, Bai CH, Yeh WT, Pan HW. C-reactive protein predicts systolic blood pressure and pulse pressure but not diastolic blood pressure: the Cardiovascular Disease Risk Factors Two-Township Study. Am J Hypertens 2013; 26:657–664. [DOI] [PubMed] [Google Scholar]
- 11.Hamer M, Chida Y. Associations of very high C-reactive protein concentration with psychosocial and cardiovascular risk factors in an ageing population. Atherosclerosis 2009; 206:599–603. [DOI] [PubMed] [Google Scholar]
- 12.Bedu-Addo G, Alicke M, Boakye-Appiah JK, Abdul-Jalil I, van der Giet M, Schulze MB, et al. In utero exposure to malaria is associated with metabolic traits in adolescence: The Agogo 2000 birth cohort study. J Infect 2017; 75:455–463. [DOI] [PubMed] [Google Scholar]
- 13.Cruickshank JK, Mzayek F, Liu L, Kieltyka L, Sherwin R, Webber LS, et al. Origins of the ‘black/white’ difference in blood pressure: roles of birth weight, postnatal growth, early blood pressure, and adolescent body size: the Bogalusa heart study. Circulation 2005; 111:1932–1937. [DOI] [PubMed] [Google Scholar]
- 14.Law CM, Shiell AW, Newsome CA, Syddall HE, Shinebourne EA, Fayers PM, et al. Fetal, infant, and childhood growth and adult blood pressure: a longitudinal study from birth to 22 years of age. Circulation 2002; 105:1088–1092. [DOI] [PubMed] [Google Scholar]
- 15.Ndao CT, Dumont A, Fievet N, Doucoure S, Gaye A, Lehesran JY. Placental malarial infection as a risk factor for hypertensive disorders during pregnancy in Africa: a case-control study in an urban area of Senegal, West Africa. Am J Epidemiol 2009; 170:847–853. [DOI] [PubMed] [Google Scholar]
- 16.Kang H, Kreuels B, Adjei O, Krumkamp R, May J, Small DS. The causal effect of malaria on stunting: a Mendelian randomization and matching approach. Int J Epidemiol 2013; 42:1390–1398. [DOI] [PubMed] [Google Scholar]
- 17.Hopstock LA, Barnett AG, Bonaa KH, Mannsverk J, Njolstad I, Wilsgaard T. Seasonal variation in cardiovascular disease risk factors in a subarctic population: the Tromso Study 1979–2008. J Epidemiol Community Health 2013; 67:113–118. [DOI] [PubMed] [Google Scholar]
- 18.Gazzinelli RT, Kalantari P, Fitzgerald KA, Golenbock DT. Innate sensing of malaria parasites. Nat Rev Immunol 2014; 14:744–757. [DOI] [PubMed] [Google Scholar]
- 19.Asgar Pour H, Yavuz M. Effects of fever on haemodynamic parameters in neurosurgical intensive care unit patients. Intensive Crit Care Nurs 2014; 30:325–332. [DOI] [PubMed] [Google Scholar]
- 20.Sampson UK, Edwards TL, Jahangir E, Munro H, Wariboko M, Wassef MG, et al. Factors associated with the prevalence of hypertension in the southeastern United States: insights from 69,211 blacks and whites in the Southern Community Cohort Study. Circ Cardiovasc Qual Outcomes 2014; 7:33–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallego-Delgado J, Rodriguez A. Malaria and hypertension. Another co-evolutionary adaptation? Front Cell Infect Microbiol 2014; 4:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwiatkowski DP. How malaria has affected the human genome and what human genetics can teach us about malaria. Am J Hum Genet 2005; 77:171–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Etyang AO, Wandabwa CK, Kapesa S, Muthumbi E, Odipo E, Wamukoya M, et al. Blood pressure and arterial stiffness in kenyan adolescents with the sickle cell trait. Am J Epidemiol 2018; 187:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain S, Tang X, Narayanan CS, Agarwal Y, Peterson SM, Brown CD, et al. Angiotensinogen gene polymorphism at -217 affects basal promoter activity and is associated with hypertension in African-Americans. J Biol Chem 2002; 277:36889–36896. [DOI] [PubMed] [Google Scholar]
- 25.Simonyte S, Kuciene R, Medzioniene J, Dulskiene V, Lesauskaite V. Renin-angiotensin system gene polymorphisms and high blood pressure in Lithuanian children and adolescents. BMC Med Genet 2017; 18:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deo SH, Holwerda SW, Keller DM, Fadel PJ. Elevated peripheral blood mononuclear cell-derived superoxide production in healthy young black men. Am J Physiol Heart Circ Physiol 2015; 308:H548–H552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dluzen DF, Noren Hooten N, Zhang Y, Kim Y, Glover FE, Tajuddin SM, et al. Racial differences in microRNA and gene expression in hypertensive women. Sci Rep 2016; 6:35815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhangadamajhi G, Mohapatra BN, Kar SK, Ranjit M. Gene polymorphisms in angiotensin I converting enzyme (ACE I/D) and angiotensin II converting enzyme (ACE2 C-->T) protect against cerebral malaria in Indian adults. Infect Genet Evol 2010; 10:337–341. [DOI] [PubMed] [Google Scholar]
- 29.Abdulazeez A, Ya’u M, Kurfi B. Association of hypertension and activity of angiotensin converting enzyme in malaria patients attending Sheik Muhammad Jidda General Hospital, Kano State, Nigeria. Nigerian J Basic Clin Sci 2017; 14:121. [Google Scholar]
- 30.Gallego-Delgado J, Baravian C, Edagha I, Ty MC, Ruiz-Ortega M, Xu W, et al. Angiotensin II moderately decreases plasmodium infection and experimental cerebral malaria in mice. PLoS One 2015; 10:e0138191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anigbogu C, Olubowale O. Effects of malaria on blood pressure, heart rate, electrocardiogram and cardiovascular response to change in posture. Nig Q J Hosp Med 2002; 12:17–20. [Google Scholar]
- 32.Bassa FK, Ouattara M, Silué KD, Adiossan LG, Baikoro N, Koné S, et al. Epidemiology of malaria in the Taabo health and demographic surveillance system, south-central Côte d’Ivoire. Malar J 2016; 15:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Righetti AA, Adiossan LG, Ouattara M, Glinz D, Hurrell RF, N’Goran EK, et al. Dynamics of anemia in relation to parasitic infections, micronutrient status, and increasing age in South-Central Côte d’Ivoire. J Infect Dis 2013; 207:1604–1615. [DOI] [PubMed] [Google Scholar]
- 34.Koné S, Baikoro N, N’Guessan Y, Jaeger FN, Silué KD, Furst T, et al. Health & demographic surveillance system profile: the Taabo Health and Demographic Surveillance System, Côte d’Ivoire. Int J Epidemiol 2015; 44:87–97. [DOI] [PubMed] [Google Scholar]
- 35.Eze IC, Esse C, Bassa FK, Knoé S, Acka F, Yao L, et al. Côte d’Ivoire Dual Burden of Disease (CoDuBu): study protocol to investigate the co-occurrence of chronic infections and noncommunicable diseases in rural settings of epidemiological transition. JMIR Res Protoc 2017; 6:e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raso G, Luginbuhl A, Adjoua CA, Tian-Bi NT, Silué KD, Matthys B, et al. Multiple parasite infections and their relationship to self-reported morbidity in a community of rural Côte d’Ivoire. Int J Epidemiol 2004; 33:1092–1102. [DOI] [PubMed] [Google Scholar]
- 37.Abba K, Deeks JJ, Olliaro P, Naing CM, Jackson SM, Takwoingi Y, et al. Rapid diagnostic tests for diagnosing uncomplicated P. falciparum malaria in endemic countries. Cochrane Database Syst Rev 2011; 7:CD008122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information Systems. Geneva: World Health Organization; 2011. [Google Scholar]
- 39.Tahar R, Sayang C, Ngane Foumane V, Soula G, Moyou-Somo R, Delmont J, et al. Field evaluation of rapid diagnostic tests for malaria in Yaounde, Cameroon. Acta Trop 2013; 125:214–219. [DOI] [PubMed] [Google Scholar]
- 40.Gurdasani D, Carstensen T, Tekola-Ayele F, Pagani L, Tachmazidou I, Hatzikotoulas K, et al. The African Genome Variation Project shapes medical genetics in Africa. Nature 2015; 517:327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choudhury I, Jothimalar R, Patra AK. Angiotensin converting enzyme gene polymorphism and its association with hypertension in South Indian Population. Indian J Clin Biochem 2012; 27:265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boiocchi C, Zorzetto M, Sbarsi I, Pirotta A, Schirinzi S, Falcone C, et al. CR1 genotype and haplotype involvement in coronary artery disease: the pivotal role of hypertension and dyslipidemia. Int J Mol Med 2009; 24:181–187. [DOI] [PubMed] [Google Scholar]
- 43.Panda AK, Panda M, Tripathy R, Pattanaik SS, Ravindran B, Das BK. Complement receptor 1 variants confer protection from severe malaria in Odisha, India. PLoS One 2012; 7:e49420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kosoy R, Ransom M, Chen H, Marconi M, Macciardi F, Glorioso N, et al. Evidence for malaria selection of a CR1 haplotype in Sardinia. Genes Immun 2011; 12:582–588. [DOI] [PubMed] [Google Scholar]
- 45.Walter EJ, Hanna-Jumma S, Carraretto M, Forni L. The pathophysiological basis and consequences of fever. Crit Care 2016; 20:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodbard S. Body temperature, blood pressure, and hypothalamus. Science 1948; 108:413–415. [DOI] [PubMed] [Google Scholar]
- 47.WHO. Enquête sur les facteurs de risque des maladies non transmissibles. Côte d’Ivoire, Régions sanitaire des Lagunes. Geneva: World Health Organization; 2005. [Google Scholar]


