ABSTRACT
Introduction:
Shock is characterized by micro- and macrovascular flow impairment contributing to acute kidney injury (AKI). Routine monitoring of the circulation regards the macrocirculation but not the renal circulation which can be assessed with Doppler ultrasound as renal resistive index (RRI). RRI reflects resistance to flow. High RRI predicts persistent AKI. Study aims were to determine whether RRI is elevated in shock and to identify determinants of RRI.
Materials and Methods:
This prospective observational cohort study included two cohorts of patients, with and without shock less than 24-h after intensive care admission. Apart from routine monitoring, three study measurements were performed simultaneously: RRI, sublingual microcirculation, and bioelectral impedance analysis.
Results:
A total of 92 patients were included (40 shock, 52 nonshock), median age was 69 [60–76] vs. 67 [59–76], P = 0.541; APACHE III was 87 [65–119] vs. 57 [45–69], P < 0.001. Shock patients had higher RRI than patients without shock (0.751 [0.692–0.788] vs. 0.654 [0.610–0.686], P < 0.001). Overall, high age, APACHE III score, lactate, vasopressor support, pulse pressure index (PPI), central venous pressure (CVP), fluid balance, and low preadmission estimated glomerular filtration rate, mean arterial pressure (MAP), creatinine clearance, and reactance/m were associated with high RRI at univariable regression (P < 0.01). Microcirculatory markers were not. At multivariable regression, vasopressor support, CVP, PPI and MAP, reactance/m, and preadmission eGFR were independent determinants of RRI (n = 92, adj. R2 = 0.587).
Conclusions:
Patients with shock have a higher RRI than patients without shock. Independent determinants of high RRI were pressure indices of the systemic circulation, low membrane capacitance, and preadmission renal dysfunction. Markers of the sublingual microcirculation were not.
Keywords: Acute kidney injury, bioimpedance, Doppler ultrasound, microcirculation, RRI, shock
Abbreviations: AKI, acute kidney injury, APACHE, acute physiology and chronic health evaluation, BIA, bioelectral impedance analysis, CI, cardiac index, CVP, central venous pressure, eGFR, estimated glomerular filtration rate, FENa, fractional excretion of sodium, FEUrea, fractional excretion of urea, HR, heart rate, HI, heterogeneity index, IC, intensive care, ICC, interclass correlation coefficient, ICU, intensive care unit, MAP, mean arterial pressure, MFI, microvascular flow index, PMM, predictive mean matching, PPI, pulse pressure index, PPV, proportion of perfused vessels, PVD, perfused vessel density, R, resistance, RRI, renal resistive index, SD, standard deviation, SDF, sidestream dark field, SE, standard error, SIRS, systemic inflammatory response syndrome, SvO2, venous oxygen saturation, VD, vessel density, VP, vasopressor, Xc, bioelectral impedance-derived reactance (membrane capacitance)
INTRODUCTION
Shock is a severe condition characterized by inadequate perfusion and subsequent injury of vital organs such as the kidney. Acute kidney injury (AKI) increases morbidity and mortality (1). Underlying mechanisms include hypoperfusion and direct membrane and cellular injury due to oxidative stress, inflammation, toxicity of drugs, and damage proteins (2).
Routine monitoring of the circulation includes pressure as well as flow measurements in the macrocirculation to guide the administration of fluids and vasoactive medication. However, it does not include the renal circulation. Nowadays, ultrasound has become an important tool to assess the circulation in critically ill patients. The renal circulation can be assessed using Doppler ultrasound, a non-invasive tool which has recently become available at the bedside (3). Renal resistive index (RRI) is a sonographic index assessing the resistance to flow in intrarenal arcuate or interlobar arteries. High RRI can be caused by vasoconstriction, decreased vascular compliance, or capillary rarefaction, and is associated with renal arterial disease (4). RRI seems a promising tool to predict the development and reversibility of AKI in critically ill patients (5–7). Up to now, there are no studies in intensive care patients showing that RRI is increased in shock. Furthermore, it is not well known to what extent RRI reflects pressure or flow indices and whether it reflects changes in the macro-, microcirculation, or fluid status (8). Before deciding whether RRI should be part of vital organ assessment in critically ill patients, it is important to identify its determinants.
The aim of this prospective observational cohort study was to determine whether RRI is higher in patients with shock than in patients without shock. The secondary aim was to identify determinants of RRI.
MATERIALS AND METHODS
The study was performed at Amsterdam UMC, Vrije Universiteit Amsterdam, Department of Intensive Care Medicine, Amsterdam, the Netherlands, and is part of a larger project that additionally determines the predictive value of RRI for the development of AKI (9). The study was approved by the Ethical Board of the VUmc, which allowed a deferred consent procedure to obtain signed consent for the use of the data (VU University Medical Centre Protocol Record METC-2015.025).
Setting and patients
Adult critically ill patients admitted to the intensive care unit (ICU) were included consecutively. Patients were subsequently allocated to the shock and nonshock group. Inclusion criteria in the shock group were shock due to sepsis, systemic inflammatory response syndrome (SIRS), and cardiac failure or hemorrhage. Inclusion criteria in the nonshock group were non- or short-term circulatory dependency of fluids and vasopressors. Exclusion criteria were severe renal insufficiency (eGFR <30 mL/min), dialysis dependency, renal transplantation, monokidney, treatment restrictions, admission after suicide attempt, and inability to obtain informed consent due to cognitive impairment. Weekend admissions were not included because investigators were not present.
Definitions
Shock was defined as vasopressor-dependent hypotension or low cardiac index (<2 L/min/m2 as measured with thermodilution or qualitatively as assessed by Doppler ultrasound) unresponsive to adequate fluid resuscitation, in the presence of perfusion abnormalities, manifest by oliguria, reduced peripheral perfusion, hyperlactatemia (lactate >2 mmol/L), or organ dysfunction (10). Patients with hypotension or low cardiac index, who were easily responsive to short-term vasopressor support or fluids, and without peripheral perfusion abnormalities, were not considered as being in shock. A low vasopressor dose during propofol sedation was therefore not diagnosed as shock. SIRS, sepsis, and septic shock were defined according to standard criteria (11). We additionally compared the RRI between subgroups using different criteria of compromised circulation: a cutoff value of serum lactate (3 mmol/L) with and without the need of vasopressors.
Study protocol
Within 24-h of ICU admission, three study measurements were performed within the time frame of 1-h: renal resistive index (RRI), the sublingual microcirculation using sidestream dark field (SDF) imaging, and bioelectral impedance analysis (BIA). All measurements were performed in accordance with relevant guidelines and regulations. The other biochemical and clinical parameters were obtained simultaneously; especially hemodynamic parameters were taken at the same time as the RRI measurements. The investigators were not blinded for inclusion criteria. All patients using vasopressors were allocated to the shock or nonshock group by the same senior investigator, intensivist, who was blinded for the RRI results, based on the aforementioned criteria.
Renal resistive index
RRI was determined using Doppler ultrasound. A transparietal 5 MHz pulsed-wave Doppler probe (C5-1 ultrasound transducer, Philips Medical Systems International B.V., Best, The Netherlands) on a CX50 ultrasound system was used. A previous study showed a good interobserver reliability between junior and senior operators: interclass correlation coefficient (ICC), 89% (95% confidence interval [CI], 82%–93%) (12). Each final RRI was based on 18 measurements. After visualizing the kidney, an interlobar or arcuate artery from the upper, middle, and lower part of each kidney was selected to obtain three pulse wave Doppler measurements for each artery. The median of these three RRI determinations was selected for each artery. For each kidney, we calculated mean RRI based on the three median RRI determinations. RRI is defined as ((peak systolic velocity−end diastolic velocity)/peak systolic velocity). Normal values are reported between 0.60 and 0.70 (13).
Sublingual microcirculation
Sublingual microcirculation was manually assessed with SDF imaging, a light-absorption-based technique using a hand-held video microscope (the Microscan. Firma) (14). Afterward, an independent investigator analyzed the videos by visual inspection using a cutoff point of 20 μm for small vessels. Flow was graded by vessel density (VD; n/mm), perfused vessel density (PVD; n/mm), proportion of perfused vessels (PPV; %), and microvascular flow index (MFI), quantifying the predominant type of flow in four quadrants: no flow [0], intermittent flow [1], slow/sluggish flow [2], and continuous flow [3]. Heterogeneity indexes (HI) were calculated for PVD, PPV, and MFI. The mean of three to five different sublingual sites was calculated.
Bioelectral impedance analysis (BIA)
Whole body BIA was measured using the phase sensitive Akern BIA 101 Anniversary edition (GLNP Life Sciences) device with an alternating current of 400 μA and a frequency of 50 kHz. BIA measures resistance (R), mainly reflecting extracellular resistance (hydration status), reactance (Xc), reflecting membrane capacitance (cellular mass and integrity), and calculates the phase angle, the arc tangens of ((Xc/R) × (180/π)). Resistance and reactance are expressed per meter. Resistance and phase angle decrease along with an increase in total body water. Reactance mainly decreases with membrane injury or decreases in cell mass (15).
Other parameters
The following standard clinical variables were collected: mean arterial pressure (MAP), heart rate (HR), cardiac index (CI), central venous pressure (CVP), arterial lactate, norepinephrine dose and central or mixed venous oxygen saturation (SvO2), demographic data, and the acute physiology and chronic health evaluation III (APACHE III) score. Pulse pressure index (PPI) was calculated as ((systolic−diastolic)/systolic pressure) obtained from continuous invasive arterial pressure monitoring. Cardiac ultrasound was performed routinely in the unit in patients with hypotension not responding to fluids. Concomitant renal function and damage were measured by creatinine clearance (4-h portion), fractional excretion of sodium (FENa) = (urinary sodium × plasma creatinine)/(plasma sodium × urinary creatinine), fractional excretion of urea (FEUrea) = (urinary urea × plasma creatinine)/(plasma urea × urinary creatinine), and albumin/creatinine ratio. In addition, preadmission glomerular filtration rate (eGFR) was estimated based on a stable serum creatinine before ICU admission. All clinical and biochemical measurements were retrieved from the patient data management system (Metavision, IMD Soft, Tel Aviv, Israel).
Sample size
Power calculations were based on an estimated mean RRI of 0.78 in the shock patients and an RRI of 0.70 in the nonshock patients with a standard deviation (SD) of 0.12 (5). To be able to detect this difference with a power of 0.80 (1−β) and a significance level of 0.05 (α), 36 patients were needed in each group. To compensate for missing data, we aimed to include at least 40 patients in each arm.
Statistical analysis
Normally distributed variables are reported as mean ± SD and not-normally distributed variables as median [interquartile range]. Normality was tested using skewness and the one-sample Kolmogorov–Smirnov test. For comparison of continuous variables between patients with and without shock, an independent two-tailed t test was used or the Mann–Whitney U test as appropriate. A P value of <0.05 was considered to be statistically significant.
To evaluate the relation between RRI (as continuous variable) and markers of the macro- and microcirculation, hydration status, membrane capacitance, and renal function, potential determinants were initially tested by univariable regression analysis. Subsequently, backward stepwise selection was computed with all variables of interest that were significantly related to RRI on univariable regression analysis. The alpha-to-remove was 0.10. Missing variables were imputed in 20 new datasets, using predictive mean matching (PMM) and a maximum of 50 iterations. Finally, the remaining independent determinants of RRI, that were presented in the pooled model, were entered in multivariable regression analysis to generate a predictive model for RRI, including a maximum of n/10 predictors (16). The reported mean adj. R2 was calculated from the 20 generated models. A sensitivity analysis was performed with the unimputed data.
RESULTS
From August 2015 until February 2016, a total of 518 patients were admitted to the ICU (Fig. 1). A total of 351 patients were not included due to weekend admission, nonavailability of the ultrasound device, inability to acquire adequate imaging, or completion of inclusion (in the nonshock group). Mean age and APACHE III scores of the nonincluded shock patients were not significantly different from the included shock patients (data not shown). Mean time to inclusion after ICU admission was 9.6-h. Time between RRI, SDF, and BIA measurements was less than 1-h.
Fig. 1.
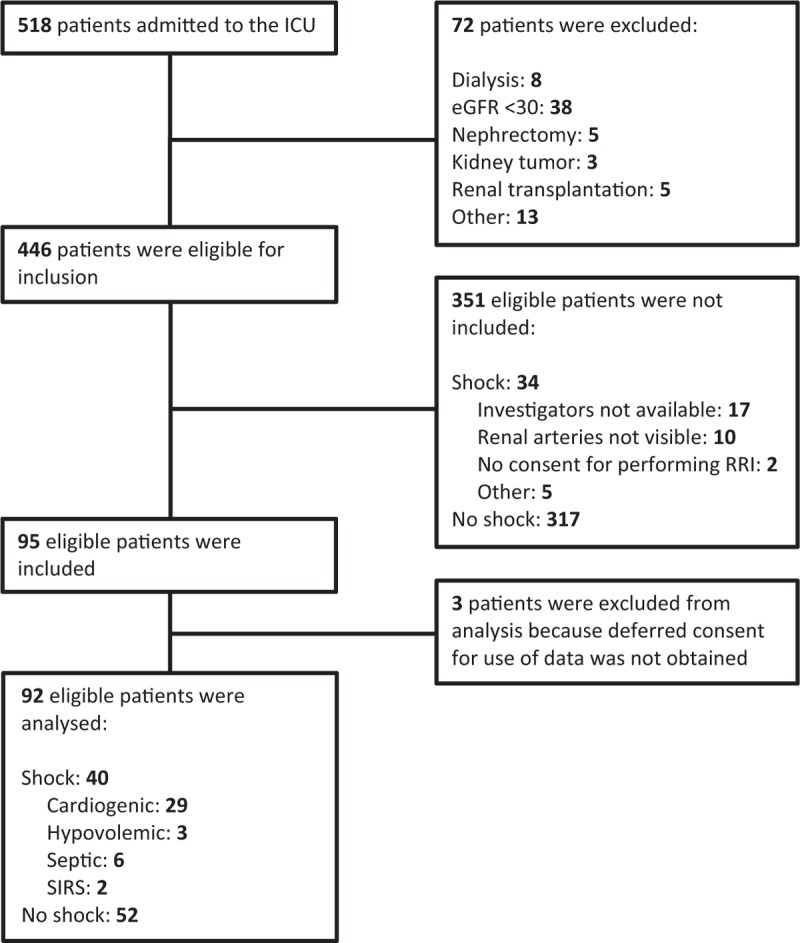
Flowchart of included patients. Inability to acquire adequate imaging was due to morbid obesity, wounds, immobility (after trauma), or agitation.
Of the 95 enrolled patients, 3 were excluded afterward because deferred consent for the use of data was not obtained. Finally, 92 patients were analyzed: 40 with shock and 52 without shock. Among the patients with shock, 29 (72.5%) had cardiogenic shock, 3 (7.5%) had hypovolemic shock, 6 (15.0%) had septic shock, and 2 (5.0%) had shock due to SIRS.
Baseline characteristics
Baseline characteristics are shown in Table 1. Patients with shock had a significantly higher APACHE III score, arterial lactate, plasma creatinine, and FEUrea, as well as a more positive fluid balance. Moreover, norepinephrine was more often administered and norepineprine dose was higher (Table 1). In the nonshock group, 5 patients, without signs of oliguria or disturbed peripheral perfusion, temporarily received low-dose norepinephrine during propofol sedation.
Table 1.
Baseline characteristics of the study population (n = 92)
| Patients with shock (n = 40) | Patients without shock (n = 52) | P | |
| Age, y | 69 [60–76] | 67 [59–76] | 0.541 |
| Sex | 0.859 | ||
| Male, % | 67.5 | 69.2 | |
| Female, % | 32.5 | 30.8 | |
| BMI, kg/m2 | 25.2 ± 4.3 | 26.2 ± 4.3 | 0.280 |
| APACHE III | 87 [65–119] | 57 [45–69] | <0.001 |
| Lactate | 2.5 [1.4–4.3] | 1.2 [0.9–2.0] | <0.001 |
| Risk factors of AKI | |||
| Chronic kidney disease*, n (%) | 8 (20.0) | 8 (15.4) | 0.563 |
| Sepsis, n (%) | 6 (15.0) | 5 (9.6) | 0.430 |
| History of hypertension, n (%) | 12 (30.0) | 21 (40.4) | 0.303 |
| Diabetes, n (%) | 8 (20.0) | 9 (17.3) | 0.742 |
| Subgroups of admission | |||
| Elective surgical, n (%) | 18 (45.0) | 32 (61.5) | 0.114 |
| Emergency surgical, n (%) | 5 (12.5) | 3 (5.8) | 0.256 |
| Medical, n (%) | 17 (42.5) | 17 (32.7) | 0.334 |
| Renal function | |||
| Serum creatinine, μmol/L | 91 [76–122] | 75 [62–84] | <0.001 |
| Pre-admission eGFR, mL/min | 71 ± 15 | 76 ± 14 | 0.102 |
| Urinary indices | |||
| FENa†, % | 0.4 [0.2–1.0] | 0.6 [0.3–1.5] | 0.314 |
| FEUrea‡, % | 31.9 [20.9–37.6] | 37.2 [27.6–44.9] | 0.024 |
| Albumin/creatinine ratio§ | 4.2 [1.2–11.4] | 2.8 [1.6–5.4] | 0.102 |
| Creatinine clearance||, mL/min | 52.3 [29.2–100.3] | 110.8 [79.5–149.6] | <0.001 |
| Hemodynamics | |||
| Heart rate (HR; bpm) | 87 [71–93] | 78 [70–88] | 0.209 |
| Mean arterial pressure (MAP; mmHg) | 70 [64–78] | 73 [66–86] | 0.171 |
| Pulse pressure index (PPI) | 0.456 ± 0.087 | 0.483 ± 0.074 | 0.121 |
| Central venous pressure¶ (CVP; cmH2O) | 10 [6–14] | 7 [4–10] | 0.007 |
| Cardiac index** (CI) | 2.1 [1.8–2.3] | 2.1 [1.9–2.8] | 0.423 |
| SvO2††, % | 66 [61–75] | 68 [65–72] | 0.403 |
| Norepinephrine‡‡, n (%) | 37 (92.5) | 5 (9.6) | <0.001 |
| Norepinephrine dose, μg/kg/min | 0.27 [0.14–0.44] | 0 [0.0–0.0] | <0.001 |
| Fluid balance§§, mL | 3075 [1466–5025] | 528 [−79 to 938] | <0.001 |
BMI, body mass index; APACHE, acute physiology and chronic health evaluation; AKI, acute kidney injury; eGFR, estimated glomerular filtration rate; FENa, fractional excretion of sodium; FEUrea, fractional excretion of urea; SvO2, central or mixed venous oxygen saturation.
Data are presented as mean ± SD or as median with [interquartile range].
*Chronic kidney disease was defined as an eGFR before ICU admission of 30 to 60 mL/min; patients with eGFR <30 mL/min on admission were not included.
†n = 40 vs. n = 49.
‡n = 40 vs. n = 48.
§n = 39 vs. n = 50.
||Creatinine clearance (4-h portion), n = 40 vs. n = 51.
¶n = 33 vs. n = 35.
**n = 16 vs. n = 14.
††n = 31 vs. n = 35.
‡‡Norepinephrine support during the measurements.
§§Fluid balance from ICU admission until measurements (<24-h after admission).
Primary endpoint: shock versus nonshock
Renal resistive index
RRI could be measured only one-sided in two patients (one with shock, one without). In 7 additional patients, one or two of the six regional RRI measurements were missing because no more vessels could be visualized. The RRI did not significantly differ between the left and right kidney. Patients with shock had a significantly higher RRI than patients without shock (0.751 [0.692–0.788] vs. 0.654 [0.610–0.686], respectively, P < 0.001) (Fig. 2 and Table 2). RRI was not significantly different between patients with septic shock (n = 6) and other shock (n = 34): 0.760 [0.647–0.789] vs. 0.751 [0.694–0.789] respectively, P = 0.956.
Fig. 2.
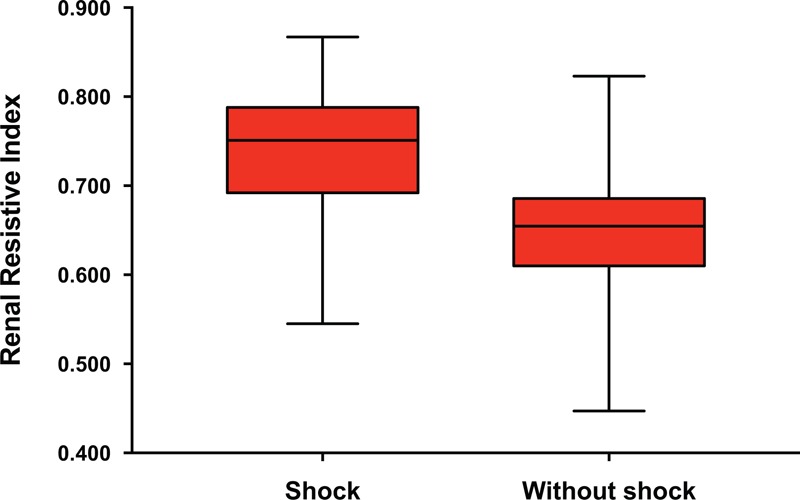
Renal resistive index of patients admitted with shock (n = 40) and without shock (n = 52).
Table 2.
Study parameters
| Patients with shock | Patients without shock | P | |
| Renal resistive index (RRI)* | |||
| Left kidney | 0.757 [0.690–0.792] | 0.656 [0.596–0.696] | <0.001 |
| Right kidney | 0.746 [0.693–0.786] | 0.656 [0.621–0.690] | <0.001 |
| Overall | 0.751 [0.692–0.788] | 0.654 [0.610–0.686] | <0.001 |
| Microcirculation (SDF)† | |||
| Vessel density (VD; n/mm) | 9.5 ± 1.6 | 9.7 ± 2.6 | 0.698 |
| Perfused vessel density (PVD; n/mm) | 7.0 ± 1.4 | 7.1 ± 2.1 | 0.747 |
| Percentage of perfused vessels (PPV; %) | 73.9 ± 12.1 | 73.1 ± 14.0 | 0.817 |
| Microvascular flow index (MFI)‡ | 2.0 ± 0.5 | 2.0 ± 0.5 | 0.926 |
| Bioelectral impedance analysis (BIA)§ | |||
| Resistance (R/m) | 285.0 [245.9–324.9] | 285.1 [255.1–326.6] | 0.585 |
| Reactance (Xc/m) | 22.1 [17.4–29.2] | 24.3 [21.6–29.8] | 0.075 |
| Phase angle | 4.3 [3.3–5.4] | 4.9 [4.0–6.1] | 0.066 |
SDF, sidestream dark field.
Data are presented as mean ± SD or as median with [interquartile range].
*n = 40 vs. n = 52.
†n = 32 vs. n = 36.
‡No flow [0], intermittent flow [1], slow/sluggish flow [2], and continuous flow [3].
§n = 39 vs. n = 50.
A comparison of RRI between subgroups using other criteria of compromised circulation is shown in Table 3.
Table 3.
Renal resistive index in different subgroups
| No. of patients | Renal resistive index | P | |
| Shock vs. nonshock* | n = 40 vs. n = 52 | 0.751 [0.692–0.788] vs. 0.654 [0.610–0.686] | <0.001 |
| Lactate ≥3 vs. <3 mmol/L | n = 22 vs. n = 70 | 0.720 [0.673–0.779] vs. 0.672 [0.626–0.735] | 0.034 |
| Lactate ≥3 mmol/L + VP support vs. other | n = 16 vs. n = 76 | 0.751 [0.687–0.781] vs. 0.674 [0.626–0.734] | 0.016 |
VP, vasopressor.
Data are presented as median with [interquartile range].
*According to the clinical criteria as described above.
Sublingual microcirculation
The sublingual microcirculation was visualized in 68/92 (73.9%) patients. In 24 patients (26.1%), the SDF measurement could not be performed due to the inability to open the mouth, instable cervical fractures, abundant secretions, or agitation. None of the microcirculatory markers differed between shock and nonshock patients (Table 2).
Bioimpedance analysis
BIA measurements were performed in 89/92 (96.7%) patients. BIA could not be performed due to the inability to position the electrodes in three patients (3.3%). Resistance/m was not significantly different between shock and nonshock patients (Table 2). However, reactance/m and phase angle tended to be lower in the shock patients.
Secondary endpoints: determinants of RRI
Univariable regression analysis
Systemic circulation. Due to the absence of a central catheter or a cardiac output device in the less severely ill patients, the number of patients with an available CI, CVP, and SvO2 was limited (Table 4). RRI was inversely related to MAP (R = −0.30) and positively to vasopressor support (R = 0.51), CVP (R = 0.50), norepinephrine dose (R = 0.39), and PPI (R = 0.36). RRI was not related to HR, CI, and SvO2 (Table 4).
Table 4.
Univariable regression analysis
| Variable | n | Regression coefficient B* | 95% CI B | SE of B | Correlation coefficient β | P |
| Systemic circulation | ||||||
| Heart rate (HR; bpm) | 92 | 0.000 | −0.001–0.001 | 0.001 | −0.07 | 0.535 |
| Mean arterial pressure (MAP; mmHg) | 92 | −0.002 | −0.004–−0.001 | 0.001 | −0.30 | 0.004 |
| Pulse pressure index (PPI) | 92 | 0.367 | 0.167–0.567 | 0.101 | 0.36 | <0.001 |
| Central venous pressure (CVP; cmH2O) | 68 | 0.009 | 0.005–0.013 | 0.002 | 0.50 | <0.001 |
| Cardiac index (CI) | 30 | −0.033 | −0.111–0.045 | 0.038 | −0.16 | 0.395 |
| Venous oxygen saturation (SvO2; %)† | 66 | 0.001 | −0.001–0.004 | 0.001 | 0.12 | 0.354 |
| Vasopressor support‡ | 92 | 0.083 | 0.054–0.113 | 0.015 | 0.51 | <0.001 |
| Norepinephrine dose, μg/kg/min | 92 | 0.144 | 0.072–0.216 | 0.036 | 0.39 | <0.001 |
| Microcirculation | ||||||
| Vessel density (VD; n/mm) | 68 | −0.004 | −0.014–0.005 | 0.005 | −0.11 | 0.370 |
| Perfused vessel density (PVD; n/mm) | 68 | −0.008 | −0.019–0.003 | 0.005 | −0.12 | 0.149 |
| Percentage of perfused vessels (PPV; %) | 68 | −0.001 | −0.002–0.001 | 0.001 | −0.13 | 0.300 |
| Microvascular flow index (MFI) | 68 | −0.032 | −0.070–0.005 | 0.019 | −0.21 | 0.092 |
| Hydration | ||||||
| Fluid balance, L§ | 92 | 0.011 | 0.005–0.017 | 0.003 | 0.34 | 0.001 |
| Resistance (R/m) | 89 | 0.000 | 0.000–0.000 | 0.000 | −0.12 | 0.281 |
| Reactance (Xc/m) | 89 | −0.003 | −0.005–−0.001 | 0.001 | −0.34 | 0.001 |
| Phase angle | 89 | −0.011 | −0.021–−0.001 | 0.005 | −0.23 | 0.031 |
| Concomitant renal function | ||||||
| Creatinine clearance, mL/min|| | 91 | −0.001 | −0.001–0.000 | 0.000 | −0.42 | <0.001 |
| FEUrea, % | 88 | −0.002 | −0.003–0.000 | 0.001 | −0.24 | 0.022 |
| FENa, % | 89 | −0.015 | −0.030–0.001 | 0.008 | −0.20 | 0.067 |
| Albumin/creatinine ratio | 89 | 0.000 | −0.001–0.000 | 0.000 | −0.05 | 0.637 |
| Other | ||||||
| Age, y | 92 | 0.002 | 0.001–0.003 | 0.001 | 0.30 | 0.004 |
| APACHE III | 92 | 0.001 | 0.000–0.001 | 0.000 | 0.37 | <0.001 |
| Lactate, mmol/L | 92 | 0.015 | 0.004–0.025 | 0.005 | 0.28 | 0.006 |
| Preadmission eGFR, mL/min | 92 | −0.002 | −0.003–−0.001 | 0.001 | −0.30 | 0.004 |
| BMI, kg/m2 | 92 | 0.000 | −0.004–0.004 | 0.002 | −0.02 | 0.834 |
SE, standard error; FEUrea, fractional excretion of urea; FENa, fractional excretion of sodium; APACHE, acute physiology and chronic health evaluation; eGFR, estimated glomerular filtration rate; BMI, body mass index.
*If X increases with 1, RRI increases with B. Variable = X.
†Central or mixed venous oxygen saturation.
‡0 = no vasopressor support; 1 = vasopressor support.
§Fluid balance from ICU admission until measurements (<24-h after admission).
||Creatinine clearance (4-h portion).
Microcirculation
None of the microcirculatory markers were significantly related to the RRI (Table 4).
Hydration and membrane capacitance
RRI was inversely related to reactance/m (R = −0.34) and phase angle (R = −0.23) and positively to fluid balance (R = 0.34). RRI was not related to resistance/m (Table 4).
Other related variables
RRI was inversely related to actual creatinine clearance (R = −0.42), preadmission eGFR (R = −0.30), and FEUrea (R = −0.24), but not to FENa and albumin/creatinine ratio. RRI was positively related to APACHE III score (R = 0.37), age (R = 0.30), and arterial lactate (R = 0.28) (Table 4).
Multivariable regression analysis
Vasopressor support, MAP, PPI, CVP, fluid balance, reactance/m, age, APACHE III score, arterial lactate, and preadmission eGFR were included for backward stepwise selection. Missing variables were imputed for CVP (24/92 (26.1%)) and reactance/m (3/92 (3.3%)). In the pooled model vasopressor support, CVP, PPI, MAP, reactance/m, and preadmission eGFR remained as independent determinants of RRI. These variables constituted the final predictive model (Table 5). Participants constant RRI was 0.601. RRI increased when vasopressors were used, along with a higher CVP, higher PPI, lower MAP, lower reactance/m, and lower preadmission eGFR. The final model explained 58.7% of the variance of RRI. When performing a sensitivity analysis on the unimputed data, vasopressor support, CVP, PPI, and MAP remained as independent predictive determinants of RRI (n = 68, adj. R2 = 0.608, standard error of estimate = 0.049). Furthermore, when forcing MFI into the model, this microcirculatory marker was removed.
Table 5.
Multivariable regression analysis
| Final model | ||||
| Regression coefficient B | 95% CI B | SE of B | P | |
| Constant | 0.601 | 0.476–0.726 | 0.064 | <0.001 |
| Systemic circulation | ||||
| Vasopressor support* | 0.059 | 0.035–0.084 | 0.012 | <0.001 |
| Central venous pressure (CVP; cmH2O) | 0.006 | 0.003–0.009 | 0.002 | <0.001 |
| Pulse pressure index (PPI) | 0.418 | 0.275–0.561 | 0.073 | <0.001 |
| Mean arterial pressure (MAP; mmHg) | −0.001 | −0.002–0.000 | 0.001 | 0.006 |
| Membrane capacitance | ||||
| Reactance (Xc/m) | −0.001 | −0.003–0.000 | 0.001 | 0.045 |
| Renal function | ||||
| Pre-admission eGFR, mL/min | −0.001 | −0.002–0.000 | 0.000 | 0.097 |
RRI = 0.601 + vasopressor support × 0.059 + CVP × 0.006 + PPI × 0.418 – MAP × 0.001 – reactance/m × 0.001 – preadmission eGFR × 0.001.
*0 = no vasopressor support; 1 = vasopressor support.
Sample size = 92; mean adjusted R2 = 0.587 [minimum 0.552 − maximum 0.638]; mean standard error of estimate = 0.053.
SE indicates standard error.
DISCUSSION
This prospective observational cohort study shows that Doppler ultrasound-derived renal resistive index (RRI) was higher in patients admitted to the ICU with shock than in patients without shock. It also shows that RRI was determined by pressure markers of the systemic circulation, membrane capacitance, and preadmission renal function and not by markers of flow in the systemic- and the microcirculation.
Our study is the first comparing the RRI of shock and nonshock patients in the ICU. Apart from a clinical definition, we additionally used other criteria of compromised circulation, including a lactate cutoff with or without the need of vasopressor support. Our results seem robust because RRI was increased in patients with shock using different criteria. A previous study from the emergency department reported that patients with hemorrhagic shock had a higher RRI than those without (mean, 0.80 ± 0.10 vs. 0.63 ± 0.03) (17). In our cohort, RRI values in the nonshock patients were comparable, but RRI in our shock patients was lower, median 0.751 [0.692–0.788], possibly due to already initiated resuscitation. On average, our patients were included 9.6-h (mean) after ICU admission.
The present study also found that high RRI was related to low MAP and vasopressor support. Previous studies in septic patients without AKI (18), septic shock (19, 20), acute circulatory failure (21), and healthy controls (22, 23) also found an inverse relation between RRI and MAP. However, in septic AKI (18) and postoperative cardiac surgery (24), MAP was not related to RRI. In six studies in mixed (5, 21, 25) and septic populations (18, 19, 26), RRI and vasopressor support were not related, whereas others found that RRI decreased when MAP was increased with norepinephrine (20). In general, RRI can increase due to intrarenal vasoconstriction if flow remains constant or due to increased flow with similar vessel diameter (3). Hypotension triggers vasoconstriction as an adaptive response and thereby increases RRI. Hypotension also triggers the clinician to give norepinephrine. Norepinephrine can lower RRI by reducing venous capacitance and increasing cardiac preload, blood pressure, and kidney perfusion (20). On the contrary, norepinephrine may increase RRI by direct vasoconstriction. This relation was found in our patients, maybe indicating that “too much” vasopressors were used or that renal vasoconstriction is not directly reversible after restoration of hypotension, as demonstrated in experimental sepsis (27) and in septic patients with AKI (18). The combined relation between MAP, norepinephrine, and RRI in our study suggests that both hypotension and vasopressor support could contribute to renal vasoconstriction.
RRI was strongly related to the PPI, the ratio of the pulse pressure to the systolic pressure. PPI reflects vascular compliance (28). In our patients, high PPI, e.g., arterial stiffness, could be due to underlying arterial disease or to vasoconstriction. A strong relation between RRI and pulse pressure was reported in isolated perfused rabbit kidneys (29) and in patients (13, 23, 30–32). In vitro, RRI was more affected by resistance when compliance was higher (33). The inverse relation between RRI and preadmission eGFR in our patients may be related to underlying arterial disease or capillary rarefaction due to preadmission renal disease. Finally, CVP appeared as a strong independent determinant of RRI. A relation between RRI and CVP has not been reported before, but has a strong pathophysiological rationale because high CVP increases resistance to flow.
Our results further suggest that RRI was not related to measured flow indices: cardiac index and the sublingual microcirculation. Unfortunately, cardiac index, which is not part of standard monitoring anymore, was only available in one-third of the patients, mainly cardiac surgery patients. Although the interrelation between flow, pressure, and RRI is complex, our study suggests that RRI is less related to markers of flow. However, we did not measure the renal microcirculation. Nowadays, the renal microcirculation can be assessed with contrast-enhanced ultrasound. A recent pilot study in patients requiring norepinephrine found great variability in pressure responsiveness of the renal microcirculation (34).
RRI was related to fluid balance on univariable but not on multivariable analysis. In some previous studies, hemodynamic changes induced by fluid challenges did not result in changes in RRI (26, 35). However, another study found that fluids decreased RRI and increased urine output (21). Interestingly, BIA-derived reactance/m (membrane capacitance) appeared as an independent determinant of RRI in our study. Reactance/m also tended to be lower in patients with shock. In our patients, low reactance may represent poor underlying condition, but also the consequence of shock, e.g., capillary leakage. This suggests that membrane injury may contribute to an increased RRI.
Strenghts. Although several studies reported relations between RRI and hemodynamic variables, this is the first study comparing RRI between patients with and without shock and analyzing the combined determinants of RRI in a mixed ICU population. Our model, including pressure markers of the systemic circulation, a marker of cell and membrane quality (reactance/m), and preadmission eGFR (a marker of preexisting vascular disease), explained about 60% of the variability of RRI. However, given the sample size of the study, there might be some overfitting. Unknown determinants were also suggested in literature (8). To minimize intraobserver variability, each final RRI was based on 18 measurements. A good interobserver reliability was already shown in a previous study (12). A third blinded investigator performed the SDF analysis, minimizing information bias. Selection bias was minimized because allocation to the shock group was performed by the senior investigator who was blinded for the RRI results. Finally, all measurements were performed in a short time frame, thereby optimizing the reliability of the relations with RRI.
Limitations. First, CI was only measured in one-third of the patients. Importantly, CI was not related to RRI on univariable analysis. There was not even a trend. Second, in the multivariable analysis we imputed for missing CVP values. However, results were similar on sensitivity analysis using unimputed data only. Third, the sample size was too small to assess the different types of shock separately. However, RRI was not different between patients with septic shock and other shock, and the final model was not influenced by excluding septic shock patients from the analysis (data not shown). Unfortunately, the proportion of septic shock patients was very low (in line with the proportion admitted to our ICU). Increased awareness of sepsis and early administration of antibiotics could be an explanation. Notably, CI and sublingual microcirculatory markers were not different between patients with or without shock. An explanation could be that during surgery a restricted fluid strategy is presently applied and that cardiac surgery patients often have low cardiac index postoperatively due to hypovolemia during rewarming while not fulfilling the shock criteria. Fourth, eligible patients were not included over the weekend. However, age and severity of illness of the nonincluded eligible shock patients were similar. Fifth, we visualized the microcirculation under the tongue, which might differ from the kidney. Furthermore, SDF could not be performed in 26.1% of the patients. Sixth, the interval between ICU admission and the measurement of RRI differed between patients. This might be a drawback; however, shock treatment and the illness duration before admission also differ between patients. Thus, lead-time bias may confound the observations but is partially inevitable. Altogether, our findings contribute to the knowledge about the renal vascular response to changes in the systemic circulation. However, a causative relation cannot be shown, nor whether the vasoconstrictive response is harmful or adaptive.
The present study explained about 60% of the variability of RRI. Previous studies found that high RRI was a predictor of AKI. Because vasopressor support showed the strongest independent relation with a high RRI and because high RRI predicts the development of AKI during the first week of ICU admission (9), our findings could indicate that too much vasopressors might unnecessarily increase renal vasoconstriction and subsequently contribute to the development of AKI. Measuring RRI using bedside ultrasound could be part of the assessment of vital organ status and function. Further studies are needed to validate our results, to find the remaining determinants of RRI, including the renal microcirculation, and to show whether RRI can be used to guide vasopressor dosing during shock resuscitation and thereby mitigate the development of AKI.
CONCLUSIONS
The present observational study shows that patients with shock have a higher RRI than critically ill patients without shock. Furthermore, independent determinants of high RRI were pressure indices of the systemic circulation, low membrane capacitance, and preadmission renal dysfunction, a surrogate marker for underlying vascular disease. Flow markers such as cardiac index and the sublingual microcirculation were not. Further studies are needed to show whether RRI can be used to guide vasopressor dosing during shock resuscitation to mitigate AKI.
Footnotes
SR and JLGH recruited all patients and performed all study measurements. SR analyzed the data and drafted the manuscript supported by HMOvS. JGR performed the analysis of all SDF recordings. PRT and PWGE assisted SR and JLGH with the ultrasound measurements. AMESdM and MCdW assisted SR and JLGH during the study when needed. JLGH, JGR, PWGE, AMESdM, PRT, and MCdW revised the manuscript. HMOvS was responsible for conceiving the study and its coordination, discussed inclusions, and helped to draft the manuscript. All authors contributed to the writing of the manuscript and approved the final version.
The authors report no conflicts of interest.
REFERENCES
- 1.Hoste EAJ, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 41 8:1411–1423, 2015. [DOI] [PubMed] [Google Scholar]
- 2.Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol 2 2:1303–1353, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le Dorze M, Bouglé A, Deruddre S, Duranteau J. Renal Doppler ultrasound: a new tool to assess renal perfusion in critical illness. Shock 37 4:360–365, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Boddi M, Natucci F, Ciani E. The internist and the renal resistive index: truths and doubts. Intern Emerg Med 10 8:893–905, 2015. [DOI] [PubMed] [Google Scholar]
- 5.Darmon M, Schortgen F, Vargas F, Liazydi A, Schlemmer B, Brun-Buisson C, Brochard L. Diagnostic accuracy of Doppler renal resistive index for reversibility of acute kidney injury in critically ill patients. Intensive Care Med 37 1:68–76, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Schnell D, Deruddre S, Harrois A, Pottecher J, Cosson C, Adoui N, Benhamou D, Vicaut E, Azoulay E, Duranteau J. Renal resistive index better predicts the occurrence of acute kidney injury than cystatin C. Shock 38 6:592–597, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Giustiniano E, Meco M, Morenghi E, Ruggieri N, Cosseta D, Cirri S, Difrancesco O, Zito PC, Gollo Y, Raimondi F. May renal resistive index be an early predictive tool of postoperative complications in major surgery? Preliminary results. Biomed Res Int 2014:917985, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lerolle N. Please don’t call me RI anymore; I may not be the one you think I am!. Crit Care 16 6:174, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haitsma Mulier JLG, Rozemeijer S, Rottgering JG, Spoelstra-de Man AME, Elbers PWG, Tuinman PR, de Waard MC, Oudemans-van Straaten HM. Renal resistive index as an early predictor and discriminator of acute kidney injury in critically ill patients: a prospective observational cohort study. PLoS One 13 6:e0197967, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bakker J, Lima A. Noninvasive monitoring of peripheral perfusion. Intensive Care Med 31:1316–1326, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RMH, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest 101:1644–1655, 1992. [DOI] [PubMed] [Google Scholar]
- 12.Schnell D, Reynaud M, Venot M, Le Maho AL, Dinic M, Baulieu M, Ducos G, Terreaux J, Zeni F, Azoulay E, et al. Resistive Index or color-Doppler semi-quantitative evaluation of renal perfusion by inexperienced physicians: results of a pilot study. Minerva Anestesiol 80 12:1273–1281, 2014. [PubMed] [Google Scholar]
- 13.Ponte B, Pruijm M, Ackermann D, Vuistiner P, Eisenberger U, Guessous I, Rousson V, Mohaupt MG, Alwan H, Ehret G, et al. Reference values and factors associated with renal resistive index in a family-based population study. Hypertension 63 1:136–142, 2014. [DOI] [PubMed] [Google Scholar]
- 14.De Backer D, Hollenberg S, Boerma C, Goedhart P, Büchele G, Ospina-Tascon G, Dobbe I, Ince C. How to evaluate the microcirculation: report of a round table conference. Crit Care 11 5:R101, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lukaski HC, Kyle UG, Kondrup J. Assessment of adult malnutrition and prognosis with bioelectrical impedance analysis: phase angle and impedance ratio. Curr Opin Clin Nutr Metab Care 20 5:330–339, 2017. [DOI] [PubMed] [Google Scholar]
- 16.Altman DG. Practical Statistics for Medical Research. 1991; London: Chapman and Hall, p. 349. [Google Scholar]
- 17.Corradi F, Brusasco C, Vezzani A, Palermo S, Altomonte F, Moscatelli P, Pelosi P. Hemorrhagic shock in polytrauma patients: early detection with renal Doppler resistive index measurements. Radiology 260 1:112–118, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Dewitte A, Coquin J, Meyssignac B, Joannes-Boyau O, Fleureau C, Roze H, Ripoche J, Janvier G, Combe C, Ouattara A. Doppler resistive index to reflect regulation of renal vascular tone during sepsis and acute kidney injury. Crit Care 16 5:R165, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lerolle N, Guérot E, Faisy C, Bornstain C, Diehl JL, Fagon JY. Renal failure in septic shock: predictive value of Doppler-based renal arterial resistive index. Intensive Care Med 32 10:1553–1559, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Deruddre S, Cheisson G, Mazoit JX, Vicaut E, Benhamou D, Duranteau J. Renal arterial resistance in septic shock: effects of increasing mean arterial pressure with norepinephrine on the renal resistive index assessed with Doppler ultrasonography. Intensive Care Med 33 9:1557–1562, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Moussa MD, Scolletta S, Fagnoul D, Pasquier P, Brasseur A, Taccone FS, Vincent J-L, De Backer D. Effects of fluid administration on renal perfusion in critically ill patients. Crit Care 19 1:250, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boddi M, Sacchi S, Lammel RM, Mohseni R, Serneri GGN. Age-related and vasomotor stimuli-induced changes in renal vascular resistance detected by Doppler ultrasound. Am J Hypertens 9 5:461–466, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Kuznetsova T, Cauwenberghs N, Knez J, Thijs L, Liu YP, Gu YM, Staessen JA. Doppler indexes of left ventricular systolic and diastolic flow and central pulse pressure in relation to renal resistive index. Am J Hypertens 28 4:535–545, 2015. [DOI] [PubMed] [Google Scholar]
- 24.Corradi F, Brusasco C, Paparo F, Manca T, Santori G, Benassi F, Molardi A, Gallingani A, Ramelli A, Gherli T, et al. Renal Doppler resistive index as a marker of oxygen supply and demand mismatch in postoperative cardiac surgery patients. BioMed Res Int 2015:763940, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darmon M, Schortgen F, Leon R, Moutereau S, Mayaux J, Di Marco F, Devaquet J, Brun-Buisson C, Brochard L. Impact of mild hypoxemia on renal function and renal resistive index during mechanical ventilation. Intensive Care Med 35 6:1031–1038, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Schnell D, Camous L, Guyomarc’h S, Duranteau J, Canet E, Gery P, Dumenil A-S, Zeni F, Azoulay E, Darmon M. Renal perfusion assessment by renal Doppler during fluid challenge in sepsis. Crit Care Med 41 5:1214–1220, 2013. [DOI] [PubMed] [Google Scholar]
- 27.Legrand M, Bezemer R, Kandil A, Demirci C, Payen D, Ince C. The role of renal hypoperfusion in development of renal microcirculatory dysfunction in endotoxemic rats. Intensive Care Med 37 9:1534–1542, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng-Lin Y, Yue-Chun L. Pulse pressure index (pulse pressure/systolic pressure) may be better than pulse pressure for assessment of cardiovascular outcomes. Med Hypotheses 72 6:729–731, 2009. [DOI] [PubMed] [Google Scholar]
- 29.Tublin ME, Tessler FN, Murphy ME. Correlation between renal vascular resistance, pulse pressure, and the resistive index in isolated perfused rabbit kidneys. Radiology 213 1:258–264, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Calabia J, Torguet P, Garcia I, Martin N, Mate G, Marin A, Molina C, Valles M. The relationship between renal resistive index, arterial stiffness, and atherosclerotic burden: The link between macrocirculation and microcirculation. J Clin Hypertens 16 3:186–191, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cauwenberghs N, Kuznetsova T. Determinants and prognostic significance of the renal resistive index. Pulse (Basel) 3 (3–4):172–178, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stea F, Sgrò M, Faita F, Bruno RM, Cartoni G, Armenia S, Taddei S, Ghiadoni L. Relationship between wave reflection and renal damage in hypertensive patients: a retrospective analysis. J Hypertens 31 12:2418–2424, 2013. [DOI] [PubMed] [Google Scholar]
- 33.Bude RO, Rubin JM. Relationship between the resistive index and vascular compliance and resistance. Radiology 211 2:411–417, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Schneider AG, Goodwin MD, Schelleman A, Bailey M, Johnson L, Bellomo R. Contrast-enhanced ultrasonography to evaluate changes in renal cortical microcirculation induced by noradrenaline: a pilot study. Crit Care 18 6:653, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lahmer T, Rasch S, Schnappauf C, Schmid RM, Huber W. Influence of volume administration on Doppler-based renal resistive index, renal hemodynamics and renal function in medical intensive care unit patients with septic-induced acute kidney injury: a pilot study. Int Urol Nephrol 48 8:1327–1334, 2016. [DOI] [PubMed] [Google Scholar]


