ABSTRACT
Introduction:
Aortic occlusion during cardiopulmonary resuscitation (CPR) increases systemic arterial pressures. Correct thoracic placement during the resuscitative endovascular balloon occlusion of the aorta (REBOA) may be important for achieving effective CPR.
Hypothesis:
The positioning of the REBOA in the thoracic aorta during CPR will affect systemic arterial pressures.
Methods:
Cardiac arrest was induced in 27 anesthetized pigs. After 7 min of CPR with a mechanical compression device, REBOA in the thoracic descending aorta at heart level (zone Ib, REBOA-Ib, n = 9), at diaphragmatic level (zone Ic, REBOA-Ic, n = 9) or no occlusion (control, n = 9) was initiated. The primary outcome was systemic arterial pressures during CPR.
Results:
During CPR, REBOA-Ic increased systolic blood pressure from 86 mmHg (confidence interval [CI] 71–101) to 128 mmHg (CI 107–150, P < 0.001). Simultaneously, mean and diastolic blood pressures increased significantly in REBOA-Ic (P < 0.001 and P = 0.006, respectively), and were higher than in REBOA-Ib (P = 0.04 and P = 0.02, respectively) and control (P = 0.005 and P = 0.003, respectively). REBOA-Ib did not significantly affect systemic blood pressures. Arterial pH decreased more in control than in REBOA-Ib and REBOA-Ic after occlusion (P = 0.004 and P = 0.005, respectively). Arterial lactate concentrations were lower in REBOA-Ic compared with control and REBOA-Ib (P = 0.04 and P < 0.001, respectively).
Conclusions:
Thoracic aortic occlusion in zone Ic during CPR may be more effective in increasing systemic arterial pressures than occlusion in zone Ib. REBOA during CPR was found to be associated with a more favorable acid–base status of circulating blood. If REBOA is used as an adjunct in CPR, it may be of importance to carefully determine the aortic occlusion level.
The study was performed following approval of the Regional Animal Ethics Committee in Linköping, Sweden (application ID 418).
Keywords: Cardiac arrest, cardiopulmonary resuscitation, hemodynamics, metabolism, resuscitative endovascular balloon occlusion of the aorta, return of spontaneous circulation
INTRODUCTION
Resuscitative endovascular balloon occlusion of the aorta (REBOA) is an emerging method to stop ongoing bleeding (1). It temporarily stabilizes the circulation in trauma patients in life-threatening hemorrhagic shock until definitive surgical repair can be accomplished (2–4). The REBOA causes a mechanical shift in the circulating blood volume from the lower part of the body to the heart and brain (5). In nontraumatic and nonhemorrhagic cardiac arrests, the coronary and cerebral perfusion pressures during CPR are the main determinants of outcome (6–8). The conventional method for increasing the coronary perfusion pressure in CPR is to administer adrenaline (epinephrine), although adrenaline might not increase the long-term survival rate (9). Previous experimental studies in animal models of cardiac arrest have shown that aortic occlusion increases systemic arterial pressures, increases cerebral and cardiac blood pressure and flow, and improves the rate of return of spontaneous circulation (ROSC) (10–19). Human studies, with the exception of some case reports, are lacking, but may be initiated in the near future because of the recent advancement of endovascular methods, in particular endovascular resuscitation, in emergency patients (20–23). In a patient with a ruptured thoracic aortic aneurysm in cardiac arrest, endovascular aortic occlusion was successfully initiated during cardiopulmonary resuscitation (CPR) (24). There is an urgent need of novel treatment modalities for nontraumatic and nonhemorrhagic out-of-hospital cardiac arrest because of a low rate of survival to hospital discharge (25, 26). In addition to increasing ROSC frequency, REBOA may be used to optimize cerebral and coronary blood flows during conventional CPR as a bridge to extracorporeal membrane oxygenation (27). However, before the initiation of clinical trials of REBOA in cardiac arrest, knowledge of optimal use and the positioning of REBOA during CPR must be gathered. The descending aorta is divided into 3 main REBOA zones. Zone I is in the thoracic descending aorta, and zones II and III are in the abdominal aorta (5). However, because of the proximity of the descending thoracic aorta to the heart, the exact position of REBOA in zone I could be of importance in CPR with chest compressions. Therefore, in this study, based on fluoroscopic appearance, the thoracic descending aorta was divided into 3 subzones: Ia, above the upper heart margin; Ib, behind the heart contour; and Ic, below the lower heart margin. We hypothesized that the exact position of REBOA in the thoracic descending aorta during CPR is of importance for generating increased systemic arterial pressures. In a porcine model of cardiac arrest and CPR, the effect on systemic arterial pressures of REBOA in zone Ib is compared with REBOA in zone Ic. The effects of REBOA on arterial metabolic status during CPR are also investigated.
MATERIALS AND METHODS
Animals
The Regional Animal Ethics Committee in Linköping approved the study (ID: 418) prior to experimentation. The animals were handled in accordance with the European Convention for Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (28). Twenty-eight male and female pigs of Swedish country breed (Hampshire and Yorkshire, 3–4 months old, mean weight of 28 kg [range 25–34 kg]) were included. The animals were bred and housed at a local farm in a 16 h/8 h day/night cycle with free access to fodder and water until the morning of experimentation. The research was performed by an experienced research team, and was supervised by a licensed veterinarian. Two simultaneous experiments were performed each day, between October 2016 and April 2017, in a laboratory equipped for animal experimentation at the Örebro University Hospital, Örebro, Sweden.
Preparation, anesthesia, ventilation, and fluid treatment
At the farm, the pigs received 200–250 mg azaperone (i.m., 40 mg ml−1; Stresnil, Elanco, Herlev, Denmark) for sedation and were thereafter transported for 30 min by road to the laboratory. On arrival, anesthesia was induced by an i.m. injection of tiletamine and zolazepam (6 mg kg−1 of each; Zoletile Forte, Virbac, Kolding, Denmark), azaperone (4 mg kg−1) and atropine (to reduce salivation; 1.5 mg; Mylan, Stockholm, Sweden). Two peripheral venous catheters (1.1 mm, Venflon Pro Safety, BD, Helsingborg, Sweden) were inserted into the pigs’ ear veins, and they received Cefuroxim (750 mg, i.v.; GSK, Solna, Sweden). If needed, Propofol (1–2 mg i.v.; Fresenius Kabi, Uppsala, Sweden) was given to facilitate oral intubation with a 6-mm endotracheal tube (Covidien, Tullamore, Ireland). Mechanical ventilation (PV 501, Breas Medical AB, Sweden) was used to achieve an arterial partial pressure of carbon dioxide (PCO2) of 4.8 kPa to 5.5 kPa with fraction of inspired oxygen of 25% to 30%. Fentanyl (20 μg kg−1 h−1 i.v; Meda, Solna, Sweden) and propofol (10 mg kg−1 h−1 i.v.; Fresenius Kabi) were continuously infused i.v. by motorized syringe pumps (Alaris CC, Cardinal Health, Rolle, Switzerland). The rates of infusion were increased if the animal showed signs of pain or awareness. 10 mL kg−1 h−1 of Ringer-Acetate (i.v.; Fresenius Kabi) and 1 mL kg−1 h−1 of 5% glucose (i.v.; Fresenius Kabi) were administered by volume pumps (Alaris GP, CareFusion, San Diego, CA). The animals were euthanized at the end of the experiment with 40 mmol of potassium chloride i.v. (Braun, Danderyd, Sweden). Circulatory arrest was confirmed with ECG and blood pressure recordings.
Surgical preparations
Five introducers, Avanti+ (Cordis, Cashel, Ireland) were inserted into surgically exposed vessels using the Seldinger technique. A 9 Fr introducer was placed in the right femoral artery for occlusion of the left anterior descending artery (LAD). A 10 Fr introducer was inserted into the left femoral artery for the REBOA catheter with a compliant balloon (Rescue Balloon, 7 Fr Tokai Medical Products Inc, Kasugai, Japan). A 5 Fr introducer was put in the right common carotid artery for blood sampling, and measurement of systemic blood pressure and heart rate. A 9 Fr introducer was placed in the right external jugular vein for the pulmonary arterial catheter (Swan-Ganz CCOmbo, 7.5 Fr, Edwards Lifesciences, Irvine, CA), which was used for measurement of central venous pressure, body temperature, and cardiac output (semicontinuously using thermodilution technique; Vigilance, Edwards Lifescience). A 10 Fr introducer was inserted into the left external jugular vein for fluid and drug administration and for a bipolar pacemaker electrode, if needed (see below). A midline abdominal incision was performed, and a 14 Fr urinary catheter (Foley, Unomedical, Flintshire, UK) was inserted into the urinary bladder. An ultrasonic flow probe (4–6 mm, PV probe, Medistim, Oslo, Norway) was fixed around the superior mesenteric artery (SMA) to measure blood flow. A 6 Fr catheter (Nutrisafe 2, Vygon, Ecouen, France) was inserted into the superior mesenteric vein for measurement of blood pressure. Thereafter, the animals received heparin (5,000 IU, i.v.; LEO Pharma, Malmö, Sweden). A warmth blanket was used to retain a normal body temperature of 38°C to 38.5°C. The defibrillator paddles were attached at heart height approximately 5 cm lateral to the sternum on both sides of the thorax. A defibrillator (Lifepak 20, Medtronic B.V., Heerlen, The Netherlands) was connected which also monitored basic ECG. An intervention-free period of at least 1 h followed the surgical preparations.
Protocol and measurements
At baseline, circulatory and respiratory data, displayed on an ICU monitor (AS/3, Datex, Helsinki, Finland), were collected. Arterial blood was analyzed for blood gases, electrolytes, and lactate by GEM 4000 (Instrumentation Laboratory, Lexington, MA). Under guidance of fluoroscopy, the REBOA catheter was inserted and positioned in the thoracic descending aorta in either zone Ib (behind the heart contour, Fig. 1B) or zone Ic (below the lower heart margin, Fig. 1C). One animal died during surgical preparation because of bleeding, which led to a total of 27 animals completing the experiment. The animals were allocated to either aortic occlusion in zone Ib (REBOA-Ib, n = 9) or in zone Ic (REBOA-Ic, n = 9) or to no aortic occlusion (n = 9, control group). The animals in the present report were part of a model-development process and therefore not randomly allocated to their particular group. Then, additional heparin (10,000 IU i.v.) was given. Thereafter, the LAD was catheterized using a 0.035″ guide wire (Abbott Vascular, Santa Clara), a 5 Fr guide catheter (Launcher Medtronic, Minneapolis, MN), a 0.014″ wire to reach the LAD, and a balloon catheter (Powerline balloon diameter 2.5 mm, balloon length 15 mm; Biosensors International, Morges, Switzerland). Subsequently, the LAD was occluded, either distally or proximally, for 6 to 30 min by inflation of the balloon (10 psi, 2.5 mm balloon diameter, Fig. 1). Coronary angiograms were obtained by contrast injection in the guide catheter to confirm correct placement and inflation of the balloon. If ventricular fibrillation (VF) did not occur (Fig. 1), it was induced by applying a voltage of 9 V at the bipolar pacemaker electrode inserted in the right ventricle. Similar model for myocardial infarction have been described (29). When VF was confirmed by ECG readings, the ventilator and infusion pumps were disabled until the start of CPR. The duration of circulatory arrest was between 2 and 8 min (Fig. 1). At the start of CPR, mechanical chest compressions by a device (Lucas, Jolife AB, Lund, Sweden) were commenced with a 50% compression phase and 50% decompression phase lasting about 0.3 s each and a compression rate of 100 min−1. Ventilation was restarted at 8 to 12 breaths min−1, with 5 L min−1 of oxygen flow into the ventilator. The CPR followed an algorithm with ECG analysis and defibrillation of 200 J (if VF or ventricular tachycardia without spontaneous circulation) every second minute and adrenaline administration i.v. (0.02–0.03 mg kg−1) every fourth minute starting after 6 min of CPR (Fig. 1). In animals allocated to aortic occlusion, the occlusion balloon was inflated after 7 min of CPR (Fig. 1). The CPR was continued for at least 30 min. During CPR, hemodynamic and respiratory data were collected, and arterial blood was analyzed intermittently (Fig. 1). If sinus rhythm was identified on the ECG and pulse waves were produced on the systemic arterial pressure recording, CPR was discontinued. If circulatory arrest reappeared, the CPR protocol was resumed. ROSC was defined in our protocol as a spontaneous mean arterial pressure (MAP) greater than 40 mmHg for at least 5 min.
Fig. 1.
Panel A shows study protocol of CPR and aortic occlusion (REBOA) in anesthetized pigs.
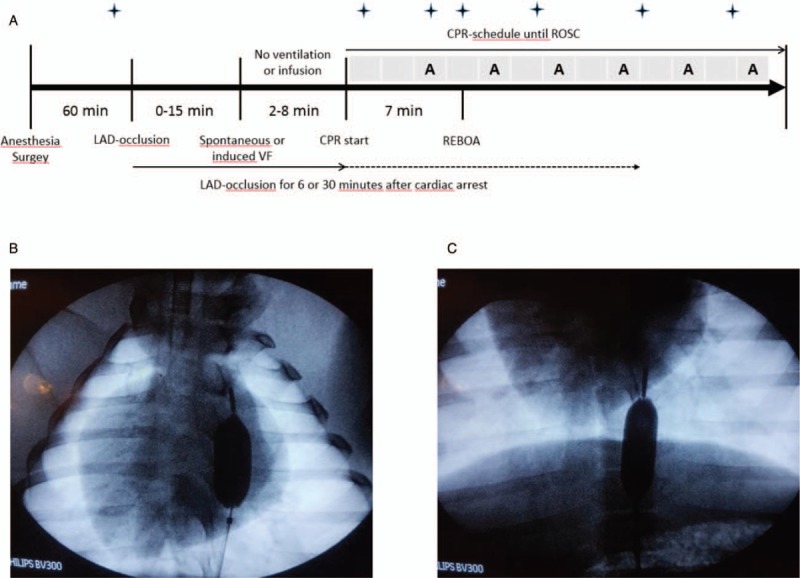
The boxes above the time line indicate 2 min of CPR with defibrillation if ventricular fibrillation or ventricular tachycardia. The letter A indicates adrenaline injection. The REBOA was inflated after 7 min of CPR.  indicates time for blood samples and hemodynamic measurements (no blood samples at 8 min of CPR). Panels B (REBOA-Ib) and C (REBOA-Ic) show the different locations of REBOA seen by fluoroscopy. CPR, cardiopulmonary resuscitation; REBOA, resuscitative endovascular balloon occlusion of the aorta.
indicates time for blood samples and hemodynamic measurements (no blood samples at 8 min of CPR). Panels B (REBOA-Ib) and C (REBOA-Ic) show the different locations of REBOA seen by fluoroscopy. CPR, cardiopulmonary resuscitation; REBOA, resuscitative endovascular balloon occlusion of the aorta.
Statistical method
The data were tested to meet the assumptions of a normal distribution, no significant outliers and homogeneity within the different groups using SPSS (SPSS version 23, IBM Corp, Armonk, NY). If the data did not strongly violate these assumptions, they were analyzed using a linear mixed model (repeated factor time, the other factor group; SPSS). If the linear mixed model identified a significant effect of interaction between group and time, multiple comparisons were performed using a Bonferroni adjusted post-hoc test (SPSS). P < 0.05 was considered significant. Data are presented as means with 95% confidence intervals.
RESULTS
Hemodynamic measurements
Before cardiac arrest, no statistical differences were detected between the groups, except that diastolic blood pressure (DBP) was slightly higher in REBOA-Ib than in REBOA-Ic (P = 0.01, Fig. 2B). During CPR but before intervention, systolic blood pressure (SBP) increased to circa 90 mmHg to 100 mmHg in all groups, indicating adequate compressions at 100 beats min−1 (Fig. 2B). In REBOA-Ic, SBP increased by approximately 42 mmHg after aortic occlusion (P < 0.001 compared with before intervention), in comparison with 17 mmHg in REBOA-Ib and 7 mmHg in the control group (Fig. 2A). In parallel, MAP and DBP were significantly higher in REBOA-Ic compared with before intervention (P < 0.001 and P = 0.006, respectively), to REBOA-Ib (P = 0.04 and P = 0.02, respectively) and to the control group (P = 0.005 and P = 0.003, respectively, Fig. 2B and C). In REBOA-Ib and the control group, there were no significant changes in systemic arterial blood pressures when comparing within the groups before and after the time of intervention (Fig. 2A-C). During CPR, but before intervention, blood flow in the SMA was present in all groups although it was substantially lower than at baseline (approximately 15–25% of baseline flow at 1 min of CPR, P < 0.001 in all groups compared with baseline, Fig. 2D). At aortic occlusion, the blood flow in the SMA decreased to almost zero in REBOA-Ib and REBOA-Ic (P = 0.001 and P = 0.001, respectively, compared with the control group; Fig. 2D). In the control group, the blood flow in the SMA decreased with the duration of CPR (Fig. 2D). There were no significant changes between the groups regarding heart rate, mean central venous pressure, mean mesenteric pressure, or body temperature throughout the experiment.
Fig. 2.
SBP (panel A), DBP (panel B), MAP (panel C), and blood flow in the SMA (panel D), during CPR in anesthetized pigs.
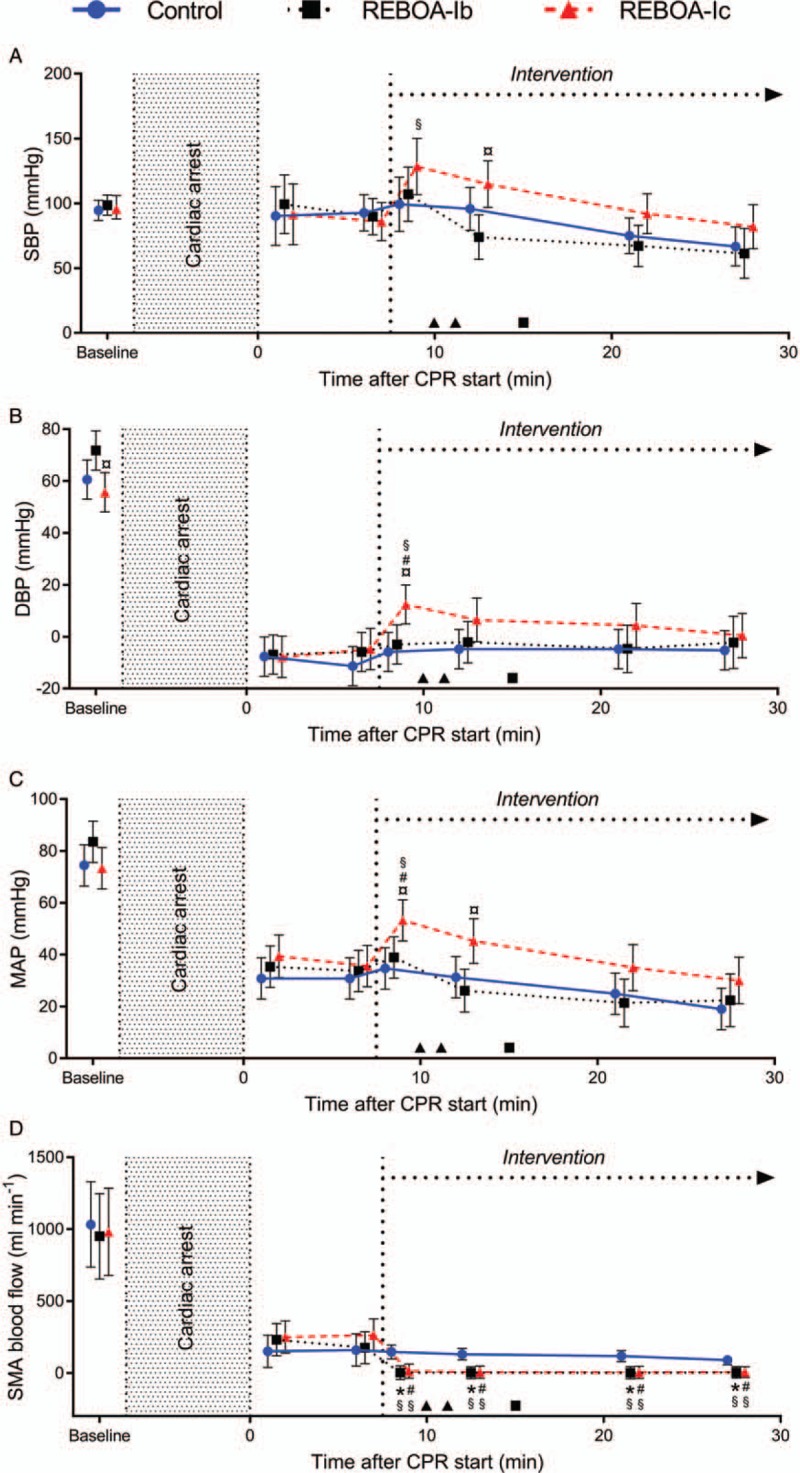
Aortic occlusion (REBOA, intervention) at heart level (REBOA-Ib, n = 9) or diaphragmatic level (REBOA-Ic, n = 9) and control (no aortic occlusion, n = 9). Values are displayed as means with 95% CI. P < 0.05 was considered as statistically significant. ∗ indicates a significant difference between control and REBOA-Ib. # indicates a significant difference between control and REBOA-Ic. ¤ indicates a significant difference between REBOA-Ib and REBOA-Ic. § indicate significant differences between the measurement before intervention (6 min) and the time points thereafter within each group. ▴ indicates ROSC in an animal with REBOA-Ic and ▪ indicates ROSC in an animal with REBOA-Ib. CI, confidence interval; CPR, cardiopulmonary resuscitation; DBP, diastolic blood pressure; MAP, mean arterial pressure; REBOA, resuscitative endovascular balloon occlusion of the aorta; ROSC, return of spontaneous circulation; SBP, systolic blood pressure; SMA, superior mesenteric artery.
Respiratory variables and blood analysis
Baseline values of the respiratory variables and arterial blood gases, including lactate, did not significantly differ between the groups (Table 1, Fig. 3). Arterial lactate concentrations were slightly higher in REBOA-Ib compared with REBOA-Ic at the time point before intervention (6 min of CPR, P = 0.004; Fig. 3C). Arterial PO2 was maintained during CPR and did not significantly differ between the groups (Table 1). Arterial acidosis was more pronounced in the control group with lower arterial pH compared with both REBOA-Ib and REBOA-Ic (P = 0.004 and P = 0.005, respectively after 21 min of CPR; Fig. 3A). There was a tendency toward decreased arterial PCO2 levels after aortic occlusion in both REBOA-Ib and REBOA-Ic compared with the control group (Fig. 3B). After intervention, arterial lactate levels were significantly lower in REBOA-Ic compared with both the control group and REBOA-Ib (P = 0.04 and P < 0.001, respectively, after 27 min of CPR, Fig. 3C).
Table 1.
Respiratory values and blood samples during CPR in anesthetized pigs
| Time (min after CPR start) | ||||||||
| Variables | Groups | Baseline | 1 | 6 | 8 | 12 | 21 | 27 |
| Fraction of inspired O2 (%) | Control | 25 (22–29) | 80 (64–97) | 77 (66–88) | 75 (69–81) | 76 (71–82) | 75 (70–80) | 76 (70–82) |
| REBOA-Ib | 25 (22–29) | 53 (36–69) | 65 (53–76) | 74 (68–81) | 71 (65–76) | 71 (65–77) | 67 (60–74) | |
| REBOA-Ic | 24 (20–27) | 75 (59–92) | 70 (59–81) | 70 (64–77) | 68 (62–74) | 71 (65–77) | 67 (61–73) | |
| Arterial PO2 (kPa) | Control | 15.3 (12.5–18.2) | 21.7 (7.8–35.6) | 26.2 (14.5–37.9) | 24.4 (15.9–32.9) | 22.0 (14.6–29.5) | 19.9 (13.1–26.7) | |
| REBOA-Ib | 16.2 (13.3–19.0) | 16.8 (3.1–30.5) | 27.3 (15.6–39.0) | 28.8 (20.1–37.5) | 24.2 (15.9–32.5) | 24.3 (15.6–33.1) | ||
| REBOA-Ic | 14.4 (11.5–17.2) | 20.1 (6.4–33.8) | 29.1 (17.4–40.8) | 25.6 (16.6–34.5) | 30.4 (22.3–38.6) | 22.2 (14.6–29.7) | ||
| Arterial base excess (mmol L−1) | Control | 6.9 (4.6–9.2) | −3.2 (−5.5−−0.9) | −9.5 (−11.8–−7.2) | −13.9 (−16.2–−11.6) | −18.1 (−20.4–−15.8) | −20.2 (−22.5–−17.8) | |
| REBOA-Ib | 7.5 (5.2–9.8) | −1.7 (−4.0–0.6) | −8.0 (−10.3–−5.7) | −10.1 (−12.4–−7.7) | −13.1 (15.7–−10.5) | −16.6 (−19.7–−13.6) | ||
| REBOA-Ic | 6.1 (3.8–8.4) | −5.6 (−7.9–−3.3) | −11.1 (−13.4–−8.8) | −11.9 (−14.3–−9.4) | −14.7 (−17.2–−12.2) | −16.7 (−19.3–−14.2) | ||
| Arterial bicarbonate (mmol L−1) | Control | 30.3 (28.5–32.1) | 22.4 (20.6–24.2) | 17.5 (15.7–19.3) | 14.1 (12.3–15.9) | 10.7 (8.9–12.5) | 9.0 (7.2–10.9) | |
| REBOA-Ib | 30.8 (29.0–32.6) | 23.5 (21.7–25.3) | 18.7 (16.9–20.5) | 17.1 (15.2–18.9) | 14.7 (12.7–16.8) | 11.9 (9.6–14.3) | ||
| REBOA-Ic | 29.7 (27.9–31.5) | 20.6 (18.8–22.4) | 16.2 (14.4–18.0) | 15.7 (13.8–17.6) | 13.5 (11.5–15.5) | 11.9 (9.9–13.9) | ||
Values are displayed as means with a 95% CI. REBOA at heart level (REBOA-Ib, n = 9) or diaphragmatic level (REBOA-Ic, n = 9) and control (no aortic occlusion, n = 9).
CI, confidence interval; CPR, cardiopulmonary resuscitation; REBOA, resuscitative endovascular balloon occlusion of the aorta.
Fig. 3.
Arterial pH (panel A), PCO2 (panel B), and lactate (panel C) during CPR in anesthetized pigs.
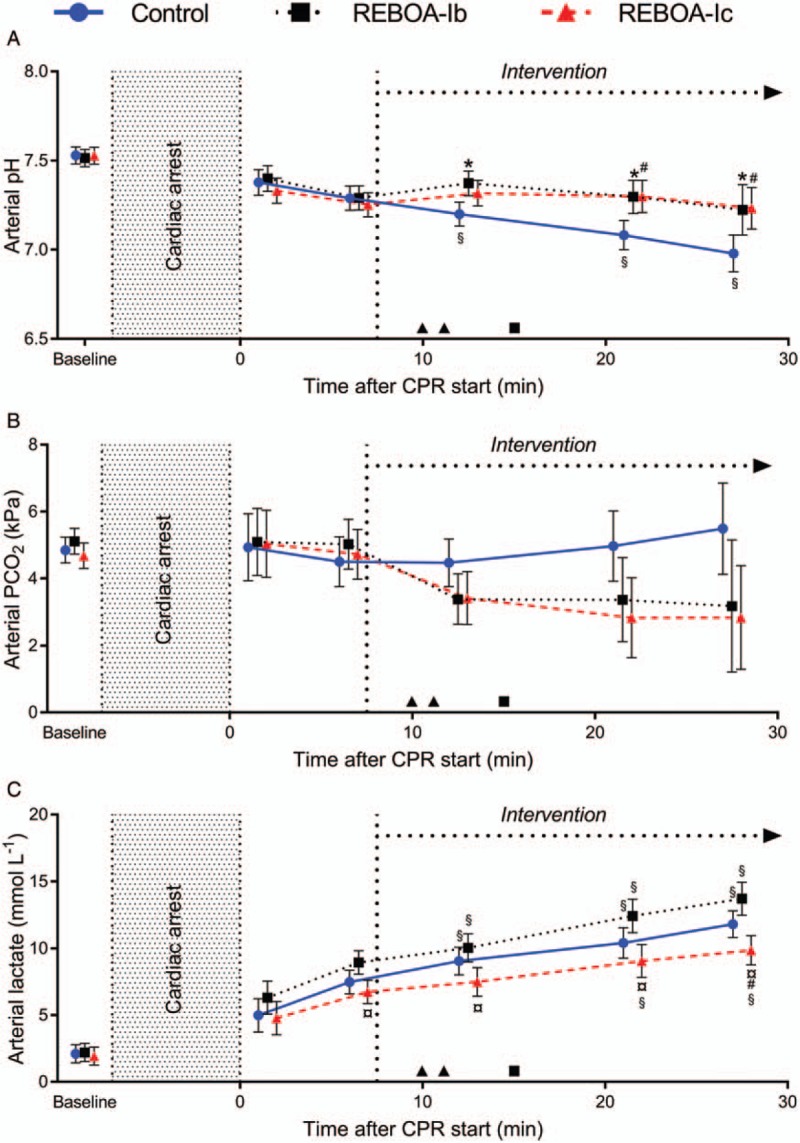
Values are displayed as means with 95% CI. Aortic occlusion (REBOA, intervention) at heart level (REBOA-Ib, n = 9) or diaphragmatic level (REBOA-Ic, n = 9) and control (no aortic occlusion, n = 9). P < 0.05 was considered as statistically significant. ∗ indicates a significant difference between control and REBOA-Ib. # indicates a significant difference between control and REBOA-Ic. ¤ indicates a significant difference between REBOA-Ib and REBOA-Ic. § indicates significant differences between the measurement before intervention (6 min) and the time points thereafter within each group. ▴ indicates ROSC in an animal with REBOA-Ic and ▪ indicates ROSC in an animal with REBOA-Ib. CI, confidence interval; CPR, cardiopulmonary resuscitation; PCO2, partial pressure of carbon dioxide; REBOA, resuscitative endovascular balloon occlusion of the aorta.
Return of spontaneous circulation
Two animals in REBOA-Ic and 1 in REBOA-Ib achieved ROSC, whereas none of the animals in the control group achieved ROSC (Fig. 2).
DISCUSSION
The results of this study suggest that the location of the REBOA affects the hemodynamic response during CPR, and that the proper placement of thoracic REBOA in CPR might be below (REBOA-Ic) and not behind (REBOA-Ib) the heart to generate increased systemic blood pressures. REBOA during CPR might be associated with less disturbed acid–base status in circulating arterial blood compared with no REBOA.
This study shows that the exact placement of REBOA in the thoracic descending aorta during CPR might be crucial. Aortic occlusion in zone Ib led to a poorer response on all the studied variables compared with occlusion at zone Ic. The increase in SBP was not statistically significant in REBOA-Ib after inflation of the balloon. However, SBP increased significantly in REBOA-Ic when comparing before and after the intervention. REBOA-Ic seems to have the greatest impact on the DBP (compared with the other groups). The DBP is an important determinant of the coronary blood flow in the myocardium during CPR (7). In addition, the increase in MAP after aortic occlusion was also statistically significant in REBOA-Ic compared with the other groups. Aortic occlusion during CPR has previously been shown to increase systemic arterial pressures and to augment the blood flow in coronary and cerebral arteries during CPR (10–19). Earlier studies have shown that the maintenance of coronary and cerebral perfusion pressures during CPR is the main determinant for survival and reduction in neurological damage after cardiac arrest (7, 8).
The present study cannot determine the reasons behind the different hemodynamic responses during CPR between REBOA-Ib and REBOA-Ic. A tentative explanation is that the inflated balloon interferes with the compression or filling of the heart during mechanical chest compressions. These findings could be of major interest in CPR of all types of cardiac arrests where REBOA might be used. By using the xiphoid process as an anatomical landmark, well-tolerated placement of REBOA in zone Ic may be clinically possible without fluoroscopy (30).
The blood flow in the SMA during CPR decreased to near zero in both the aortic occlusion groups. This means that there was almost total ischemia in the organs below the aortic occlusion, which is harmful and the duration of occlusion should therefore be limited (31). However, the animals in REBOA-Ic achieved ROSC 3 to 4 min after inflation of the balloon. Then, the balloon was slowly deflated for 5 min to prevent a circulatory collapse when the blood volume redistributes, which is probably the greatest risk factor for return of cardiac arrest. The total time of occlusion was less than 10 min, which is a very short time compared with the management of hemorrhagic shock, where the median time of occlusion was 63 min (32). If occlusion time is short, the hypotension following unclamping is temporary and normal pressure is restored within a short time (33). In the control group, despite no aortic occlusion, the blood flow in the SMA was less than one-fifth of baseline flow which is below the critical limit for aerobic metabolism (34).
Interestingly, acidosis was less pronounced in the REBOA groups compared with the control group, which might seem paradoxical. The explanation is probably that the ischemic organs distal to the occlusion did not have perfusion which led to accumulation of the ischemic substances in the tissues but not in circulating blood. The major part of the ischemic metabolites will reach the circulating blood volume first at reperfusion. In contrast, in the control group, an existing but inadequate perfusion caused partial hypoxic tissues leading to a constant production of acidotic substances to the circulating volume. A short aortic occlusion period during CPR compared with no aortic occlusion might be more favorable in maintaining arterial pH, lactate, and electrolytes closer to the normal range, which would enhance the probability to achieve ROSC. However, following the release of the occlusion, the ischemic substances that have gathered in the occluded tissues are released to the body through the replenished blood flow (35).
This study has some obvious limitations. It was part of a model-development process, which means that some experimental factors (dose of adrenaline, location and duration of LAD occlusion, and duration of circulatory arrest) were varied slightly between the experiments to find the optimal model. Nevertheless, we believe that these variations do not interfere with our major conclusions. The most important factors for hemodynamic and metabolic impact during CPR (e.g., duration of circulatory arrest) were similar in all the groups. In fact, the mean duration of circulatory arrest was almost identical across groups (6.8, 6.9, and 6.7 min in the control group, REBOA-Ib and REBOA-Ic, respectively). The relatively small number of animals in each group is a limiting factor, but the study emphasizes that greater knowledge of optimal use of REBOA in cardiac arrest and CPR is needed before initiating clinical trials. Another limitation of the study is that research on healthy animals is not directly transferable to diseased humans. However, pigs are the ideal animals for modeling cardiac arrest because of their similarities to humans in the anatomy of their heart and abdominal organs (36). Also, the anesthetic drugs could depress the hemodynamic results, however, all animals were anesthetized with the same protocol which enables comparison between the groups.
Previous studies have concluded that REBOA increase ROSC frequency during nontraumatic cardiac arrest (10, 13, 15). This study was not designed, nor had the power to compare the rates of ROSC between the groups.
CONCLUSION
Aortic occlusion in the thoracic descending aorta during CPR in zone Ic might generate higher systemic arterial pressures compared with aortic occlusion in zone Ib and no occlusion. REBOA during CPR was found to be associated with a more favorable acid–base status of circulating blood. If REBOA is used as an adjunct in traumatic or nontraumatic CPR, it might be of importance to carefully determine the aortic occlusion level prior to inflation of the balloon. REBOA in cardiac arrest seems to be a promising therapeutic intervention to be investigated in humans, as a primary intervention to achieve ROSC or as a bridge to more definitive care like percutaneous coronary intervention or/and extracorporeal membrane oxygenation. However, the optimal use of REBOA must be further explored in randomized studies.
Acknowledgments
The authors would like to express their gratitude for excellent assistance in the animal experimentation by the registered nurses: Nina Adolfsson, Monica Clomén, Erika Martell, Johan Josefsson, and Jonas Berlin. We would also like to thank Mr Jon Kimber for language revision. The study was funded by the Research Committee of Region Örebro County, Nyckelfonden, at Örebro University Hospital, ALF Grants (Agreement concerning research and education of doctors), Region Örebro County, and the Swedish Society for Medical Research.
Footnotes
Sources of Support: The study was funded by the Research Committee of Region Örebro County, Nyckelfonden, at Örebro University Hospital, ALF Grants (Agreement concerning research and education of doctors), Region Örebro County, and the Swedish Society for Medical Research.
Conflicts of Interest: The authors report no conflicts of interest.
REFERENCES
- 1.Morrison JJ, Ross JD, Rasmussen TE, Midwinter MJ, Jansen JO. Resuscitative endovascular balloon occlusion of the aorta: a gap analysis of severely injured UK combat casualties. Shock 41:388–393, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Horer TM, Skoog P, Pirouzram A, Nilsson KF, Larzon T. A small case series of aortic balloon occlusion in trauma: lessons learned from its use in ruptured abdominal aortic aneurysms and a brief review. Eur J Trauma Emerg Surg 42:585–592, 2016. [DOI] [PubMed] [Google Scholar]
- 3.Morrison JJ, Ross JD, Houston R, 4th, Watson JD, Sokol KK, Rasmussen TE. Use of resuscitative endovascular balloon occlusion of the aorta in a highly lethal model of noncompressible torso hemorrhage. Shock 41:130–137, 2014. [DOI] [PubMed] [Google Scholar]
- 4.Russo RM, Neff LP, Johnson MA, Williams TK. Emerging endovascular therapies for non-compressible torso hemorrhage. Shock 46 3 Suppl 1:12–19, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stannard A, Eliason JL, Rasmussen TE. Resuscitative endovascular balloon occlusion of the aorta (REBOA) as an adjunct for hemorrhagic shock. J Trauma 71:1869–1872, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Gheeraert PJ, Henriques JP, De Buyzere ML, Voet J, Calle P, Taeymans Y, Zijlstra F. Out-of-hospital ventricular fibrillation in patients with acute myocardial infarction: coronary angiographic determinants. J Am Coll Cardiol 35:144–150, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Paradis NA, Martin GB, Rivers EP, Goetting MG, Appleton TJ, Feingold M, Nowak RM. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA 263:1106–1113, 1990. [PubMed] [Google Scholar]
- 8.Shaffner DH, Eleff SM, Brambrink AM, Sugimoto H, Izuta M, Koehler R, Traystman RJ. Effect of arrest time and cerebral perfusion pressure during cardiopulmonary resuscitation on cerebral blood flow, metabolism, adenosine triphosphate recovery, and pH in dogs. Crit Care Med 27:1335–1342, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Olasveengen TM, Sunde K, Brunborg C, Thowsen J, Steen PA, Wik L. Intravenous drug administration during out-of-hospital cardiac arrest: a randomized trial. JAMA 302:2222–2229, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Gedeborg R, Rubertsson S, Wiklund L. Improved haemodynamics and restoration of spontaneous circulation with constant aortic occlusion during experimental cardiopulmonary resuscitation. Resuscitation 40:171–180, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Spence PA, Lust RM, Chitwood WR, Jr, Iida H, Sun YS, Austin EH., 3rd Transfemoral balloon aortic occlusion during open cardiopulmonary resuscitation improves myocardial and cerebral blood flow. J Surg Res 49:217–221, 1990. [DOI] [PubMed] [Google Scholar]
- 12.Wesley RC, Jr, Morgan DB. Effect of continuous intra-aortic balloon inflation in canine open chest cardiopulmonary resuscitation. Crit Care Med 18:630–633, 1990. [DOI] [PubMed] [Google Scholar]
- 13.Rubertsson S, Bircher NG, Alexander H. Effects of intra-aortic balloon occlusion on hemodynamics during, and survival after cardiopulmonary resuscitation in dogs. Crit Care Med 25:1003–1009, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Sesma J, Labandiera M, Espila S, Arteche A, Saez J. Effect of intra-aortic occlusion balloon in external thoracic compressions during CPR in pigs. Am J Emerg Med 20:453–462, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Tang W, Weil MH, Noc M, Sun S, Gazmuri RJ, Bisera J. Augmented efficacy of external CPR by intermittent occlusion of the ascending aorta. Circulation 88:1916–1921, 1993. [DOI] [PubMed] [Google Scholar]
- 16.Gedeborg R, Silander H, Rubertsson R, Wiklund L. Cerebral ischaemia in experimental cardiopulmonary resuscitation: comparison of epinephrine and aortic occlusion. Resuscitation 50:319–329, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Nozari A, Rubertsson S, Gedeborg R, Nordgren A. Maximisation of cerebral blood flow during experimental cardiopulmonary resuscitation does not ameliorate post-resuscitation hypoperfusion. Resuscitation 40:27–35, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Nozari A, Rubertsson S, Wiklund L. Improved cerebral blood supply and oxygenation by aortic balloon occlusion combined with intra-aortic vasopressin administration during experimental cardiopulmonary resuscitation. Acta Anaesthesiol Scand 44:1209–1219, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Nozari A, Rubertsson S, Wiklund L. Intra-aortic administration of epinephrine above an aortic balloon occlusion during experimental CPR does not further improve cerebral blood flow and oxygenation. Resuscitation 44:119–127, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Daley J, Morrison J, Sather J, Hile L. The role of resuscitative endovascular balloon occlusion of the aorta (REBOA) as an adjunct to ACLS in non-traumatic cardiac arrest: a review of key concepts, physiology, current evidence, and future directions. Am J Emerg Med 35:731–736, 2017. [DOI] [PubMed] [Google Scholar]
- 21.Aslanger E, Golcuk E, Oflaz H, Yilmaz A, Mercanoglu F, Bugra Z, Umman B, Nisanci Y. Intraaortic balloon occlusion during refractory cardiac arrest. A case report. Resuscitation 80:281–283, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Deakin CD, Barron DJ. Haemodynamic effects of descending aortic occlusion during cardiopulmonary resuscitation. Resuscitation 33:49–52, 1996. [DOI] [PubMed] [Google Scholar]
- 23.McGreevy D, Dogan E, Toivola A, Bilos L, Pirouzram A, Nilsson KF, Hörer TM. View of endovascular resuscitation with aortic balloon occlusion in non-trauma cases: first use of ER-REBOA in Europe. JEVTM 1:42–49, 2017. [Google Scholar]
- 24.Larzon T, Jansson H, Holmstrom B, Lund P, Norgren L, Arfvidsson B, Berggren L, Nydahl A, Eriksson T, Jonsson T, et al. Salvage of an acutely ruptured thoracic aortic aneurysm during CPR. J Endovasc Ther 9 Suppl 2:II67–II71, 2002. [PubMed] [Google Scholar]
- 25.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 131:e29–e322, 2015. [DOI] [PubMed] [Google Scholar]
- 26.Berdowski J, Berg RA, Tijssen JG, Koster RW. Global incidences of out-of-hospital cardiac arrest and survival rates: systematic review of 67 prospective studies. Resuscitation 81:1479–1487, 2010. [DOI] [PubMed] [Google Scholar]
- 27.Chouihed T, Kimmoun A, Lauvray A, Laithier FX, Jaeger D, Lemoine S, Maureira JP, Nace L, Duarte K, Albizzati S, et al. Improving patient selection for refractory out of hospital cardiac arrest treated with extracorporeal life support. Shock 49:24–28, 2018. [DOI] [PubMed] [Google Scholar]
- 28.Anonymous: European convention for protection of vertebrate animals used for experimental and other scientific purposes. European Treaty Series - No. 123. Council of Europe, Strasbourg, France, 1986. [Google Scholar]
- 29.Ristagno G, Fumagalli F, Russo I, Tantillo S, Zani DD, Locatelli V, De Maglie M, Novelli D, Staszewsky L, Vago T, et al. Postresuscitation treatment with argon improves early neurological recovery in a porcine model of cardiac arrest. Shock 41:72–78, 2014. [DOI] [PubMed] [Google Scholar]
- 30.Okada Y, Narumiya H, Ishi W, Iiduka R. Anatomical landmarks for safely implementing resuscitative balloon occlusion of the aorta (REBOA) in zone 1 without fluoroscopy. Scand J Trauma Resusc Emerg Med 25:63, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Markov NP, Percival TJ, Morrison JJ, Ross JD, Scott DJ, Spencer JR, Rasmussen TE. Physiologic tolerance of descending thoracic aortic balloon occlusion in a swine model of hemorrhagic shock. Surgery 153:848–856, 2013. [DOI] [PubMed] [Google Scholar]
- 32.Morrison JJ, Galgon RE, Jansen JO, Cannon JW, Rasmussen TE, Eliason JL. A systematic review of the use of resuscitative endovascular balloon occlusion of the aorta in the management of hemorrhagic shock. J Trauma Acute Care Surg 80:324–334, 2016. [DOI] [PubMed] [Google Scholar]
- 33.Spyrou P, Jones N, Matsumoto T. Pathophysiology and management of hypotension following declamping of abdominal aorta. Ann Surg 176:805–808, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pargger H, Staender S, Studer W, Schellscheidt O, Mihatsch MJ, Scheidegger D, Skarvan K. Occlusive mesenteric ischemia and its effects on jejunal intramucosal pH, mesenteric oxygen consumption and oxygen tensions from surfaces of the jejunum in anesthetized pigs. Intensive Care Med 23:91–99, 1997. [DOI] [PubMed] [Google Scholar]
- 35.Gelman S. The pathophysiology of aortic cross-clamping and unclamping. Anesthesiology 82:1026–1060, 1995. [DOI] [PubMed] [Google Scholar]
- 36.Xanthos T, Lelovas P, Vlachos I, Tsirikos-Karapanos N, Kouskouni E, Perrea D, Dontas I. Cardiopulmonary arrest and resuscitation in Landrace/Large White swine: a research model. Lab Anim 41:353–362, 2007. [DOI] [PubMed] [Google Scholar]


