Summary
Objective
Early weight loss is a strong predictor of longer‐term and clinically meaningful weight loss but has not been studied in the context of mobile health (‘mHealth’) interventions.
Methods
GoalTracker was a randomized trial among adults (21–65 years) with overweight or obesity comparing three 12‐week standalone mHealth interventions for weight loss. All arms received a free commercial mobile app (MyFitnessPal) for daily self‐monitoring of diet and/or weight and a goal to lose 5% of weight by 3 months. Collapsing across arms, this analysis examined participants with a 1‐month weight (n = 84), categorizing them as either early responders (≥2% weight loss at 1 month) or early non‐responders (<2% weight loss at 1 month).
Results
Early responders – 36% of participants – had greater per cent weight change at 3 months (−5.93% [95% confidence interval: −6.82%, −5.03%]) than early non‐responders (−1.45% [−2.15%, −0.75%]), which was sustained at 6 months (−5.91% [−7.33%, −4.48%] vs. −1.28% [−2.37%, −0.19%]; ps < 0.0001). Over half (57%) of early responders achieved ≥5% weight loss at 3 months vs. 11% of early non‐responders. At 4 weeks, self‐monitoring frequency (weight/diet) was significantly greater among early responders, which continued across 12 weeks.
Conclusion
Responding early to an mHealth treatment is associated with higher engagement and greater likelihood of achieving clinically meaningful weight loss.
Keywords: lifestyle intervention, mHealth, obesity treatment, weight loss
Introduction
Behavioural weight loss treatment delivered via mobile technology is efficacious 1, 2, with roughly 25–44% of participants achieving clinically significant weight loss of 5% in interventions that are standalone (i.e. those without a counselling component) 3, 4, 5. Early weight loss is a robust predictor of clinically meaningful weight loss 6, 7, 8 and has been used as a trigger in stepped‐care behavioural interventions to implement rescue efforts for individuals not responding early to obesity treatment 6, 9, 10, 11. A 12‐week internet‐based weight loss study found a strong positive relation of early weight loss at 1 month predicting weight loss at 3, 6 and 12 months 12. However, no studies of mobile health (‘mHealth’) interventions – i.e. behavioural treatments delivered via modalities such as smartphone applications (‘apps’) or text messaging – have examined the ability of early weight loss success to predict longer‐term weight loss. Given the increasing evidence base for obesity treatment delivered via mHealth strategies 1, 2, research is needed that examines whether early weight loss success in mHealth interventions predicts future weight loss and intervention engagement.
Standalone mHealth interventions offer a unique treatment experience as their high portability enables self‐monitoring to occur at the time of actual food and beverage consumption, which can prompt real‐time tailored feedback, and they have high dissemination potential given that 77% of US adults own a smartphone 13. Further, mHealth interventions have unparalleled reach, providing treatment to those who are currently without care. The predictive utility of early weight loss is especially important in mHealth interventions: patients who successfully lose weight in these remotely delivered interventions can do so with lower healthcare costs, while those who need additional support can quickly be diverted to higher levels of care.
The GoalTracker trial compared self‐monitoring strategies for weight loss in a 12‐week commercial app‐based intervention that was remotely delivered 14. In intent‐to‐treat analyses, there were no differences between treatment arms in weight change at 1 (range: −0.80 to −1.76 kg), 3 (range: −2.43 to −2.75 kg) or 6 months (range: −1.88 to −3.05 kg).
The current study examines early weight loss and its predictive ability in a mobile app‐based intervention that emphasized self‐monitoring for weight loss (the GoalTracker trial). Hypotheses included that early weight loss (at 1 month) would predict greater weight loss at 3 and 6 months, as well as greater intervention engagement.
Methods
GoalTracker was a randomized controlled trial designed to promote weight loss via a 12‐week standalone intervention delivered via a smartphone app 14. Devised from self‐regulation theories, all three treatment arms received the following core intervention components: MyFitnessPal (a free commercial app) for self‐monitoring, a goal to lose 5% of initial weight by 12 weeks, a tailored daily calorie goal and weekly weight loss goal, and in‐app daily push reminders that would be automatically sent if tracking had not occurred by the evening. These reminders were programmed by the study staff in the MyFitnessPal app during the baseline visit.
Procedures
Participants (N = 105) were randomized equally to one of three treatment arms: (i) a Simultaneous self‐monitoring arm in which participants simultaneously tracked body weight and dietary intake daily and received lessons on nutrition/behaviour modification, corresponding action plans, visual tips on using the app and tailored feedback on goal progress – all sent weekly via email; (ii) a Sequential self‐monitoring arm that included all of the same components but that delayed diet tracking until week 5 in order to promote mastery and self‐efficacy (rooted in Carver's control theory 15 and Bandura's social cognitive theory 16); or (iii) an App‐Only arm in which participants tracked diet daily but did not receive additional behaviour change techniques. The action plans were accessed via a Qualtrics survey link and guided participants in identifying current behaviours related to that week's lesson (e.g. reducing sugar and managing emotional eating), assessing reasons for making changes and confidence levels, detailing the specifics of making a change and brainstorming potential barriers and ways to avoid or handle them; principles of motivational interviewing 17 and problem solving 18 were used.
In‐person evaluation visits occurred at baseline, 1 month and 3 months, and self‐reported weight was collected via email or text message at 6 months. Recruitment occurred between April and September 2017 in central North Carolina, and data collection ended in March 2018. No intervention content was delivered between 3 and 6 months. In order to easily access participants' MyFitnessPal self‐monitoring data, a Duke‐developed software engine (Prompt) was used. Study staff created both MyFitnessPal and Fitbit accounts for all participants at the baseline visit and linked these accounts. Fitbit is a free, commercially available platform for self‐monitoring. Prompt used Fitbit's application programming interface to retrieve the MyFitnessPal data. Participants were not given a Fitbit device nor were they instructed to use this account. Duke University Institutional Review Board approved the study procedures.
Participants
Five participants became ineligible during the 12‐week intervention due to pregnancy (n = 3), cancer diagnosis (n = 1) or previously undisclosed eating disorder (n = 1). Among the remaining eligible participants, 84 had a 1‐month weight (28 per arm) and were included in this secondary analysis – an approach used previously 12. One additional participant became ineligible between 3 and 6 months due to pregnancy and was excluded from the 6‐month analyses. Of those, 73 and 71 participants provided weight data at 3 and 6 months, respectively. Eligibility included ages 21–65 years, body mass index 25.0–45.0 kg/m2, interest in losing weight through dietary changes, iPhone or Android smartphone ownership, willingness to download a mobile app on their phone and not track diet or body weight using any other modality (e.g. other health apps, websites and paper diaries) for the duration of the intervention, access to a bathroom scale, English fluency, no current enrolment in another weight loss intervention, no use of the MyFitnessPal app in the past 6 months, no use of a weight loss medication or weight loss ≥10 lb in the past 6 months and no medical or psychiatric contraindications that may necessitate more intensive treatment (e.g. cancer, uncontrolled hypertension, eating disorder, pregnancy or <12 months postpartum).
Measures
All self‐report measures were administered in English via a Qualtrics survey via a desktop computer. At baseline, sociodemographic and clinical characteristics were collected, and health literacy was assessed with the Newest Vital Sign, which depicts a nutrition label. Scores on this measure range from 0 to 6 with limited health literacy defined as scores of 0–3 while adequate health literacy defined as scores of 4–6 19, 20. Also assessed was whether the MyFitnessPal app was downloaded on the phone prior to the study.
Anthropometric data
At baseline, 1 month and 3 months (our primary outcome), study staff measured participants' body weight using a calibrated electronic scale (SECA 876); shoes and heavy clothing were removed. At 6 months, study staff collected self‐reported body weight, asking participants to send a photo with their feet on the scale. Height was measured at baseline to the nearest 0.1 cm using a calibrated, wall‐mounted stadiometer (SECA 222). Also assessed was the proportion of participants at 3 and 6 months who achieved weight loss of ≥3% 21 and ≥5%, 22 thresholds used to define clinically meaningful weight loss.
Intervention engagement
Objective self‐monitoring data from MyFitnessPal were collected using Fitbit's application programming interface, retrieved via the Prompt software engine. Data were separated into the percentage of days that self‐monitoring entries were recorded during weeks 1–4, weeks 5–12 and the entire 12‐week intervention. Diet entries were considered complete if they contained ≥800 kcal day−1 23. Through objective online survey data, the percentage of action plans completed during the intervention was assessed, ranging from 0% to 100% completion (the latter of which indicates 11 of 11 action plans completed).
Statistical analysis
Because weight change did not differ between treatment arms at 1 or 3 months in the parent study 14, data were collapsed across arms in the current analysis. Consistent with previous behavioural weight loss research 6, 12, participants were categorized into early responders (i.e. ≥2% weight loss at 1 month) and early non‐responders (i.e. <2% weight loss at 1 month). Chi‐squared tests and analysis of variance were used to examine differences in baseline characteristics by early weight loss status. Fisher's exact tests were used with small cell counts.
Linear regression and Pearson correlation were used to examine the association between per cent weight loss at 1 month and either 3 or 6 months, controlling for baseline weight. Six‐month self‐reported weight values were adjusted; for weight values sent via photo, 0.172 kg (0.4 lb) was subtracted to account for participants holding a device on the scale to take the photo. To account for underestimation of self‐reported weights when no photo was sent, a regression model from Jain 24 was used to adjust for age, gender and race/ethnicity.
Linear mixed models with random intercepts and slopes and restricted maximum likelihood estimates were used to examine changes in per cent weight over time, by early weight loss status. Logistic regression with firth correction was used to examine the odds of achieving clinically significant weight loss (i.e. ≥3% or ≥5%) at 3 or 6 months by early weight loss status.
Given non‐normal distributions of intervention engagement data, medians and interquartile ranges were reported, and the Wilcoxon Mann–Whitney U‐test was used to examine differences by early weight loss status. For each time interval, only participants who were instructed to self‐monitor an item during that period were included; for instance, App‐Only participants were excluded from weight tracking data analyses because they were never instructed to track weight. p < 0.05 was considered statistically significant. Analyses were conducted using sas 9.4 (SAS Institute, Cary, NC).
Results
The 84 participants in the current analysis were predominantly female (81%) and college educated (85%), with a mean (SD) age of 43.7 years (11.6). A majority of the sample had obesity (58%). Roughly one‐third (36%; n = 30) of participants were categorized as early responders (i.e. achieving ≥2% weight loss at 1 month), while 64% (n = 54) were early non‐responders. Baseline characteristics did not differ by early weight loss status (Table 1).
Table 1.
Baseline characteristics by 1‐month weight loss
| Total (N = 84) | Early responders ≥ 2% at 1 month (n = 30) | Early non‐responders < 2% at 1 month (n = 54) | P value | |
|---|---|---|---|---|
| Age, mean (SD), years | 43.7 (11.6) | 43.0 (12.3) | 44.1 (11.4) | 0.66 |
| Gender, no. (%) | 0.78 | |||
| Male | 16 (19.1) | 5 (16.7) | 11 (20.4) | |
| Female | 68 (81.0) | 25 (83.3) | 43 (79.6) | |
| Marital status, no. (%) | 0.42 | |||
| Married or living with partner | 57 (67.9) | 22 (73.3) | 35 (64.8) | |
| Not married or living with partner | 27 (32.1) | 8 (26.7) | 19 (35.2) | |
| Race/ethnicity, no. (%) | 0.46 | |||
| Non‐Hispanic White | 60 (71.4) | 22 (73.3) | 38 (70.4) | |
| Non‐Hispanic Black | 16 (19.1) | 4 (13.3) | 12 (22.2) | |
| Hispanic (all races) | 1 (1.2) | 0 (0.0) | 1 (1.9) | |
| Non‐Hispanic other | 7 (8.3) | 4 (13.3) | 3 (5.6) | |
| Education, no. (%) | 0.40 | |||
| Less than college graduate | 13 (15.5) | 6 (20.0) | 7 (13.0) | |
| College graduate or above | 71 (84.5) | 24 (80.0) | 47 (87.0) | |
| Employment status, no. (%) | 0.07 | |||
| Employed, full time | 55 (65.5) | 15 (50.0) | 40 (74.1) | |
| Employed, part time | 11 (13.1) | 6 (20.0) | 5 (9.3) | |
| Not employed | 18 (21.4) | 9 (30.0) | 9 (16.7) | |
| Annual household income, no. (%) | 0.32 | |||
| $0–$49,999 | 19 (23.5) | 7 (24.1) | 12 (23.1) | |
| $50,000–$99,999 | 30 (37.0) | 7 (24.1) | 23 (44.2) | |
| $100,000 or greater | 32 (39.5) | 15 (51.7) | 17 (32.7) | |
| Weight, mean (SD), kg | 89.8 (16.8) | 88.4 (14.5) | 90.5 (18.0) | 0.58 |
| BMI, mean (SD), kg/m2 | 31.7 (4.5) | 31.7 (4.3) | 31.8 (4.7) | 0.94 |
| BMI category, no. (%) | 0.50 | |||
| Overweight, 25–29.9 kg/m2 | 35 (41.7) | 13 (43.3) | 22 (40.7) | |
| Class I obesity, 30–34.9 kg/m2 | 32 (38.1) | 11 (36.7) | 21 (38.9) | |
| Class II obesity, 35–39.9 kg/m2 | 13 (15.5) | 6 (20.0) | 7 (13.0) | |
| Class III obesity, 40+ kg/m2 | 4 (4.8) | 0 (0.0) | 4 (7.4) | |
| Prediabetes, no. (%) | 7 (8.3) | 4 (13.3) | 3 (5.6) | 0.24 |
| Limited health literacy, no. (%)† | 4 (4.8) | 1 (3.3) | 3 (5.6) | 1.00 |
| MyFitnessPal app already on phone prior to study, no. (%) | 17 (20.2) | 4 (13.3) | 13 (24.1) | 0.27 |
Limited health literacy, measured at baseline using the 6‐item Newest Vital Sign, was defined as scores of 0–3, while adequate health literacy was defined as scores of 4–6.19
BMI, body mass index; SD, standard deviation.
Weight change
Per cent weight change at 1 month was positively associated with per cent weight change at 3 (r = 0.77) and 6 months (r = 0.51), with 58% and 25% of the variance accounted for by 1‐month per cent weight change, respectively. For every 1% weight loss at 1 month, there was a 1.50% and 1.38% increase in weight loss at 3 and 6 months, respectively.
Early responders had significantly greater per cent weight loss at 1, 3 and 6 months, compared with early non‐responders (ps < 0.0001; Table 2). The odds of achieving ≥3% and ≥5% weight loss at 3 months were 21.86 (95% confidence interval [CI]: 5.83, 81.99) and 9.72 (95% CI: 3.02, 31.34) times higher, respectively, among early responders, compared with early non‐responders. Similarly, at 6 months, the odds of achieving clinically significant weight loss were significantly greater among early responders than early non‐responders (for ≥3% weight loss: odds ratio, 6.26; 95% CI: 2.14, 18.28; for ≥5% weight loss: odds ratio, 6.93; 95% CI: 2.33, 20.62).
Table 2.
Outcomes of early responders vs. early non‐responders
| N | Early responders ≥ 2% at 1 month (n = 30) | N | Early non‐responders < 2% at 1 month (n = 54) | P value of between‐group difference | |
|---|---|---|---|---|---|
| % weight change from baseline, mean (95% CI), kg | |||||
| 1 month | 30 | −3.37 (−3.88%, −2.85%) | 54 | −0.43 (−0.81%, −0.04%) | <0.0001 |
| 3 months | 28 | −5.93 (−6.82%, −5.03%) | 45 | −1.45 (−2.15%, −0.75%) | <0.0001 |
| 6 months | 26 | −5.91 (−7.33%, −4.48%) | 45 | −1.28 (−2.37%, −0.19%) | <0.0001 |
| Intervention engagement, median [IQR] | |||||
| % action plans completed† | 17 | 72.7 [27.3] | 39 | 45.5 [72.7] | 0.02 |
| % of days tracked weight† | |||||
| Weeks 1–4 | 17 | 96.4 [10.7] | 39 | 85.7 [42.9] | 0.0018 |
| Weeks 5–12 | 17 | 92.7 [29.1] | 39 | 67.3 [76.4] | 0.0063 |
| Weeks 1–12 | 17 | 90.4 [19.3] | 39 | 73.5 [57.8] | 0.0027 |
| % of days tracked diet | |||||
| Weeks 1–4‡ | 24 | 100.0 [5.4] | 32 | 78.6 [71.4] | 0.0006 |
| Weeks 5–12 | 30 | 73.6 [50.9] | 54 | 27.3 [78.2] | 0.0027 |
| Weeks 1–12§ | 24 | 82.5 [34.3] | 32 | 46.4 [66.9] | 0.008 |
Among participants in the Simultaneous and Sequential arms only (n = 56), because App‐Only arm was not asked to track body weight or complete action plans.
Among participants in the Simultaneous and App‐Only arms only (n = 56), because Sequential arm was not asked to track dietary intake during this period.
Among participants in the Simultaneous and App‐Only arms only (n = 56), because Sequential arm was not asked to track dietary intake during the entire period.
CI, confidence interval; IQR, interquartile range.
Most (89.3%) early responders went on to achieve ≥3% weight loss at 3 months, compared with 24.4% of early non‐responders. In terms of ≥5% weight loss at 3 months, 57.1% of early responders and 11.1% of early non‐responders achieved this threshold. Similar patterns were observed at 6 months (see Figure 1).
Figure 1.
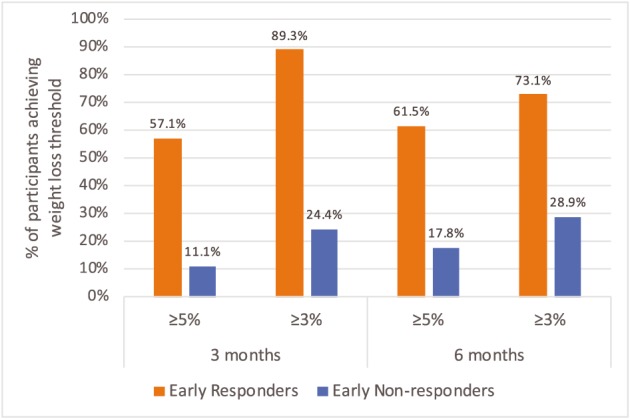
Proportion of participants achieving clinically significant weight loss, by early weight loss status
Intervention engagement
As shown in Table 2, early responders completed more action plans than did early non‐responders (p = 0.02) and self‐monitored body weight and dietary intake more frequently at all time intervals (ps < 0.01). For instance, over the course of the 12‐week intervention, early responders tracked weight 90% of days, while early non‐responders tracked it 74% of days.
Discussion
In a standalone app‐based intervention focused on self‐monitoring, over half of early responders met their goal of losing 5% of initial weight by 3 months and maintained this clinically significant weight loss at 6 months. In contrast, over 8 in 10 participants who did not respond early to treatment were unsuccessful in achieving 5% weight loss at 3 or 6 months. These findings illustrate the importance of early weight loss, which is consistent with past studies of behavioural interventions for weight loss 6, 7, 8. The GoalTracker trial is the first study to demonstrate this relation in the context of a standalone mHealth intervention, which holds promise as a less resource‐intensive and more scalable initial intervention strategy in stepped‐care approaches.
No gold standard exists for when to intervene with non‐responders in behavioural weight loss interventions. While later time points of assessing early weight loss (e.g. in months 2 and 3) are also predictive of overall weight loss 7, 25, past trials have found that intervening with non‐responders at 3 months post‐randomization does not translate to improved outcomes 9, 26, perhaps reflecting that waiting longer to intervene may be ineffective among those who feel discouraged about their poor early treatment response. Our findings support previous research that suggests 1 month may be an advantageous point at which to intervene 12 and that engagement likely drives early weight loss success 12, 25, 27. Future studies are needed that experimentally test the impact of intervening with non‐responders at this early time point.
The results of the present study suggest that practitioners consider the following strategies to optimize early weight loss for their patients who are interested in a standalone intervention: (i) discuss realistic expectations for weight loss in a standalone mHealth intervention, (ii) set both an overall weight loss goal (e.g. 3% or 5% weight loss by 3 months) and a weekly weight loss goal (e.g. 0.5–2 lb week−1), (iii) decide whether to track diet, body weight or both in the initial month and commit to tracking daily, (iv) encourage patients to set reminders, such as automated in‐app push reminders, in order to prompt oneself to track each day, (v) check weight loss progress at the 1‐month mark and (vi) consider altering treatment if less than 2% weight loss was achieved by this point, discussing together other solutions (e.g. refer to a dietitian, use pre‐portioned meals, adhere to a ketogenic or DASH diet, join an in‐person weight loss group or meet with a psychologist to treat maladaptive eating behaviours), if needed. Given that the difference between responding and not responding to treatment in the first month was just a few extra days of self‐monitoring, clinicians should emphasize that daily tracking in the first month will likely prove meaningful and maintain accountability.
Strengths of our study include the collection of objective self‐monitoring data from a commercial mobile app and objective action plan completion data. A limitation of the data is that the smaller sample size yielded wide CIs in the odds of achieving 3% or 5% weight loss by early response status; future studies with larger sample sizes and longer follow‐up should replicate these analyses. Further, because of logistical constraints, in‐person weights at 6 months were unable to be collected, and instead, self‐report was used; relying on self‐report may underestimate weight values. Nevertheless, the response rate at 6 months was consistent with that at 3 months despite no intervention contact during this period, indicating that this remote weight collection method holds promise for use in research with real‐world dissemination potential as it reduces time and effort demands for both participants and staff.
Conclusion
Overall, early weight loss success in a standalone smartphone app‐based intervention predicted clinically significant weight loss and intervention engagement among adults with overweight or obesity. Interventionists and clinicians should discuss upfront with patients the importance of early weight loss in mHealth interventions and emphasize the utility of high levels of engagement (e.g. self‐monitoring daily or almost daily). In clinical settings, measuring patients' early weight loss response would allow providers and patients to make informed decisions together about whether to continue, stop or alter treatment.
Conflicts of Interest Statement
G. G. B. serves on the Scientific Advisory Board of Nutrisystem and Interactive Health and holds equity in Coeus Health. The remaining authors declare no competing interests.
Funding
The research described in this paper was supported by grants to the first author from the American Psychological Association, the Duke Interdisciplinary Behavioral Research Center and the Aleane Webb Dissertation Research Award provided by The Graduate School at Duke University.
Acknowledgements
M. L. P. and G. G. B. conceived and designed the study. M. L. P. and C. M. H. delivered the intervention and collected data. M. L. P. conducted statistical analyses and drafted the manuscript. All authors were involved in writing the paper and had final approval of the submitted and published versions. M. L. P. was affiliated with Duke University at the time of the trial and is currently affiliated with the Stanford University School of Medicine. We sincerely thank all who participated in GoalTracker.
Patel M. L., Hopkins C. M., and Bennett G. G. (2019) Early weight loss in a standalone mHealth intervention predicting treatment success, Obesity Science & Practice. 5, 231–237, 10.1002/osp4.329.
References
- 1. Semper H, Povey R, Clark‐Carter D. A systematic review of the effectiveness of smartphone applications that encourage dietary self‐regulatory strategies for weight loss in overweight and obese adults. Obes Rev 2016; 17: 895–906. [DOI] [PubMed] [Google Scholar]
- 2. Schippers M, Adam P, Smolenski D, Wong H, Wit J. A meta‐analysis of overall effects of weight loss interventions delivered via mobile phones and effect size differences according to delivery mode, personal contact, and intervention intensity and duration. Obes Rev 2017; 18: 450–459. [DOI] [PubMed] [Google Scholar]
- 3. Burke LE, Zheng Y, Ma Q, et al. The SMARTER pilot study: testing feasibility of real‐time feedback for dietary self‐monitoring. Prev Med Rep 2017; 6: 278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Svetkey LP, Batch BC, Lin P‐H, et al. Cell phone intervention for you (CITY): a randomized, controlled trial of behavioral weight loss intervention for young adults using mobile technology. Obesity (Silver Spring) 2015; 23: 2133–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Turner‐McGrievy GM, Wilcox S, Boutté A, et al. The Dietary Intervention to Enhance Tracking with Mobile Devices (DIET Mobile) study: a 6‐month randomized weight loss trial. Obesity (Silver Spring) 2017; 25: 1336–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Unick JL, Pellegrini CA, Demos KE, Dorfman L. Initial weight loss response as an indicator for providing early rescue efforts to improve long‐term treatment outcomes. Curr Diab Rep 2017; 17: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barnes RD, Ivezaj V, Pittman BP, Grilo CM. Early weight loss predicts weight loss treatment response regardless of binge‐eating disorder status and pretreatment weight change. Int J Eat Disord 2018; 51: 558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. James B, Roe L, Loken E, Rolls B. Early predictors of weight loss in a 1‐year behavioural weight‐loss programme. Obes Sci Pract 2018; 4: 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jakicic JM, Tate DF, Lang W, et al. Effect of a stepped‐care intervention approach on weight loss in adults: a randomized clinical trial. JAMA 2012; 307: 2617–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carels RA, Selensky JC, Rossi J, Solar C, Hlavka R. A novel stepped‐care approach to weight loss: the role of self‐monitoring and health literacy in treatment outcomes. Eat Behav 2017; 26: 76–82. [DOI] [PubMed] [Google Scholar]
- 11. Sherwood NE, Butryn ML, Forman EM, et al. The BestFIT trial: a SMART approach to developing individualized weight loss treatments. Contemp Clin Trials 2016; 47: 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Unick JL, Leahey T, Kent K, Wing RR. Examination of whether early weight loss predicts 1‐year weight loss among those enrolled in an Internet‐based weight loss program. Int J Obes (Lond) 2015; 39: 1558–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mobile Fact Sheet (2018). Pew Research Center: internet & technology. [WWW document]. URL http://www.pewinternet.org/fact‐sheet/mobile/.
- 14. Patel ML, Hopkins CM, Brooks TL, Bennett GG. Comparing self‐monitoring strategies for weight loss in a smartphone app: A randomized controlled trial. JMIR mHealth and uHealth 2019; 10.2196/12209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carver CS, Scheier MF. Control theory: a useful conceptual framework for personality–social, clinical, and health psychology. Psychol Bull 1982; 92: 111–135. [PubMed] [Google Scholar]
- 16. Bandura A. Health promotion from the perspective of social cognitive theory. Psychol Health 1998; 13: 623–649. [Google Scholar]
- 17. Armstrong M, Mottershead T, Ronksley P, Sigal R, Campbell T, Hemmelgarn B. Motivational interviewing to improve weight loss in overweight and/or obese patients: a systematic review and meta‐analysis of randomized controlled trials. Obes Rev 2011; 12: 709–723. [DOI] [PubMed] [Google Scholar]
- 18. Perri MG, Nezu AM, McKelvey WF, Shermer RL, Renjilian DA, Viegener BJ. Relapse prevention training and problem‐solving therapy in the long‐term management of obesity. J Consult Clin Psychol 2001; 69: 722–726. [PubMed] [Google Scholar]
- 19. Pfizer . (2011). The Newest Vital Sign: a health literacy assessment tool. [WWW document]. URL https://www.pfizer.com/sites/default/files/health/nvs_flipbook_english_final.pdf
- 20. Weiss BD, Mays MZ, Martz W, et al. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med 2005; 3: 514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011; 34: 1481–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. JACC 2014; 63: 2985–3023. [DOI] [PubMed] [Google Scholar]
- 23. Wharton CM, Johnston CS, Cunningham BK, Sterner D. Dietary self‐monitoring, but not dietary quality, improves with use of smartphone app technology in an 8‐week weight loss trial. J Nutr Educ Behav 2014; 46: 440–444. [DOI] [PubMed] [Google Scholar]
- 24. Jain R. Regression models to predict corrected weight, height and obesity prevalence from self‐reported data: data from BRFSS 1999–2007. Int J Obes (Lond) 2010; 34: 1655–1664. [DOI] [PubMed] [Google Scholar]
- 25. Unick JL, Neiberg RH, Hogan PE, et al. Weight change in the first 2 months of a lifestyle intervention predicts weight changes 8 years later. Obesity (Silver Spring) 2015; 23: 1353–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carels RA, Young KM, Coit CB, et al. The failure of therapist assistance and stepped‐care to improve weight loss outcomes. Obesity (Silver Spring) 2008; 16: 1460–1462. [DOI] [PubMed] [Google Scholar]
- 27. Nackers LM, Ross KM, Perri MG. The association between rate of initial weight loss and long‐term success in obesity treatment: does slow and steady win the race? Int J Behav Med 2010; 17: 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]


