Summary
Tumour infiltration by regulatory T (Treg) cells contributes to suppression of the anti‐tumour immune response, which limits the efficacy of immune‐mediated cancer therapies. The phosphoinositide 3‐kinase (PI3K) pathway has key roles in mediating the function of many immune cell subsets, including Treg cells. Treg function is context‐dependent and depends on input from different cell surface receptors, many of which can activate the PI3K pathway. In this review, we explore how PI3Kδ contributes to signalling through several major immune cell receptors, including the T‐cell receptor and co‐stimulatory receptors such as CD28 and ICOS, but is antagonized by the immune checkpoint receptors CTLA‐4 and PD‐1. Understanding how PI3Kδ inhibition affects Treg signalling events will help to inform how best to use PI3Kδ inhibitors in clinical cancer treatment.
Keywords: signal transduction, regulatory T cell, T cell, tumour immunology
Introduction
CD4+ Foxp3+ regulatory T (Treg) cells can act as a barrier to the effective implementation of cancer therapy.1, 2 Initially identified as a CD25hi T‐cell subset with immunosuppressive function, Treg cells assume critical roles in the prevention of autoimmunity.3 Among the numerous immune cell subsets now known to form the immunosuppressive barrier within the tumour microenvironment, including macrophages and myeloid‐derived suppressor cells, Treg cells remain the most intensely studied.4, 5, 6, 7, 8 The therapeutic targeting of tumour Treg cells to enhance anti‐tumour immunity is therefore an active area of research.7, 8 The lack of a truly unique surface marker that identifies Treg cells, and the fundamental requirement of Treg cells to prevent lethal autoimmunity, makes the strategy of Treg cell depletion challenging. However, recent advancements using antibodies against CD25 or CCR4, a chemokine receptor that is strongly up‐regulated on tumour Treg cells, are showing promise.9, 10
Alongside the developments in antibody therapies, modulation of cell signalling pathways through small‐molecule inhibitors has also gained ground within the immunotherapy field. The functional profiles of immune cells are necessarily shaped in response to environmental cues, which are conveyed to the cellular machinery through a myriad of distinct but overlapping signalling cascades. Many of these pathways are driven by post‐translational phosphorylation of downstream targets by kinases, several of which have been targeted with some success in oncology.11 In particular, the phosphoinositide‐3‐kinase (PI3K) pathway has a uniquely extensive role in transducing signals from key cell surface receptors in the immune system, influencing many important aspects of the immune response.12 Inhibition of the PI3K pathway represents a promising approach to the therapeutic manipulation of Treg cell function. Here, we explore the role of the PI3K pathway in Treg cell biology, particularly in the context of tumour immunosuppression.
A regulatory T‐cell subset
Evidence of immune‐suppressive T cells, then proposed as ‘T suppressor’ cells, first emerged half‐century ago,13, 14 but detailed study of a defined T‐cell subset was only made possible when, 25 years later, Sakaguchi et al.3 described a CD4+ CD25+ population, with a critical role in maintaining immune homeostasis and preventing autoimmunity. The identification of the forkhead box P3 (Foxp3) transcription factor as a master regulator of the Treg cell lineage15, 16 revealed a unique transcriptional programme driving a suppressive profile. Loss‐of‐function mutations in the FOXP3 gene result in lethal multi‐organ autoimmunity, known as immunodysregulation polyendocrinopathy enteropathy X‐linked (IPEX) syndrome in humans,17 and as scurfy in mice.18 A plethora of studies since their initial discovery have shown that Treg cells play prominent roles in preventing pathogenic autoimmunity,19 and in controlling rejection and graft‐versus‐host‐disease in transplant recipients,20, 21 but can contribute to the persistence of infections.22
In addition to Treg cells originating in the thymus, naive conventional CD4 T cells can also be ‘induced’ by environmental cues in the periphery, most prominently transforming growth factor‐β, to express Foxp3 and take on a suppressive phenotype.23, 24 Both thymic Treg cells and peripherally‐derived Treg cells employ a broad range of suppressive mechanisms; their application and relative importance appear to depend heavily on the specific physiological context.25
Treg cells in the immunosuppressive tumour environment
The proportion of Treg cells within the tumour immune infiltrate often far outstrips homeostatic proportions in circulation – although 5–10% of CD4+ T cells in the spleen or lymph nodes are Foxp3+, in many types of tumour this can be up to 30–50%.26 In certain types of tumour, most notably colorectal cancers, it has been argued that a large Treg cell infiltrate is beneficial and indicates a favourable prognosis, due to its role in dampening the inflammation driving oncogenic progression.27, 28 In other cancers, however, increased Treg cell infiltration correlates with a poor prognosis,29, 30 and therapies that reduce Treg cell numbers in the tumour have produced positive results.31, 32
Accumulation of Treg cells is a crucial part of immune evasion by the tumour where cytokines and chemokines, produced by both tumour cells and stromal cells, contribute to Treg cell recruitment, in situ proliferation, and conversion from conventional CD4+ T cells.33 Treg‐mediated immunosuppression has been held accountable for the reduced anti‐tumour functionality of CD8+ and CD4+ conventional T cells in the tumour.34, 35 Tumour Treg cells have also been implicated in the recruitment of myeloid‐derived suppressor cells, playing an accessory role in the formation of the tumour immunosuppressive environment.36
The first attempts to treat cancer by depleting Treg cells targeted the interleukin‐2 (IL‐2) receptor, which is highly expressed by Treg cells. Denileukin diftitox, a fusion molecule combining IL‐2 and diphtheria toxin originally designed for the treatment of cutaneous T‐cell leukaemia, was demonstrated to deplete Treg cells and enhance anti‐tumour immunity in mouse B16 melanoma,37 but failed to provide clinical benefit in human ovarian or breast cancers.38, 39 A depleting antibody against the IL‐2 receptor α subunit, also known as CD25, can mediate tumour rejection in mice when administered before or shortly after tumour implantation, but is not effective against established tumours, possibly because highly activated conventional T cells required for tumour elimination also up‐regulate CD25.40 This approach may still prove effective, however; anti‐CD25 with an engineered antibody Fc region to mediate enhanced antibody‐dependent cell‐mediated cytotoxicity by tumour‐resident macrophages can combine with anti‐programmed death‐1 (anti‐PD‐1) to effectively eliminate tumours in mice.9
Beyond CD25, Treg‐selective cell surface markers amenable to antibody targeting are highly sought after, but have remained largely elusive – most markers are also shared with activated T effector cells. Meanwhile, a greater understanding of the phenotypic profile of activated, highly suppressive effector Treg cells – describing the great majority of Treg cells within the tumour – is forming the basis for alternative approaches to Treg cell modulation. Although genetic aberrations that disrupt the development or maintenance of the homeostatic ‘resting’ Treg cell pool can result in catastrophic autoimmunity, effector Treg cells depend on a partially distinct set of transcriptional, metabolic and signalling conditions to maintain high functionality in specific contexts, such as the tumour microenvironment.41, 42 Tumour‐infiltrating Treg cells adapt to an environment characterized by a myriad of cytokines and chemokines, low oxygen availability, and high glucose demand, among other factors.43, 44 Nuclear factor‐κB activation through the tumour necrosis factor receptor super family has been shown to have special significance in the effector Treg cell population,45 as has the promotion of glycolysis through the adenosine‐monophosphate‐activated protein kinase.46 Pharmacological manipulation of these cellular pathways therefore holds promise for the therapeutic targeting of tumour Treg cells.
PI3K in the immune system
One cellular signalling pathway that has become increasingly prominent as a pharmaceutical target is the PI3K pathway. One of the master signalling pathways with critical roles in all mammalian cells, PI3K signalling has been shown to be involved in processes including cell survival, proliferation, differentiation and mobility.47
Class I PI3Ks are activated by the recruitment of the catalytic p110 subunit to the plasma membrane, through the binding of a regulatory subunit (p85 in the case of the class 1A PI3Ks: p110α, β or δ; p55 or p101 for the class 1B PI3K: p110γ) to a phosphorylated tyrosine on the intracellular domain of a cell surface receptor.12, 47 On the inner surface of the cell membrane, the p110 subunit phosphorylates phosphatidylinositol‐4,5‐bisphosphate (PIP2) to generate phosphatidylinositol‐3,4,5‐trisphosphate (PIP3), a second messenger molecule propagating the signal to downstream effectors.48 The phosphorylation cascade of the PI3K pathway is regulated at several points by phosphatases, the best‐known of which is the phosphatase and tensin homologue conversion of PIP3 back into PIP2.49 PIP3 recruits phosphoinositide‐dependent kinase 1 (PDK1) and protein kinase B/AKT, the latter of which is phosphorylated and activated by the former.50 Both PDK1 and AKT phosphorylate and activate multiple downstream targets, among which the activation of mammalian target of rapamycin is known to be a central modulator of many transcriptional and metabolic processes.51
In contrast to non‐immune cells, which dominantly express the p110α or p110β catalytic isoform, immune cells largely depend on the p110δ or p110γ isoforms for PI3K signal transduction.12 Many principle receptors in lymphocyte biology, e.g. the T‐cell receptor (TCR), the B‐cell receptor, the IL‐2 receptor and various co‐stimulatory receptors, activate p110δ (hereafter PI3Kδ) upon ligand binding, making it an integral component in mounting a coherent immune response to extracellular cues.12 PI3Kγ is more predominant in myeloid cells and can also play a key role in tumour immune suppression.52, 53, 54
Dysregulation of PI3Kδ signalling leads to altered lymphocyte development and function.12 Mice with a knock‐in kinase‐inactivating D910A point mutation in p110δ (PI3Kδ D910A) have a profound defect in B‐cell development and function.55 Remarkably, constitutive activation of PI3Kδ also leads to B‐cell dysfunction, resulting in symptoms of immunodeficiency such as recurrent respiratory infections.56 PI3Kδ D910A mice show a reduced CD8+ T‐cell response to pathogenic Listeria monocytogenes infection, but improved bacterial clearance due to an enhancement of the innate immune response.57 Under specific circumstances, however, the attenuated phenotype of PI3Kδ‐deficient CD8+ T cells paradoxically results in an overall improvement of the desired immune response – in vitro treatment of T cells with inhibitors of the PI3K/Akt pathway has been shown to improve in vivo persistence and anti‐tumour efficacy when transfused into tumour‐bearing mice, by favouring a central‐memory phenotype over terminal differentiation as effectors.58, 59, 60
A requirement for PI3Kδ in Treg‐mediated tumour immunosuppression
Treg cell development has been widely reported to be enhanced under PI3K/AKT pathway inhibition.61, 62, 63 Suppression of the PI3K signal has been shown to be necessary for normal Treg cell differentiation,63 and PI3Kδ D910A mice have increased numbers of Treg cells in the thymus.61 In circulation, however, Treg cells are reduced in PI3Kδ D910A compared with wild‐type,61 and deletion of Foxo1, a transcription factor inhibited by AKT, abrogates normal Treg cell function.64 It may be surmised that optimal Treg cell development and homeostatic maintenance require dynamic regulation of PI3K activity (Fig. 1).
Figure 1.
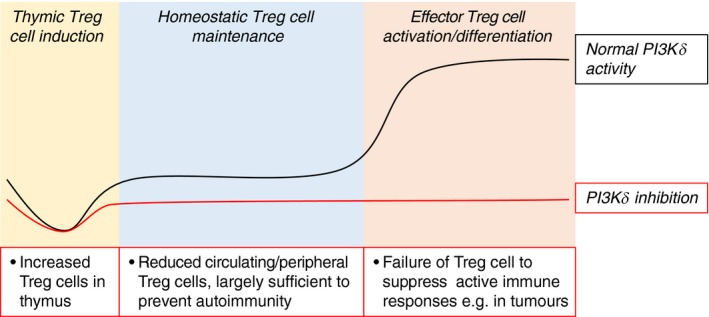
Regulatory T (Treg) cells require different levels of phosphoinositide‐3‐kinase (PI3Kδ) activity during different phases of development and function. An increase in Treg cells in the thymi of mice with inactive PI3Kδ may indicate a required repression of PI3Kδ signalling in early Treg cell development. In the periphery, a basal level of PI3Kδ activity is needed to maintain a homeostatic Treg cell population, such that PI3Kδ inhibition results in reduced circulating Treg cells in the resting state. For Treg cells to differentiate into fully activated effector Treg cells, the PI3Kδ pathway is required to transduce an array of signals (see Fig. 2); loss of PI3Kδ activity can therefore lead to an acute impairment of Treg‐mediated suppression at sites of active inflammation.
PI3Kδ activity is necessary for Treg cell suppressive function. PI3Kδ D910A mice can develop intestinal inflammation, due to a breach in immune tolerance against colon microflora.55, 61 Several patients with genetic loss of PI3Kδ have also been described, who suffer from colon and liver inflammation.65, 66 With the use of PI3Kδ‐specific inhibitor idelalisib in the treatment of chronic lymphocytic leukaemia, colitis and transaminitis have been reported as common adverse effects in patients, indicating immune dysregulation.67 A recent study identified clear defects in human Treg cell activation and suppressive function under PI3Kδ inhibition, both in vitro and in idelalisib‐treated patients.68
Additionally, we and others have shown that PI3Kδ‐inactivated Treg cells are impaired in mediating tumour immunosuppression. Loss of PI3Kδ activity, especially by specific deletion in Treg cells, can restrict the growth of transplanted tumours in mice.69, 70, 71 Whereas PI3Kδ inhibition has been reported to preferentially incapacitate Treg cells over effector T cells in the anti‐tumour immune response,59 a different study has shown that loss of PI3Kδ activity abrogates tumour elimination by CD8+ T cells,72 so the overall effect of PI3Kδ inactivation on tumour growth may depend on a balance of the impact on effector and regulatory T‐cell subsets. We have demonstrated that enhancement of anti‐tumour immunity by PI3Kδ inactivation occurs in spite of concurrent impairment of CD8+ T‐cell cytotoxicity,69 and correlates with the dependence of the tumour on Treg‐mediated immunosuppression.70 Supporting a requirement for PI3Kδ activity in tumour Treg cell function, constitutive nuclear localization of Foxo1 – as would be the case in PI3Kδ inactivation – blocks Treg cell tumour infiltration and boosts anti‐tumour immunity.73
We therefore suggest that Treg cells have varying requirements for PI3Kδ signalling throughout their lifespan, benefiting from reduced activity during development, but depending on the PI3Kδ pathway in delivering immune suppression as mature regulators.74 This bifurcation represents an intriguing therapeutic potential to selectively target highly activated effector Treg cells – for example, in the tumour, or less desirably, in the gut – while leaving the homeostatic pool of resting Treg cells intact.
PI3Kδ downstream of core T‐cell receptors
Treg cells are thought to develop within a narrow window of TCR–MHC binding affinity above the threshold of negative selection, thus possessing a TCR repertoire skewed towards self‐recognition.75 The signal immediately downstream of the TCR is constricted in Treg cells compared with conventional T cells,76 protecting, perhaps, against stimulation‐induced apoptosis – but rendering them especially vulnerable to inhibition of the TCR signal (Tanaka et al., under review).
Stimulation of the TCR acutely activates PI3Kδ.55, 77 Once activated, it is thought that Treg cells are capable of suppressing in a TCR‐independent manner,78 but tumour‐infiltrating Treg cells are enriched for tumour antigen‐specific clones, indicating an influence of antigen‐binding within the tumour.79 The TCR can also play a larger coordinating role beyond direct signalling from its own intracellular domains, as a focal point for the formation of immunological synapses, within which many of the receptors we will go on to discuss receive and transduce their respective signals.80
Alongside the TCR, T cells need a second signal81 through co‐stimulatory receptors to achieve full activation (Fig. 2). A canonical example of such a receptor is CD28, which binds CD80 and CD86 on antigen‐presenting cells. Within Treg cells, CD28 binding is required for the up‐regulation of key functional proteins cytotoxic T lymphocyte antigen‐4 (CTLA‐4), PD‐1 and CCR6, the lack of which reduces effector Treg cell suppressive capacity and abrogates the ability to home to tissues.82 CD28 ligation has been reported to signal through PI3Kδ,83 although in naive T cells CD28 plays a more important role in amplifying the PI3K signal downstream of the TCR.77 However, there is evidence that memory T cells can activate PI3K via CD28 in a TCR‐independent manner to promote migration into tissues.84 CD28‐dependent activation of PI3K was recently shown to regulate glycolysis by increasing the expression of glucokinase. Interestingly, this was not required for Treg‐mediated suppression, but rather for Treg cell migration to non‐lymphoid tissues, such as the skin.85 It will be intriguing to know of CD28‐dependent glucokinase expression also regulates Treg cell migration to tumours. CD28 is also a major activator of the nuclear factor‐κB pathway, which may be more central to its co‐stimulatory function.86, 87
Figure 2.
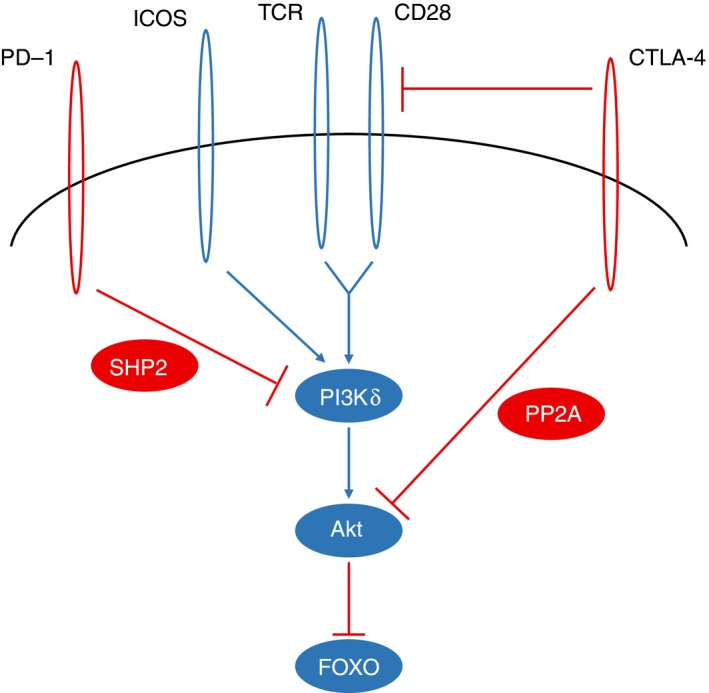
Antigen‐binding, co‐stimulatory and co‐inhibitory receptors shape regulatory T (Treg) cell function through phosphoinositide‐3‐kinase (PI3Kδ) signalling. T‐cell receptor (TCR) stimulation strongly activates PI3Kδ, and binding of CD28 amplifies this signal. ICOS, another co‐stimulatory receptor highly up‐regulated in effetor Treg cells, can independently activate PI3Kδ. Through phosphorylation of PIP2 to produce PIP3, PI3Kδ then activates AKT, which inhibits FOXO, a transcription factor critical to Treg cell function, in part through its regulation of the Foxp3 gene. CTLA‐4, constitutively expressed in Treg cells, is known to attenuate the co‐stimulatory signal by competition with CD28 for its ligands, but can also antagonize AKT activity through activation of the PP2A phosphatase. PD‐1, which is up‐regulated in Treg cells as well as conventional T cells in the tumour, can restrict the T‐cell activation signal through the SHP2 phosphatase, dephosphorylating tyrosine residues required for PI3Kδ recruitment and activation.
By contrast to CD28, the inducible co‐stimulatory (ICOS) signals almost exclusively through PI3Kδ to enhance T‐cell activation and function, and is instrumental in T helper cell differentiation.88, 89 CD4+ T cells, especially, show an almost exclusive dependence on PI3K signalling downstream of ICOS, whereas PI3K‐independent signals could mediate some degree of ICOS co‐stimulation in CD8+ T cells.90, 91 ICOS expression in Treg cells correlates with a highly activated Blimp‐1+ IL‐10+ effector Treg cell population,92, 93 and is thought to promote a Foxp3‐driven transcriptional programme over an effector T‐cell phenotype upon cell activation.94
A third signal critical to the development and function of Treg cells is IL‐2.95, 96, 97 Interleukin‐2 can signal through a receptor composed of the β (CD122) and γ (CD132) subunits, which binds the ligand with moderate affinity; the addition of the α subunit, also known as CD25, forms the high‐affinity receptor for IL‐2.98 With constitutively higher expression of CD25 on Treg cells compared with other T‐cell subsets, the competitive sequestration of IL‐2 from the environment has been proposed as a mechanism of suppression against conventional T cells.99 The IL‐2 receptor primarily activates the Janus kinase–signal transducer and activator of transcription (JAK/STAT) pathways but can also lead to TCR‐dependent PI3K signalling.100
To summarize, there is known PI3Kδ involvement in signalling downstream of the TCR, CD28, ICOS, and possibly the IL‐2 receptor, each of which regulates Treg cell homeostasis and function (Fig. 2). The observation that PI3Kδ‐deficient Treg cells appear phenotypically normal and are only modestly reduced in number suggests that they are functionally incapacitated in a manner that largely manifests itself in loss of immune tolerance to commensal microorganisms and in the context of tumour immunology. The precise Treg‐mediated suppressive mechanism that is impaired by PI3Kδ inhibition remains to be firmly established, as do the receptors that activate PI3Kδ to this end.
PI3Kδ downstream of checkpoint receptors
Checkpoint receptor molecules, such as CTLA‐4 and PD‐1, have gained much publicity as targets of breakthrough cancer immunotherapies, as evidenced by this year's Nobel Prize in Medicine or Physiology awarded to James Allison and Tasuko Honjo. Checkpoint receptors are so named for their role in restricting the cytotoxic or inflammatory functions of effector T cells, and are often up‐regulated in highly activated effector T cells to limit collateral damage to self tissue in the wake of pathogen‐induced immune responses, as well as to prevent autoimmune damage.101 The list of checkpoint receptors continues to grow; this section will focus primarily on the founding members CTLA‐4 (also known as CD152) and PD‐1 (CD279) (Fig. 2).
CTLA‐4 shares its ligands with the co‐stimulatory receptor CD28, binding both CD80 and CD86 with higher affinity than CD28. Its presence on both activated conventional T cells and Treg cells is thought to abrogate the co‐stimulatory signal through competitive binding,102 and has indeed been proposed to remove these ligands from the surface of antigen‐presenting cells through trogocytosis and transendocytosis.103 In contrast with conventional T cells, Treg cells constitutively express CTLA‐4 at a high level;104 whereas the anti‐CTLA‐4 antibody ipilimumab was primarily developed to relieve the ‘checkpoint’‐induced suppression on cytotoxic CD8+ T cells in the tumour,105 it has since been reported that Treg cells remain the cell population expressing the highest levels of CTLA‐4 within the tumour,106 and that the efficacy of ipilimumab treatment can at least partially be attributed to antibody‐dependent cell‐mediated cytotoxicity in the Treg cell population.107
Although there are questions about the capacity of the intracellular domain of the CTLA‐4 receptor to transduce signals108 (and some suggestion that the influence of CTLA‐4 binding is limited to its own cycling between cytosolic vesicles and the cell surface109), it has also been proposed that CTLA‐4 binding can antagonize the activation signal in a cell‐intrinsic manner by attenuating the PI3K pathway, by activating the protein phosphatase 2A (PP2A).110
PD‐1 is up‐regulated in both activated conventional T cells and effector Treg cells – such as those found in the tumour – and binds ligands PD‐L1 and PD‐L2 expressed by a variety of immune and non‐immune cell types.111 PD‐1 binding stimulates SHP2, a phosphatase that antagonizes a range of cell‐activating signals – including PI3K – so exerting an inhibitory effect on T‐cell function.110, 112, 113 Like conventional T cells, Treg cells lacking PD‐1 show enhanced proliferation; however, PD‐1‐deficient Treg cells have reduced expression of Bcl2, and may have reduced viability or stability.114 Intriguingly, PI3Kδ‐deficient tumour‐infiltrating Treg cells – but not CD8+ T cells – express markedly lower levels of PD‐1 than PI3Kδ‐sufficient controls;70 whether a consequent loss of Treg cell stability can explain the anti‐tumour effects of PI3Kδ inactivation remains to be determined.
Mice with inactive PI3Kδ become unresponsive to anti‐CTLA‐4 or anti‐PD‐L1 treatment, even in tumour models that are usually sensitive to checkpoint blockade.70 As both receptors are implicated in reducing PI3Kδ signalling, we hypothesize that loss of PI3Kδ may at least partially nullify the benefits of concurrent checkpoint blockade therapy, which act in part by increasing PI3Kδ signalling in CD8+ T cells. These results do not preclude combination of pharmacological PI3Kδ inhibition and checkpoint blockade therapy, however, as others have reported success with this strategy.115, 116 The discrepancy between studies using a genetic model of PI3Kδ inactivation and those using pharmacological inhibition points to important differences in the underlying mechanism of these approaches, warranting additional investigation to inform clinical use. Intriguingly, an intermittent dosing schedule for PI3Kδ inhibition is reported to yield even better enhancement of anti‐tumour immunity, relieving Treg cell suppression without compromising effector CD8+ T‐cell function,117 raising the attractive possibility that PI3Kδ inhibition can be further refined to improve synergy with other therapies.
PI3Kδ in the recruitment and localization of Treg cells in tumours
Cells rely on chemokine signals for navigational cues, and the tumour exploits a wide range of chemokines to recruit various immune cell subsets.118 The chemokine receptors CCR4 (binding CCL17 and CCL22) and CCR8 (binding CCL1) have both been identified as tumour Treg‐specific markers,10, 119, 120 and are the targets of ongoing efforts to selectively deplete tumour‐infiltrating Treg cells.
The PI3K pathway is involved in several aspects of cell mobility. A major consequence of the constitutive nuclear localization of FOXO1 is the failure of Treg cells to down‐regulate lymphoid homing receptors such as CD62L, hampering tumour infiltration.73 PI3Kδ‐deficient T cells also fail to down‐regulate CD62L and CCR7, well‐established markers of naive T cells important for lymphoid homing, even upon strong stimulation in vitro.121 PI3Kδ inactivation also impairs the activation of LFA‐1 in CD4+ T cells, leading to defects in cell–cell interaction, which could affect the adhesive contacts necessary for tissue infiltration, or for contact‐dependent suppression122 – particularly as LFA‐1 is involved in CTLA‐4 transendocytosis of CD80 and CD86 from antigen‐presenting cells.103
However, a comparison of tumour‐infiltrating Treg cells between wild‐type and PI3Kδ D910A mice does not show a consistent quantitative reduction in PI3Kδ‐deficient mice.69 We have also not observed gross‐level differences in the intratumoural localization of Treg cells with inactivated PI3Kδ, even though a number of chemokine receptors may have lower expression in the absence of PI3Kδ (unpublished observations). Further investigation will be required to determine how prominent this factor is in mediating the effects of PI3Kδ inhibition in tumour Treg cells.
PI3Kδ in cytokine‐driven adaptation in tumour Treg cells
As Foxp3 functions as a master lineage regulator in Treg cells, so too can the T helper type 1 (Th1), Th2 and Th17 subsets of CD4+ T helper cells be identified by their expression of T‐bet, GATA‐3 and RORγt, respectively.123, 124 However, Foxp3+ Treg cells can also up‐regulate the lineage‐regulating transcription factors of T helper subsets in response to appropriate cytokine stimulation,125 and such expression has been recognized as a mark of adaptation in Treg cells, allowing them to co‐localize with their targets and function optimally within the same environmental conditions.124, 126 Follicular regulatory T cells, for example, share with T follicular helper cells the expression of lineage transcription factor Bcl6, allowing residency in the germinal centre through expression of the chemokine receptor CXCR5.127, 128
Tumour‐infiltrating Treg cells have been widely reported to up‐regulate T helper lineage transcription factors, especially T‐bet, and in some cases GATA‐3.129, 130, 131 Both T‐bet‐expressing and GATA‐3‐expressing Treg cells were shown to have enhanced suppression of anti‐tumour CD8+ T cells, without up‐regulating inflammatory cytokines characteristic of their Foxp3− counterparts. Strikingly, Treg cells have been reported to rely on granzyme expression in mediating tumour immunosuppression132, 133 but not in the control of graft‐versus‐host disease,134 and it is plausible that an adaptive Th1‐like transcriptional programme driven by the expression of T‐bet is required for the manifestation of these context‐specific suppressive features.
We have observed that PI3Kδ‐deficient tumour Treg cells have reduced expression of T‐bet at the transcriptional level, and further that addition of a PI3Kδ inhibitor to in vitro Treg cell cultures with interferon‐γ and IL‐12 abrogates the acquisition of T‐bet expression (unpublished observations). Further study will reveal whether, and how, loss of PI3Kδ activity disrupts the tumour‐adapted functional profile of Treg cells, to the enhancement of anti‐tumour immunity.
Summary
Treg cells in the tumour context lose suppressive capacity in the absence of PI3Kδ activity, indicating a central role for PI3Kδ signalling in facilitating tumour Treg cell function. As one of the most pleiotropic signalling cascades in immune cells, the precise sequence of events, leading from stimulus to suppression, which is perturbed in PI3Kδ‐deficient Treg cells is not straightforward to pinpoint. Indeed, considering the wide‐ranging but subtle phenotypic differences observed in PI3Kδ‐inactivated tumour‐infiltrating Treg cells, it is distinctly possible that the impairment of suppressive function is not due to the abrogation of any single pathway, but a cumulative result of incomplete restrictions in numerous signalling cascades. We have explored some of the better‐studied pathways among these, presenting a picture that we hope will serve as a starting point for understanding how PI3Kδ signals are integrated to shape Treg cells within the tumour environment.
Disclosure
KO is on the Scientific Advisory Board of Karus Therapeutics and has received consultancy payments from Gilead, GSK and Azeria Therapeutic.
Contributor Information
Ee Lyn Lim, Email: eelynlim@ifrec.osaka-u.ac.jp.
Klaus Okkenhaug, Email: ko256@cam.ac.uk.
References
- 1. Liu Z, Kim JH, Falo LD, You Z. Tumor regulatory T cells potently abrogate antitumor immunity. J Immunol 2009; 182:6160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res 2017; 27:109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self‐tolerance maintained by activated T cells expressing IL‐2 receptor alpha‐chains (CD25). Breakdown of a single mechanism of self‐tolerance causes various autoimmune diseases. J Immunol Baltim Md1950 1995; 155:1151–64. [PubMed] [Google Scholar]
- 4. Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour‐associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 2017; 14:399–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gabrilovich DI, Bronte V, Chen S‐H, Colombo MP, Ochoa A, Ostrand‐Rosenberg S et al The terminology issue for myeloid‐derived suppressor cells. Cancer Res 2007; 67:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qian B, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell 2010; 141:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol 2014; 27:1–7. [DOI] [PubMed] [Google Scholar]
- 8. Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol 2006; 6:295–307. [DOI] [PubMed] [Google Scholar]
- 9. Arce Vargas F, Furness AJS, Solomon I, Joshi K, Mekkaoui L, Lesko MH et al Fc‐optimized anti‐CD25 depletes tumor‐infiltrating regulatory T cells and synergizes with PD‐1 blockade to eradicate established tumors. Immunity 2017; 46:577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sugiyama D, Nishikawa H, Maeda Y, Nishioka M, Tanemura A, Katayama I et al Anti‐CCR10 mAb selectively depletes effector‐type FoxP3+ CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci USA 2013; 110:17945–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gotwals P, Cameron S, Cipolletta D, Cremasco V, Crystal A, Hewes B et al Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer 2017; 17:286–301. [DOI] [PubMed] [Google Scholar]
- 12. Okkenhaug K. Signaling by the phosphoinositide 3‐kinase family in immune cells. Annu Rev Immunol 2013; 31:675–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nishizuka Y, Sakakura T. Thymus and reproduction: sex‐linked dysgenesia of the gonad after neonatal thymectomy in mice. Science 1969; 166:753–5. [DOI] [PubMed] [Google Scholar]
- 14. Gershon RK, Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology 1970; 18:723–37. [PMC free article] [PubMed] [Google Scholar]
- 15. Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003; 4:330–6. [DOI] [PubMed] [Google Scholar]
- 16. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003; 299:1057–61. [DOI] [PubMed] [Google Scholar]
- 17. Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L et al The immune dysregulation, polyendocrinopathy, enteropathy, X‐linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet 2001; 27:20–1. [DOI] [PubMed] [Google Scholar]
- 18. Khattri R, Cox T, Yasayko S‐A, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol 2003; 4:337–42. [DOI] [PubMed] [Google Scholar]
- 19. Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol 2007; 8:191–7. [DOI] [PubMed] [Google Scholar]
- 20. Beres A, Drobyski W. The role of regulatory T cells in the biology of graft versus host disease. Front Immunol 2013; 4:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Walsh PT, Taylor DK, Turka LA. Tregs and transplantation tolerance. J Clin Invest 2004; 114:1398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sanchez AM, Yang Y. The role of natural regulatory T cells in infection. Immunol Res 2011; 49:124–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen W, Jin W, Hardegen N, Lei K, Li L, Marinos N et al Conversion of peripheral CD4+ CD25− naive T cells to CD4+ CD25+ regulatory T cells by TGF‐β induction of transcription factor Foxp3. J Exp Med 2003; 198:1875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF‐β induces a regulatory phenotype in CD4+ CD25− T cells through Foxp3 induction and down‐regulation of Smad7. J Immunol 2004; 172:5149–53. [DOI] [PubMed] [Google Scholar]
- 25. Vignali DAA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol 2008; 8:523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM‐CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest 2006; 116:1935–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hu G, Li Z, Wang S. Tumor‐infiltrating FoxP3+ Tregs predict favorable outcome in colorectal cancer patients: a meta‐analysis. Oncotarget 2017; 8:75361–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou Y, Shao N, Aierken N, Xie C, Ye R, Qian X et al Prognostic value of tumor‐infiltrating Foxp3+ regulatory T cells in patients with breast cancer: a meta‐analysis. J Cancer 2017; 8:4098–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P et al Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004; 10:942–9. [DOI] [PubMed] [Google Scholar]
- 30. Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F et al Intraepithelial CD8+ tumor‐infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA 2005; 102:18538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rasku MA, Clem AL, Telang S, Taft B, Gettings K, Gragg H et al Transient T cell depletion causes regression of melanoma metastases. J Transl Med 2008; 6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ladoire S, Arnould L, Apetoh L, Coudert B, Martin F, Chauffert B et al Pathologic complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor‐infiltrating Foxp3+ regulatory T cells. Clin Cancer Res 2008; 14:2413–20. [DOI] [PubMed] [Google Scholar]
- 33. Ondondo BO, Gallimore A, Jones EG, Godkin A. Home sweet home: the tumor microenvironment as a haven for regulatory T Cells. Front Immunol 2013; 4:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thomas DA, Massagué J. TGF‐β directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 2005; 8:369–80. [DOI] [PubMed] [Google Scholar]
- 35. Nishikawa H, Jäger E, Ritter G, Old LJ, Gnjatic S. CD4+ CD25+ regulatory T cells control the induction of antigen‐specific CD4+ helper T cell responses in cancer patients. Blood 2005; 106:1008–11. [DOI] [PubMed] [Google Scholar]
- 36. Holmgaard RB, Zamarin D, Li Y, Gasmi B, Munn DH, Allison JP et al Tumor‐expressed IDO recruits and activates MDSCs in a Treg‐dependent manner. Cell Rep 2015; 13:412–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Drerup J, Liu A, Hurez V, Curiel T. Anti‐CD25 antibody and denileukin diftitox deplete regulatory T cells in ID8 ovarian cancer equally, but αCD25 promotes more T cell cytotoxicity that correlates with better survival (VAC12P.1112). J Immunol 2015;194(1 Suppl):213.3. [Google Scholar]
- 38. Curiel T, Thibodeaux S, Wall S, Pandeswara SL, Daniel B, Drerup J et al Denileukin diftitox depletes regulatory T cells without clinical benefit in advanced stage epithelial ovarian carcinoma (VAC3P.945). J Immunol 2014;192(1 Suppl):73.7.24277699 [Google Scholar]
- 39. Luke JJ, Zha Y, Matijevich K, Gajewski TF. Single dose denileukin diftitox does not enhance vaccine‐induced T cell responses or effectively deplete Tregs in advanced melanoma: immune monitoring and clinical results of a randomized phase II trial. J Immunother Cancer 2016; 4:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti‐CD25 (interleukin‐2 receptor α) monoclonal antibody. Cancer Res 1999; 59:3128–33. [PubMed] [Google Scholar]
- 41. Savage PA, Malchow S, Leventhal DS. Basic principles of tumor‐associated regulatory T cell biology. Trends Immunol 2013; 34:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Magnuson AM, Kiner E, Ergun A, Park JS, Asinovski N, Ortiz‐Lopez A et al Identification and validation of a tumor‐infiltrating Treg transcriptional signature conserved across species and tumor types. Proc Natl Acad Sci USA 2018; 115:E10672–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol 2017; 17:559–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Eales KL, Hollinshead KER, Tennant DA. Hypoxia and metabolic adaptation of cancer cells. Oncogenesis 2016; 5:e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vasanthakumar A, Liao Y, Teh P, Pascutti MF, Oja AE, Garnham AL et al The TNF receptor superfamily‐NF‐κB axis is critical to maintain effector regulatory T cells in lymphoid and non‐lymphoid tissues. Cell Rep 2017; 20:2906–20. [DOI] [PubMed] [Google Scholar]
- 46. Kunisada Y, Eikawa S, Tomonobu N, Domae S, Uehara T, Hori S et al Attenuation of CD4+ CD25+ regulatory T cells in the tumor microenvironment by metformin, a type 2 diabetes drug. EBioMedicine 2017; 25:154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3‐kinases as regulators of growth and metabolism. Nat Rev Genet 2006; 7:606–19. [DOI] [PubMed] [Google Scholar]
- 48. Auger KR, Serunian LA, Soltoff SP, Libby P, Cantley LC. PDGF‐dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell 1989; 57:167–75. [DOI] [PubMed] [Google Scholar]
- 49. Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5‐trisphosphate. J Biol Chem 1998; 273:13375–8. [DOI] [PubMed] [Google Scholar]
- 50. Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto‐oncogene product by phosphatidylinositol‐3,4‐bisphosphate. Science 1997; 275:665–8. [DOI] [PubMed] [Google Scholar]
- 51. Dibble CC, Cantley LC. Regulation of mTORC1 by PI3K signaling. Trends Cell Biol 2015; 25:545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kaneda MM, Messer KS, Ralainirina N, Li H, Leem CJ, Gorjestani S et al PI3Kγ is a molecular switch that controls immune suppression. Nature 2016; 539:437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Foubert P, Kaneda MM, Varner JA. PI3Kγ activates integrin α4 and promotes immune suppressive myeloid cell polarization during tumor progression. Cancer Immunol Res 2017; 5:957–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. De Henau O, Rausch M, Winkler D, Campesato LF, Liu C, Cymerman DH et al Overcoming resistance to checkpoint blockade therapy by targeting PI3Kγ in myeloid cells. Nature 2016; 539:443–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Okkenhaug K, Bilancio A, Farjot G, Priddle H, Sancho S, Peskett E et al Impaired B and T Cell antigen receptor signaling in p110δ PI 3‐kinase mutant mice. Science 2002; 297:7–18. [DOI] [PubMed] [Google Scholar]
- 56. Angulo I, Vadas O, Garçon F, Banham‐Hall E, Plagnol V, Leahy TR et al Phosphoinositide 3‐kinase δ gene mutation predisposes to respiratory infection and airway damage. Science 2013; 342:866–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pearce VQ, Bouabe H, MacQueen AR, Carbonaro V, Okkenhaug K. PI3Kδ regulates the magnitude of CD8+ T cell responses after challenge with Listeria monocytogenes . J Immunol Author Choice 2015; 195:3206–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bowers JS, Majchrzak K, Nelson MH, Aksoy BA, Wyatt MM, Smith AS et al PI3Kδ inhibition enhances the antitumor fitness of adoptively transferred CD8+ T cells. Front Immunol 2017; 8:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Eid RA, Ahmad S, Lin Y, Webb M, Berrong Z, Shrimali R et al Enhanced therapeutic efficacy and memory of tumor‐specific CD8 T cells by ex vivo PI3K‐δ inhibition. Cancer Res 2017; 77:4135–45. [DOI] [PubMed] [Google Scholar]
- 60. Majchrzak K, Nelson MH, Bowers JS, Bailey SR, Wyatt MM, Wrangle JM et al β‐catenin and PI3Kδ inhibition expands precursor Th17 cells with heightened stemness and antitumor activity. JCI Insight 2017; 2:e90547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Patton DT, Garden OA, Pearce WP, Clough LE, Monk CR, Leung E et al Cutting Edge: the phosphoinositide 3‐kinase p110δ Is critical for the function of CD4+ CD25+ Foxp3+ regulatory T cells. J Immunol 2006; 177:6598–602. [DOI] [PubMed] [Google Scholar]
- 62. Haxhinasto S, Mathis D, Benoist C. The AKT–mTOR axis regulates de novo differentiation of CD4+ Foxp3+ cells. J Exp Med 2008; 205:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M et al T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci USA 2008; 105:7797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ouyang W, Liao W, Luo CT, Yin N, Huse M, Kim MV et al Novel Foxo1‐dependent transcriptional programs control Treg cell function. Nature 2012; 491:554–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cohen SB, Bainter W, Johnson JL, Lin T‐Y, Wong JCY, Wallace JG et al Human primary immunodeficiency caused by expression of a kinase‐dead p110δ mutant. J Allergy Clin Immunol 2019; 143:797–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sogkas G, Fedchenko M, Dhingra A, Jablonka A, Schmidt RE, Atschekzei F. Primary immunodeficiency disorder caused by phosphoinositide 3–kinase δ deficiency. J Allergy Clin Immunol 2018; 142:1650–3.e2. [DOI] [PubMed] [Google Scholar]
- 67. Cheah CY, Fowler NH. Idelalisib in the management of lymphoma. Blood 2016; 128:331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chellappa S, Kushekhar K, Munthe LA, Tjønnfjord GE, Aandahl EM, Okkenhaug K et al The PI3K p110δ isoform inhibitor idelalisib preferentially inhibits human regulatory T cell function. J Immunol 2019; 202:1397–405. [DOI] [PubMed] [Google Scholar]
- 69. Ali K, Soond DR, Piñeiro R, Hagemann T, Pearce W, Lim EL et al Inactivation of PI(3)K p110δ breaks regulatory T‐cell‐mediated immune tolerance to cancer. Nature 2014; 510:407–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lim EL, Cugliandolo FM, Rosner DR, Gyori D, Roychoudhuri R, Okkenhaug K. Phosphoinositide 3‐kinase δ inhibition promotes antitumor responses but antagonizes checkpoint inhibitors. JCI Insight 2018; 3:e120626 URL: https://insight.jci.org/articles/view/120626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gyori D, Lim EL, Grant FM, Spensberger D, Roychoudhuri R, Shuttleworth SJ et al Compensation between CSF1R+ macrophages and Foxp3+ Treg cells drives resistance to tumor immunotherapy. JCI Insight 2018; 3:e120631 URL: https://insight.jci.org/articles/view/120631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Putz EM, Prchal‐Murphy M, Simma OA, Forster F, Koenig X, Stockinger H et al PI3Kδ Is essential for tumor clearance mediated by cytotoxic T lymphocytes. PLoS ONE 2012; 7:e40852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Luo CT, Liao W, Dadi S, Toure A, Li MO. Graded Foxo1 activity in Treg cells differentiates tumour immunity from spontaneous autoimmunity. Nature 2016; 529:532–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Soond DR, Slack EC, Garden OA, Patton DT, Okkenhaug K. Does the PI3K pathway promote or antagonize regulatory T cell development and function? Front Immunol 2012; 3:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hsieh C‐S, Lee H‐M, Lio C‐WJ. Selection of regulatory T cells in the thymus. Nat Rev Immunol 2012; 12:157–67. [DOI] [PubMed] [Google Scholar]
- 76. Yan D, Farache J, Mingueneau M, Mathis D, Benoist C. Imbalanced signal transduction in regulatory T cells expressing the transcription factor FoxP3. Proc Natl Acad Sci USA 2015; 112:14942–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Garçon F, Patton DT, Emery JL, Hirsch E, Rottapel R, Sasaki T et al CD28 provides T‐cell costimulation and enhances PI3K activity at the immune synapse independently of its capacity to interact with the p85/p110 heterodimer. Blood 2008; 111:1464–71. [DOI] [PubMed] [Google Scholar]
- 78. Levine AG, Hemmers S, Baptista AP, Schizas M, Faire MB, Moltedo B et al Suppression of lethal autoimmunity by regulatory T cells with a single TCR specificity. J Exp Med 2017; 214:609–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sainz‐Perez A, Lim A, Lemercier B, Leclerc C. The T‐cell receptor repertoire of tumor‐infiltrating regulatory T lymphocytes is skewed toward public sequences. Cancer Res 2012; 72:3557–69. [DOI] [PubMed] [Google Scholar]
- 80. Dustin ML. The immunological synapse. Cancer Immunol Res 2014; 2:1023–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bretscher PA. A two‐step, two‐signal model for the primary activation of precursor helper T cells. Proc Natl Acad Sci USA 1999; 96:185–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhang R, Borges CM, Fan MY, Harris JE, Turka LA. Requirement for CD28 in effector regulatory T cell differentiation, CCR83 induction, and skin homing. J Immunol 2015; 195:4154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rudd CE, Taylor A, Schneider H. CD28 and CTLA‐4 coreceptor expression and signal transduction. Immunol Rev 2009; 229:12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mirenda V, Jarmin SJ, David R, Dyson J, Scott D, Gu Y et al Physiologic and aberrant regulation of memory T‐cell trafficking by the costimulatory molecule CD28. Blood 2007; 109:2968–77. [DOI] [PubMed] [Google Scholar]
- 85. Kishore M, Cheung KCP, Fu H, Bonacina F, Wang G, Coe D et al Regulatory T cell migration is dependent on glucokinase‐mediated glycolysis. Immunity 2017; 47:875–89.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Thaker YR, Schneider H, Rudd CE. TCR and CD28 activate the transcription factor NF‐κB in T‐cells via distinct adaptor signaling complexes. Immunol Lett 2015; 163:113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Roncagalli R, Cucchetti M, Jarmuzynski N, Grégoire C, Bergot E, Audebert S et al The scaffolding function of the RLTPR protein explains its essential role for CD28 co‐stimulation in mouse and human T cells. J Exp Med 2016; 213:2437–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Rolf J, Bell SE, Kovesdi D, Janas ML, Soond DR, Webb LMC et al Phosphoinositide 3‐kinase activity in T cells regulates the magnitude of the germinal center reaction. J Immunol Baltim Md1950 2010; 185:4042–52. [DOI] [PubMed] [Google Scholar]
- 89. Gigoux M, Shang J, Pak Y, Xu M, Choe J, Mak TW et al Inducible costimulator promotes helper T‐cell differentiation through phosphoinositide 3‐kinase. Proc Natl Acad Sci USA 2009; 106:20371–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Li J, Heinrichs J, Leconte J, Haarberg K, Semple K, Liu C et al Phosphatidylinositol 3‐kinase‐independent signaling pathways contribute to ICOS‐mediated T cell costimulation in acute graft‐versus‐host disease in mice. J Immunol Baltim Md1950 2013; 191:200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chen H, Fu T, Suh W‐K, Tsavachidou D, Wen S, Gao J et al CD4 T cells require ICOS‐mediated PI3K signaling to increase T‐bet expression in the setting of anti‐CTLA‐4 therapy. Cancer Immunol Res 2014; 2:167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cretney E, Xin A, Shi W, Minnich M, Masson F, Miasari M et al The transcription factors Blimp‐1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat Immunol 2011; 12:304–11. [DOI] [PubMed] [Google Scholar]
- 93. Cretney E, Leung PS, Trezise S, Newman DM, Rankin LC, Teh CE et al Characterization of Blimp‐1 function in effector regulatory T cells. J Autoimmun 2018; 91:73–82. [DOI] [PubMed] [Google Scholar]
- 94. Chen Q, Mo L, Cai X, Wei L, Xie Z, Li H et al ICOS signal facilitates Foxp3 transcription to favor suppressive function of regulatory T cells. Int J Med Sci 2018; 15:666–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL‐2 receptor β‐dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol 2007; 178:280–90. [DOI] [PubMed] [Google Scholar]
- 96. Chinen T, Kannan AK, Levine AG, Fan X, Klein U, Zheng Y et al An essential role for the IL‐2 receptor in Treg cell function. Nat Immunol 2016; 17:1322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL‐2Rβ‐deficient mice: implications for the nonredundant function of IL‐2. Immunity 2002; 17:167–78. [DOI] [PubMed] [Google Scholar]
- 98. Kim HP, Imbert J, Leonard WJ. Both integrated and differential regulation of components of the IL‐2/IL‐2 receptor system. Cytokine Growth Factor Rev 2006; 17:349–66. [DOI] [PubMed] [Google Scholar]
- 99. Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation‐mediated apoptosis of effector CD4+ T cells. Nat Immunol 2007; 8:1353–62. [DOI] [PubMed] [Google Scholar]
- 100. Ross SH, Cantrell DA. Signaling and function of interleukin‐2 in T lymphocytes. Annu Rev Immunol 2018; 36:411–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 2015; 27:450–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Krummel MF, Allison JP. CD28 and CTLA‐4 have opposing effects on the response of T cells to stimulation. J Exp Med 1995; 182:459–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM et al Trans‐endocytosis of CD80 and CD86: a molecular basis for the cell‐extrinsic function of CTLA‐4. Science 2011; 332:600–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N et al Immunologic self‐tolerance maintained by CD25+ CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte‐associated antigen 4. J Exp Med 2000; 192:303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wolchok JD, Hodi FS, Weber JS, Allison JP, Urba WJ, Robert C et al Development of ipilimumab: a novel immunotherapeutic approach for the treatment of advanced melanoma. Ann N Y Acad Sci 2013; 1291:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ha D, Tanaka A, Kibayashi T, Tanemura A, Sugiyama D, Wing JB et al Differential control of human Treg and effector T cells in tumor immunity by Fc‐engineered anti–CTLA‐4 antibody. Proc Natl Acad Sci USA 2018; 11:609–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Arce Vargas F, Furness AJS, Litchfield K, Joshi K, Rosenthal R, Ghorani E et al Fc Effector function contributes to the activity of human anti‐CTLA‐4 antibodies. Cancer Cell 2018; 33:649–63.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Rowshanravan B, Halliday N, Sansom DM. CTLA‐4: a moving target in immunotherapy. Blood 2018; 131:58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Walker LSK, Sansom DM. Confusing signals: recent progress in CTLA‐4 biology. Trends Immunol 2015; 36:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV et al CTLA‐4 and PD‐1 receptors inhibit T‐cell activation by distinct mechanisms. Mol Cell Biol 2005; 25:9543–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Okazaki T, Honjo T. PD‐1 and PD‐1 ligands: from discovery to clinical application. Int Immunol 2007; 19:813–24. [DOI] [PubMed] [Google Scholar]
- 112. Bardhan K, Patsoukis N, Weaver J, Freeman G, Li L, Boussiotis VA. PD‐1 inhibits the TCR signaling cascade by sequestering SHP‐2 phosphatase, preventing its translocation to lipid rafts and facilitating Csk‐mediated inhibitory phosphorylation of Lck. J Immunol 2016; 196(1 Supplement):128.15. [Google Scholar]
- 113. Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA et al T cell costimulatory receptor CD28 is a primary target for PD‐1‐mediated inhibition. Science 2017; 355:1428–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Asano T, Meguri Y, Yoshioka T, Kishi Y, Iwamoto M, Nakamura M et al PD‐1 modulates regulatory T‐cell homeostasis during low‐dose interleukin‐2 therapy. Blood 2017; 129:2186–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Koblish HK, Wang L‐C, Hansbury M, Zhang Y, Yang G, Burn T et al Abstract C103: the combination of PI3kδ‐selective inhibition and immunomodulation shows efficacy in solid tumor models. Mol Cancer Ther 2015; 14(12 Supplement 2):C103. [Google Scholar]
- 116. Wang L‐CS, Koblish H, Hansbury M, Zhang Y, Yang G, Burn T et al Pharmacological inactivation of PI3Kδ in the tumor microenvironment enhances efficacy of other immunotherapeutic agents. J Immunother Cancer 2015; ;3(Suppl 2):P377. [Google Scholar]
- 117. Carnevalli LS, Sinclair C, Taylor MA, Gutierrez PM, Langdon S, Coenen‐Stass AML et al PI3Kα/δ inhibition promotes anti‐tumor immunity through direct enhancement of effector CD8+ T‐cell activity. J Immunother Cancer 2018; 6:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Franciszkiewicz K, Boissonnas A, Boutet M, Combadière C, Mami‐Chouaib F. Role of chemokines and chemokine receptors in shaping the effector phase of the antitumor immune response. Cancer Res 2012; 72:6325–32. [DOI] [PubMed] [Google Scholar]
- 119. Villarreal DO, L'Huillier A, Armington S, Mottershead C, Filippova EV, Coder BD et al Targeting CCR120 induces protective antitumor immunity and enhances vaccine‐induced responses in colon cancer. Cancer Res 2018; Jan:1;canres.1119.2018. [DOI] [PubMed] [Google Scholar]
- 120. Plitas G, Konopacki C, Wu K, Bos PD, Morrow M, Putintseva EV et al Regulatory T cells exhibit distinct features in human breast cancer. Immunity 2016; 45:1122–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Sinclair LV, Finlay D, Feijoo C, Cornish GH, Gray A, Ager A et al Phosphatidylinositol‐3‐OH kinase and nutrient‐sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol 2008; 9:513–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Garçon F, Okkenhaug K. PI3Kδ promotes CD4+ T‐cell interactions with antigen‐presenting cells by increasing LFA‐1 binding to ICAM‐1. Immunol Cell Biol 2016; 94:486–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Dong C, Flavell RA. Control of T helper cell differentiation – in search of Master Genes. Sci Signal 2000;2000:pe1. [DOI] [PubMed] [Google Scholar]
- 124. Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ et al The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL‐17+ T helper cells. Cell 2006; 126:1121–33. [DOI] [PubMed] [Google Scholar]
- 125. Duhen T, Duhen R, Lanzavecchia A, Sallusto F, Campbell DJ. Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells. Blood 2012; 119:4430–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Levine AG, Mendoza A, Hemmers S, Moltedo B, Niec RE, Schizas M et al Stability and function of regulatory T cells expressing the transcription factor T‐bet. Nature 2017; 546:421–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF et al Foxp3+ follicular regulatory T cells control T follicular helper cells and the germinal center response. Nat Med 2011; 17:975–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Chung Y, Tanaka S, Chu F, Nurieva R, Martinez GJ, Rawal S et al Follicular regulatory T (Tfr) cells with dual Foxp3 and Bcl6 expression suppress germinal center reactions. Nat Med 2011; 17:983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Halim L, Romano M, McGregor R, Correa I, Pavlidis P, Grageda N et al An atlas of human regulatory T helper‐like cells reveals features of Th2‐like Tregs that support a tumorigenic environment. Cell Rep 2017; 20:757–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Redjimi N, Raffin C, Raimbaud I, Pignon P, Matsuzaki J, Odunsi K et al CXCR131+ T regulatory cells selectively accumulate in human ovarian carcinomas to limit type I immunity. Cancer Res 2012; 72:4351–60. [DOI] [PubMed] [Google Scholar]
- 131. Kachler K, Holzinger C, Trufa DI, Sirbu H, Finotto S. The role of Foxp3 and Tbet co‐expressing Treg cells in lung carcinoma. OncoImmunology 2018; 7:e1456612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica‐Worms DR et al Granzyme B and perforin are important for regulatory T cell‐mediated suppression of tumor clearance. Immunity 2007; 27:635–46. [DOI] [PubMed] [Google Scholar]
- 133. Kelley TW, Efimova O. Induction of granzyme B expression in human regulatory T cells requires TCR and CD28 triggering but not IL‐2 and is suppressed by inhibitors of the PI3K‐mTOR pathway. J Immunol 2009; 1(Suppl):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Cai SF, Cao X, Hassan A, Fehniger TA, Ley TJ. Granzyme B is not required for regulatory T cell‐mediated suppression of graft‐versus‐host disease. Blood 2010; 115:1669–77. [DOI] [PMC free article] [PubMed] [Google Scholar]


