Abstract
Acute myeloid leukemia (AML) with an internal tandem duplication in Fms-related tyrosine kinase 3 (FLT3-ITD) is identified as a subgroup with poor outcome and intrinsic resistance to chemotherapy and therefore urgent need for development of novel therapeutic strategies.
Methods: The antitumor effects of melatonin alone or combined with sorafenib were evaluated via flow cytometry and immunoblotting assays in FLT-ITD AML cells. Also, the ex vivo and in vivo models were used to test the synergistic effects of melatonin and sorafenib against leukemia with FLT3/ITD mutation.
Results: Our study shows for the first time that melatonin inhibits proliferation and induces apoptosis in FLT3/ITD-positive leukemia cells. Mechanistically, melatonin preferentially causes overproduction of reactive oxygen species (ROS) and ultimately massive cell death in FLT3-ITD AML cells. Moreover, melatonin significantly enhances the cytotoxicity induced by the FLT3 tyrosine kinase inhibitor sorafenib in AML cells with FLT3/ITD through redox modification. Importantly, combination of melatonin and sorafenib exhibited highly synergistic therapeutic activity in MV4-11 xenografts and a murine model bearing FLT3/ITD leukemia.
Conclusion: This study indicates that melatonin, alone or in combination with sorafenib, has potential to improve the therapeutic outcome of AML patients with FLT3-ITD mutation that merits further investigation.
Keywords: Melatonin, Sorafenib, FLT3-ITD, Leukemia, Redox modification
Introduction
Nearly one third of acute myeloid leukemia (AML) patients are characterized with activation mutation in the juxta membrane domain of type III receptor Fms-like tyrosine kinase 3 (FLT3) 1, 2. Constitutive internal tandem duplication (ITD) mutation of FLT3 receptors results in activation of multiple downstream pathways including MAPK, STAT and PI3K-AKT and ultimately unrestricted proliferation and cancer cells 3-5. Consequently, AML patients with FLT3-ITD mutation represent a subgroup with poor prognosis as well as intrinsic resistance to conventional chemotherapy 6. It was therefore reasonable that many efforts have been made to develop novel therapeutic modality targeting FLT3-ITD and several clinical trials testing efficiency of small molecule inhibitors in AML patients are ongoing 7. However, none of them could satisfy the clinical demands due to poor response rate as single agent 8, which suggests urgent needs for novel therapeutic agents that could be used in combination to overcome chemoresistance effectively and safely in AML patients with FLT3-ITD mutation.
Melatonin (N-acetyl-5-methoxytryptamine) secreted by the endocrine pineal gland plays vital roles in modulation of circadian rhythm 9. It exerts both multiple biological or pharmacological effects and antitumor activity in various cancers, including lung, breast, and gastrointestinal tumors and so on 10, 11. Recent studies showed that melatonin synergizes the chemotherapeutic effects in pancreatic and colon cancer by suppressing the PI3K/AKT and NF-κB signaling pathways 12, 13. Melatonin was also reported to induce caspase-dependent apoptosis in human leukemia Molt-3 cells 14, and inhibit MLL-rearranged leukemia through suppressing the RBFOX3/hTERT and NF-κB/COX-2 signaling pathways 15. Melatonin can also enhance anti-leukemia activity of retinoic acid in HL60 cells 16. However, whether melatonin has selective effects against FLT3-ITD-positive AML cells remain unclear and have attracted our research interest.
This study was aimed to clarify whether melatonin treatment could inhibit growth and induce apoptosis in FLT3-ITD-mutated AML cells and to determine whether the combination of melatonin and sorafenib has synergistic effects in FLT-ITD AML cells and in mice bearing the FLT3-ITD leukemia model. We found that melatonin enhances the sorafenib-induced cytotoxicity in FLT3-ITD AML cells by redox modification both in vitro and in vivo. Our results suggest that the combination of melatonin and FLT3 inhibitors is an appealing approach that warrants further investigation in clinical settings for FLT3-ITD-mutated AML patients.
Materials and methods
Cell lines and cell culture
FLT3 wild type (ML-1 and HL-60) and ITD mutated (MOLM-13 and MV4-11) leukemia cells were obtained from ATTC (Manassas, VA, USA) and DSMZ (Braunschweig, Germany), respectively. BaF3 and BaF3/ITD were provided by Dr. Donald Small (Johns Hopkins University School of Medicine) and are described as previous report 17, 18. All cell lines were cultured at 37°C with 5% CO2 in RPMI-1640 medium with 10% fetal bovine serum (FBS). Recombinant murine IL-3 (Cell Signaling Technology, Beverly, MA, USA) was added to the medium (10 ng/mL) for BaF3 cell culture. All cell lines were tested for negative mycoplasma contamination and authenticated by short tandem repeat (STR) fingerprinting at the Medicine Lab of Forensic Medicine Department of Sun Yat-sen University (Guangzhou, China) before use.
Reagents
Reagents used in our study were as follows: melatonin (#S1204), cytarabine (Ara-C, #S1648) and sorafenib (#S7397) from Selleck Chemicals (Houston, TX, USA), N-Acetylcysteine (NAC, #A0737) from Sigma-Aldrich (St. Louis, MO, USA) and CM-H2DCF-DA (#C6827) and Rhodamine-123 (#R302) from Invitrogen Life Technologies (Carlsbad, CA, USA). Melatonin was dissolved in DMSO at 2 M in stock solution, and sorafenib was dissolved in DMSO at 5 mM in stock solution. For in vivo experiments, sorafenib was dissolved in a DMSO-PEG solution (5% DMSO+45% PEG 400) and diluted with distilled water (50%), while melatonin was dissolved in distilled water. Cell Proliferation Assay (MTS, #G3580) and Caspase 3/7 Glo assay kit (#G8093) were purchased from Promega (Madison, WI, USA).
Mitochondrial transmembrane potential and cell apoptosis analysis
Mitochondrial transmembrane potential and cell apoptosis were detected by a BD Biosciences FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) as described in our previous publication 19, 20. Briefly, cells were seeded in 6-well plates, treated with melatonin, sorafenib or combination with different concentration for indicated time. For mitochondrial transmembrane potential analysis, AML cells were incubating with 1 μM Rhodamine-123 at 37 °C for 60 min, protected from light. After incubation, cells were harvested, washed twice with PBS and evaluated by flow cytometry. For cell apoptosis analysis, AML cells were harvested, washed twice and resuspended in 500 µl of PBS plus Annexin V-FITC and propidium iodide (PI) (BD Biosciences, Franklin Lakes, NJ, USA), and then evaluated by flow cytometry.
Determination of cellular ROS and GSH/GSSG levels
The levels of cellular ROS were determined by flow cytometry as described in our previous publication 20, 21. Briefly, cells were seeded in 6-well plates, treated with melatonin, sorafenib or combination with different concentration for indicated time, incubated with 3 µM CM-H2DCF-DA in PBS at 37 °C for 30 min protected from light. After incubation, cells were harvested, washed twice with PBS and evaluated by flow cytometry. Flow cytometric analysis was performed using an excitation/emission wavelength of 488/525 nm for DCFH-DA. The intracellular levels of GSH/GSSG were measured with a GSH/GSSG-Glo Assay kit (Promega, WI, USA) respectively, according to the manufacturer's instructions.
Immunoblotting analysis
Immunoblotting analysis was conducted with standard procedures. Briefly, cells were collected and lysed with radioimmunoprecipitation (RIPA) buffer. The extracted proteins were separated by SDS-PAGE and transferred onto a PVDF membrane (Millipore, Billerica, MA, USA). After incubation with specific primary antibodies and peroxidase-conjugated secondary antibodies, proteins in the membranes were visualized using Hyperfilm ECL. Antibodies used for immunoblotting or immunohistochemical analysis were as follows: Cytochrome c (1:1000, #4272), HSP90 (1:1000, #4877), VDAC (1:1000, #4866), Ki67 (1:400, #9027), Cleaved caspase-3 (1:300, #9664) and β-Actin (1:5000, #3700) (Cell Signaling, Beverly, MA, USA).
Animal study
All animals (4-5 week old, 13-15 g female BABL/c mice) were obtained from used in our study were purchased from the Animal Center of Guangdong province (Guangzhou, China). Mice were kept for one week in the animal facility for quarantine and acclimatization before the experiment start. The animal experiments were approved by the Institutional Animal Care and Use Committee of Sun Yat-Sen University.
The xenograft mouse model was used to evaluate therapeutic value of melatonin and sorafenib. Briefly, equal amounts of MV4-11 cells (2×106/mice) mixed in 100 µL of Matrigel were inoculated into the mouse flank subcutaneously. When the tumor had grown to approximate 50 mm3 in volume, mice were randomly assigned to four groups (6 mice/group): the control group (received an equal volume of solvent control), the melatonin-treated group (20 mg/Kg daily, i.p.), the sorafenib-treated group (3 mg/kg daily, oral gavage) or the combination group (melatonin plus sorafenib) by referring to published literature 13, 18, 22, 23. Melatonin, sorafenib or solvent were administrated to mice by gavage or intraperitoneal (i.p.) injection in a total volume of 200 μL. The tumor volume was monitored every four days with a caliper using the formula (V=length×width2/2). After four weeks of treatment, the tumors were extracted from the sacrificed mice and the weight was recorded. All dissected tissues were embedded in paraffin and sectioned for hematoxylin and eosin (H&E) staining or immunohistochemical analysis, while apoptosis percentage was determined with a TUNEL (TdT-mediated dUTP Nick-End Labeling) kit (Roche, Basel, Switzerland) following the manufacturer's instructions.
To evaluate the leukemic burden and conduct survival analysis, BaF3/ITD cells (2×106/mice) were injected intravenously and all the mice were randomly divided into four groups (15 mice each group). The treatment as described above was initiated five days after cell injection. After 2 weeks treatment, five mice from each group were killed by cervical dislocation. Wright-Giemsa staining on the peripheral blood smears obtained from the retro-orbital sinuses and hematoxylin and eosin staining on the paraffin embedded spleens and livers was conducted, respectively. All the slides were investigated by professional pathologist blind with respect to the treatment group for leukemic burden. Survival curves were illustrated with the product-limit estimator of Kaplan-Meier methods and log-rank statistics for the other 10 mice.
Statistical analysis
All data are presented as the mean ± SD. The Student's unpaired t test was used to compare the statistical difference between groups using the GraphPad Prism (Version 6; La Jolla, CA, USA). The Calcusyn software (Biosoft, Ferguson, MO) was used to calculate the synergistic or antagonistic interactions between melatonin and sorafenib. All differences were considered statistically significant when a P value less than 0.05 were reached.
Results
Melatonin inhibits the proliferation and induces apoptosis in FLT3/ITD-positive leukemia cells
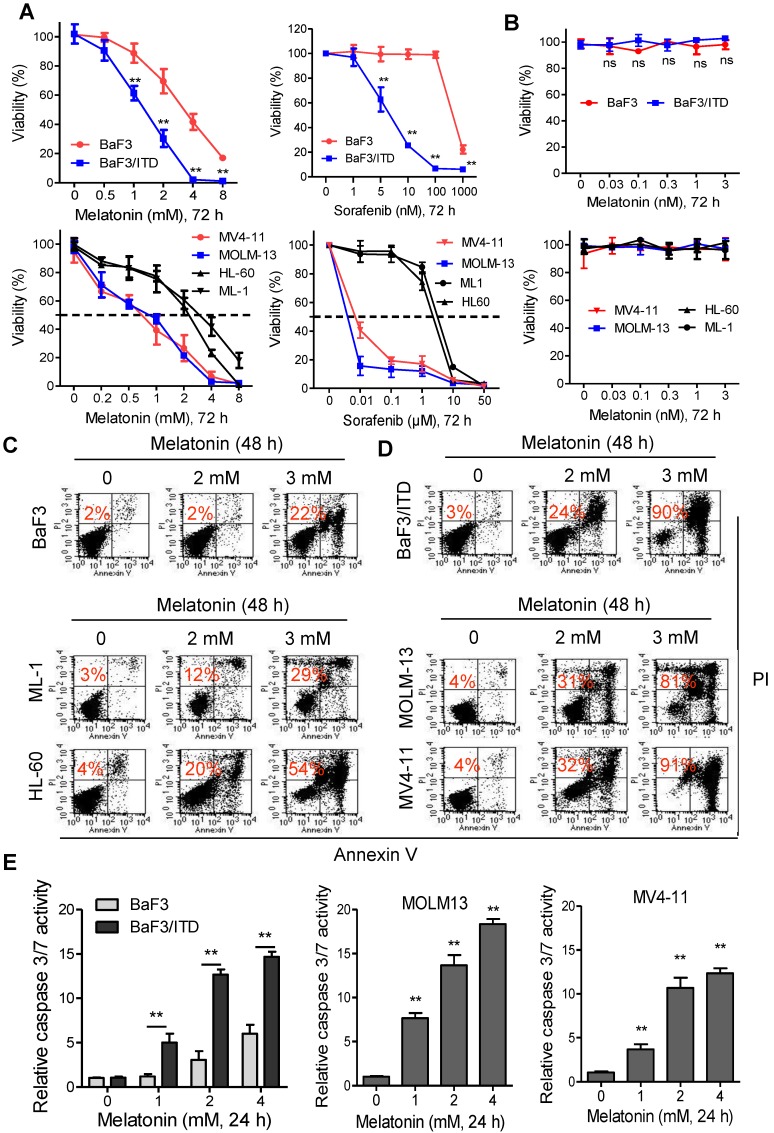
The effects of melatonin, alone or in combination with sorafenib on cell viability of mouse Ba/F3 cells or isogenic BaF3/ITD cells was evaluated with MTS assays. Sorafenib was reported to selectively kill leukemia blasts in AML patients with FLT3/ITD 22, 24. As shown in Figure 1A, both melatonin and sorafenib treatment for 72 h caused significant reduction of cell viability in BaF3/ITD cells, while only marginal decrease in the parental BaF3 cells was observed. Similar results were obtained in the human FLT3-ITD mutated MV4-11 and MOLM-13 leukemia cells and FLT3/wild type HL-60 and ML-1 leukemia cells (Figure 1A).
Figure 1.
Melatonin inhibits the proliferation and induces apoptosis in FLT3/ITD-positive leukemia cells. (A) The cell viabilities of BaF3 and BaF3/ITD cells incubated with melatonin and sorafenib were determined by MTS assay. (B) The cell viabilities of the AML cells incubated with melatonin and sorafenib were determined by MTS assay. (C, D) Representative images of cell apoptosis in the indicated cells treated with melatonin for 48 h were determined by Annexin-V/propidium iodide (PI) assay (red numbers indicate subpopulation of cells negative for Annexin V/PI). (E) Caspase 3/7 activity was measured in indicated cells treated with melatonin for 48 h. The results are presented as a fold increase relative to the untreated sample. Data are presented as the mean±SD (n=3). **P<0.01 for indicated comparison (Student's unpaired t-test).
However, melatonin under physiological concentrations (≤3 nM) had no effects on the leukemia cell viability (Figure 1B). Moreover, we found that treatment with 2 or 3 mM melatonin for 48 h effectively induced cell apoptosis (Annexin V/PI-positive cell) in BaF3/ITD, MV4-11 and MOLM-13 cells, whereas BaF3, HL-60 and ML-1 cells were notably less sensitive to the same treatment (Figure 1C and D) in the apoptosis assays. Further study also confirmed that melatonin selectively induced activation of caspase 3/7 in BaF3/ITD, MV4-11 and MOLM13 cells in a dose-dependent manner (Figure 1E). Altogether, these results indicate that melatonin may selectively kill leukemia cells with FLT3/ITD at pharmacologic concentrations.
Melatonin induces apoptosis through ROS-mediated damage in FLT3/ITD-positive cells
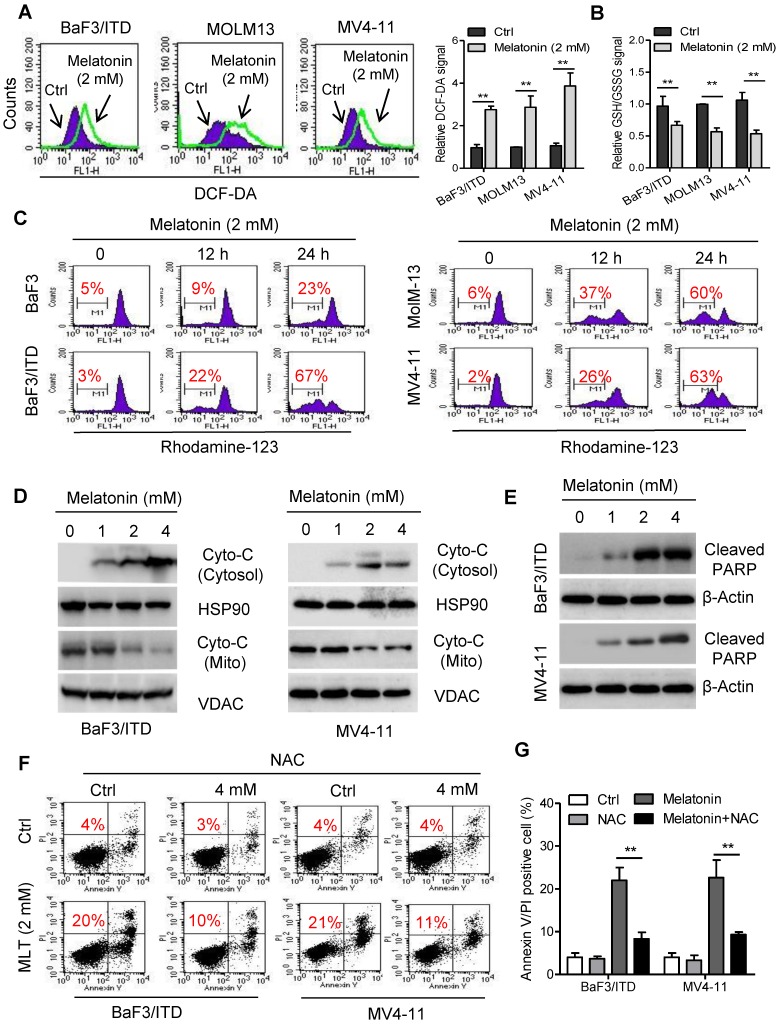
Although melatonin is well-known as an antioxidant molecule, it has been found that treatment with melatonin also stimulates ROS generation in some cancer cell types, such as cervical cancer and hepatocellular carcinoma cells 25, 26. Overproduction of ROS could induce mitochondrial dysfunction and ultimately lead to cell death 27. To test whether oxidative stress mediated melatonin induced cell apoptosis, ROS production was measured in BaF3/ITD, MOLM-13 and MV4-11 cells with flow cytometry. As shown melatonin induced significant increase of cellular ROS and decrease of cellular GSH/GSSG levels in these cells after incubation with 2 mM melatonin for 12 h (Figure 2A and B). This result was consistent with previous report that high melatonin concentrations (1-10 mM) induced ROS generation and GSH depletion 28. The ROS-induced apoptosis was reported to be associated with the mitochondrial membrane leakage, release of cytochrome c and activation of apoptotic cascades 29. We therefore conducted assays to determine whether melatonin-induced apoptosis could be attributed to increased release of cytochrome c from the mitochondria in AML cells. Indeed, obvious loss of mitochondrial transmembrane potential in BaF3/ITD, MOLM-13 and MV4-11 cells treated with 2 mM melatonin for 12 or 24 h was observed as detected by the fluorescent dye rhodamine-123. In contrast, only a small portion of the BaF3 cells exhibited a loss of rhodamine-123 signal (Figure 2C). Immunoblotting of the mitochondrial and cytosolic protein fractions further confirmed that melatonin treatment increased the release of cytochrome c from the mitochondria to the cytosol. Additionally, expression of cleaved PARP in BaF3/ITD and MV4-11 cells was upregulated after melatonin treatment (Figure 2D and E). To determine the causality between overproduction of ROS and increase of apoptosis, cell viabilities of melatonin-treated BaF3/ITD and MV4-11 cells were assessed after pre-incubation with the ROS scavenger NAC (4 mM). The results showed that pretreatment with NAC could rescue cells from cell death induced by melatonin (Figure 2F and G). These observations suggest that melatonin induces cell apoptosis through ROS-mediated damage in human leukemia cells with FLT3/ITD.
Figure 2.
Melatonin induces apoptosis through ROS-mediated damage in FLT3/ITD-positive cells. (A) Representative histograms and quantification of cellular ROS levels in indicated cells treated with melatonin (2 mM) for 12 h, as detected by the fluorescent probe DCF-DA. (B) Cellular GSH/GSSG levels were measured in the indicated AML cells treated with melatonin (2 mM) for 12 h (GSH, reduced glutathione; GSSG, oxidized glutathione) (C) Comparison of the mitochondrial transmembrane potential loss in the indicated cells after treatment with 2 mM oxaliplatin for 12 or 24 h as measured by the potential-sensitive probe Rhodamine-123 (red number indicates subpopulation of cells that lost transmembrane potential). (D) Immunoblotting analysis of Cyto-C in subcellular fraction cytoplasm isolated from the indicated BaF3/ITD and MV4-11 cells after treatment of melatonin (1, 2, 4 mM) for 48 h. The protein HSP90 and VDAC were used as markers of cytoplasm and mitochondria, respectively. (E) Immunoblotting analysis of cleaved PARP in the indicated cells treated with melatonin for 48 h. β-Actin was used as the loading control. (F, G) Reversion of Melatonin-induced cell death by the antioxidant NAC (N-acetyl-L-cysteine). BaF3/ITD and MV4-11 cells were treated with melatonin (2 mM) alone or a combination NAC (4 mM) for 48 h, then determined by Annexin-V/propidium iodide (PI) assay (red numbers indicate subpopulation of cells negative for Annexin V/PI). Representative dot plots and quantitative bar graph are shown. Data in A, B and G are presented as the mean±SD (n=3). **P<0.01 for indicated comparison (Student's unpaired t-test).
Melatonin sensitizes FLT3/ITD cells to the FLT3 inhibitor sorafenib
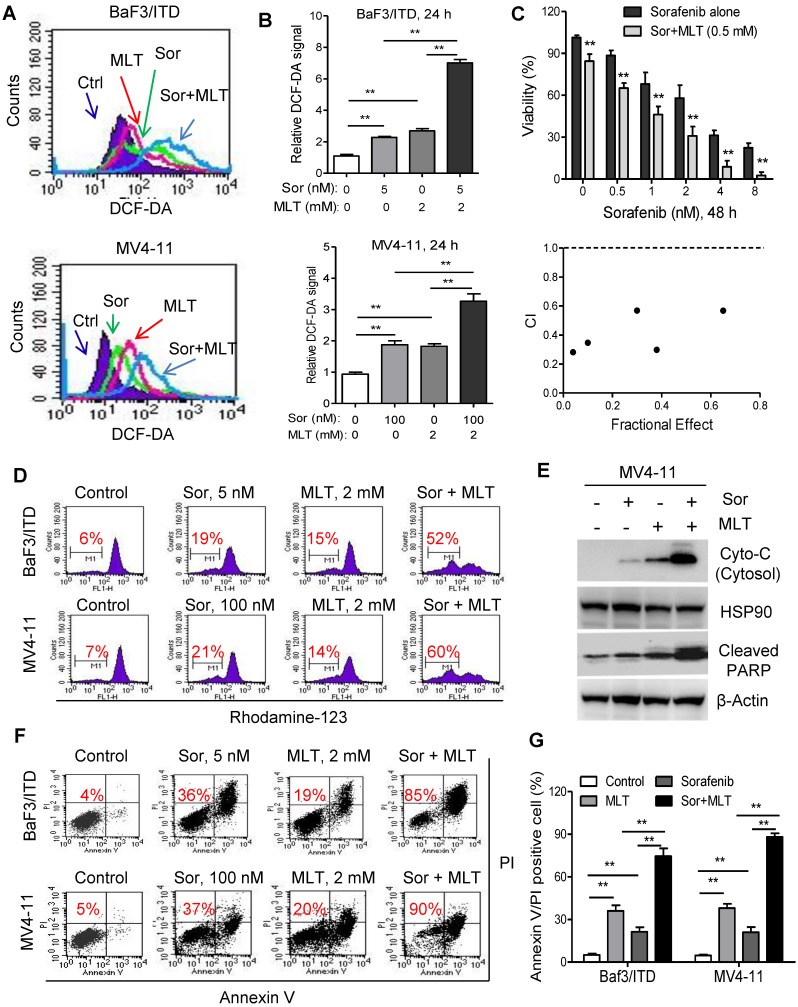
ROS might function as a double-edged sword. Previous reports have demonstrated that adequate amount of ROS is essential for regulation of physiological event, while excessive production could lead to cell death 27, 30, 31. Besides inhibition of tyrosine receptor signaling, induction of ROS in tumor cells also underlies the antitumor activity of sorafenib 32, 33, which prompted us to explore whether melatonin could significantly enhance the anti-leukemia activity of sorafenib though redox modification and achieve a synergistic therapeutic effects. Firstly, ROS level was significantly upregulated in leukemia cells after treatment with melatonin, sorafenib or both with flow cytometry analysis (Figure 3A). Moreover, combination of melatonin and sorafenib induced significantly higher intracellular ROS compared with either agent alone (Figure 3A and B). Secondly, melatonin suppressed the leukemic cell viability when used in combination with sorafenib as demonstrated in the MTS analysis. The combination index calculated by the Calcusyn software was less than 1, indicating a synergistic interaction between melatonin and sorafenib (Figure 3C). Thirdly, treatment with melatonin and sorafenib resulted in significantly higher loss of the mitochondrial membrane potential and subsequently increased release of cytochrome c from the mitochondria to the cytosol compared with that of either single-agent treatments (Figure 3D and E). Further flow analysis showed that melatonin could enhance the pro-apoptosis effects of sorafenib in BaF3/ITD and MV4-11 cells (Figure 3F and G). In summary, these results suggest that melatonin significantly enhances the antitumor activity of FLT3/ITD cells to FLT3 inhibitor sorafenib through accumulation of intracellular ROS generation.
Figure 3.
Melatonin sensitizes FLT3/ITD cells to the FLT3 inhibitor sorafenib. (A, B) Representative histograms and quantification of cellular ROS levels in the BaF3/ITD and MV4-11 cells treated with melatonin, sorafenib or combination for 24 h. (C) BaF3/ITD cells were incubated with various concentrations of sorafenib with or without melatonin (0.5 mM) for 48 h. The effect on cell viability was determined by MTS assay, and the combination index (CI) of sorafenib and melatonin treatment in BaF3/ITD cells was analyzed by a median dose-effect method using Calcusyn software (Biosoft). CI<1 indicates a synergistic effect. CI>1 indicates an antagonist effect. (D) Comparison of the mitochondrial transmembrane potential loss in the indicated cells treated with melatonin, sorafenib or combination for 12 h (red number indicates subpopulation of cells that lost transmembrane potential). (E) Immunoblotting analysis of cytoplastic Cyto-C and cleaved PARP in indicated cells treated with melatonin, sorafenib or combination for 48 h. The protein HSP90 and β-Actin were used as loading control. (F, G) Representative images and quantification of cell apoptosis in the indicated cells treated with melatonin, sorafenib or combination for 48 h (red numbers indicate subpopulation of cells negative for Annexin V/PI). Data in B, C and G are presented as the mean±SD (n=3). **P<0.01 for indicated comparison (Student's unpaired t-test).
Melatonin enhances the therapeutic effect of sorafenib in primary leukemia cells and MV4-11 xenografts in vivo
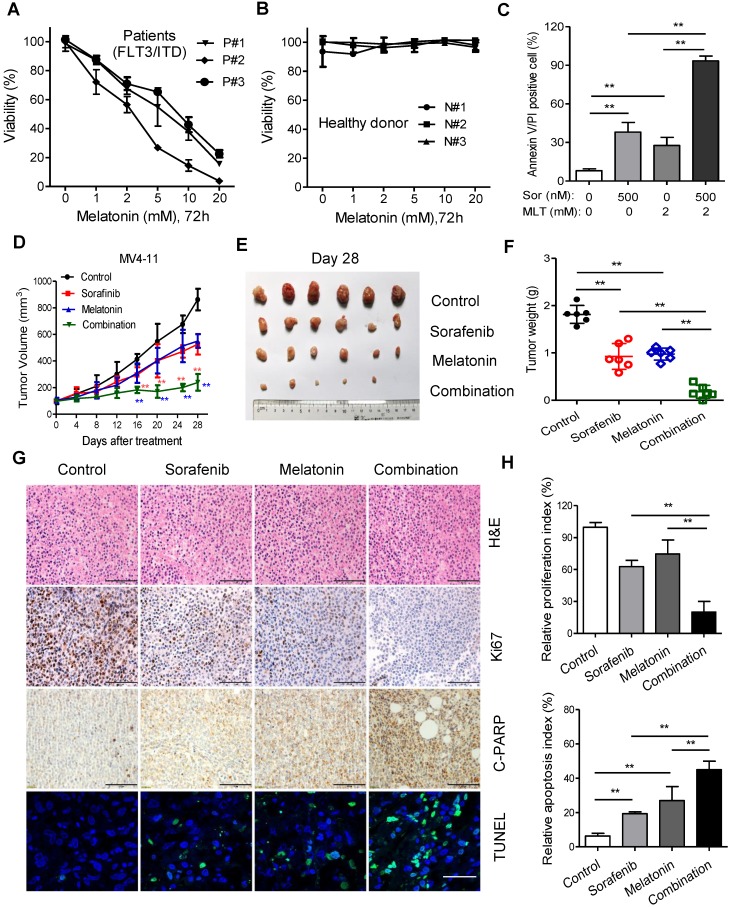
Finally, ex vivo and in vivo models were used to test the synergistic effects of melatonin and sorafenib against leukemia with FLT3/ITD mutation. The peripheral blood from AML patients with FLT3/ITD and healthy donors were collected and prepared for isolation of mononuclear cells, namely the primary FLT3/ITD AML cells used for subsequent analysis.
The cell viability of these primary cells was substantial inhibited by treatment with melatonin for 72 h in a dose-dependent manner with no signs of cytotoxicity to the healthy donor samples (Figure 4A and B). Flow cytometry analysis demonstrated that this combination induced remarkable increase of apoptotic percentage in primary FLT3/ITD AML cells compared with sorafenib or melatonin alone (Figure 4C). Then, MV4-11 cells were inoculated into the nude mice to test the synergistic effects of melatonin plus sorafenib at clinically relevant doses in vivo. Twenty mice were randomly assigned to four groups (n = 6/group) for therapeutic analysis. After treatment lasting for 4 weeks, the mice treated with melatonin and sorafenib showed a significantly larger reduction in tumor burden in comparison with the mice treated with melatonin alone or sorafenib alone, which was evidenced by lower tumor weight and slowed tumor growth rate (Figure 4D-F). Additionally, the combination of melatonin and sorafenib resulted in the greatest decreased in cell proliferation index (Ki67-positive) and enhanced more cell apoptosis as determined by cleaved PARP and TUNEL staining compared with melatonin or sorafenib alone (Figure 4G and H). Altogether, these results suggest that the combination of melatonin and sorafenib represents an effective approach to eradicate the primary AML cells with FLT3/ITD and exhibits significant anti-proliferation and pro-apoptosis activity in vivo.
Figure 4.
Melatonin enhances the therapeutic effect of sorafenib in primary leukemia cells and MV4-11 xenografts in vivo. (A, B) Comparison of cytotoxicity induced by treatment of melatonin for 72 h in healthy donors (n=3) and AML patients with FLT3/ITD (n=3) using MTS assay. (C) Comparison of cell death of AML with FLT3/ITD (n=3) induced by 500 nM sorafenib (Sor), 2 mM melatonin (MLT) or their combination for 48 h. The percentage of cell death was determined by Annexin-V/PI assay. (D) The mice subcutaneously injected with MV4-11 cells (2×106) were treated as indicated when the tumor volume reached 100 mm3. Tumor volumes recorded on the indicated days are shown. (E, F) Photograph and comparison of excised tumor size, and the tumor weights of the indicated group on day 28 were measured. These animal experiments were repeated once. (G) Paraffin-embedded tumor sections derived from the different treatment groups were stained with hematoxylin and eosin (H&E) or Ki67 and cleaved PARP antibodies (scale bar: 100 µm). Apoptotic cells were visualized by TUNEL staining (green) and counterstained with DAPI (blue) (scale bar: 10 µm). (H) The proliferation index (Ki67 staining) and apoptotic index (TUNEL staining) in tumor sections were also quantified. **P<0.01 for the indicated comparison (Student's unpaired t-test).
Melatonin enhances the therapeutic effect of sorafenib in mice bearing FLT3-ITD leukemia
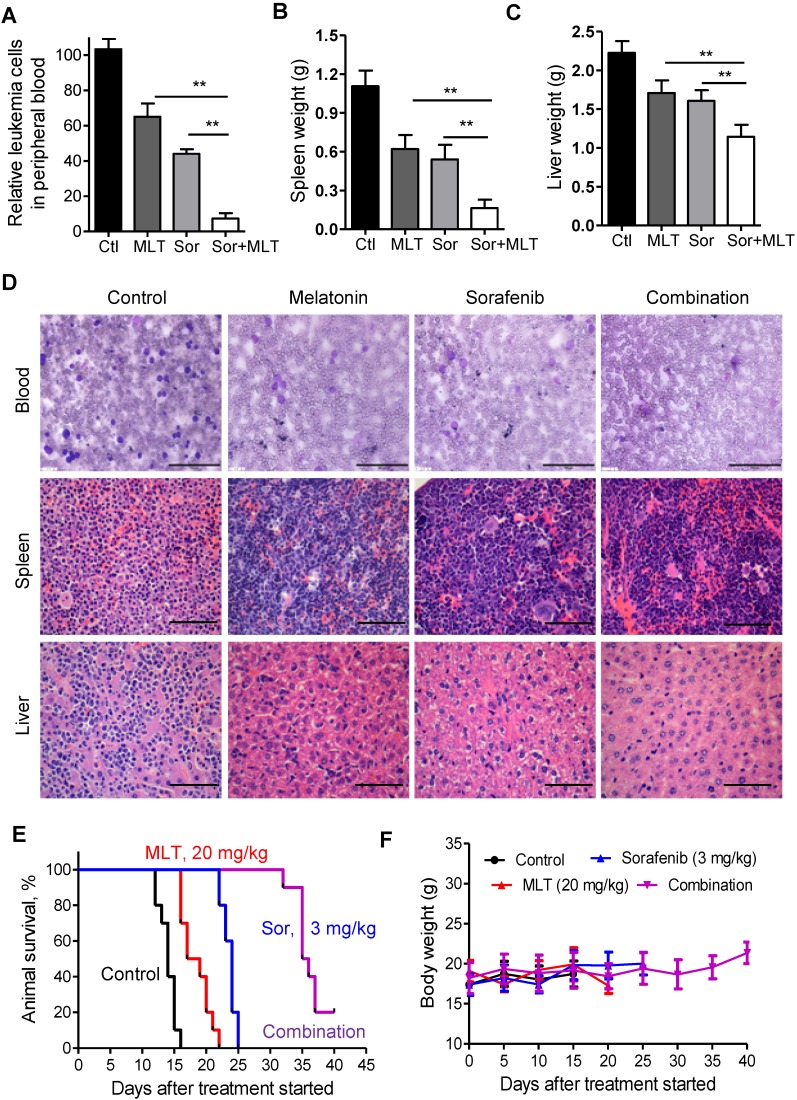
Furthermore, the previously reported BaF3/ITD leukemia model was used 18, 34. Two weeks after injection, Wright-Giemsa staining of the peripheral blood smears from the mice was conducted to evaluate the leukemic burden. As shown in Figure 5A, combination of melatonin and sorafenib almost completely depleted the leukemia cells in the peripheral blood. At the time of sacrifice, spleen and liver weights were significantly lower in the combination treatment group compared to those of the melatonin and sorafenib alone groups, indicating that melatonin could enhance the anti-leukemic effects of sorafenib (Figure 5B and C). Highly infiltrated immature forms/blasts, including cells with deep nuclear indentations could be observed in the peripheral blood smears from the control group, while no immature forms/blasts were seen in the melatonin and sorafenib-treated group as shown in the representative Wright-Giemsa staining images (Figure 5D). In addition, H&E staining of the spleen and liver tissues showed an aggressive and disseminated disease burden in the control group, which could be prohibited by melatonin and sorafenib treatment (Figure 5D). Furthermore, melatonin plus sorafenib (median survival, 35.5 days) significantly prolonged the median survival over melatonin alone (median survival, 18 days) or sorafenib alone (median survival, 24 days) in the survival analysis (Figure 5E). More importantly, no significant weight loss or any other signs of toxicity were observed in any mouse during treatment (Figure 5F). Overall, melatonin, in combination with sorafenib exhibited highly synergistic therapeutic activity against the AML cells with FLT3/ITD not only in vitro but also in vivo.
Figure 5.
Melatonin enhances the therapeutic effect of sorafenib in mice bearing FLT3-ITD leukemia. (A) Relative leukemia cell counts in the peripheral blood smears from leukemic mice (n=5) with different treatments. (B, C) Comparison of the spleen and liver weights from leukemic mice (n=5) with different treatments. (D) Representative peripheral blood smear and hematoxylin and eosin staining of the spleens and livers of FLT3/ITD mice (scale bar: 100 µm). (E) The survival rate of the combination group was significantly different from that of the control (P<0.001), melatonin alone (P<0.001) and sorafenib alone (P<0.001). **P<0.01 for the indicated comparison (Student's unpaired t-test). (G) The leukemic mice were weighed every 5 days for 40 days to estimate toxicity. *P<0.01 for the indicated comparison (Student's unpaired t-test).
Discussion
AML patients with FLT3 mutation are characterized with high relapse rate and short survival. These mutations could be classified into ITD in the juxta membrane domain (~25%) and point mutations in the tyrosine kinase domain (~5%), all of which induce activation of downstream signaling 1. Patients with AML with FLT3-ITD have a high relapse rate and short relapse-free and overall survival after chemotherapy and after transplant 2. Inhibitors of FLT3 signaling, alone or with traditional chemotherapy have attracted the attention of investigators. Various tyrosine kinase inhibitors (TKIs) have been developed targeting FLT3. Currently, the second-generation TKIs are showing highly potent and selective than chemotherapeutic agents 35. However, due to less clinical response and rapidly developed chemoresistance, monotherapy has been substituted by combinational strategies. Addition of chemicals could enhance the efficacy of FLT3 inhibitors, reduce the side effects or reverse the acquired resistance. Such modalities include epigenetic modulators, proteasome inhibitors, kinase inhibitors, phosphatase activators, and other drugs that interrupt downstream signaling 36. Thus, new generations of TKIs might be feasible for use in combination therapy for FLT3-ITD AML patients.
Sorafenib is an orally administered, multitargeted small-molecule TKI. Sorafenib exhibits potent antitumor activity through inhibition of several kinase including RAF, VEGF, and FLT3-ITD and has been approved for treatment in late stage hepatocellular carcinoma. Moreover, sorafenib could effectively kill AML cells with FLT3-ITD mutation 37. Both metabolic abnormalities and impaired redox homeostasis are prominent features of cancer. Recent studies show that both glycolytic and glutaminolytic pathways are clinically actionable therapeutic targets when combined with sorafeinib or specific TKIs 18, 38. In the current study, we reported that melatonin as a potent endogenous free radical scavenge enhances sorafenib-induced cytotoxicity in FLT3-ITD acute myeloid leukemia cells by redox modification (Figure 6), which merits further investigation in the future.
Figure 6.
Proposed working model. Melatonin enhances sorafenib-induced cytotoxicity in FLT3-ITD acute myeloid leukemia cells by redox modification.
Melatonin is characterized with pleiotropic activities, such as physiological circadian regulation and antitumor properties 39. Melatonin has been reported to enhance the antitumor activity of multiple chemotherapeutic agents, including gemcitabine and 5-fluorouracil 12, 13. Melatonin can also enhance anti-leukemia activity of retinoic acid in HL60 cells 15. Previous reports demonstrated that melatonin acts as an anti-oxidant to efficiently scavenge free radicals in the protection against injuries in normal cells 40. Interestingly, pro-oxidant roles of melatonin have been found in tumor cells 25, 26, 28, stressing the link among ROS, mitochondria and apoptosis in cancer. Excessive levels of ROS stress beyond a threshold that exceeds the antioxidant capacity is deleterious to cells 27. Consistently, our current study showed that melatonin induces cell apoptosis through ROS-mediated damage in human leukemia cells with FLT3/ITD. Our previous studies have shown addition of redox-modulation chemicals may further enhance antitumor activity and selectivity of traditional chemotherapy without any toxic side effects 30. Here, we also proposed a novel therapeutic combination to kill AML cells with FLT3/ITD by modulation of the redox homeostasis. Based on our results, we demonstrated that the combination of melatonin and sorafenib showed highly synergistic effects against AML cells with FLT3/ITD in vitro and in vivo.
In this study, we report that combination of melatonin and sorafenib provides a significant therapeutic benefit in AML patients. More importantly, FLT3/ITD could be used as the molecular biomarker to select patients who would benefit from this novel modality. Our results support the notion that modulation of redox status may represent a novel strategy that could potentially improve the outcome of FLT3/ITD leukemia and highlight the necessity of testing this novel therapeutic strategy in preclinical and clinical trials.
Acknowledgments
This work was supported by funds from the Sci-Tech Project Foundation of Guangzhou City, China (201607020038), the Science and Technology Planning Project of Guangdong Province, China (2014A020212132), the National Natural Science Foundation of China, China (81702761, 81772925), the Natural Science Foundation of Guangdong Province, China (2016A03031100, 2015A030313018).
References
- 1.Thiede C, Steudel C, Mohr B, Schaich M, Schakel U, Platzbecker U. et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–35. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 2.Whitman SP, Archer KJ, Feng L, Baldus C, Becknell B, Carlson BD. et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: a cancer and leukemia group B study. Cancer Res. 2001;61:7233–39. [PubMed] [Google Scholar]
- 3.Mizuki M, Fenski R, Halfter H, Matsumura I, Schmidt R, Muller C. et al. Flt3 mutations from patients with acute myeloid leukemia induce transformation of 32D cells mediated by the Ras and STAT5 pathways. Blood. 2000;96:3907–14. [PubMed] [Google Scholar]
- 4.Brandts CH, Sargin B, Rode M, Biermann C, Lindtner B, Schwable J. et al. Constitutive activation of Akt by Flt3 internal tandem duplications is necessary for increased survival, proliferation, and myeloid transformation. Cancer Res. 2005;65:9643–50. doi: 10.1158/0008-5472.CAN-05-0422. [DOI] [PubMed] [Google Scholar]
- 5.Rocnik JL, Okabe R, Yu JC, Lee BH, Giese N, Schenkein DP. et al. Roles of tyrosine 589 and 591 in STAT5 activation and transformation mediated by FLT3-ITD. Blood. 2006;108:1339–45. doi: 10.1182/blood-2005-11-011429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith CC, Wang Q, Chin CS, Salerno S, Damon LE, Levis MJ. et al. Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature. 2012;485:260–63. doi: 10.1038/nature11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konig H, Levis M. Targeting FLT3 to treat leukemia. Expert Opin Ther Targets. 2015;19:37–54. doi: 10.1517/14728222.2014.960843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weisberg E, Barrett R, Liu Q, Stone R, Gray N, Griffin JD. FLT3 inhibition and mechanisms of drug resistance in mutant FLT3-positive AML. Drug Resist Updat. 2009;12:81–89. doi: 10.1016/j.drup.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Claustrat B, Leston J. Melatonin: Physiological effects in humans. Neurochirurgie. 2015;61:77–84. doi: 10.1016/j.neuchi.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Su SC, Hsieh MJ, Yang WE, Chung WH, Reiter RJ, Yang SF. Cancer metastasis: Mechanisms of inhibition by melatonin. J Pineal Res. 2017;62:e12370. doi: 10.1111/jpi.12370. [DOI] [PubMed] [Google Scholar]
- 11.Xin Z, Jiang S, Jiang P, Yan X, Fan C, Di S. et al. Melatonin as a treatment for gastrointestinal cancer: a review. J Pineal Res. 2015;58:375–87. doi: 10.1111/jpi.12227. [DOI] [PubMed] [Google Scholar]
- 12.Gao Y, Xiao X, Zhang C, Yu W, Guo W, Zhang Z. et al. Melatonin synergizes the chemotherapeutic effect of 5-fluorouracil in colon cancer by suppressing PI3K/AKT and NF-kappaB/iNOS signaling pathways. J Pineal Res. 2017;62:e12380. doi: 10.1111/jpi.12380. [DOI] [PubMed] [Google Scholar]
- 13.Ju HQ, Li H, Tian T, Lu YX, Bai L, Chen LZ. et al. Melatonin overcomes gemcitabine resistance in pancreatic ductal adenocarcinoma by abrogating nuclear factor-kappaB activation. J Pineal Res. 2016;60:27–38. doi: 10.1111/jpi.12285. [DOI] [PubMed] [Google Scholar]
- 14.Perdomo J, Cabrera J, Estevez F, Loro J, Reiter RJ, Quintana J. Melatonin induces apoptosis through a caspase-dependent but reactive oxygen species-independent mechanism in human leukemia Molt-3 cells. J Pineal Res. 2013;55:195–206. doi: 10.1111/jpi.12062. [DOI] [PubMed] [Google Scholar]
- 15.Tang YL, Sun X, Huang LB, Liu XJ, Qin G, Wang LN. et al. Melatonin inhibits MLL-rearranged leukemia via RBFOX3/hTERT and NF-kappaB/COX-2 signaling pathways. Cancer Lett. 2019;443:167–78. doi: 10.1016/j.canlet.2018.11.037. [DOI] [PubMed] [Google Scholar]
- 16.Krestinina O, Fadeev R, Lomovsky A, Baburina Y, Kobyakova M, Akatov V. Melatonin Can Strengthen the Effect of Retinoic Acid in HL-60 Cells. Int J Mol Sci. 2018;19:e2873. doi: 10.3390/ijms19102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levis M, Allebach J, Tse KF, Zheng R, Baldwin BR, Smith BD. et al. A FLT3-targeted tyrosine kinase inhibitor is cytotoxic to leukemia cells in vitro and in vivo. Blood. 2002;99:3885–91. doi: 10.1182/blood.v99.11.3885. [DOI] [PubMed] [Google Scholar]
- 18.Ju HQ, Zhan G, Huang A, Sun Y, Wen S, Yang J. et al. ITD mutation in FLT3 tyrosine kinase promotes Warburg effect and renders therapeutic sensitivity to glycolytic inhibition. Leukemia. 2017;31:2143–50. doi: 10.1038/leu.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ju HQ, Lu YX, Wu QN, Liu J, Zeng ZL, Mo HY. et al. Disrupting G6PD-mediated Redox homeostasis enhances chemosensitivity in colorectal cancer. Oncogene. 2017;36:6282–92. doi: 10.1038/onc.2017.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ju HQ, Lu YX, Chen DL, Zuo ZX, Liu ZX, Wu QN. et al. Modulation of Redox Homeostasis by Inhibition of MTHFD2 in Colorectal Cancer: Mechanisms and Therapeutic Implications. J Natl Cancer Inst. 2018 doi: 10.1093/jnci/djy160. doi: 10.1093/jnci/djy160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu YX, Wu QN, Chen DL, Chen LZ, Wang ZX, Ren C. et al. Pharmacological Ascorbate Suppresses Growth of Gastric Cancer Cells with GLUT1 Overexpression and Enhances the Efficacy of Oxaliplatin Through Redox Modulation. Theranostics. 2018;8:1312–26. doi: 10.7150/thno.21745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang W, Konopleva M, Shi YX, McQueen T, Harris D, Ling X. et al. Mutant FLT3: a direct target of sorafenib in acute myelogenous leukemia. J Natl Cancer Inst. 2008;100:184–98. doi: 10.1093/jnci/djm328. [DOI] [PubMed] [Google Scholar]
- 23.Chen YT, Yang CC, Shao PL, Huang CR, Yip HK. Melatonin-mediated downregulation of ZNF746 suppresses bladder tumorigenesis mainly through inhibiting the AKT-MMP-9 signaling pathway. J Pineal Res. 2019;66:e12536. doi: 10.1111/jpi.12536. [DOI] [PubMed] [Google Scholar]
- 24.Auclair D, Miller D, Yatsula V, Pickett W, Carter C, Chang Y. et al. Antitumor activity of sorafenib in FLT3-driven leukemic cells. Leukemia. 2007;21:439–45. doi: 10.1038/sj.leu.2404508. [DOI] [PubMed] [Google Scholar]
- 25.Prieto-Dominguez N, Ordonez R, Fernandez A, Mendez-Blanco C, Baulies A, Garcia-Ruiz C. et al. Melatonin-induced increase in sensitivity of human hepatocellular carcinoma cells to sorafenib is associated with reactive oxygen species production and mitophagy. J Pineal Res. 2016;61:396–407. doi: 10.1111/jpi.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pariente R, Pariente JA, Rodriguez AB, Espino J. Melatonin sensitizes human cervical cancer HeLa cells to cisplatin-induced cytotoxicity and apoptosis: effects on oxidative stress and DNA fragmentation. J Pineal Res. 2016;60:55–64. doi: 10.1111/jpi.12288. [DOI] [PubMed] [Google Scholar]
- 27.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–91. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 28.Osseni RA, Rat P, Bogdan A, Warnet JM, Touitou Y. Evidence of prooxidant and antioxidant action of melatonin on human liver cell line HepG2. Life Sci. 2000;68:387–99. doi: 10.1016/s0024-3205(00)00955-3. [DOI] [PubMed] [Google Scholar]
- 29.Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Updat. 2004;7:97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Ju HQ, Lu YX, Chen DL, Tian T, Mo HY, Wei XL. et al. Redox Regulation of Stem-like Cells Though the CD44v-xCT Axis in Colorectal Cancer: Mechanisms and Therapeutic Implications. Theranostics. 2016;6:1160–75. doi: 10.7150/thno.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li S, Zhuang Z, Wu T, Lin JC, Liu ZX, Zhou LF. et al. Nicotinamide nucleotide transhydrogenase-mediated redox homeostasis promotes tumor growth and metastasis in gastric cancer. Redox Biol. 2018;18:246–55. doi: 10.1016/j.redox.2018.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Connor AE, Mc Gee MM, Likar Y, Ponomarev V, Callanan JJ, O'Shea D F. et al. Mechanism of cell death mediated by a BF2-chelated tetraaryl-azadipyrromethene photodynamic therapeutic: dissection of the apoptotic pathway in vitro and in vivo. Int J Cancer. 2012;130:705–15. doi: 10.1002/ijc.26073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wan J, Liu T, Mei L, Li J, Gong K, Yu C. et al. Synergistic antitumour activity of sorafenib in combination with tetrandrine is mediated by reactive oxygen species (ROS)/Akt signaling. Br J Cancer. 2013;109:342–50. doi: 10.1038/bjc.2013.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tse KF, Mukherjee G, Small D. Constitutive activation of FLT3 stimulates multiple intracellular signal transducers and results in transformation. Leukemia. 2000;14:1766–76. doi: 10.1038/sj.leu.2401905. [DOI] [PubMed] [Google Scholar]
- 35.Engen CB, Wergeland L, Skavland J, Gjertsen BT. Targeted Therapy of FLT3 in Treatment of AML-Current Status and Future Directions. J Clin Med. 2014;3:1466–89. doi: 10.3390/jcm3041466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larrosa-Garcia M, Baer MR. FLT3 Inhibitors in Acute Myeloid Leukemia: Current Status and Future Directions. Mol Cancer Ther. 2017;16:991–1001. doi: 10.1158/1535-7163.MCT-16-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mathew NR, Baumgartner F, Braun L, O'Sullivan D, Thomas S, Waterhouse M. et al. Sorafenib promotes graft-versus-leukemia activity in mice and humans through IL-15 production in FLT3-ITD-mutant leukemia cells. Nat Med. 2018;24:282–91. doi: 10.1038/nm.4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallipoli P, Giotopoulos G, Tzelepis K, Costa ASH, Vohra S, Medina-Perez P. et al. Glutaminolysis is a metabolic dependency in FLT3(ITD) acute myeloid leukemia unmasked by FLT3 tyrosine kinase inhibition. Blood. 2018;131:1639–53. doi: 10.1182/blood-2017-12-820035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xia Y, Chen S, Zeng S, Zhao Y, Zhu C, Deng B. et al. Melatonin in macrophage biology: Current understanding and future perspectives. J Pineal Res. 2019;66:e12547. doi: 10.1111/jpi.12547. [DOI] [PubMed] [Google Scholar]
- 40.Galano A, Tan DX, Reiter RJ. Melatonin: A Versatile Protector against Oxidative DNA Damage. Molecules. 2018;23:e530. doi: 10.3390/molecules23030530. [DOI] [PMC free article] [PubMed] [Google Scholar]