Abstract
This 2-week randomized, double-blind, placebo-controlled fixed-dose study (NCT02919501) explored the potential of accelerating onset of antidepressant efficacy and plasma exposure with single-dose intravenous vortioxetine at oral vortioxetine treatment initiation. Outpatients (ages 18–65 years) with major depressive disorder and a current depressive episode (Montgomery Åsberg Depression Rating Scale total score ≥30) were randomized to an initial single dose of either intravenous vortioxetine 17 mg (n = 27) or intravenous placebo (n = 28), both treatments followed by 2 weeks of oral vortioxetine (10 mg/day). From baseline to day 7, both groups exhibited fast and substantial improvements by approximately 14 Montgomery Åsberg Depression Rating Scale points, with no statistically significant treatment difference for this primary endpoint. Improvements were substantial already within 24 hours, with numerical treatment differences of 1.3 and 1.6 points at days 1 and 3, respectively, in favour of intravenous vortioxetine + oral vortioxetine. Pharmacokinetic data confirmed that intravenous vortioxetine facilitated reaching steady-state plasma concentration within 24 hours. Intravenous vortioxetine + oral vortioxetine was safe and well-tolerated, with nausea as the most common adverse event. This study supported intravenous vortioxetine as a means of rapidly reaching therapeutic vortioxetine blood levels.
Keywords: antidepressant, fast onset of action, intravenous administration, depression
Introduction
Major depressive disorder (MDD) is a disabling illness with significant negative impact on patients’ daily functioning and quality of life (American Psychiatric Association, 2016). In spite of a wide range of pharmacotherapeutic options, most currently available antidepressants require some weeks to induce clinically relevant symptomatic improvement (Machado-Vieira et al., 2008). Lagged onset of therapeutic effect may have negative implications for short- and long-term clinical outcomes, for example by compromising adherence and timely changing of treatment, as well as increasing the risk of suicide (Machado-Vieira et al., 2008; Witkin et al., 2018). Antidepressant treatments that provide immediate symptomatic relief may therefore help reduce the overall burden of disease, for the individual patient, and in a global public health perspective (World Health Organization, 2017). This unmet need in the treatment of depression is reflected in the current focus in drug development on potentially fast-acting antidepressant compounds, mainly targeting glutamatergic pathways, and with a few in late-stage development (Witkin et al., 2018; Daly et al., 2018).
Vortioxetine is a serotonin (5-HT)3, 5-HT7 and 5-HT1D receptor antagonist, a 5-HT1B receptor partial agonist, a 5-HT1A receptor agonist, and an inhibitor of the serotonin transporter (SERT) that has demonstrated robust antidepressant efficacy and a favourable tolerability profile broadly across patient populations (Katona et al., 2012; Thase et al., 2016; Baldwin et al., 2016b). Pre-clinical studies indicate that vortioxetine may have potential to accelerate the onset of effect, theoretically by its 5-HT1A receptor agonism, which may lead to rapid desensitizing of autoreceptors in the raphe nucleus (Bétry et al., 2013). In addition, its 5-HT3 receptor antagonism may reduce GABAergic input from hippocampal interneurons, which may in turn increase serotonin-mediated activation of glutamatergic neurons in the frontal cortex (Artigas et al., 2018). Thereby, vortioxetine may counteract the initial suppression of 5-HT neurotransmission following inhibition of the SERT thought to account for the 2–3 weeks’ lag of effect seen with selective serotonin reuptake inhibitors (SSRIs) (Bétry et al., 2013; Artigas et al., 2018).
Despite the potential for faster antidepressant onset of action suggested by pre-clinical data, there is no documentation of such effects in available clinical study data. However, vortioxetine has a relatively long half-life, on average 66 hours, with steady-state plasma concentration levels reached after approximately 2 weeks, which may delay the onset of beneficial pharmacological effects (Areberg et al., 2014). Moreover, approved dosages of vortioxetine for the treatment of MDD comprise 5–20 mg/day administered perorally, with recommended starting doses of 5–10 mg/day. Consequently, plasma exposure of vortioxetine is low on the first day after dosing. Methods to attain steady-state levels faster and increase initial exposure may potentially provide faster symptomatic relief. Intravenous (IV) administration of the drug directly into systemic circulation, thereby bypassing first-pass metabolism, may be one method to increase initial plasma exposure. Vortioxetine has previously been administered intravenously to healthy subjects (Areberg et al., 2012).
The aim of this study was to explore the early efficacy, the pharmacokinetics (PK), and the safety and tolerability, of a single initial, IV dose of vortioxetine added to daily oral vortioxetine, with the purpose of reducing the time to onset of antidepressant effect from 2–3 weeks to 7 days or less.
Methods
Study design and patients
In this multi-site, parallel-group, fixed-dose study, patients were randomized 1:1 to double-blind treatment with either a single dose of IV vortioxetine 17 mg (0.068 mg/ml solution infused over 2 hours) plus 2 weeks of open-label oral vortioxetine 10 mg/day (IV vortioxetine + oral vortioxetine), or a single dose of IV saline infusion plus 2 weeks of open-label oral vortioxetine 10 mg/day (IV placebo + oral vortioxetine).
The study included outpatients aged 18–65 years with recurrent MDD (per DSM-5), who experienced a current major depressive episode (classification code 296.3x). Patients had to be severely depressed, with a Montgomery Åsberg Depression Rating Scale (MADRS) total score ≥30 at screening and at baseline, and with the current depressive episode lasting at least 3 months. Patients with any psychiatric comorbidity, including alcohol or other substance abuse/dependence, a history of mania, hypomania, or any psychotic disorder, were excluded, as were patients who were pregnant, at risk of suicide, or who had any impairments likely to compromise the validity of the assessments. Patients with a current depressive episode resistant to two antidepressants treatments at recommended doses and of at least 6 weeks’ duration, were excluded to avoid including patients with treatment-resistant depression.
Patients were hospitalized for observation for 24 hours after the IV infusion, after which period they continued the study as outpatients. A safety follow-up was conducted approximately 4 weeks after the end of treatment. Two blinded safety and tolerability evaluations were conducted internally after randomization of 8 and 20 patients, before continuing the study.
The dose of 17 mg vortioxetine to be administered intravenously was determined based on PK simulations showing that the steady-state plasma drug level reached after 2–3 weeks of treatment with a conventional dose of 10 mg/day oral vortioxetine (Cmax and AUC0-24 values of 14 ng/ml and 326 ng*h/ml, respectively [Areberg et al., 2014]), would be obtained within 24 hours with the combined doses of 17 mg IV and 10 mg oral vortioxetine. A standard 10 mg/day oral dose of vortioxetine was used as comparison, adding placebo (IV saline) to ensure blinding against IV vortioxetine. All patients received first oral dose immediately before the infusion.
The study was conducted between September 2016 and April 2017 at seven sites in Estonia and Finland. Study protocols and amendments were approved by the independent ethics committee of each trial site. The trial was conducted in accordance with the International Conference on Harmonization Good Clinical Practices guidelines and with the ethical principles of the Declaration of Helsinki. The trial is registered at ClinicalTrials.gov (NCT02919501).
Assessments
Efficacy and safety data were collected at day 0 (baseline), and at days 1, 3, 7 and 14 post-baseline; adverse events in addition at 2 hours (i.e., immediately after the IV infusion). Blood samples for vortioxetine assessments were collected on day 1 (three samples; 2, 8 and 24 hours post-IV infusion) and day 14 (one sample; no specific sampling time specified).
The primary efficacy measure was the MADRS (Montgomery and Asberg, 1979), a 10-item clinician-reported rating scale assessing depressive symptom severity. In this study, the MADRS was modified to assess symptoms within the time since previous visit, i.e., for the past 1, 2 or 4 days at days 1, 3 and 7, respectively. MADRS total score (possible score range 0–60) was calculated as the sum of scores on single items. A six-item subscale score of the MADRS (MADRS-6) was calculated as the sum of the following items: Apparent sadness, reported sadness, inner tension, lassitude, inability to feel and pessimistic thoughts (item numbers 1, 2, 3, 7, 8 and 9). The MADRS-6 subscale was developed to provide a more unidimensional representation of the core symptoms of depression compared with the full scale and has demonstrated higher sensitivity to detect treatment differences (Bech et al., 2002; Bech, 2006; Thase et al., 2012). For the MADRS scales, higher scores indicate worse symptom severity.
Disease severity and improvement were recorded by clinicians using the Clinical Global Impressions-Severity of Illness (CGI-S) and CGI-Improvement (CGI-I) (Guy, 1976). Both scales range from 1 to 7, with higher CGI scores indicating worse status/worsening of illness, a CGI-I score of four reflecting neither improvement nor worsening relative to baseline.
The Hospital Anxiety and Depression Scale (HADS) was used to assess patient-rated symptoms of depression (HADS-D) and anxiety (HADS-A) (Zigmond and Snaith, 1983). Total score for each of the seven-items subscales was calculated as the sum of scores on single items (possible score range 0–21), higher scores indicating higher symptom severity.
Safety and tolerability assessments included reported adverse events, vital signs, clinical safety laboratory tests, electrocardiograms (ECGs) and physical examinations. Suicidality was rated and monitored using the Columbia-Suicide Severity Rating Scale (Posner et al., 2011).
Statistical analyses
Safety analyses comprised all randomized patients who received IV infusion or took at least one tablet (treated patients), with treatment-emergent adverse events (TEAEs) summarized using descriptive statistics. Efficacy analyses comprised all patients who received infusion and took at least one tablet, and who had a valid MADRS total score assessment at baseline and at least one valid MADRS assessment before or at day 7 post-baseline (the full-analysis set).
The primary efficacy endpoint assessing the change from baseline at day 7 in MADRS total score was analysed using a restricted maximum likelihood-based mixed model for repeated measurements (MMRM), including study site, day and treatment as factors, baseline MADRS total score as a covariate, and interactions for treatment-by-day and baseline MADRS total score-by-day. The mean difference between treatment groups at day 7 was estimated based on the least squares mean for the treatment-by-day interaction in the MMRM-model.
Key secondary endpoints, the changes from baseline in MADRS total score at days 14, 3 and 1, respectively, were analysed using the same approach. Mean changes from baseline and mean treatment differences with standard errors are reported, unless otherwise stated.
To account for multiplicity, the primary and key secondary endpoints were tested according to the following, hierarchically ordered sequence:
(1) change from baseline to day 7 in MADRS total score (primary endpoint);
(2) change from baseline to day 14 in MADRS total score;
(3) change from baseline to day 3 in MADRS total score;
(4) change from baseline to day 1 in MADRS total score.
If an endpoint in the sequence did not reach statistical significance at an alpha level of 5%, the testing strategy was stopped, and subsequent P-values were considered nominal.
Other endpoints were analysed using MMRM models similar to that specified for the primary endpoint, with CGI-S baseline score included as a covariate in the analyses of outcomes for CGI-I and the change from baseline in MADRS-6 subscale score analysed post hoc.
All statistical tests are two-sided. Data were analysed using SAS, Version 9.4 statistical software.
Sample size determination
Assuming an effect size at day 7 of 4.7 points in MADRS total score with an SD of 6.0, a total of 54 patients (27 per treatment arm) would provide a power of approximately 80% at an alpha level of 5%.
Based on pre-clinical data supporting the potential for fast onset (Bétry et al., 2013; Sanchez et al., 2015), a steady-state exposure level obtained at day 1 with the IV 17 mg dose was hypothesized to result in an effect size at day 7 that would correspond to the effect size seen after 2–3 weeks with 10 mg oral vortioxetine. The assumed effect size of 4.7 MADRS points at day 7 was based on the theoretical change from baseline with the conventional 10 mg oral dose of vortioxetine (approximately 5 points) versus week 3 (approximately 10 points).
Population pharmacokinetic analysis
The sampled plasma concentrations of vortioxetine were analysed using non-linear mixed effect methods, based on a previously developed model for vortioxetine (Areberg et al., 2014). The population PK model was used to simulate full individual plasma concentration-time profiles and estimate area under the curve and Cmax parameters for days 0, 3, 7 and 14.
Results
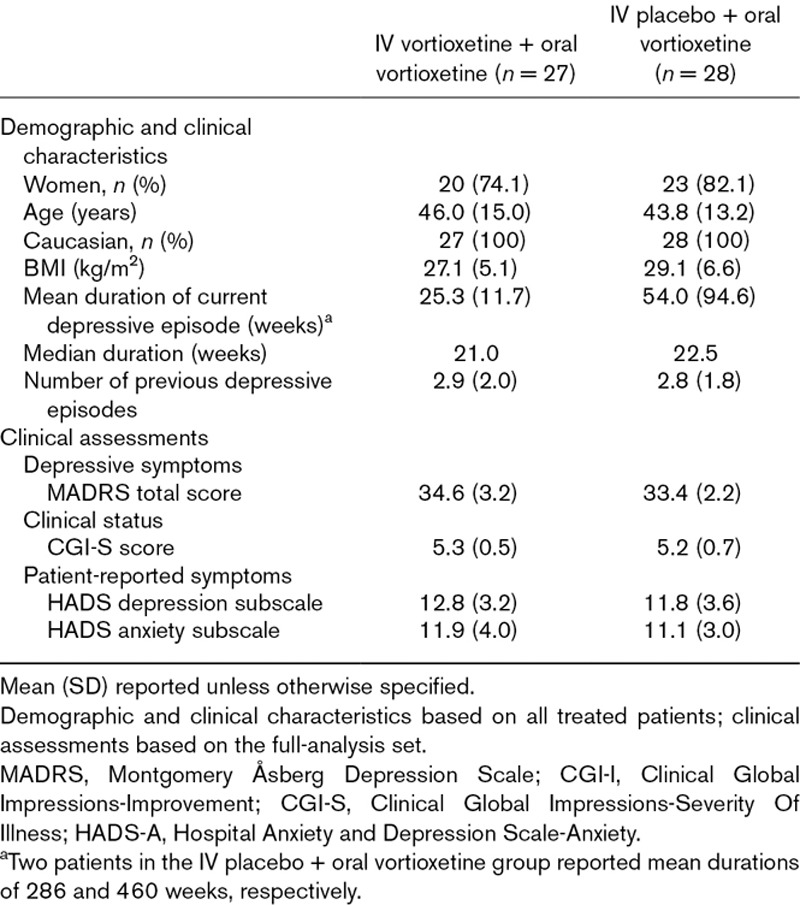
Of 55 patients randomized, 54 (98.2%) completed the study (Fig. 1). The mean age of the total sample was approximately 45 years, and most patients were women (74.1% and 82.1% in the IV vortioxetine and IV placebo group, respectively; Table 1). The mean MADRS total score at baseline was approximately 34. No notable differences between the treatment groups in demographic characteristics or baseline clinical assessments were seen, except for a substantially higher mean duration of the current depressive episode for the IV placebo + oral vortioxetine group (54.0 versus 25.3 weeks). This difference was driven by two patients in the IV placebo group (cf. Table 1). The median duration of the current depressive episode was approximately 22 weeks in both treatment groups.
Fig. 1.
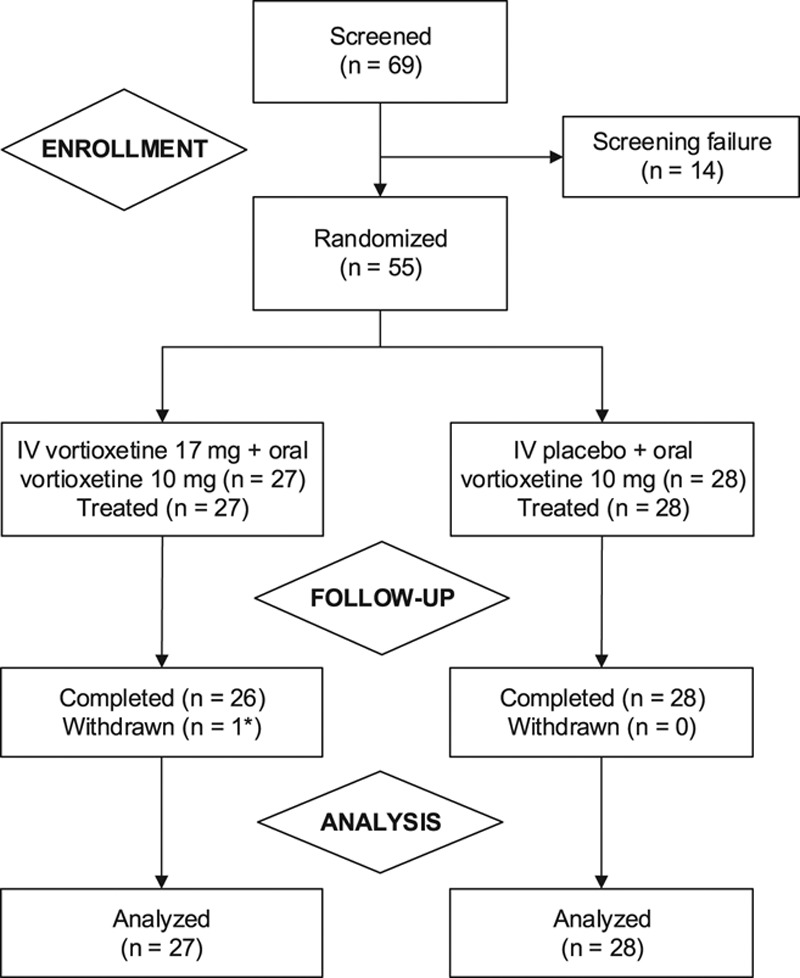
Patient disposition. *Lost to follow-up.
Table 1.
Demographic and baseline clinical characteristics
Efficacy
From baseline to day 7, patients in both treatment groups showed considerable improvements, with a reduction of approximately 14 points in MADRS total score, and no statistically significant treatment difference (Fig. 2). The primary endpoint was therefore not met, and subsequent P-values were considered nominal, in accordance with the testing strategy.
Fig. 2.
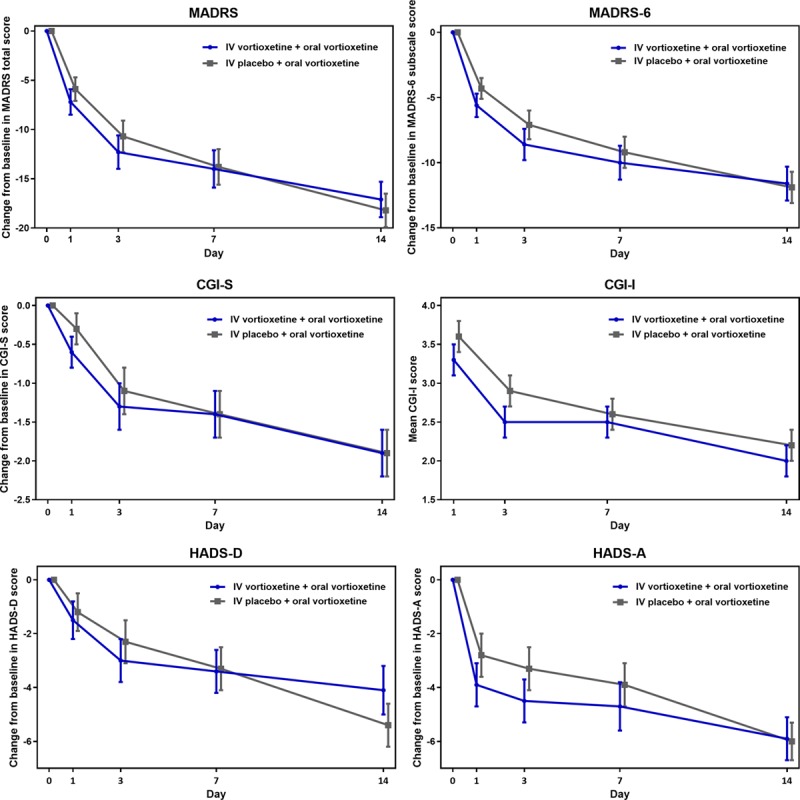
Efficacy assessments across study period (FAS, MMRM). IV vortioxetine + oral vortioxetine, n = 27; IV placebo + oral vortioxetine, n = 28. Treatment differences are based on the least squares means; error bars represent standard errors. CGI-I, Clinical Global Impressions-Improvement; CGI-S, Clinical Global Impressions-Severity of Illness; FAS, full-analysis set; HADS-A, Hospital Anxiety and Depression Scale-Anxiety; HADS-D, Hospital Anxiety and Depression Scale-Depression; IV, intravenous; MADRS, Montgomery Åsberg Depression Scale; MMRM, mixed model for repeated measurements.
Across the 2-week study period, mean MADRS total score steadily decreased in both treatment groups. The improvement was most pronounced within the first 3 days, with a MADRS total score change from baseline at day 1 of −7.2 points for IV vortioxetine + oral vortioxetine and −5.9 points for IV placebo + oral vortioxetine, and with numerical treatment differences of 1.3 and 1.6 in favour of IV vortioxetine at days 1 and 3, respectively. Similarly, MADRS-6 subscale score, and CGI-S and CGI-I scores decreased in both treatment groups across the study period; overall, these numerical improvements were more pronounced in the IV vortioxetine group.
Similar patterns were seen for other endpoints assessing patient-reported symptoms of depression and anxiety as measured by HADS-D and HADS-A, respectively; in particular, patients in both treatment groups reported substantial reduction in anxiety levels throughout the study. Patients treated with IV vortioxetine reported numerically larger improvement in anxiety up to day 7, with the changes from baseline for the treatment groups converging at day 14.
Pharmacokinetics
The concentration of vortioxetine in plasma, as reflected in Cmax, of 12 ng/ml at day 0 among patients receiving IV vortioxetine, were close to the expected steady-state levels of 14 ng/ml predicted by a simulation model (Fig. 3). Cmax was on average 4.3, 1.4 and 1.1 times higher at days 0, 3 and 7, respectively, for IV vortioxetine + oral vortioxetine versus IV placebo + oral vortioxetine, and converged at day 14.
Fig. 3.
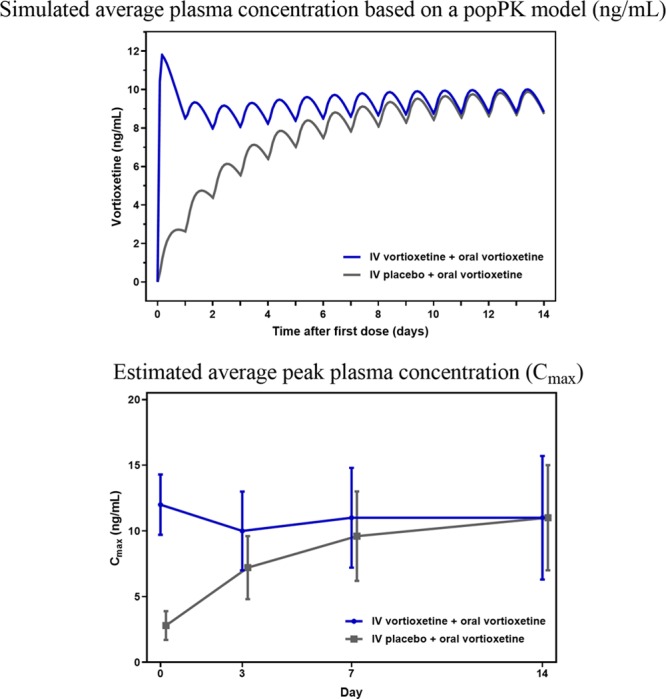
Simulated and estimated plasma concentration. IV vortioxetine + oral vortioxetine, n = 27; IV placebo + oral vortioxetine, n = 28. Cmax, maximum concentrations; IV, intravenous; PopPK, population pharmacokinetics.
Safety
During the treatment period, 78% (n = 21) of patients treated with IV vortioxetine + oral vortioxetine and 64% (n = 18) of those treated with IV placebo + oral vortioxetine reported TEAEs. TEAEs were predominantly mild or moderate, with a total of two patients, both in the IV vortioxetine group, reporting severe TEAEs (anxiety and fatigue, respectively; Table 2)
Table 2.
Summary of treatment emergent adverse events, and treatment emergent adverse events with an incidence of ≥5% in either treatment group in the 2-week treatment period
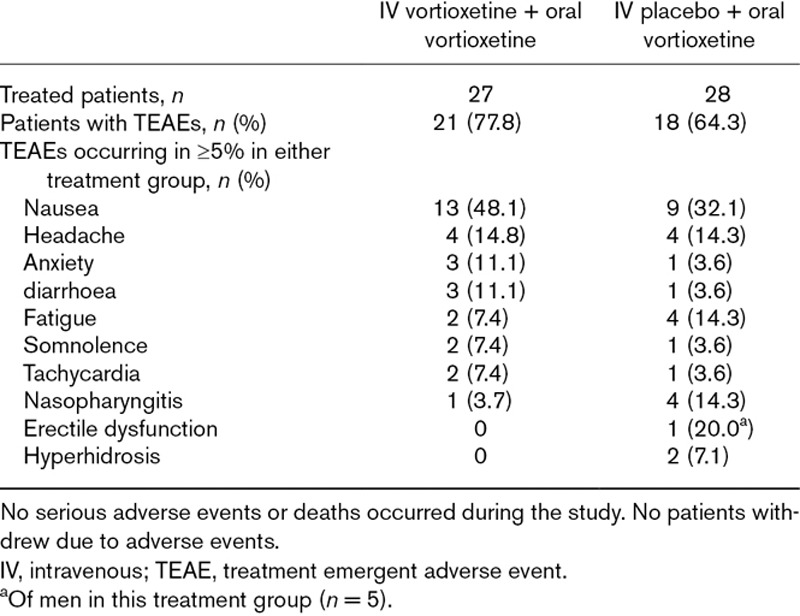
The most common TEAE in both treatment groups was nausea, reported by 48% (n = 13) of patients in the IV vortioxetine group and 32% (n = 9) in the IV placebo group. All events of nausea were mild or moderate (moderate-intensity nausea reported by one patient in the IV placebo group and two in the IV vortioxetine group), with comparable severity levels between the groups. No patients reported vomiting. Among patients treated with IV vortioxetine who experienced nausea, most (nine of 13) TEAEs of nausea began within 24 hours after the IV dose, with no new incidences after day 2, and a mean duration of 4.5 days. Among patients treated with IV placebo, events of nausea began relatively later (mean onset approximately 2 days) and were of longer duration (mean = 12.5 days).
One patient in the IV placebo group reported treatment-emergent sexual dysfunction (erectile dysfunction) while no patients reported insomnia or sedation. No deaths or serious adverse events occurred during the study, and none of the patients withdrew because of an adverse event. There were no notable findings in the clinical safety laboratory tests, vital signs or ECG parameters.
Discussion
In this study, all patients showed immediate and substantial symptomatic improvement in MADRS total score, with no statistically significant treatment difference at day 7. Hence, the primary endpoint was not met, and P-values are considered nominal. At days 1 and 3, numerically larger improvements were seen with IV vortioxetine versus IV placebo. Similar patterns were seen across outcomes on MADRS-6, CGI and HADS scales.
PK results showed that an initial, single-dose infusion of vortioxetine accelerated attainment of steady-state concentrations, as the Cmax values highly approximated the prediction of plasma exposure level of vortioxetine reached within 24 hours to correspond to steady-state levels for 10 mg/day oral dosing reached after approximately 14 days. The early clinical response seen in the IV vortioxetine group was consistent with exposure levels. Also, based on PK, the symptomatic response in the groups would be predicted to converge at day 14, as observed for the majority of endpoints.
Historically, IV formulations to accelerate onset of therapeutic action have been investigated for a number of existing antidepressants, including the TCA clomipramine and the SSRI citalopram, with mixed results (Guelfi et al., 2000; Moukaddam and Hirschfeld, 2004; Norman and Olver, 2004). The success of compounds currently in development in consistently achieving fast onset of action is yet to be seen (Freedman et al., 2018). These studies typically used repeated IV administrations whereas this study administered a single initial IV dose followed by oral dosing. Considering that the antidepressant effect of vortioxetine has been demonstrated at lower SERT occupancy compared with SSRIs (Stenkrona et al., 2013; Sanchez et al., 2015), and the rapid desensitizing of 5-HT autoreceptors related to 5-HT1A receptor agonism and 5-HT3 receptor antagonism (Bétry et al., 2013), the mode of action of vortioxetine may plausibly carry a potential for fast therapeutic action.
The symptomatic improvement among patients who received IV placebo + oral vortioxetine was both larger and earlier than anticipated, although it should be noted that the existing knowledge on very early efficacy (<5 days) with fast-acting antidepressants is limited. Still, in previous short-term studies with oral vortioxetine 10 mg/day, the mean changes from baseline at 1 week after first dose were in the order of five MADRS points or less (Alvarez et al., 2012, Katona et al., 2012). Although changes from baseline to day 7 of approximately five and 10 points were expected a priori for IV placebo + oral vortioxetine and IV vortioxetine + oral vortioxetine, respectively, both treatment groups improved by 14 points from baseline to day 7. Various factors may have contributed to this finding: first, the hospitalization procedure of the study prioritized safety precautions against the risk of effects arising from the hospital care and environment; second, the IV procedure was likely to augment the perceived potency–and thus expectancy-driven placebo-response–of the treatment, due to a more invasive procedure relative to oral treatment; likewise, patients’ expectations of an early effect would be likely to produce early-onset placebo-response. Finally, the repeated assessments at short intervals could increase the placebo response, as might the absence of a placebo-only treatment arm, implying certainty of receiving an active treatment (Schedlowski et al., 2015; Carlino and Vase, 2018).
Across assessments, the numerical treatment differences were largest up to day 3 and converged from day 7. At the time of study, the optimal endpoint time for vortioxetine as an IV formulation was not known but the results suggest that initial boosting with IV vortioxetine may potentially translate into a therapeutic effect already within 24 hours after administration, and that day 7 may therefore be too late to capture the effect. However, the numerical treatment differences that were seen at day 7 for depressive symptoms, as measured by the MADRS-6 subscale and the patient-rated HADS-D, indicate that assessment method–in particular the sensitivity of the rating scale–may also play a role. Further, the similarity in results for the MADRS-6 subscale to those for MADRS total score support this briefer scale as sufficient to capture treatment changes in core depressive symptoms.
Vortioxetine as oral and IV formulations showed overall good safety and tolerability profiles. The differential patterns of TEAEs of nausea in the respective treatment groups appeared to reflect vortioxetine exposure, with indications of earlier onset and shorter duration of nausea with IV vortioxetine compared to IV placebo; however, it should be kept in mind that these descriptions were based on a small number of patients reporting nausea (n = 22). Overall, TEAEs of nausea observed in this study align with general results for the program, showing that vortioxetine-induced nausea is transient (Baldwin et al., 2016a; Vieta et al., 2017). Moreover, the indication of exposure-dependent onset and duration might support nausea following IV vortioxetine as centrally mediated, since peripheral mediation via the gastro-intestinal system would require longer time to onset. This is consistent with the mechanism of action and tolerability profile already described for the oral formulation (Salagre et al., 2018; Baldwin et al., 2016a).
Study limitations included the small sample size, and the absence of a placebo-only arm, for which reason it was not possible to quantify the response attributable to IV placebo alone. Finally, although PK results were as expected, the optimal therapeutic dose for this new formulation of vortioxetine was not well defined, and it might be considered if the IV dose of 17 mg used in this study was too low. Still, the findings from this study may inform future studies, which are warranted to further investigate the potential of vortioxetine to induce fast antidepressant relief, using other routes than per oral administration while aiming at minimizing placebo effects.
Conclusion
In this study, a single, initial dose of IV vortioxetine 17 mg added to oral vortioxetine 10 mg/day was not superior to IV placebo plus oral vortioxetine 10 mg/day in improving depressive symptoms as measured by MADRS total score at day 7. Substantial symptomatic improvements in both treatment groups were seen already within 24 hours after the IV doses, with numerical treatment differences at days 1 and 3 in favour of vortioxetine. PK data confirmed IV vortioxetine to result in steady-state blood concentration levels within 24 hours. Vortioxetine as oral and IV formulations was well-tolerated, with nausea as the most common TEAE.
Acknowledgements
We thank all participants in the study, as well as the investigators and sites involved in conducting the trial.
Medical writing support was provided by Hanne-Lise F. Eriksen, PhD, employee of H. Lundbeck A/S. This study was supported by H. Lundbeck A/S.
Conflicts of interest
E.V. has received grants and served as consultant, advisor or CME speaker for the following entities: AB-Biotics, Allergan, Angelini, AstraZeneca, Bristol-Myers Squibb, Farmindustria, Ferrer, Forest Research Institute, Gedeon Richter, Glaxo-Smith-Kline, Janssen, Lundbeck, Otsuka, Pfizer, Roche, SAGE, Sanofi-Aventis, Servier, Shire, Sunovion, Takeda, the Brain and Behaviour Foundation, the Spanish Ministry of Science and Innovation (CIBERSAM), the Seventh European Framework Programme (ENBREC), and the Stanley Medical Research Institute. I.F., S.N.S., J.A., and A.E. are employees of Lundbeck.
References
- Alvarez E, Perez V, Dragheim M, Loft H, Artigas F. A double-blind, randomized, placebo-controlled, active reference study of Lu AA21004 in patients with major depressive disorder. Int J Neuropsychopharmacol. 2012; 15:589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 20165th ed Washington, DC: American Psychiatric Association. [Google Scholar]
- Areberg J, Petersen KB, Chen G, Naik H. Population pharmacokinetic meta-analysis of vortioxetine in healthy individuals. Basic Clin Pharmacol Toxicol. 2014; 115:552–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Areberg J, Sogaard B, Hojer AM. The clinical pharmacokinetics of Lu AA21004 and its major metabolite in healthy young volunteers. Basic Clin Pharmacol Toxicol. 2012; 111:198–205. [DOI] [PubMed] [Google Scholar]
- Artigas F, Bortolozzi A, Celada P. Can we increase speed and efficacy of antidepressant treatments? Part I: general aspects and monoamine-based strategies. Eur Neuropsychopharmacol. 2018; 28:445–456. [DOI] [PubMed] [Google Scholar]
- Baldwin DS, Chrones L, Florea I, Nielsen R, Nomikos GG, Palo W, Reines E. The safety and tolerability of vortioxetine: analysis of data from randomized placebo-controlled trials and open-label extension studies. J Psychopharmacol. 2016a; 30:242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin DS, Florea I, Jacobsen PL, Zhong W, Nomikos GG. A meta-analysis of the efficacy of vortioxetine in patients with major depressive disorder (MDD) and high levels of anxiety symptoms. J Affect Disord. 2016b; 206:140–150. [DOI] [PubMed] [Google Scholar]
- Bech P. Rating scales in depression: limitations and pitfalls. Dialogues Clin Neurosci. 2006; 8:207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bech P, Tanghøj P, Andersen H, Overø K. Citalopram dose-response revisited using an alternative psychometric approach to evaluate clinical effects of four fixed citalopram doses compared to placebo in patients with major depression. Psychopharmacology. 2002; 163:20–25. [DOI] [PubMed] [Google Scholar]
- Bétry C, Pehrson AL, Etiévant A, Ebert B, Sánchez C, Haddjeri N. The rapid recovery of 5-HT cell firing induced by the antidepressant vortioxetine involves 5-HT3 receptor antagonism. Int J Neuropsychopharmacol. 2013; 16:1115–1127. [DOI] [PubMed] [Google Scholar]
- Carlino E, Vase L. Can knowledge of placebo and nocebo mechanisms help improve randomized clinical trials? Int Rev Neurobiol. 2018; 138:329–357. [DOI] [PubMed] [Google Scholar]
- Daly EJ, Singh JB, Fedgchin M, Cooper K, Lim P, Shelton RC, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018; 75:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Brown AS, Cannon TD, Druss BG, Earls FJ, Escobar J, et al. Can a framework be established for the safe use of ketamine? Am J Psychiatry. 2018; 175:587–589. [DOI] [PubMed] [Google Scholar]
- Guelfi JD, Strub N, Loft H. Efficacy of intravenous citalopram compared with oral citalopram for severe depression. Safety and efficacy data from a double-blind, double-dummy trial. J Affect Disord. 2000; 58:201–209. [DOI] [PubMed] [Google Scholar]
- Guy W. Guy W. Clinical global impressions. In: ECDEU Assessment Manual for Psychopharmacology. 1976Revised ed Rockville, MD: National Institute of Mental Health. [Google Scholar]
- Katona C, Hansen T, Olsen CK. A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol. 2012; 27:215–23. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R, Salvadore G, Luckenbaugh DA, Manji HK, Zarate CA., Jr Rapid onset of antidepressant action: a new paradigm in the research and treatment of major depressive disorder. J Clin Psychiatry. 2008; 69:946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979; 134:382–9. [DOI] [PubMed] [Google Scholar]
- Moukaddam NJ, Hirschfeld RMA. Intravenous antidepressants: a review. Depress Anxiety. 2004; 19:1–9. [DOI] [PubMed] [Google Scholar]
- Norman TR, Olver JS. New formulations of existing antidepressants: advantages in the management of depression. CNS Drugs. 2004; 18:505–520. [DOI] [PubMed] [Google Scholar]
- Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, et al. The Columbia–Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011; 168:1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salagre E, Grande I, Sole B, Sanchez-Moreno J, Vieta E. Vortioxetine: a new alternative for the treatment of major depressive disorder. Rev Psiquiatr Salud Ment. 2018; 11:48–59. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Asin KE, Artigas F. Vortioxetine, a novel antidepressant with multimodal activity: review of preclinical and clinical data. Pharmacol Ther. 2015; 145:43–57. [DOI] [PubMed] [Google Scholar]
- Schedlowski M, Enck P, Rief W, Bingel U. Neuro-bio-behavioral mechanisms of placebo and nocebo responses: implications for clinical trials and clinical practice. Pharmacol Rev. 2015; 67:697–730. [DOI] [PubMed] [Google Scholar]
- Stenkrona P, Halldin C, Lundberg J. 5-HTT and 5-HT1A receptor occupancy of the novel substance vortioxetine (Lu AA21004). A PET study in control subjects. Eur Neuropsychopharmacol. 2013; 23:1190–1198. [DOI] [PubMed] [Google Scholar]
- Thase ME, Bowden CL, Nashat M, Eudicone JM, Marcus R, Mcquade RD, Carlson BX. Aripiprazole in bipolar depression: a pooled, post-hoc analysis by severity of core depressive symptoms. Int J Psychiatry Clin Pract. 2012; 16:121–131. [DOI] [PubMed] [Google Scholar]
- Thase ME, Mahableshwarkar AR, Dragheim M, Loft H, Vieta E. A meta-analysis of randomized, placebo-controlled trials of vortioxetine for the treatment of major depressive disorder in adults. Eur Neuropsychopharmacol. 2016; 26:979–993. [DOI] [PubMed] [Google Scholar]
- Vieta E, Loft H, Florea I. Effectiveness of long-term vortioxetine treatment of patients with major depressive disorder. Eur Neuropsychopharmacol. 2017; 27:877–884. [DOI] [PubMed] [Google Scholar]
- Witkin JM, Knutson DE, Rodriguez GJ, Shi S. Rapid-acting antidepressants. Curr Pharm Des. 2018; 24:2556–2563. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates. 2017. Geneva, Switzerland: World Health Organization. [Google Scholar]
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983; 67:361–370. [DOI] [PubMed] [Google Scholar]


