Abstract
Background:
Results from the HPTN 065 study showed that financial incentives (FI) were associated with significantly higher viral load suppression and higher levels of engagement in care among patients at HIV care sites randomized to FI versus sites randomized to standard of care (SOC). We assessed HIV viral suppression and continuity in care after intervention withdrawal to determine the durability of FI on these outcomes.
Setting:
A total of 37 HIV test and 39 HIV care sites in the Bronx, New York, and Washington, DC, participated in the study.
Methods:
Laboratory data reported to the US National HIV Surveillance System were used to determine site-level viral suppression and continuity in care outcomes. Postintervention effects were assessed for the 3 quarters after discontinuation of FI. Generalized estimation equations were used to compare FI and SOC site-level outcomes after intervention withdrawal.
Results:
After FI withdrawal, a trend remained for an increase in viral suppression by 2.7% (−0.3%, 5.6%, P = 0.076) at FI versus SOC sites, decreasing from the 3.8% increase noted during implementation of the intervention. The significant increase in continuity in care during the FI intervention was sustained after intervention with 7.5% (P = 0.007) higher continuity in care at FI versus SOC sites.
Conclusions:
After the withdrawal of FI, findings at the 9-months postintervention withdrawal from this large study showed evidence of durable effects of FI on continuity in care, with trend for continued higher viral suppression. These findings are promising for adoption of such interventions to enhance key HIV-related care outcomes.
Key Words: financial incentives, viral suppression, continuity in care, durability
INTRODUCTION
Financial incentives (FI) are intended to motivate and influence behaviors to achieve desired health outcomes. Studies have evaluated the effects of FI on a wide array of health behaviors including tobacco cessation, weight reduction, chronic disease management, prevention of HIV infection, voluntary medical male circumcision, and adherence with a variety of medications including anticoagulants and antiretroviral therapy (ART).1–10 It is widely recognized that adherence to ART is critical for achieving viral suppression, leading to decreased HIV-related morbidity and mortality for the HIV-infected person as well as societal benefit through prevention of HIV transmission to sexual partners.11,12
HPTN 065, a large community-based multicomponent study conducted by the HIV Prevention Trials Network (HPTN), examined the feasibility of a test, link-to-care, plus treat strategy for HIV prevention in the Bronx, NY, and Washington, DC.13 One component involved the evaluation, through a large site-randomized study, of the effectiveness of FI for viral suppression among HIV-positive patients engaged in care. Results showed that FI were associated with significantly higher levels of viral load suppression and higher levels of engagement in care among patients at sites randomized to FI compared to those randomized to standard of care (SOC).14 Economic analyses also demonstrated the cost-effectiveness of the two-year FI intervention as used in HPTN 065.15 An important remaining question is whether these effects were sustained after FI were withdrawn. In this analysis, we assess the durability of the increase in viral suppression and continuity in care in the 9 months after the end of FI.
METHODS
A total of 37 (20 Bronx, NY/17 Washington, DC) HIV care sites with 51,782 patients in care (28,439 Bronx, NY/23,343 Washington, DC) were randomized to FI or SOC. At sites randomized to FI, from February 2011 through January 2013, patients on ART could earn a $70 gift card quarterly if they were virally suppressed. Postintervention effects were assessed for the 3 quarters after discontinuation of FI (April to December 2013).
The 2 outcomes for the study, viral suppression and continuity in care, were assessed using viral load laboratory data that were routinely reported from the laboratories to local health departments and uploaded in the US National HIV Surveillance System. Viral suppression was assessed each calendar quarter for each study site, and was defined as the proportion of patients at a site with a viral load of <400 copies/ml among those currently engaged in care, ie, those who had site visits in 2 of the prior 5 quarters. Continuity in care was defined as the proportion having evidence of a clinical visit (ie, a CD4+ cell count or viral load test data in the Surveillance Database) in 4 of the prior 5 quarters. We used the generalized estimation equation method to compare FI and SOC site-level outcomes after withdrawal of the intervention, adjusted for preintervention viral suppression and weighted by the number of patients at each site. Four a priori site subgroups were defined: Bronx versus Washington DC, community clinics versus hospital-based sites, sites with lower versus high baseline viral suppression, and larger versus smaller clinics (< or >median).
RESULTS
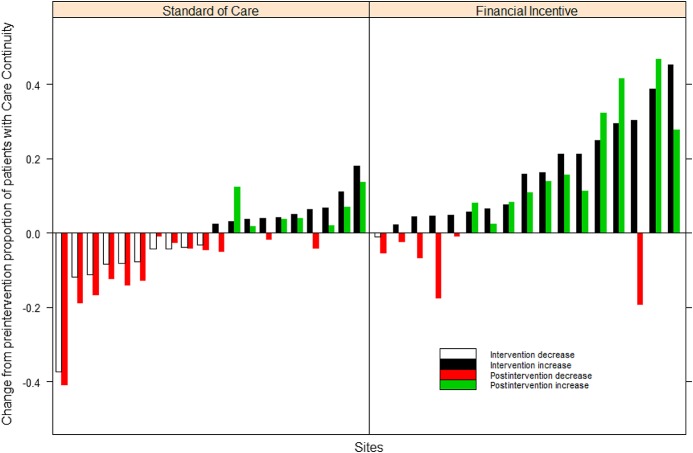
Figure 1 shows changes from baseline in viral suppression and continuity in care during and after FI intervention at sites randomized to FI versus SOC during the 9 months after intervention. In the postintervention period, the increase in viral suppression was 2.7% (−0.3%, 5.6%, P = 0.076) at FI versus SOC sites (Table 1). This trend in higher viral suppression noted at FI sites decreased from the significantly higher viral suppression of 3.8% achieved during the implementation of the intervention.
FIGURE 1.
Effect of FI postintervention HIV viral suppression and continuity care at standard of care and FI sites.
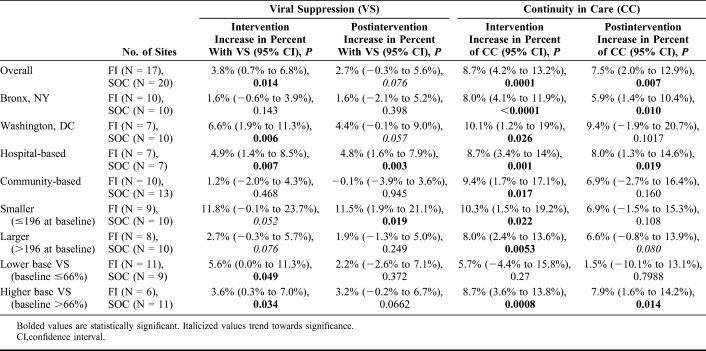
TABLE 1.
Effects of FI During and After Intervention on Viral Suppression and Continuity in Care
At hospital-based sites, viral suppression was sustained at 4.8% higher at former FI sites, P = 0.003 (reduced from 4.9% during the intervention period, P = 0.007). Similar trends of sustained albeit slightly reduced differences were seen in 2 other subsets of sites: in Washington, DC, viral suppression remained 4.4% higher, P = 0.057 (compared to 6.6%, P = 0.006); and at sites with higher baseline viral suppression, viral suppression remained 3.2% higher, P = 0.066 (compared to 3.6%, P = 0.03).
The increase achieved in continuity of care at sites randomized to FI was durable, with a 7.5% (P = 0.007) higher proportion of patients continuing regular visits at FI sites, compared to an 8.7% (P = 0.0001) increase during the intervention period (Table 1). The positive effect on continuity in care persisted within the following subset of sites: the Bronx, NY (P = 0.010), at hospital-based sites (P = 0.019), and at sites with higher baseline viral suppression (P = 0.014) (Table 1).
DISCUSSION
Optimizing the HIV care continuum is critically important to achieve the potential benefits of ART in both prevention and treatment.16 However, gaps remain in this continuum, and interventions based on behavioral economics may motivate behavioral change across the prevention and care continua.17 FI have been evaluated in studies to improve each step of the care continuum: HIV testing, linkage to care, and viral load suppression.5,18 Multiple types of FI have been studied to motivate adherence with ART and viral suppression, including cash financial transfers, noncash FI, vouchers, and commitment contracts.19 Four US-based studies have assessed FI on viral suppression. Two studies among persons who inject drugs showed the benefit of contingency management with voucher prizes on adherence to ART, whereas 3 studies did not show benefits on viral suppression.10,20–22 A recent small study among HIV-positive patients in the United States also failed to show benefits of provider visit incentives or visit incentives plus a commitment contract for achievement of viral suppression.23 However, HPTN 065, the largest study to date to assess the effectiveness of FI on viral load suppression and continuity in care, demonstrated a significant increase in viral suppression and continuity in care.
The ultimate goal of an intervention such as FI is to move adherence behaviors from being externally motivated to internally motivated, for example, by developing new clinic visit and pill-taking habits, or experiencing the self-reinforcing benefits of remaining in care and viral load suppression.24,25 However, despite the positive effects of FI noted in some of the studies, a common limitation is the absence of data on the durability of the effects noted beyond the period of the intervention. In the few studies that have assessed durability of the effect of FI, the postintervention assessment period was only for a short time after withdrawal of FI (eg, 3 months). In a study by Alsan et al,23 a beneficial effect was noted 3 months after withdrawal of the FI, whereas none was noted during the intervention period; however, the study had limited sample size and the follow-on assessment was a post hoc analysis. In another study that evaluated individual versus group-based FI for weight loss, the beneficial effect was durable 3 months after withdrawal of the intervention.26
In the HPTN 065 study, we report durability of the benefits of FI on viral suppression and continuity in care for a full 9 months after withdrawal of the intervention. Reasons for persistence of benefits may be gleaned from qualitative work conducted among staff and patients at sites randomized to FI in HPTN 065.27,28 In interviews with recipients of FI and staff involved in the intervention at those sites, the incentives were widely welcomed and associated with strong bidirectional emotional benefits, ie, for both the providers and recipients of care. The durability of significant effect of FI on continuity in care demonstrates deeper engagement of patients with their providers and offers an opportunity to garner benefits from ongoing contact with their providers. Participants reported being disappointed that the FI were not maintained beyond the intervention period. Thus, it is noteworthy to observe the sustained increase in contact with providers and trend toward durability of positive effects of the FI after they ended.
The durability of the FI effect noted in our study is an important finding from the perspective of policy makers, program managers, and funders. Common concerns cited regarding FI is their associated costs and the feasibility of sustaining the systems to provide this intervention, particularly in the context of a chronic condition such as HIV disease which requires lifelong engagement in care and adherence to treatment. Our findings demonstrate that after 2 years of FI, HIV-positive patients had sustained improvements in their adherence and care behaviors through 9 months after withdrawal of FI.
CI, confidence interval.
Footnotes
The HIV Prevention Trials Network (HPTN) 065 study is sponsored by the National Institute of Allergy and Infectious Diseases, the National Institute of Mental Health, and the National Institute on Drug Abuse, of the US National Institutes of Health, under Cooperative Agreements #UM1 AI 068619 and UM1 AI 068617, as well as the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention.
Presented in part as a poster presentation at the Conference on Retroviruses and Opportunistic Infections (CROI); March 4–7, 2018; Boston, MA.
The authors have no conflicts of interest to disclose.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases, the National Institutes of Health, or the Centers for Disease Control and Prevention.
REFERENCES
- 1.Volpp KG, Troxel AB, Pauly MV, et al. A randomized, controlled trial of financial incentives for smoking cessation. N Engl J Med. 2009;360:699–709. [DOI] [PubMed] [Google Scholar]
- 2.John LK, Loewenstein G, Troxel AB, et al. Financial incentives for extended weight loss: a randomized, controlled trial. J Gen Intern Med. 2011;26:621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volpp KG, John LK, Troxel AB, et al. Financial incentive-based approaches for weight loss: a randomized trial. JAMA. 2008;300:2631–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.VanEpps EM, Troxel AB, Villamil E, et al. Financial incentives for chronic disease management: results and limitations of 2 randomized clinical trials with New York Medicaid patients. Am J Health Promot. 2018;32:1537–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pettifor A, MacPhail C, Nguyen N, et al. Can money prevent the spread of HIV? A review of cash payments for HIV prevention. AIDS Behav. 2012;16:1729–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.deWalque D, Dow WH, Nathan R, et al. Incentivising safe sex: a randomised trial of conditional cash transfers for HIV and sexually transmitted infection prevention in rural Tanzania. BMJ Open. 2012;2:e000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nyqvist MB, Corno L, deWalque D, et al. Using Lotteries to Incentivize Safer Sexual Behavior: Evidence from a Randomized Controlled Trial on HIV Prevention. World Bank Group; 2015. WPS7215. Available at: https://openknowledge.worldbank.org/bitstream/handle/10986/21654/WPS7215.pdf. Accessed March 20, 2018. [Google Scholar]
- 8.Thirumurthy H, Masters SH, Rao S, et al. Effect of providing conditional economic compensation on uptake of voluntary medical male circumcision in Kenya: a randomized clinical trial. JAMA. 2014;312:703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimmel SE, Troxel AB, Loewenstein G, et al. Randomized trial of lottery-based incentives to improve warfarin adherence. Am Heart J. 2012;164:268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metsch LR, Feaster DJ, Gooden L, et al. Effect of patient navigation with or without financial incentives on viral suppression among hospitalized patients with HIV infection and substance use: a randomized clinical trial. JAMA. 2016;316:156–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lundgren JD, Babiker AG, Gordin F, et al. ; INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen MS, Chen YQ, McCauley M, et al. ; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gamble T, Branson B, Donnell D, et al. ; HPTN 065 Study Team. Design of the HPTN 065 (TLC-Plus) study: a study to evaluate the feasibility of an enhanced test, link-to-care, plus treat approach for HIV prevention in the United States. Clin Trials. 2017;14:322–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Sadr WM, Donnell D, Beauchamp G, et al. ; HPTN 065 Study Team. Financial incentives for linkage to care and viral suppression among HIV-positive patients: a randomized clinical trial (HPTN 065). JAMA Intern Med. 2017;177:1083–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adamson BJ, Donnell D, Dimitrov D, et al. The Cost-effectiveness of financial incentives for viral suppression in HPTN 065. Presented at: CROI 2017; February 13–17, 2017; Seattle, WA. [DOI] [PMC free article] [PubMed]
- 16.McNairy ML, El-Sadr WM. The HIV care continuum: no partial credit given. AIDS. 2012;26:1735–1738. [DOI] [PubMed] [Google Scholar]
- 17.Bassett IV, Wilson D, Taaffe J, et al. Financial incentives to improve progression through the HIV treatment cascade. Curr Opin HIV AIDS. 2015;10:451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maughan-Brown B, Smith P, Kuo C, et al. A conditional economic incentive fails to improve linkage to care and antiretroviral therapy initiation among HIV-positive adults in Cape Town, South Africa. AIDS Patient Care STDS. 2018;32:70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galárraga O, Genberg BL, Martin RA, et al. Conditional economic incentives to improve HIV treatment adherence: literature review and theoretical considerations. AIDS Behav. 2013;17:2283–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosen MI, Dieckhaus K, McMahon TJ, et al. Improved adherence with contingency management. AIDS Patient Care STDS. 2007;21: 30–40. [DOI] [PubMed] [Google Scholar]
- 21.Sorensen JL, Haug NA, Delucchi KL, et al. Voucher reinforcement improves medication adherence in HIV-positive methadone patients: a randomized trial. Drug Alcohol Depend. 2007;88:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rigsby MO, Rosen MI, Beauvais JE, et al. Cue-dose training with monetary reinforcement: pilot study of an antiretroviral adherence intervention. J Gen Intern Med. 2000;15:841–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alsan M, Beshears J, Armstrong WS, et al. A commitment contract to achieve virologic suppression in poorly adherent patients with HIV/AIDS. AIDS. 2017;31:1765–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deci EL, Ryan RM. The support of autonomy and the control of behavior. J Pers Soc Psychol. 1987;53:1024–1037. [DOI] [PubMed] [Google Scholar]
- 25.Mantzari E, Vogt F, Shemilt I, et al. Personal financial incentives for changing habitual health-related behaviors: a systematic review and meta-analysis. Prev Med. 2015;75:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kullgren JT, Troxel AB, Loewenstein G, et al. Individual- versus group-based financial incentives for weight loss: a randomized, controlled trial. Ann Intern Med. 2013;158:505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tolley EE, Taylor J, Pack A, et al. The role of financial incentives along the antiretroviral therapy adherence continuum: a qualitative sub-study of the HPTN 065 (TLC-Plus) study. AIDS Behav. 2018;22:245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greene E, Pack A, Stanton J, et al. “It Makes You Feel like Someone Cares” acceptability of a financial incentive intervention for HIV viral suppression in the HPTN 065 (TLC-Plus) study. PLoS One. 2017;12:e0170686. [DOI] [PMC free article] [PubMed] [Google Scholar]