Abstract
Background:
Metoclopramide is used to relieve gastrointestinal symptoms, however, it could cause adverse reactions of motor disorders. The aim of this study was to investigate whether metoclopramide treatment has a duration–response or dose–response effect and to estimate the risk of developing Parkinsonism following different and specific durations of treatment.
Methods:
A cohort study of newly diagnosed type 2 diabetes mellitus in 45- to 79-year-old patients, between 1999 and 2008, was selected using the Longitudinal Health Insurance Database 2005. A nested case–control study was conducted in the diabetes cohort in which all incident cases of Parkinsonism were identified. We randomly matched each case with up to 10 controls from the risk set. Conditional logistic regression was utilized to estimate odds ratio of Parkinsonism associated with metoclopramide use.
Results:
A total of 34,685 patients with diabetes were assembled as the cohort, and 541 incident Parkinsonism cases were identified. There were duration–response and dose–response effects on the risk of developing Parkinsonism. Compared with never-use patients, the adjusted odds ratios (ORs) of continuing therapy for 0–1 month, 1–2 months, 2–3 months, 3–5 months, and more than 5 months were 1.17 [95% confidence interval (CI) 0.93–1.45], 1.44 (95% CI 1.04–2.00), 1.74 (95% CI 1.14–2.65), 1.90 (95% CI 1.23–2.93), and 2.17 (95% CI 1.50–3.12), respectively.
Conclusions:
With metoclopramide treatment, regardless of less or more than 3 months of use, the risk of developing Parkinsonism in patients with newly diagnosed diabetes escalated with the duration of therapy. Therefore, we recommend close monitoring for the development of Parkinsonism in patients treated with metoclopramide, particularly (but not limited to) those with prolonged exposure.
Keywords: metoclopramide, Parkinsonism, type 2 diabetes, Taiwan
Introduction
Parkinsonism, one of the most common movement disorders in the elderly, is characterized by a constellation of rigidity, bradykinesia, impaired postural reflex, and tremor.1 Although idiopathic Parkinson’s disease is the most common etiology of Parkinsonism, drug-induced Parkinsonism accounts for the second most common cause.2,3 Recognizing drug-induced Parkinsonism is important because it could be reversible if the medicine was discontinued in time.4–6
Diabetes is the leading cause of mortality and morbidity worldwide, and it is associated with a variety of complications. Gastrointestinal complications usually manifest as esophageal dysmotility, gastroparesis, constipation, and diarrhea. In addition, many glucose-lowering agents have potential gastrointestinal side effects.7,8 Prokinetic agents, such as metoclopramide, are commonly used to relieve gastrointestinal symptoms in diabetes patients.9 In 2009, the United States Food and Drug Administration (FDA) issued a boxed warning for the use metoclopramide citing the risk of irreversible tardive dyskinesia.10 Following the black box warning, 23.7% of patients with gastroparesis were still prescribed metoclopramide in the North-Shore University health system, Illinois, United States.11 Both drug-induced Parkinsonism and tardive dyskinesia are drug-induced movement disorders, but the clinical manifestations are different. In addition, both of them could be the adverse reactions of metoclopramide treatment.12 Therefore, the boxed warning for irreversible tardive dyskinesia was adapted for drug-induced Parkinsonism in this study.
Metoclopramide has been reported to cause Parkinsonism with long-term use13,14 because it readily crosses the blood–brain barrier to inhibit dopamine D2 group receptors.15 Indeed, the prescription of metoclopramide in patients with diabetes to relieve gastroparesis is a drug safety issue. To the best of the authors’ knowledge, there has been no study investigating the risk of metoclopramide-induced Parkinsonism specifically in a diabetic population. Therefore, we conducted a nested case–control study to investigate whether metoclopramide treatment had a dose–response effect or duration effect on developing Parkinsonism and to estimate if the risk is increased among those taking metoclopramide for less than 3 months.
Methods
Data source
Taiwan launched a single-payer National Health Insurance (NHI) program in 1995; nearly all of Taiwan’s population is enrolled. NHI provides all necessary medical services including hospitalization, outpatient care, rehabilitation, Chinese medicine, dental treatment, vaccination, and all other essential services for all insured individuals. The National Health Insurance Research Database (NHIRD) is a digitalized database containing demographic information, date of medical service, diagnosis, item, and cost of all kinds of medical orders for all claims. However, the dataset lacks the results of all kinds of examination and personal information such as habits of tobacco or alcohol use, family history, and body height and weight. The Longitudinal Health Insurance Database 2005 (LHID 2005), a subset of NHIRD containing all data of original claim of 1 million beneficiaries enrolled in 2005, was used for this study. There is no significant difference between NHIRD and LHID 2005 for age and sex distribution or average insured payroll-related amount.16 De-identified LHID 2005 data was obtained following a double encryption process. This study was approved by the Institutional Review Board of Changhua Christian Hospital. Informed consent was waived because this study used de-identified data.
Study cohort
We established a diabetes cohort by retrieving from LHID 2005 all newly diagnosed patients with mild to moderate type 2 diabetes, aged 45–79 years, during 1999–2008. The diagnosis of diabetes was defined as having at least three outpatient visit or one admission records with diagnosis of the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) code 250.xx or A181 and prescriptions of antidiabetic medication for over 60 days. Individuals who had insulin prescription within 1 year of the diagnosis of diabetes were excluded to avoid type 1 or advanced diabetes, and patients with malignancy were also excluded. The rationale for the inclusion and exclusion criteria includes: type 1 diabetes usually begins before age 40 while Parkinsonism usually begins after age 50. In addition, advanced stage of diabetes and malignancy more often have various complications that are likely confounders.
We defined the date of first diabetes diagnostic date as the cohort entry. We also excluded those who had Parkinsonism diagnosed within 1 year of diabetes. Parkinsonism is a slowly progressive disorders, which might lead to a delayed diagnosis. In addition, stroke may cause vascular Parkinsonism, and drugs used to treat mood disorders might cause drug-induced Parkinsonism. Therefore, we excluded those who had a stroke, mood disorders, and cancers before the cohort entry. These exclusions ensured a follow-up period of over 1 year and reduced confounding factors.
Selection of cases and controls
A nested case–control study was conducted within the diabetic cohort. The ICD-9-CM code 332.0, indicating the code of Parkinson’s disease whereas the ICD-9-CM code 332.1 meant secondary Parkinsonism. Because it is often challenging to distinguish idiopathic Parkinson disease from secondary Parkinsonism. Therefore, both 332.0 and 332.1 were selected as cases of Parkinsonism in our study. All selected cases had at least three records of neurology outpatient visits or one neurology admission. We randomly matched each Parkinsonism case with up to 10 controls from the risk set by sex, age, calendar year of cohort entry, and follow-up duration. The index date was defined as the date of Parkinsonism diagnosed for the first time. For controls, the index date was set as the same date as their matched Parkinsonism case.
Potential confounding factors
Some risk factors that might influence the occurrence of Parkinsonism were identified in this study, including the age of diabetes onset, sex, duration from diabetes to the index date, some comorbidities, and use of statin [Anatomical Therapeutic Chemical (ATC) classification C10AA] and antipsychotic drugs (ATC classification N05A). Comorbidities comprised stroke (ICD-9-CM 430-434), hypertension (ICD-9-CM 401), episodic mood disorders (ICD-9-CM 296), schizophrenic disorders (ICD-9-CM 295), and hyperlipidemia (ICD-9-CM 272). The diagnosis of comorbidities was identified with at least three outpatient visits or one admission claim before index date. (The detailed coding of diseases and medication are listed in the online supplementary material Table S3.)
Exposure to metoclopramide
Information for the dose of metoclopramide prescriptions was acquired from the NHI website. We identified all prescriptions of metoclopramide dispensed to patients during the period from cohort entry to the index date, and we calculated the correlated indicators, such as the duration from first to last prescription, cumulative drug days, and cumulative dosage of metoclopramide use for each participant. Further, while patients were dispensed metoclopramide continuously over 3 months, the period was defined as overuse.
Statistical analyses
In order to examine whether the frequency of metoclopramide use is higher in diabetes than in hypertension population, we assembled overall metoclopramide records for all patients who were diagnosed diabetes or hypertension according to the calendar year.
Descriptive statistics were used to summarize the characteristics of study cases and matched controls. Chi-squared test was performed to test the difference between categorical variables. Independent t test was used to test the mean difference for continuous variables between cases and matched controls.
We used conditional logistic regression to estimate odds ratio along with corresponding 95% confidence intervals of Parkinsonism associated with the use of metoclopramide. We counted patients without exposure to metoclopramide as ‘never’ between cohort entry and index date. We split duration–response and dose–response variables into four categories by tertiles based on the distribution of metoclopramide use of control group. We evaluated the duration–response and dose–response relationship and used the transformed ordinal variables as continuous variables in the model to test whether there were a linear relationship between the metoclopramide dose and the risk of Parkinsonism.
Furthermore, we collected all metoclopramide prescriptions between cohort entry and index date. Then we calculated the duration of the consecutive prescriptions. If the gap between consecutive prescriptions was less than 7 days, we treated the prescriptions as consecutive. However, if the gap was more than 7 days, the treatments were considered nonconsecutive. Subsequently, we selected the maximum duration of metoclopramide for each user and divided the consecutive duration into less than 1 month, 1–2 months, 2–3 months, 3-5 months, and over 5 months. The FDA does not recommend the continuous use of metoclopramide for over 3 months; therefore, patients who were dispensed metoclopramide over 3 months were treated as overuse. In addition, we collected dose per day in concordance with continuous duration of metoclopramide use. All statistical analyses were performed with R version 3.2.3.17,18
Results
Comparing the utilization of metoclopramide between diabetes and hypertension population
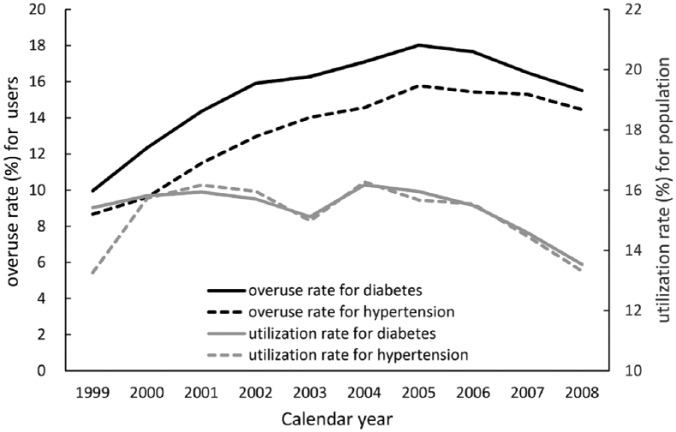
The utilization of metoclopramide in diabetes and hypertension population is similar, however, the overuse rate of metoclopramide in diabetes was higher than in hypertension population. (Figure 1 and the detailed results are listed in the online supplementary material Tables S1 and S2)
Figure 1.
The utilization and overuse rate of metoclopramide for diabetes and hypertension population by year.
Baseline characteristics of study subjects
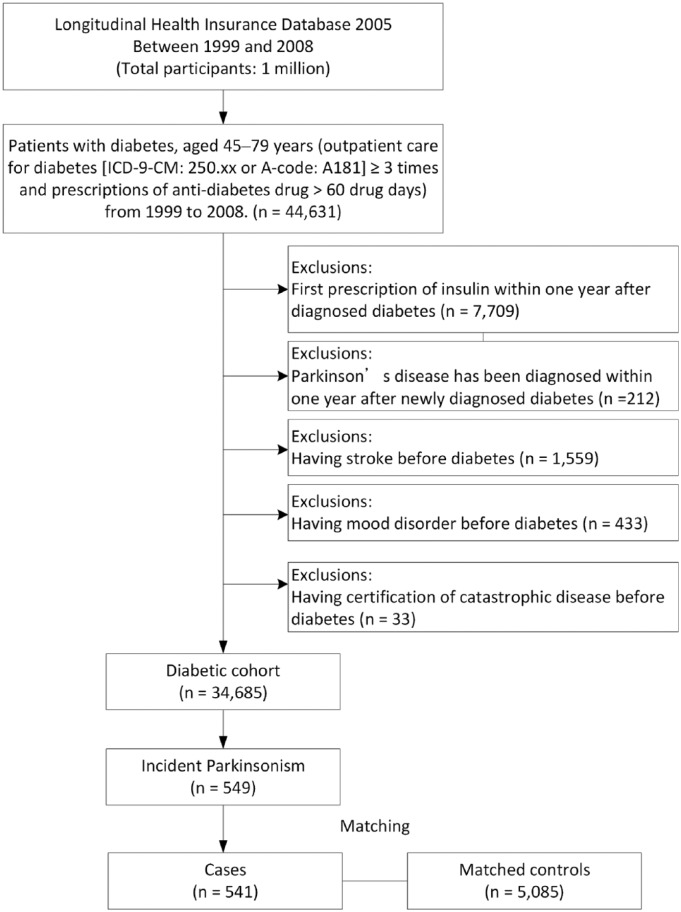
A diabetic cohort was assembled consisting of a total of 34,685 patients with mild–moderate type 2 diabetes and aged 45–79 years (Figure 2). After risk set sampling, there were 541 incident Parkinsonism and 5085 matched controls. Eight cases of incident Parkinsonism were not included because of no eligible controls. The mean age was 66.8 (range: 46–80) years and 44% were male participants. As listed in Table 1, patients and matched controls were similar in distributions of comorbidities and use of statin and antipsychotics.
Figure 2.
Flow chart of the study participants.
ICD-9-CM, International Classification of Diseases, 9th Revision, Clinical Modification.
Table 1.
Demographic characteristics of Parkinson’s disease and matched controls.
| Case | Control | p value | |||
|---|---|---|---|---|---|
| N | % | n | % | ||
| Number of subjects | |||||
| Gendera | |||||
| Male | 244 | 45.1 | 2258 | 44.41 | 0.7566 |
| Female | 297 | 54.9 | 2827 | 55.59 | |
| Agea,b | 66.93 | 6.8 | 66.79 | 6.91 | 0.6560 |
| <65 years old | 175 | 32.35 | 1741 | 34.24 | 0.3777 |
| ⩾65 years old | 366 | 67.65 | 3344 | 65.76 | |
| Duration of follow up (years)a,b | 5.24 | 2.46 | 5.25 | 2.44 | 0.8928 |
| <5 | 266 | 49.17 | 2473 | 48.63 | 0.8129 |
| ⩾5 | 275 | 50.83 | 2612 | 51.37 | |
| Hypertension | |||||
| No | 365 | 67.47 | 3585 | 70.5 | 0.1424 |
| Yes | 176 | 32.53 | 1500 | 29.5 | |
| Hyperlipidemia | |||||
| No | 467 | 86.32 | 4378 | 86.1 | 0.8854 |
| Yes | 74 | 13.68 | 707 | 13.9 | |
| Any statin use | |||||
| No | 501 | 92.61 | 4791 | 94.22 | 0.1314 |
| Yes | 40 | 7.39 | 294 | 5.78 | |
| Any antipsychotics use | |||||
| No | 503 | 92.98 | 4821 | 94.81 | 0.0722 |
| Yes | 38 | 7.02 | 264 | 5.19 | |
Matched variables.
Mean and standard deviation.
Metoclopramide use and the risk of Parkinsonism
Table 2 lists the relationship between metoclopramide and the development of Parkinsonism. Overall, the use of metoclopramide was associated with an increased risk of Parkinsonism, with adjusted odds ratio (OR) 1.39 [95% confidence interval (CI) 1.16–1.67]. Table 2 also demonstrated a duration–response relationship observed in both durations between first and last prescription (p for trend <0.001) and cumulative days (p < 0.001). In addition, a linear dose–response effect was also detected in the cumulative dosage of metoclopramide (p for trend <0.001).
Table 2.
Crude and adjusted odds ratios (ORs) of Parkinsonism associated with the use of metoclopramide.
| Case | Control | Crude | Adjusteda | |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | |||
| Metoclopramide use | ||||
| Never | 256 (47.3) | 2,811 (55.3) | 1.00 | 1.00 |
| Ever | 285 (52.7) | 2,274 (44.7) | 1.40 (1.17–1.68) | 1.39 (1.16–1.67) |
| Duration between first and last prescriptionb | ||||
| No | 256 (47.3) | 2,811 (55.3) | 1.00 | 1.00 |
| 1–28 | 84 (15.5) | 797 (15.7) | 1.19 (0.91–1.54) | 1.18 (0.91–1.54) |
| 29–677 | 91 (16.8) | 705 (13.9) | 1.43 (1.11–1.84) | 1.42 (1.10–1.83) |
| >677 | 110 (20.3) | 772 (15.2) | 1.63 (1.27–2.11) | 1.63 (1.26–2.10) |
| p for trend | <0.001 | |||
| Cumulative duration of use (days)b | ||||
| No | 256 (47.3) | 2,811 (55.3) | 1.00 | 1.00 |
| 1–8 | 77 (14.2) | 812 (16.0) | 1.06 (0.81–1.39) | 1.06 (0.81–1.39) |
| 9–41 | 82 (15.2) | 696 (13.7) | 1.32 (1.02–1.73) | 1.31 (1.00–1.71) |
| >41 | 126 (23.3) | 766 (15.1) | 1.88 (1.48–2.38) | 1.87 (1.47–2.37) |
| p for trend | <0.001 | |||
| Cumulative dose of metoclopramideb | ||||
| No | 256 (47.3) | 2,811 (55.3) | 1.00 | 1.00 |
| 1–103 mg | 76 (14.1) | 766 (15.1) | 1.11 (0.84–1.45) | 1.10 (0.84–1.45) |
| 104–449 mg | 79 (14.6) | 735 (14.5) | 1.21 (0.92–1.58) | 1.20 (0.92–1.57) |
| >449 mg | 130 (24.0) | 773 (15.2) | 1.92 (1.52–2.43) | 1.91 (1.51–2.42) |
| p for trend | <0.001 | |||
Each model adjusted for sex, age, duration from diabetes to index date, hypertension, hyperlipidemia, ever use of statin, and antipsychotics.
Categories based on the distribution of metoclopramide use of control group.
CI, confidence interval.
Dose–response and duration–response effect on metoclopramide use
There was no significant difference between never use of metoclopramide and continuous use for less than 1 month (OR 1.17; 95% CI 0.93–1.45). However, compared with the never-use group, the adjusted ORs of the continuous durations 1–2 months, 2–3 months, 3–5 months, and ⩾5 months were 1.44 (95% CI 1.04–2.00), 1.74 (95% CI 1.14–2.65), 1.90 (95% CI 1.23–2.93) and 2.17 (95% CI 1.50–3.12), respectively (Table 3). In addition, the adjusted ORs of dose per day for <10 mg, 10–20 mg, 20–30 mg, and ⩾30 mg were 0.90 (0.57–1.40), 1.43 (1.17–1.76), 1.57 (1.09–2.26), and 1.55 (1.08–2.21), respectively.
Table 3.
Crude and adjusted odds ratios (ORs) of Parkinsonism associated with continuous duration and corresponding dose per day of metoclopramide between cohort entry and index date.
| Metoclopramide use | Case | Control | Crude OR (95% CI) |
Adjusteda
OR (95% CI) |
|---|---|---|---|---|
| Continuous duration | ||||
| No | 256 (47.3) | 2811 (55.3) | 1.00 | 1.00 |
| <1 month | 138 (25.5) | 1321 (26.0) | 1.17 (0.94–1.46) | 1.17 (0.93–1.45) |
| 1–2 months | 50 (9.2) | 391 (7.7) | 1.45 (1.04–2.01) | 1.44 (1.04–2.00) |
| 2–3 months | 28 (5.2) | 177 (3.5) | 1.75 (1.15–2.67) | 1.74 (1.14–2.65) |
| 3–5 months | 27 (5.0) | 169 (3.3) | 1.88 (1.22–2.90) | 1.90 (1.23–2.93) |
| ⩾5 months | 42 (7.8) | 216 (4.3) | 2.18 (1.52–3.14) | 2.17 (1.50–3.12) |
| p for trend | <0.001 | |||
| Dose per day | ||||
| No | 256 (47.3) | 2811 (55.3) | 1.00 | 1.00 |
| <10 mg | 23 (4.3) | 283 (5.6) | 0.91 (0.58–1.42) | 0.90 (0.57–1.40) |
| 10–20 mg | 183 (33.8) | 1426 (28.0) | 1.43 (1.17–1.76) | 1.43 (1.17–1.76) |
| 20–30 mg | 38 (7.0) | 270 (5.3) | 1.59 (1.10–2.29) | 1.57 (1.09–2.26) |
| ⩾30 mg | 41 (7.6) | 295 (5.8) | 1.55 (1.09–2.21) | 1.55 (1.08–2.21) |
| p for trend | <0.001 |
Models were adjusted for sex, age, duration from diabetes to index date, hypertension, hyperlipidemia, ever use of statin, and antipsychotics.
Discussion
Our results showed the overuse rate of metoclopramide in diabetes was higher than in hypertension population. Whilst metoclopramide-induced Parkinsonism is well recognized,13,19 this study demonstrated for the first time the duration–response and dose–response effects in patients with mild-to-moderate type 2 diabetes.
Tardive dyskinesia is a movement disorder mainly caused by the prolonged use of dopamine receptor-blocking agents such as antipsychotics or antiemetic drugs. The duration of therapy is one of the major risk factors.20 The FDA recommend not prescribing metoclopramide for longer than 12 weeks to decrease the risk. In contrast to tardive dyskinesia, to the best of the authors’ knowledge, there are still no recommendations so far regarding the specific duration of metoclopramide treatment to avoid the risk of drug-induced Parkinsonism in patients with type 2 diabetes. Our study clearly demonstrated that the risk for Parkinsonism in patients who overused metoclopramide was higher (OR 2.05; 95% CI 1.53–2.76) than those who never used, while longer consecutive use of metoclopramide also increased the risk of developing Parkinsonism, with a remarkable duration–response and dose–response effects.
Drug-induced Parkinsonism is a major health problem.4 Initially, it was reported as a complication of typical antipsychotics but later recognized as an adverse effect of some of other medicines, including gastrointestinal prokinetics, antidepressants, calcium channel antagonists, and antiepileptic drugs.21 Among these medicines, metoclopramide is especially important because of its frequent use. The FDA estimates over 2 million Americans are using the medicine.22 Therefore, we selected a hypertension cohort to compare the utilization and overuse rate of metoclopramide use with the diabetes cohort in the online supplementary material. The trends of patients taking metoclopramide in both diabetes and hypertension cohorts from 1999 to 2008 were around 13–16%, but the overuse proportion of metoclopramide users for diabetes was higher than for hypertension (Tables S1 and S2 in the online supplementary material). Metoclopramide is still commonly used to treat gastrointestinal complications in diabetes patients.
A recent retrospective cohort study coming from Taiwan using the same database found that high metoclopramide exposure users had higher incidence of Parkinsonism than standard exposure users. Patients received metoclopramide more than 30 mg per day or a prescription of duration more than 5 days were defined as high-exposure users.23 Our study additionally assembled the cumulative dose and duration in the study period to assess the dose–response and duration–response effect in the metoclopramide use and the risk of Parkinsonism. Furthermore, we estimated the risk for the continuous period of metoclopramide use. Therefore, our study may provide practical information for clinician.
This study has several strengths. First, it used the LHID 2005 database, which is a large population database containing all original claim data for as many as 1 million beneficiaries. This large number enabled us to establish a mild–moderate type 2 diabetes cohort with as many as 34,685 patients and to enroll as many as 541 Parkinsonism cases. In addition, the diagnostic accuracy of type 2 diabetes in our study is highly reliable owing to selecting the patients with three or more visits of consistent ICD-9 CM code as well as antidiabetic medication administration for over 60 days. It has already been established that the diagnostic accuracy of NHIRD for diabetes increases with the number of outpatient visits.24 Population-based studies need to pay more attention to time-relative biases, for example, immortal time bias, selection bias, and confounding bias.25,26 Therefore, we conducted a nested case–control study and matched the duration of follow up among cases to controls to deal with the exposure to metoclopramide over time.27
However, the study also has some limitations. First, the diagnosis of NHIRD relied on ICD-9-CM codes. An administrative database in the United States using the same ICD-9-CM codes revealed that this coding system was limited in its ability to differentiate Parkinsonism of variable causes.28 Not only in diagnosis coding but in clinical practice, it is often challenging to distinguish idiopathic Parkinson disease from secondary Parkinsonism. Therefore, we rely on the neurologist’s at least three consistent outpatient diagnosis to improve the accuracy. We also enrolled one neurology admission of Parkinsonism, hospitalization increases the probability of accurate diagnosis. We only recruited patients with Parkinsonism from neurology clinics. Second, patients were newly diagnosed diabetics, and thus would not necessarily be expected to have developed significant neuropathic complications requiring metoclopramide use. Third, owing to the nature of the study, we have no data on adherence. These might underestimate the risk of metoclopramide on Parkinsonism. Finally, a limitation is the possibility of information bias, that is, that increasing duration of metoclopramide exposure may present more opportunities for the diagnosis of Parkinsonism owing to prolonged duration of symptoms and more clinic visits.
In conclusion, patients with diabetes who are being considered for treatment with metoclopramide for gastrointestinal symptoms should be made aware of the risks. There were a remarkable duration–response and dose–response effects on the risk of developing Parkinsonism. We recommend close monitoring for the development of Parkinsonism in patients treated with metoclopramide, particularly (but not limited to) those with prolonged exposure.
Supplemental Material
Supplemental material, esupp_file-20181002 for Metoclopramide as a prokinetic agent for diabetic gastroparesis: revisiting the risk of Parkinsonism by Chien-Hsu Lai, Yi-Chun Yeh and Yen-Yu Chen in Therapeutic Advances in Drug Safety
Acknowledgments
This study is based in part on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health and managed by National Health Research Institutes (registered number NHIRD-104-323). The interpretation and conclusions contained herein do not represent those of National Health Insurance Administration, Department of Health, or National Health Research Institutes.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Supplemental material: Supplemental material for this article is available online.
ORCID iD: Yen-Yu Chen  https://orcid.org/0000-0001-5380-3147
https://orcid.org/0000-0001-5380-3147
Contributor Information
Chien-Hsu Lai, Department of Neurology, Changhua Christian Hospital, Changhua, Taiwan.
Yi-Chun Yeh, Research Education and Epidemiology Center, Changhua Christian Hospital, Changhua, Taiwan.
Yen-Yu Chen, Department of Neurology, Changhua Christian Hospital, No. 135, Nan-Hsiao St., Changhua City, Changhua County 500, Taiwan Research Education and Epidemiology Center, Changhua Christian Hospital, Changhua, Taiwan Institute of Clinical Medicine, National Yang-Ming University, Taipei, Taiwan.
References
- 1. Nutt JG. Management of Parkinsonism and treatment of associated complications. Curr Opin Neurol 1995; 8: 327–330. [DOI] [PubMed] [Google Scholar]
- 2. Rajput AH, Offord KP, Beard CM, et al. Epidemiology of Parkinsonism: incidence, classification, and mortality. Ann Neurol 1984; 16: 278–282. [DOI] [PubMed] [Google Scholar]
- 3. Barbosa MT, Caramelli P, Maia DP, et al. Parkinsonism and Parkinson’s disease in the elderly: a community-based survey in Brazil (the Bambui study). Mov Disord 2006; 21: 800–808. [DOI] [PubMed] [Google Scholar]
- 4. Alvarez MV, Evidente VG. Understanding drug-induced Parkinsonism: separating pearls from oy-sters. Neurology 2008; 70: e32–e34. [DOI] [PubMed] [Google Scholar]
- 5. Lim TT, Ahmed A, Itin I, et al. Is 6 months of neuroleptic withdrawal sufficient to distinguish drug-induced Parkinsonism from Parkinson’s disease? Int J Neurosci 2013; 123: 170–174. [DOI] [PubMed] [Google Scholar]
- 6. Lopez-Sendon JL, Mena MA, de Yebenes JG. Drug-induced Parkinsonism in the elderly: incidence, management and prevention. Drugs Aging 2012; 29: 105–118. [DOI] [PubMed] [Google Scholar]
- 7. American Diabetes Association. 9. Microvascular complications and foot care. Diabetes Care 2016; 39: S72–S80. [DOI] [PubMed] [Google Scholar]
- 8. Choung RS, Locke GR, 3rd, Schleck CD, et al. Risk of gastroparesis in subjects with type 1 and 2 diabetes in the general population. Am J Gastroenterol 2012; 107: 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee A, Kuo B. Metoclopramide in the treatment of diabetic gastroparesis. Expert Rev Endocrinol Metab 2010; 5: 653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. American Diabetes Association. 7. Approaches to glycemic treatment. Diabetes Care 2016; 39: S52–S59. [DOI] [PubMed] [Google Scholar]
- 11. Ehrenpreis ED, Deepak P, Sifuentes H, et al. The metoclopramide black box warning for tardive dyskinesia: effect on clinical practice, adverse event reporting, and prescription drug lawsuits. Am J Gastroenterol 2013; 108: 866–872. [DOI] [PubMed] [Google Scholar]
- 12. Horn S. Drug-induced movement disorders. Continuum 2004; 10: 142–153. [Google Scholar]
- 13. Grimes JD. Parkinsonism and tardive dyskinesia associated with long-term metoclopramide therapy. N Engl J Med 1981; 305: 1417. [DOI] [PubMed] [Google Scholar]
- 14. Yamamoto M, Ujike H, Ogawa N. Metoclopramide-induced Parkinsonism. Clin Neuropharmacol 1987; 10: 287–289. [DOI] [PubMed] [Google Scholar]
- 15. Jolliet P, Nion S, Allain-Veyrac G, et al. Evidence of lowest brain penetration of an antiemetic drug, metopimazine, compared to domperidone, metoclopramide and chlorpromazine, using an in vitro model of the blood-brain barrier. Pharmacol Res 2007; 56: 11–17. [DOI] [PubMed] [Google Scholar]
- 16. National Health Insurance Research Database Taiwan. http://nhird.nhri.org.tw/en/index.htm, http://nhird.nhri.org.tw/en/Data_Subsets.html (2015, accessed 13 August 2015).
- 17. Therneau T. A package for survival analysis in R_. version 2.38, http://CRAN.R-project.org/package=survival (accessed 12 October 2015).
- 18. R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, http://www.R-project.org/ (accessed 10 May 2015).
- 19. Indo T, Ando K. Metoclopramide-induced Parkinsonism. Clinical characteristics of ten cases. Arch Neurol 1982; 39: 494–496. [DOI] [PubMed] [Google Scholar]
- 20. Woerner MG, Alvir JM, Saltz BL, et al. Prospective study of tardive dyskinesia in the elderly: rates and risk factors. Am J Psychiatry 1998; 155: 1521–1528. [DOI] [PubMed] [Google Scholar]
- 21. Lopez-Sendon J, Mena MA, de Yebenes JG. Drug-induced Parkinsonism. Expert Opin Drug Saf 2013; 12: 487–496. [DOI] [PubMed] [Google Scholar]
- 22. Rao AS, Camilleri M. Review article: metoclopramide and tardive dyskinesia. Aliment Pharmacol Ther 2010; 31: 11–19. [DOI] [PubMed] [Google Scholar]
- 23. Tsai SC, Sheu SY, Chien LN, et al. High exposure compared with standard exposure to metoclopramide associated with a higher risk of Parkinsonism: a nationwide population-based cohort study. Br J Clin Pharmacol 2018; 84: 2000–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin CC, Lai MS, Syu CY, et al. Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J Formos Med Assoc 2005; 104: 157–163. [PubMed] [Google Scholar]
- 25. Smiechowski B, Azoulay L, Yin H, et al. The use of metformin and colorectal cancer incidence in patients with type II diabetes mellitus. Cancer Epidemiol Biomarkers Prev 2013; 22: 1877–1883. [DOI] [PubMed] [Google Scholar]
- 26. Targownik LE, Suissa S. Understanding and avoiding immortal-time bias in gastrointestinal observational research. Am J Gastroenterol 2015; 110: 1647–1650. [DOI] [PubMed] [Google Scholar]
- 27. Suissa S, Dell’aniello S, Vahey S, et al. Time-window bias in case-control studies: statins and lung cancer. Epidemiology 2011; 22: 228–231. [DOI] [PubMed] [Google Scholar]
- 28. Swarztrauber K, Anau J, Peters D. Identifying and distinguishing cases of Parkinsonism and Parkinson’s disease using ICD-9 CM codes and pharmacy data. Mov Disord 2005; 20: 964–970. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, esupp_file-20181002 for Metoclopramide as a prokinetic agent for diabetic gastroparesis: revisiting the risk of Parkinsonism by Chien-Hsu Lai, Yi-Chun Yeh and Yen-Yu Chen in Therapeutic Advances in Drug Safety