Abstract
Objectives
The prevalence of infections caused by OXA-48-like carbapenemase-producing organisms in Ireland has increased dramatically since 2011 and is an urgent public health issue. Genome-based high-resolution genotyping was used to analyse clinical isolates submitted to the Irish Carbapenemase-Producing Enterobacteriaceae Reference Laboratory Service for a 13 month period (2016–17).
Methods
A total of 109 OXA-48-producing non-duplicate clinical isolates from 16 submitting centres were sequenced. Using a gene-by-gene approach, isolate genomes were characterized by MLST and core genome MLST, and the presence of antimicrobial resistance determinants was determined. Reference mapping and a novel plasmid MLST-type approach was applied to determine plasmid background.
Results
The OXA-48-like-producing isolates were Escherichia coli (n = 56), Klebsiella spp. (n = 46) and Enterobacter cloacae (n = 7). Amongst the E. coli isolates there were 37 different STs and amongst the Klebsiella spp. isolates there were 27 different STs. blaOXA-48 was present in 105/109 (96.3%) of isolates. Based on mapping analysis and detection of the pOXA-48 IncL-type plasmid replicon and backbone genes, a pOXA-48-like plasmid was identified in 93/109 isolates (85.3%). The remaining isolates (n = 16; 14.7%) harboured blaOXA-48-like genes in unknown environments. Using a gene-by-gene approach two pOXA-48-like plasmid groups with 2/71 pOXA-48-like locus differences between them were identified.
Conclusions
In Ireland we found a diversity of genotypes associated with OXA-48-like-producing clinical isolates with the IncL pOXA-48 plasmid type predominating as the blaOXA-48 genetic environment. A plasmid MLST approach can rapidly identify plasmids associated with outbreaks and monitor spread of types temporally and geographically.
Introduction
Carbapenems are broad-spectrum antibiotics that play a key role in therapy of infection caused by many antimicrobial-resistant Enterobacteriaceae. Global dissemination of carbapenem-resistant Enterobacteriaceae threatens the therapeutic efficacy of carbapenems. Bacteria of the Enterobacteriaceae family that produce one of a number of carbapenemase enzymes [carbapenemase-producing Enterobacteriaceae (CPE)] constitute a major public health concern. CPE have been reported in Ireland since 2009 and are detected with increasing frequency.1
The OXA-48-type carbapenemase first emerged in the mid-2000s in Turkey and has since been found across Europe and globally.2–4 CPE infections became notifiable in Ireland in 2011. Voluntary enhanced surveillance of CPE bloodstream infection was also implemented the same year. The first description of OXA-48-producing Enterobacteriaceae in Ireland (in this case Klebsiella pneumoniae) was in 2011 in five hospital patients.5 Since then the number of confirmed cases of OXA-48 has increased dramatically. In 2014, the National CPE Reference Laboratory confirmed 14 OXA-48 isolates, which increased to 274 just 2 years later.1 OXA-48 accounted for 77% of CPE isolates in 2016 with KPC isolates the next most prevalent at 14%. There were four OXA-48-related outbreaks reported in Irish hospitals in 2016 and almost half of the cases were attributed to one Dublin hospital experiencing an extended outbreak.1,6
The blaOXA-48 gene is believed to have originated on the chromosome of the environmental organism Shewanella.7 Clinical cases are predominantly associated with K. pneumoniae and Escherichia coli.1,8 The gene has been able to spread rapidly given its genetic environment. The gene is nested within a transposon (Tn1999), of which there are several variants, which is in turn predominantly carried within IncL-type plasmids 62–66 kb in size.9,10 Chromosomally integrated fragmented versions of the transposon have also been identified.11 This complexity hampers efforts to characterize bacterial isolates and also resistance element-bearing plasmids even with the availability of WGS technology. Careful characterization of isolates will help clarify the extent of the diversity of spread of these organisms and particularly the spread of transmissible elements such as plasmids.
Here we present the molecular epidemiology and genomic characterization, including a novel plasmid MLST-type approach, of OXA-48-like-producing clinical isolates submitted to the Irish National Reference Laboratory Service for CPE during a period of 13 months.
Materials and methods
The National Reference Laboratory Service for CPE is based at University Hospital Galway and has provided reference services since October 2012. All isolates from confirmed CPE cases, detected either diagnostically from infection and/or colonization or through screening, are submitted to the CPE Reference Laboratory. The laboratory received a total of 274 OXA-48-like isolates in 2016.1 Of these OXA-48-like isolates 126 were from a single hospital (Hospital A) as it was experiencing an extended outbreak in this time period. Of these 126 isolates, 53 non-duplicate isolates that spanned the time period and encompassed the different species identified were selected for WGS. A further 56 non-duplicate isolates were selected to represent the diversity of other submitting hospital clinical microbiology laboratories (n = 15) around the country, also across the study time period. Therefore, a total of 109 non-duplicate OXA-48-like CPE isolates from individual patients from between 6 January 2016 and 31 January 2017 were analysed in this study. For all submitted isolates, initial species identification was confirmed using MALDI-TOF MS (Bruker). The MIC of meropenem was determined by Etest in accordance with EUCAST criteria.12 Routine phenotypic detection and characterization of suspect CPE isolates was carried out using the ROSCO KPC/MBL and OXA-48 Confirm Kit (ROSCO, Denmark). All suspect CPE isolates submitted to the laboratory were confirmed by real-time PCR for the most common genes associated with CPE (blaOXA-48, blaKPC, blaNDM, blaVIM and blaIMP).13,14
WGS and analysis
The isolates were sequenced (paired-end sequencing, read length 150 bp) using the Illumina NextSeq 500 platform (UCL Genomics, London, UK). Resulting short reads were quality checked and assembled de novo using Spades within the BioNumerics (Applied Maths) genomics software platform. Genomic analyses were performed using BioNumerics and the BIGSdb platform.15 Core genome MLST (cgMLST) was carried out within BIGSdb (https://pubmlst.org/escherichia/) using an E. coli cgMLST scheme downloaded from the EnteroBase database (http://enterobase.warwick.ac.uk/) and for Klebsiella spp. within the Pasteur Klebsiella BIGSdb database (http://bigsdb.pasteur.fr/klebsiella). The Genome Comparator tool in BIGSdb was used to investigate relationships amongst the isolates. This was done by carrying out pairwise allele comparisons amongst the genomes according to different sets of loci: first, cgMLST comparisons were based on 2513 loci for E. coli and 694 loci for Klebsiella spp.;16 and second, for plasmid comparisons using 71 loci identified in the archetypal pOXA-48 plasmid (JN626286) (Table S1, available as Supplementary data at JAC Online). The two IS1999 transposase genes in JN626286 were excluded from the pOXA-48 MLST analysis due to the difficulty in assembly of short reads for these genes. Other pOXA-48 plasmid reference sequences used in the plasmid MLST analysis, for comparison purposes, were pRA35 (LN864821), pE71T (KC335143) and pKpn-E1.Nr7 (KM406491).9,17,18Klebsiella spp. reference genomes were used for comparison with Klebsiella phylogroups.19 Distance matrices generated were used to create NeighborNet diagrams using Splitstree version 4.14.2.20 The GrapeTree plugin in BIGSdb was used to create a minimum-spanning tree of the pOXA-48 locus differences amongst isolates.21 The ResFinder database was used to identify plasmid replicon types and antibiotic resistance-associated genes (https://cge.cbs.dtu.dk/services/ResFinder).22 The Enterobacter cloacae seven-locus MLST scheme hosted on the PubMLST database was used for speciation and to obtain ST (http://pubmlst.org/ecloacae). Species identification for Klebsiella spp. isolate genomes was carried out using the rMLST.org website based on exact matches to ribosomal protein gene alleles.23 MEGA version 7.0 was used for sequence alignment.24 The National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov/BLAST/) was used for sequence queries.
For plasmid reference mapping analysis, short reads were mapped against known complete plasmids: pOXA-48 (JN626286, 61.8 kb) and pOXA-181 (KP400525, 51 kb).25,26 Short-read reference mapping and processing was carried out using SMALT version 0.7.4 and Samtools version 0.1.11.27,28 Resulting processed mapped reads and annotated reference sequences were visualized using Artemis version 16.0.0.29
Results
Characterization of OXA-48-like isolates
There were a total of 56 E. coli, 46 Klebsiella spp. and 7 E. cloacae isolates analysed. Within the 46 Klebsiella spp. isolates there were 35 K. pneumoniae, 8 Klebsiella quasipneumoniae and 3 Klebsiella variicola isolates identified (Table S2). There was a genotypically diverse set of seven-locus MLST STs within each species (Table 1 and Figures 1 and 2). Amongst the E. coli isolates, there were 37 STs (Achtman scheme), of which ST131 was the most prevalent (n = 9, 16.1%) followed by ST38 (n = 4) and ST10 (n = 3). Amongst the Klebsiella spp. isolates there were 27 STs, of which K. quasipneumoniae ST1308 was the most common (n = 6, 13.0%) followed by ST20 and ST11. Amongst the E. cloacae isolates there were four ST66 isolates and one each of ST108, ST110 and ST135.
Table 1.
Genotyping of blaOXA-48-like-harbouring isolates from Ireland (n = 109)
| Species | Number of isolates | OXA-48-like alleles (number) | STs (number)a | Plasmid replicons | β-Lactamase genes (number) | Other resistance genes |
|---|---|---|---|---|---|---|
| E. coli | 56 | OXA-48 (55), OXA-244 (1) | 131 (9), 38 (4), 10 (3), 12 (2), 58 (2), 155 (2), 357 (2), 1049 (2), 7401 (2) | IncL, IncFII, IncX1, ColRNAI, Col156, Col, Col8282 | bla TEM-1 (16), blaCTX-M-24 (3), blaCTX-M-15 (2), blaCTX-M-14 (2), blaCTX-M-9 (1), blaCTX-M-3 (1), blaOXA-1 (1), blaCMY-2 (1), blaDHA-1 (1) | aac(3)-Iia, aac(3)-Iid, aac(6')Ib-cr, aadA1, aadA2, aadA5, catA1, catB3, dfrA1, dfrA12, dfrA14, dfrA17, erm(B), mph(A), qnrB4, qnrS1, strA, strB, sul1, sul2, tet(A) |
| Klebsiella spp. | 46 | OXA-48 (44), OXA-181 (2) | 1308 (6), 20 (5), 11 (4), 37 (4), 309 (2), 336 (2), 922 (2), 2978 (2) | IncL, IncX3, IncR, ColRNAI, ColKP3 | bla CTX-M-15 (14), blaCTX-M-14 (1), blaTEM-1 (6), blaSHV-1 (6), blaSHV-11 (12), blaSHV-27 (1), blaSHV-164 (1), blaSHV-38 (1), blaNDM-5 (1), blaACC-1 (1), blaLEN12 (2), blaLEN24 (1), blaOKP-A-8 (2), blaOKP-A-8 (1), blaOKP-B-2 (1) | aac(3)-Iia, aac(3)-Iid, aac(6')Ib-cr, aadA1, aadA2, aadA5, aph(3')-Ia, ARR-3, catA1, catA2, catB3, dfrA1, dfrA12, dfrA14, dfrA17, floR, fosA, mph(A), oqxA, oqxB, qnrB66, qnrB7, qnrS1, strA, strB, sul1, sul2, tet(A), tet(D) |
| E. cloacae | 7 | OXA-48 (6), OXA-181 (1) | 66 (4), 108 (1), 110 (1), 135 (1) | IncL, TrfA, IncX3, IncR, ColRNAI | bla OXA-1 (6), blaCTX-M-15 (4), blaCTX-M-9 (3), blaSHV-12 (1), blaTEM-1 (1), blaACT-7 (5), blaACT-14 (1), blaACT-15 (1) | aacA4, aac(3)-IIa, aac(6')Ib-cr, aadA1, aadA2, aadB, catA1, catB3, dfrA14, dfrA16, fosA, mph(A), qnrA1, qnrB1, strA, strB, sul1, sul2, tet(A) |
Only STs with number >1 are given for E. coli and Klebsiella spp.
Figure 1.
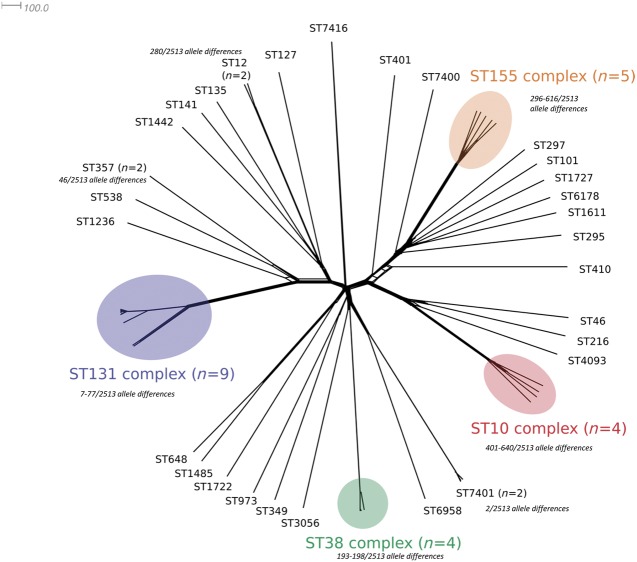
NeighborNet diagram based on pairwise allele comparisons of core genome locus alleles (n = 2513) amongst the genomes of OXA-48 E. coli isolates (n = 56) in Ireland. STs and major clonal complexes are indicated. The scale bar represents the number of locus allele differences. Allele differences between closely related isolates are indicated. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Figure 2.
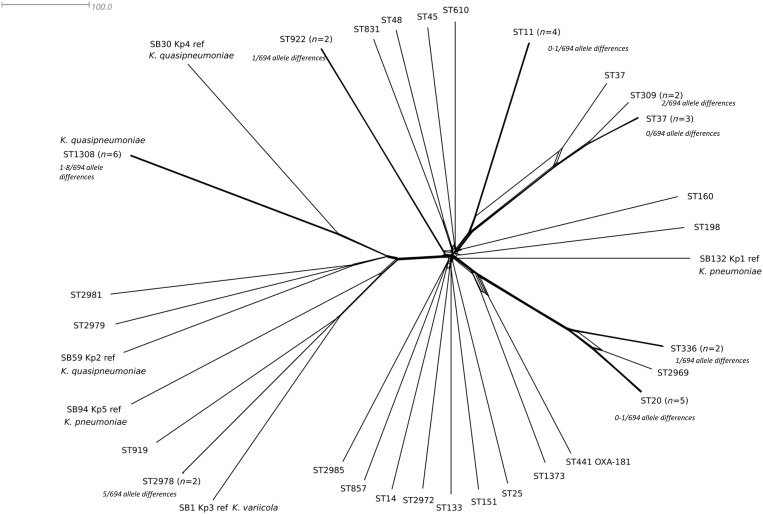
NeighborNet diagram based on pairwise allele comparisons of core genome locus alleles (n = 694) amongst the genomes of OXA-48 Klebsiella spp. isolates (n = 46) in Ireland. STs and reference isolate genomes of Klebsiella spp. phylogroups are indicated. The scale bar represents the number of locus allele differences. Allele differences between closely related isolates are indicated.
cgMLST schemes for E. coli and K. pneumoniae were used to determine genetic relationships amongst isolates (Figures 1 and 2). There were between 2 and 2495 out of 2513 core genome locus differences amongst E. coli genomes. One hospital (Hospital A), which contributed 48.6% (n = 53) of the isolates, had a diverse set of bacterial genotypes that appeared to be circulating over the time period. In Hospital A, amongst the E. coli isolates (n = 33) there were 26 different STs and amongst the Klebsiella spp. isolates (n = 20) there were 12 different STs. Some of the major E. coli lineages were found across all sites, but some were more localized, suggestive of local transmission. For example, E. coli ST131 and ST38 were found distributed throughout the country, while K. quasipneumoniae ST1308 and K. pneumoniae ST20 were only found in Hospitals A and B, respectively.
The blaOXA-48 gene was present in 105/109 (96.3%) of isolates, while the remaining 4 isolates harboured blaOXA-181 [K. pneumoniae (n = 1), K. quasipneumoniae (n = 1) and E. cloacae (n = 1)] and blaOXA-244 [E. coli (n = 1)]. One K. pneumoniae isolate (id 5493, Pasteur Klebsiella BIGSdb database) harboured a blaNDM-5 gene as well as blaOXA-48. Many of the isolates also contained genes encoding ESBLs (Table 1). A total of nine E. coli isolates (16.1%) harboured a blaCTX-M gene [blaCTX-M-24 (n = 3), blaCTX-M-15 (n = 2), blaCTX-M-14 (n = 2), blaCTX-M-9 (n = 1) and blaCTX-M-3 (n = 1)]. A total of 15 Klebsiella spp. isolates (32.6%) harboured a blaCTX-M gene variant [blaCTX-M-15 (n = 14) and blaCTX-M-14 (n = 1)]. All E. cloacae isolates harboured a blaCTX-M gene [blaCTX-M-9 (n = 3) and blaCTX-M-15 (n = 4)]. For the three blaOXA-181-harbouring isolates [Klebsiella spp. (n = 2) and E. cloacae (n = 1)] the quinolone resistance gene qnrS1 was found on the same assembled contig sequence and therefore the same plasmid as blaOXA-181.
Genomic context of OXA-48-like genes
A variety of plasmid replicon types were detected, including those of Inc-type plasmids (L, X1, X3, R, IncFII) and Col-type plasmids (RNAI, KP3, 156, 8282) (Table 1). A total of 93/109 isolates (85.3%) harboured the replicon and three backbone genes characteristic of the archetypal IncL-type pOXA-48 plasmid, repA, traU and parA.25 Of the isolates with blaOXA-48 but without the IncL-type plasmid replicon (n = 11), two (both E. coli) did not contain repA, traU and parA, and nine contained just parA [E. coli (n = 8) and K. pneumoniae (n = 1)]. No plasmid replicons were found in four isolates (3.7%, all E. coli) that harboured blaOXA-48. These four isolates contained the same 29 of the 71 loci identified in the reference pOXA-48 plasmid.
Read mapping to plasmid reference sequences was carried out for all isolates. Of the 93 harbouring repA, traU and parA, and the IncL replicon, all except 1 mapped to the whole length of the ∼63 kb reference pOXA-48 (JN626286) plasmid sequence. They harboured all the other 71 pOXA-48 loci. The exception (id 113, E. coli BIGSdb) appeared to be truncated and only mapped to a stretch of ∼51 kb of the pOXA-48 reference sequence as well as harbouring just 51/71 pOXA-48 loci (loci consecutive). Another isolate genome (id 144, E. coli ST12) containing the IncL pOXA-48 replicon but which was missing the traU locus appeared to be truncated and contained 39/71 pOXA-48 loci (loci consecutive). Mapping revealed a region of the reference sequence ∼28 kb long to which short reads did not map. The unmapped region included the plasmid mobilization genes mobA and mobB and plasmid transfer protein genes traH to traU. The three blaOXA-181-harbouring isolates mapped to the 51 kb pOXA-181 IncX3-type plasmid reference sequence (KP400525). Three of the isolates’ plasmid genes were identical at all 62 loci of the KP400525 pOXA-181 reference plasmid, while the fourth isolate differed at 4 loci (two transposases, a type IV secretion protein gene and a plasmid replication gene).
All nine isolates harbouring only parA but harbouring blaOXA-48 [E. coli (n = 8) and K. pneumoniae (n = 1)] mapped to an ∼23 kb consecutive region of the reference pOXA-48 (JN626286) sequence (Table S1). This region spanned from the IS1999delta-IS1 at the upstream part of the transposon and the second IS1 element after korC and orf25. The isolates spanned the time period of the study and were from eight different submitting centres. The isolates were eight E. coli (ST131, 38, 12, 69, 12) and one K. pneumoniae (ST11). All nine isolates contained the same 29/71 pOXA-48 loci (Table S1). The blaOXA-244 isolate (id 104, E. coli ST1722) also mapped to an ∼23 kb region of the pOXA-48 plasmid and harboured the same 29/71 loci.
One isolate (id 97, E. coli ST410) appeared to have blaOXA-48 integrated into a Tn1999.3 variant within a Col-type plasmid as the Col replicon and blaOXA-48 were on the same assembled contig sequence. A further isolate (id 101, E. coli ST38) also appeared to have a truncated part of the pOXA-48 plasmid as short reads mapped only to an ∼7 kb region in the pOXA-48 reference sequence around the Tn1999 element from the IS1999 element downstream of OXA-48 and upstream to mucA. Isolates id 97 and id 101 harboured just 3 and 4 of 71 pOXA-48 loci, respectively, including blaOXA-48 itself. Isolates id 97 and id 101 had Col and IncF replicons detected, respectively, but no IncL replicon.
Plasmid molecular epidemiology using pOXA-48 gene-by-gene approach
There were two groupings of pOXA-48 plasmid types based on pOXA-48 MLST allele profiles (Figure 3). A total of 44 isolates (47.8%) within the first group (Group 1) and 27 isolates (29.3%) in the second group (Group 2) were identical at 69 loci of the pOXA-48 plasmid reference (JN626286). These two groups differed from each other at two loci; one was a hypothetical protein (orf10 in pOXA-48 annotation) and the other a putative restriction endonuclease (orf16 in pOXA-48 annotation). Isolate plasmids of Group 2 were identical at all 71 loci with the pRA35 plasmid. No isolate plasmids were identical at all 71 loci to the reference pOXA-48 plasmid.
Figure 3.

Minimum-spanning tree of pOXA-48 plasmid (JN626286) locus (n = 71) allele differences amongst isolates with an IncL pOXA-48-type plasmid (n = 92). Each circle (node) contains isolates with identical profiles at the 71 locus alleles. Lines (edges) connecting nodes indicate the number of locus allele differences between nodes. Nodes are divided in pie-chart form for individual isolates. Nodes are coloured by isolate-submitting hospital. Four complete reference plasmids (coloured white in nodes) were used in the analysis for comparison: pOXA-48 (JN626286), pRA35 (LN864821), pE71T (KC335143) and pKpn-E1.Nr7 (KM406491). The minimum-spanning tree was constructed using the GrapeTree plugin in BIGSdb. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Each group had isolates from the whole time span of the collection and were represented by at least three species (Figure S1). Each group contained E. coli and K. pneumoniae isolates, but Group 1 contained all K. quasipneumoniae and K. variicola isolates, while all E. cloacae were within Group 2. The majority (41/44) of isolates from Group 1 were from Hospital A, while the majority of Group 2 were from Hospital B (15/25), which indicates some local homogeneity of plasmid types in particular within Hospital A. Group 2 had a more diverse geographical spread with isolates encompassing (if including the single locus variants of the main cluster) all four provinces of the country.
Discussion
The number of patients colonized with CPE detected in Ireland has increased dramatically since 2012 (n = 5). In 1 year between 2015 and 2016 the increase was 156% (139 to 356 cases), though it is likely that increased ascertainment is one factor contributing to the apparent increase.1 An outbreak in one major hospital has contributed a high proportion (48.6%) of the isolates studied.
In common with several other countries, in Ireland the blaOXA-48 gene was associated with several species and with several lineages or clonal groups within each species. There were, however, two groups of pOXA-48-like plasmids and a number of variants related to these groups. One of these plasmid types was the predominant blaOXA-48 carrier in a large hospital with an extended outbreak. The variety of species and genotypes that bore this plasmid in this case leads to characterization of this as a ‘plasmid outbreak’. This is an important distinction, as failure to recognize that CPE isolates of different species may be linked by carriage of a common plasmid may lead to delay in recognizing an outbreak.
While the IncL-type pOXA-48 plasmid was the predominant carrier for the carbapenemase gene, there was an indication that the gene was integrated within a pOXA-48 plasmid fragment with an unknown genetic background for a number of isolates (n = 12). This would have to be verified with long-read sequencing using PacBio SMRT or Minion technologies to span the whole region. However, previous studies have shown that the gene can be maintained chromosomally at a low level in the population.9–11,30 Most of the isolates we examined with the pOXA-48 plasmid fragment belonged to globally distributed extraintestinal pathogenic E. coli lineages ST131, ST38 and ST127, in which the gene has been identified chromosomally previously.9,11,31 One of the isolates possessed the single peptide variant blaOXA-244 (E. coli).30 As with plasmid-associated OXA-48, there does not appear to be a single bacterial clone associated with these OXA-48 isolates with unknown genetic background, as several clones disseminated throughout Ireland. The range of plasmid types associated with blaOXA-48 appears to be widening, including truncated and much larger versions of the IncL-type pOXA-48 plasmid as well as other plasmid family types.32–34 In Ireland there were a small number of isolates that appeared to have either truncated IncL-type plasmids or other plasmid types such as Col. Again it must be noted that gene-by-gene approaches and mapping analysis are hindered in their ability to detect multiple copies of similar plasmids and rearrangements within and between them. Therefore, long-read sequencing should be used to bring further clarity to the chromosomal and accessory genome contexts.
The dissemination of blaOXA-48 has been aided by the particular propensity of the pOXA-48 IncL-type plasmid for horizontal genetic transfer (HGT) and within-host transfer of the plasmid amongst Enterobacteriaceae species is likely to occur since co-colonization and infection with multiple species harbouring the gene is known.10,35,36 It is likely that in a Catch 22-like scenario, colonization is prolonged by HGT and HGT is aided by prolonged colonization.37 Duration of colonization with OXA-48-producing organisms lasts for at least several months, increasing the likelihood of spread within the healthcare environment and within the community.37,38 While carriage prevalence of OXA-48-producing organisms in the community is as yet not well studied, in Western Europe it is currently thought to be low.39,40 However, this is likely to change over time with the apparent increase in this CPE in Europe from its original and now endemic reservoir in the Near and Middle East and North Africa.41
Hospital outbreaks of OXA-48 are becoming more common in Ireland and elsewhere as organisms carrying the plasmid may spread in hospital from individuals who are colonized/infected.5,42–44 A critical element in the control of an outbreak or epidemic is recognition of the outbreak and an understanding of the underlying biology. This work contributes to the growing acceptance that the traditional paradigm of an outbreak or epidemic as a phenomenon characterized by a series of closely related isolates is not always applicable. This paradigm is not applicable to outbreaks and epidemics of acquired antimicrobial resistance encoded on highly mobile genetic elements that transmit to several lineages and species. Rapid genomic analysis such as the gene-by-gene MLST-type approach to plasmids applied here can support rapid characterization and improved focus on tracking the mobile genetic elements that are the actual driver of the outbreak.
Supplementary Material
Acknowledgements
We thank all of the clinical laboratories in Ireland who continue to support the National CPE Reference Laboratory. We acknowledge bioinformatics support from Tim Downing [Dublin City University (DCU)] and computational facilities from DCU’s Enhancing Performance fund.
Funding
This study was supported by internal funding.
Transparency declarations
None to declare.
References
- 1.National Carbapenemase Producing Enterobacteriaceae (CPE) Reference Laboratory Service. CPEaRLS ANNUAL REPORT 2016 2016.
- 2. Albiger B, Glasner C, Struelens MJ. et al. Carbapenemase-producing Enterobacteriaceae in Europe: assessment by national experts from 38 countries, May 2015. Euro Surveill 2015; 20: pii=30062. [DOI] [PubMed] [Google Scholar]
- 3. Mathers AJ, Hazen KC, Carroll J. et al. First clinical cases of OXA-48-producing carbapenem-resistant Klebsiella pneumoniae in the United States: the “menace” arrives in the New World. J Clin Microbiol 2013; 51: 680–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carrër A, Poirel L, Yilmaz M. et al. Spread of OXA-48-encoding plasmid in Turkey and beyond. Antimicrob Agents Chemother 2010; 54: 1369–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O’Brien DJ, Wrenn C, Roche C. et al. First isolation and outbreak of OXA-48-producing Klebsiella pneumoniae in an Irish hospital, March to June 2011. Euro Surveill 2011; 16: pii=19921. [PubMed] [Google Scholar]
- 6.Health Protection Surveillance Centre (HPSC). Carbapenemase-producing Carbapenem-Resistant Enterobacteriaceae (CRE) in Ireland: Quarters 1 & 2 2017 2017.
- 7. Poirel L, Héritier C, Nordmann P.. Chromosome-encoded Ambler class D β-lactamase of Shewanella oneidensis as a progenitor of carbapenem-hydrolyzing oxacillinase. Antimicrob Agents Chemother 2004; 48: 348–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Findlay J, Hopkins KL, Loy R. et al. OXA-48-like carbapenemases in the UK: an analysis of isolates and cases from 2007 to 2014. J Antimicrob Chemother 2017; 72: 1340–9. [DOI] [PubMed] [Google Scholar]
- 9. Beyrouthy R, Robin F, Delmas J. et al. IS1R-mediated plasticity of IncL/M plasmids leads to the insertion of blaOXA-48 into the Escherichia coli chromosome. Antimicrob Agents Chemother 2014; 58: 3785–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Skalova A, Chudejova K, Rotova V. et al. Molecular characterization of OXA-48-like-producing Enterobacteriaceae in the Czech Republic and evidence for horizontal transfer of pOXA-48-like plasmids. Antimicrob Agents Chemother 2017; 61: e01889-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Turton JF, Doumith M, Hopkins KL. et al. Clonal expansion of Escherichia coli ST38 carrying chromosomally-integrated OXA-48 carbapenemase gene. J Med Microbiol 2016; 65: 538–46. [DOI] [PubMed] [Google Scholar]
- 12.EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 6.0 2016. http://www.eucast.org.
- 13. Swayne RL, Ludlam HA, Shet VG. et al. Real-time TaqMan PCR for rapid detection of genes encoding five types of non-metallo- (class A and D) carbapenemases in Enterobacteriaceae. Int J Antimicrob Agents 2011; 38: 35–8. [DOI] [PubMed] [Google Scholar]
- 14.CDC. Multiplex Real-Time PCR Detection of Klebsiella pneumoniae Carbapenemase (KPC) and New Delhi Metallo-β-Lactamase (NDM-1). https://www.cdc.gov/hai/pdfs/labsettings/KPC-NDM-protocol-2011.pdf.
- 15. Jolley KA, Maiden MCJ.. BIGSdb: scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics 2010; 11: 595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bialek-Davenet S, Criscuolo A, Ailloud F. et al. Genomic definition of hypervirulent and multidrug-resistant Klebsiella pneumoniae clonal groups. Emerg Infect Dis 2014; 20: 1812–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Power K, Wang J, Karczmarczyk M. et al. Molecular analysis of OXA-48-carrying conjugative IncL/M-like plasmids in clinical isolates of Klebsiella pneumoniae in Ireland. Microb Drug Resist 2014; 20: 270–4. [DOI] [PubMed] [Google Scholar]
- 18. Carattoli A, Seiffert SN, Schwendener S. et al. Differentiation of IncL and IncM plasmids associated with the spread of clinically relevant antimicrobial resistance. PLoS One 2015; 10: e0123063.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hæggman S, Löfdahl S, Paauw A. et al. Diversity and evolution of the class A chromosomal β-lactamase gene in Klebsiella pneumoniae. Antimicrob Agents Chemother 2004; 48: 2400–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huson DH, Bryant D.. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol 2006; 23: 254–67. [DOI] [PubMed] [Google Scholar]
- 21. Zhou Z, Alikhan N-F, Sergeant MJ. et al. GrapeTree: visualization of core genomic relationships among 100,000 bacterial pathogens. Genome Res 2018; 28: 1395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zankari E, Hasman H, Cosentino S. et al. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 2012; 67: 2640–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jolley KA, Bliss CM, Bennett JS. et al. Ribosomal multilocus sequence typing: universal characterization of bacteria from domain to strain. Microbiology 2012; 158: 1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kumar S, Stecher G, Tamura K.. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol 2016; 33: 1870–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Poirel L, Bonnin RA, Nordmann P.. Genetic features of the widespread plasmid coding for the carbapenemase OXA-48. Antimicrob Agents Chemother 2012; 56: 559–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu Y, Feng Y, Wu W. et al. First report of OXA-181-producing Escherichia coli in China and characterization of the isolate using whole-genome sequencing. Antimicrob Agents Chemother 2015; 59: 5022–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. SMALT Version 0.7.4 2013. ftp://ftp.sanger.ac.uk/pub/resources/software/smalt/smalt-manual-0.7.4.pdf.
- 28. Li H, Handsaker B, Wysoker A. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009; 25: 2078–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carver T, Harris SR, Berriman M. et al. Artemis: an integrated platform for visualization and analysis of high-throughput sequence-based experimental data. Bioinformatics 2012; 28: 464–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Potron A, Poirel L, Dortet L. et al. Characterisation of OXA-244, a chromosomally-encoded OXA-48-like β-lactamase from Escherichia coli. Int J Antimicrob Agents 2016; 47: 102–3. [DOI] [PubMed] [Google Scholar]
- 31. Beyrouthy R, Robin F, Cougnoux A. et al. Chromosome-mediated OXA-48 carbapenemase in highly virulent Escherichia coli. J Antimicrob Chemother 2013; 68: 1558–61. [DOI] [PubMed] [Google Scholar]
- 32. Solgi H, Badmasti F, Giske CG. et al. Molecular epidemiology of NDM-1- and OXA-48-producing Klebsiella pneumoniae in an Iranian hospital: clonal dissemination of ST11 and ST893. J Antimicrob Chemother 2018; 73: 1517–24. [DOI] [PubMed] [Google Scholar]
- 33. Dimou V, Dhanji H, Pike R. et al. Characterization of Enterobacteriaceae producing OXA-48-like carbapenemases in the UK. J Antimicrob Chemother 2012; 67: 1660–5. [DOI] [PubMed] [Google Scholar]
- 34. Zautner AE, Bunk B, Pfeifer Y. et al. Monitoring microevolution of OXA-48-producing Klebsiella pneumoniae ST147 in a hospital setting by SMRT sequencing. J Antimicrob Chemother 2017; 72: 2737–44. [DOI] [PubMed] [Google Scholar]
- 35. Potron A, Poirel L, Nordmann P.. Derepressed transfer properties leading to the efficient spread of the plasmid encoding carbapenemase OXA-48. Antimicrob Agents Chemother 2014; 58: 467–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Manageiro V, Ferreira E, Pinto M. et al. First description of OXA-48 carbapenemase harbored by Escherichia coli and Enterobacter cloacae from a single patient in Portugal. Antimicrob Agents Chemother 2014; 58: 7613–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Haverkate MR, Dautzenberg MJD, Ossewaarde TA. et al. Within-host and population transmission of blaOXA-48 in K. pneumoniae and E. coli. PLoS One 2015; 10: e0140960.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van Hattem JM, Arcilla MS, Bootsma MCJ. et al. Prolonged carriage and potential onward transmission of carbapenemase-producing Enterobacteriaceae in Dutch travelers. Future Microbiol 2016; 11: 857–64. [DOI] [PubMed] [Google Scholar]
- 39. Reuland EA, Overdevest I, Naiemi N. et al. High prevalence of ESBL-producing Enterobacteriaceae carriage in Dutch community patients with gastrointestinal complaints. Clin Microbiol Infect 2012; 19: 542–9. [DOI] [PubMed] [Google Scholar]
- 40. Zurfluh K, Nüesch-Inderbinen M, Poirel L. et al. Emergence of Escherichia coli producing OXA-48 β-lactamase in the community in Switzerland. Antimicrob Resist Infect Control 2015; 4: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Poirel L, Potron A, Nordmann P.. OXA-48-like carbapenemases: the phantom menace. J Antimicrob Chemother 2012; 67: 1597–606. [DOI] [PubMed] [Google Scholar]
- 42. Guo L, An J, Ma Y. et al. Nosocomial outbreak of OXA-48-producing Klebsiella pneumoniae in a Chinese hospital: clonal transmission of ST147 and ST383. PLoS One 2016; 11: e0160754.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thomas CP, Moore LSP, Elamin N. et al. Early (2008–2010) hospital outbreak of Klebsiella pneumoniae producing OXA-48 carbapenemase in the UK. Int J Antimicrob Agents 2013; 42: 531–6. [DOI] [PubMed] [Google Scholar]
- 44. Dautzenberg MJ, Ossewaarde JM, de Kraker ME. et al. Successful control of a hospital-wide outbreak of OXA-48 producing Enterobacteriaceae in the Netherlands, 2009 to 2011. Euro Surveill 2014; 19: pii=20723. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.