Abstract
Oxandrolone, a testosterone analog, is used to counteract the catabolic effects of burn injury. Recent animal studies suggest a possible hormonal association with heterotopic ossification (HO) development postburn. This work examines oxandrolone administration and HO development by exploring historical clinical data bridging the introduction of oxandrolone into clinical practice. Additionally, we examine associations between oxandrolone administration and HO in a standardized mouse model of burn/trauma-related HO. Acutely burned adults admitted between 2000 and 2014, survived through discharge, and had a HO risk factor of 7 or higher were selected for analysis from a single burn center. Oxandrolone administration, clinical and demographic data, and elbow HO were recorded and were analyzed with logistic regression. Associations of oxandrolone with HO were examined in a mouse model. Mice were administered oxandrolone or vehicle control following burn/tenotomy to examine any potential effect of oxandrolone on HO and were analyzed by Student’s t test. Subjects who received oxandrolone had a higher incidence of elbow HO than those that did not receive oxandrolone. However, when controlling for oxandrolone administration, oxandrolone duration, postburn day oxandrolone initiation, HO risk score category, age, sex, race, burn size, and year of injury, there was no significant difference between rates of elbow HO between the two populations. In agreement with the review, in the mouse model, while there was a trend toward the oxandrolone group developing a greater volume of HO, this did not reach statistical significance.
An important innovation in burn treatment is the use of oxandrolone, a synthetic analog of testosterone, to counteract the catabolic effects of the burn. In children, oxandrolone has been shown to have numerous positive effects including increasing rate of protein synthesis, reversal of growth arrest when combined with propranolol, and decreasing length of hospital stay.1–3 In adult patients, however, large multicenter randomized controlled studies are more limited in number.4 Oxandrolone is associated with reduced mortality rates of severely burned adults compared to a similar group of controls and decreases length of stay in hospital.5,6 Few studies on oxandrolone in adults have focused on potential adverse effects. Of those studies, liver function and androgenic activity have been areas of interest, though both were found to occur at significantly lower rates in oxandrolone patients than in similar patients treated with human growth hormone, which has also been used to combat increased metabolism in burn patients.7
Heterotopic ossification (HO) is a serious but rare complication of burns, trauma, or traumatic brain injury that results in the formation of ectopic extra-skeletal bone. HO affects a small fraction (2–5%) of the burn survivor population but presents substantial problems for these individuals.8 HO can severely impede range of motion, affecting function, and often requires surgical intervention.9 There are scant data to support prophylactic measures in burn patients.10–13 Moreover, once HO has formed, there is no pharmaceutical treatment available, and the only treatment to improve range of motion that is supported by clinical evidence is surgical intervention.12,14 For this reason, there is a clinical need to both identify those at risk for HO and to lower the risk for HO formation following trauma. Investigators recently developed an HO risk calculator based on factors known at admission. The authors found that burn size, and need for autografting to the arms, head or neck, and trunk were predictors of HO development.15
Estrogens and androgens have been well characterized for their role in postnatal bone development, especially in the obstetrics and gynecology literature in the context of postmenopausal decreases in trabecular and cortical bone after loss of these steroids16,17 corroborated by mouse models of orchiectomy/ovariectomy.18,19 It is this association between steroids and their pro-osteogenic20 and metabolic effects that serves as the rationale for extended oxandrolone therapy in burn patients who are at risk for osteo- and sarcopenia during their hospital course and recovery.21 Despite substantial data on sex steroids in bone development, maintenance, and repair, little has been studied on the role of estrogen and androgens in HO development.22–25 Interestingly, in posttraumatic patients, we have observed a discrepancy in incidence of HO by sex. For instance, male sex is an important predictor of the occurrence of HO of the hip in patients following spinal cord injury26 and an increased incidence of scapular HO in patients after rotator cuff injuries.27 This is further recapitulated in our traumatic injury model of HO in the mouse, where male mice developed a greater volume of HO following a burn/tenotomy than females after the same procedure.28 We hypothesized that this observation may be due to the differences in estrogen and androgen levels between male and females. The direct role of sex steroids on this finding, however, was not explored. Given the indirect evidence suggesting a possible link between testosterone and bone growth/HO, as well as the severe impairments caused by HO, there is a clinical need to further investigate this potential relationship. In this study, we hypothesize that sex steroids play a role in postburn/trauma-induced HO and that manipulation of sex steroids can be used to alter HO formation in mice. Additionally, we examine if early institution of oxandrolone therapy in humans at high risk for developing HO further increases their risk of developing the complication.
METHODS
Ethics Statements
Study approval was obtained from Massachusetts General Hospital institutional review board, the University Committee on Use and Care of Animals at the University of Michigan-Ann Arbor (Protocols: 5909/7930) using the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Retrospective Review of Human Experience
Data Collection and Study Design.
Patients were selected from the Massachusetts General Hospital Research Patient Data Registry. Patients, age ≥18 years, with acute burns admitted between 2000 and 2014 and who survived their acute hospitalization were screened for study inclusion. Records were reviewed, and all individuals were assigned a risk score for the development of HO using a previously validated scoring system.15 The HO Risk Scoring System, derived using the Burn Model Systems National Database and externally validated, assigns point values based on burn size and deep wounds to the upper extremities, trunk, and head and neck. All subjects with a HO risk score of ≥7 were included. A score of 7 was chosen as a cutoff as this is the lowest score at which the risk for HO (4.88%) was greater than the sample average (3.06%).
Medical records, including inpatient hospitalizations and outpatient visits, were examined for patient demographic (age, sex, race) and clinical data. Clinical characteristics included burn size, year of burn, duration, and early (on or before postburn day 7) vs late institution of oxandrolone therapy. For this study, HO was defined as a diagnosis of HO in an elbow as the number of patients with HO elsewhere was small. This was done in an attempt to eliminate variables related to potential unknown differences in mechanism of HO formation in trauma/crush vs a topical burn injury. To ascertain presence of elbow HO, the following medical records were reviewed: discharge summaries, operative notes, progress notes, outpatient notes, and radiologic evidence including plain films, CT scans, or MRIs. Patients with a HO risk score of ≥7 that did not meet the criteria for the elbow HO group were included as the comparison group. Subjects who developed only non-elbow HO, or who had a diagnosis of HO prior to the burn were excluded from further analyses.
The period which the subjects were on oxandrolone was defined as the period between the time oxandrolone was first prescribed and the time it was discontinued or the date of discharge, whichever came first. As was standard of care, patients treated with oxandrolone were administered 10 mg twice per day orally. Due to inconsistencies in notation in the medication administration records, missed doses were not accounted for in the total days on oxandrolone calculation. A second reviewer verified all data.
Statistical Analysis of Human Experience.
Descriptive demographic and clinical statistics are reported. The data were analyzed using logistic regression where the outcome variable was presence of elbow HO. Possible covariates included: oxandrolone administration, oxandrolone duration, postburn day oxandrolone initiation, HO risk score category, age, sex, race, TBSA, and year of injury. For each quantitative variable, linearity was checked via examination of lowess graphs.29 If any curvature was seen, it was then modeled using polynomials, piecewise models or restricted cubic splines, as appropriate. The c-statistic, area under the receiver operating characteristic curve, was examined for each model to assess model discrimination. A P-value < .05 was considered significant. As part of a sensitivity analysis, analogous regression was performed including patients with non-elbow HO and results were qualitatively compared.
Mouse Study of Effect of Oxandrolone on HO
Mice received presurgical analgesia consisting of subcutaneously administered 0.06 mg/kg buprenorphine, followed by inhaled isoflurane anesthesia, and close postoperative monitoring and additional subcutaneous 0.06 mg/kg buprenorphine administrations for 48 hours postoperatively. Six-week-old C57BL/6J male mice underwent Achilles’ tendon transection and dorsal burn injury to cause trauma-induced HO formation as previously described.30 Experimental mice were gavaged with 100 μg oxandrolone (1 mg/ml) (University of Michigan Pharmacy) and concurrently control mice were gavaged 100 µl of phosphate-buffered saline (PBS) for three times a week for 9 weeks. Mice were euthanized at age 15 weeks. Mice body weights were measured prior to euthanasia and seminal vesicles were weighted after the euthanasia. The average body weight for a C57BL/6J mouse was determined following the Jackson Laboratory website ((initial weight, 6 weeks + final weight, 15 weeks)/2).31 For comparison to human doses, the average adult male body weight 88.8 kg was taken into consideration (20 years and over, all races and Hispanic origin groups in United States between 2011 and 2014).32
MicroCT Analysis
Left legs were scanned in microCT (Bruker SkyScan 1176). Bone volumes were determined utilizing micro computerized topography imaging at the following parameters: resolution of 35 μM, beam energy of 357 μA, beam current of 70 kV, exposed for 520 ms. Scans were analyzed using calibrated imaging protocol as previously described by MicroView micro CT viewer (GE Health Care and Parallax Innovations).33 Bone reconstructions were calculated at 800 HU depicting representative means of treatment groups. Ectopic bone was manually splined and measured at 0, 800, and 1250 Hounsfield unit (HU) thresholds. Ectopic bone volumes were characterized as total volume, proximal, distal, floating, and bone associated. Bone associated HO at the calcaneus (S4) was analyzed further with trabecular bone volume (0 HU–1250 HU), bone porosity (1 − (1250 HU/0 HU)), ectopic shell (1250 HU), and bone volume to total volume ratio (BV/TV, 1250 HU/0 HU). Tibial cortical volume was measured just above the tibia-fibula fuse point on the medial side as performed in previous literature.34 Tibial trabecular bone volume BV/TV was calculated from manually splined tibial bone and calculated from quantified volumes (1250 HU/0 HU).
Liver Measurements
Blood was collected with cardiac puncture. Serum was isolated with BD Microtainer 365967. One hundred microliters of serum was submitted to University of Michigan Animal Core and Liver Panel was run by the Core Facility.
Statistical Analysis for Mouse Study
Statistical analyses were performed in IBM SPSS Statistics 24. Graphs were created in Prism GraphPad 7 for Windows. Shapiro–Wilk test and Levene’s test were performed to determine the appropriate inferential statistics test. Two-way independent t tests were performed on parametric data at α = 0.05. Mann–Whitney U test was performed on nonparametric data at α = 0.05.
RESULTS
Retrospective Review of Human Experience
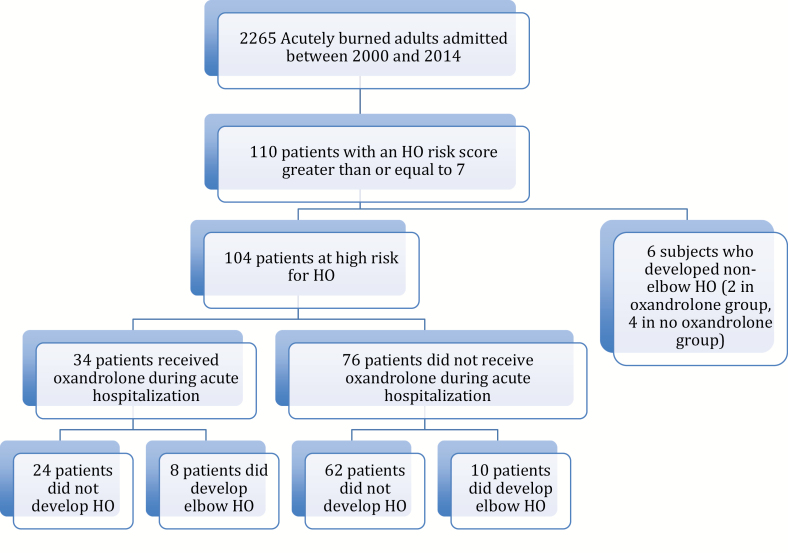
Out of 2265 patients, 110 acutely burned subjects were identified with an HO risk score ≥7 (4.8%). Of these patients, six had HO at a site other than the elbow (such as in an amputated residual limb or HO associated with previous trauma) and were excluded from further analysis, leaving a total study population of 104 subjects (Figure 1). Age (mean ± SD) at burn was 39 ± 14 years, burn size was 51 ± 20% (range 21–95% TBSA); 74% were male, and 82% were white (Table 1). Elbow HO developed in 23% (24/104 subjects). These diagnoses were confirmed by clinical evidence and imaging studies. Patients that did not develop HO were followed for a median of 529.5 days (range 18–6635 days) to ensure that there was no record of late HO development.
Figure 1.
Flow chart representing the study population. HO = heterotopic ossification.
Table 1.
Demographic and clinical information
| No Oxandrolone | Yes Oxandrolone | Total | P-Value | |
|---|---|---|---|---|
| N (%) | 72 (69.2) | 32 (30.8) | 104 (100) | |
| Burn size, mean %TBSA burned (SD) | 49.6 (20.1) | 55.0 (18.2) | 51.3 (19.6) | .19 |
| Race = white | 52 (80.0) | 23 (85.2) | 75 (81.5) | .56 |
| Gender = male, N (%) | 54 (75.0) | 20 (62.5) | 74 (71.2) | .19 |
| Age at time of burn, mean years (SD) | 37 (14) | 44 (13) | 39 (14) | 0.01 |
| Burn year, mean (SD) | 2004 (3) | 2009 (2) | 2005 (3) | .00 |
| Days on oxandrolone during acute stay, mean (SD) | 0.00 (0) | 55 (57) | N/A | N/A |
| Started oxandrolone within 7 days, N (%) | 0 (0.00) | 20 (62.5) | 20 (19.2) | .00 |
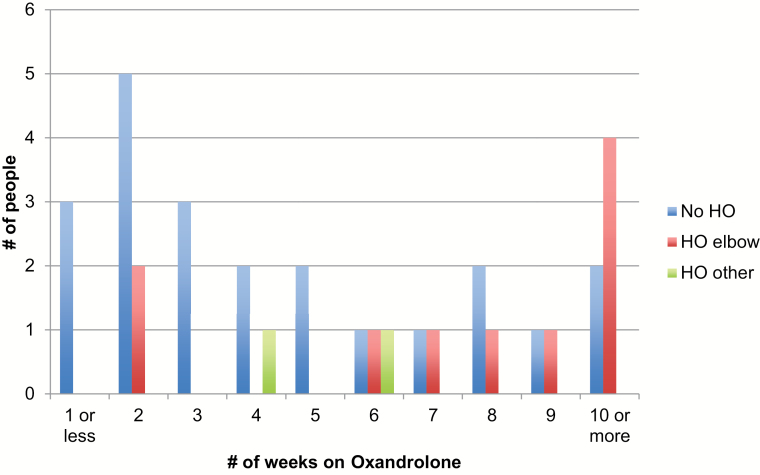
Approximately one third (32/104) of patients were treated with oxandrolone during their acute hospitalization. Patients who received oxandrolone were burned in 2005 or later. After 2008, all subjects received oxandrolone. Patients who received oxandrolone were older (44 vs 37 years, P = .01). Otherwise, the clinical and demographic characteristics between groups were similar (Table 1). Length of oxandrolone treatment and diagnosis of HO are illustrated in Figure 2.
Figure 2.
Incidence of heterotopic ossification (HO) in high-risk burn patients who received oxandrolone during acute hospitalization.
Subjects who received oxandrolone had a higher incidence of HO (10/32, 31%) than those that did not receive oxandrolone (14/72, 19%), Table 2. However, when controlling for oxandrolone administration, oxandrolone duration, postburn day oxandrolone initiation, HO risk score category, age, sex, race, burn size, and year of injury, there was no significant difference between the rates of elbow HO between the two populations. We looked at various models including unadjusted (odds ratio = 1.88, 95% CI: 0.73, 4.86; P-value: .191, c-statistic: 0.5708) and fully adjusted (odds ratio = 2.78, 95% CI: 0.66, 11.68; P-value: .163; c-statistic: 0.7714) and some models that dropped candidate predictors that were not statistically significant at the .05 level. Although odds ratios for these models demonstrated an association between oxandrolone and HO development, P-values were not significant (P-values ranged from .15 to .20). There were no qualitative differences in the regression analysis results when patients with HO at sites other than the elbow were included in the analysis.
Table 2.
Number of patients with elbow heterotopic ossification (HO) described by oxandrolone administration and HO risk score
| No Oxandrolone | Oxandrolone | |||||
|---|---|---|---|---|---|---|
| HO Risk Score | No HO | HO | Total | No HO | HO | Total |
| 7 | 17 | 4 | 21 | 3 | 0 | 3 |
| 8 | 11 | 1 | 12 | 5 | 0 | 5 |
| 9 | 10 | 3 | 13 | 9 | 0 | 9 |
| 10 | 12 | 1 | 13 | 5 | 6 | 11 |
| 11 | 5 | 3 | 8 | 0 | 2 | 2 |
| 12 | 2 | 1 | 3 | 0 | 2 | 2 |
| 13 | 1 | 1 | 2 | 0 | 0 | 0 |
| Total | 58 | 14 | 72 | 22 | 10 | 32 |
Results of the Mouse Study
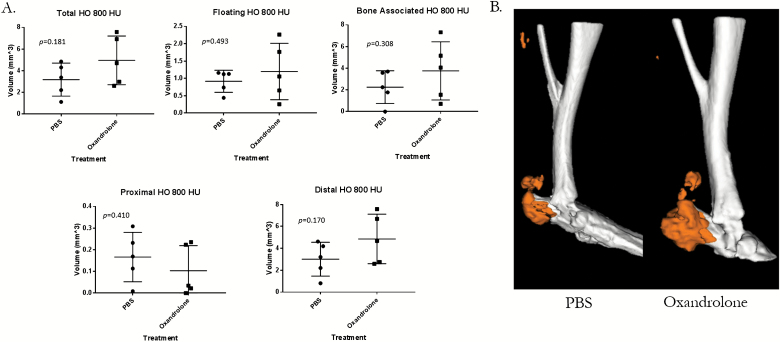
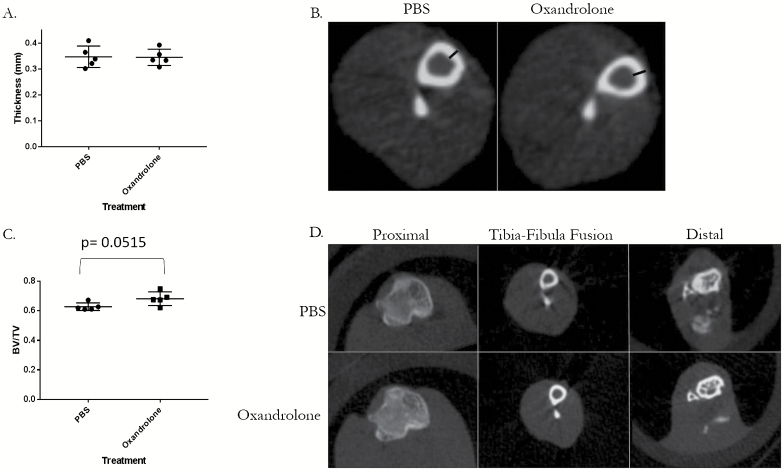
Oxandrolone is standard of care thereby making randomization of burned human subjects difficult. To mitigate this challenge, we examined the use of oxandrolone in our proven mouse HO model. Specifically, five mice underwent a burn/tenotomy followed by oral treatment with oxandrolone or vehicle control. Though not statistically significant, oxandrolone groups trended consistently higher than their PBS counterparts in total, bone-associated, and floating HO with large to medium effect (hedges g = 0.93, 0.69, and 0.45, respectively). There also was no statistical difference in the ectopic shell, porosity, or trabecular thickness of the HO that was formed between the oxandrolone- or control-treated mice (Figure 3 and Supplementary Figure 1). We also did not observe any difference in the tibial cortical thickness (P = .928) and in the tibial BV/TV, although there was a trend toward oxandrolone group (P = .0515) (Figure 4A and B) having increased tibial cortical thickness. The oxandrolone-treated group had less body weight compared to their control mice (P = .0312). Unthresholded segmented regions show no differences in volume between treatments (Supplementary Figure 2). Seminal vesicle weight was normal, indicating that the treatment did not affect the androgen status (Supplementary Figure 3).35 Liver measurements were also similar between treated and control animals (Supplementary Figure 4).
Figure 3.
Oxandrolone-treated mice exhibited similar ectopic bone volumes to control mice. A. MicroCT analysis of the left leg in 15-week-old male mice treated with oxandrolone or phosphate-buffered saline (PBS) 9 weeks following B/T. B. Image of representative means of each treatment group from microCT scans. Hounsfield unit (HU). Whiskers indicate ± 1 SD and the central line indicates mean. *P < .05 by independent samples t test assuming equal variance, n = 5.
Figure 4.
Oxandrolone treatment shows no difference in tibial cortical thickness and tibial BV/TV compared to phosphate-buffered saline (PBS)-treated male mice at 15 weeks of age following B/T. A. Tibial cortical thickness measured with microCT. B. Image of representative mean of each treatment group with black bars indicating measured distance. C. Tibial BV/TV measured in whole tibia with microCT at 15 weeks of age in male mice following B/T with either oxandrolone or PBS administration. D. Images of representative means of each treatment group at labeled cross-sections. Whiskers indicate ± 1 SD and the central line indicates mean. *P < .05 by independent samples t test assuming equal variance, n = 5. BV/TV = bone volume to total volume ratio.
DISCUSSION
Androgens and estrogens have a known central role in bone development, maintenance, and repair.22–25 However, their role in the formation of heterotopic bone after trauma has not been well characterized. Oxandrolone, a synthetic analog of testosterone, has been a crucial innovation in burn care in adults and has previously been shown to improve mortality, to reduce length of hospital stay, and to play a role in preventing bone loss during burn recovery.5,6 Deficiency of sex steroids as well as chronic inflammation and burn injuries are known to decrease bone mass. Though the cause of HO is unknown, one current theory and area of research is acute inflammation.36 Additional studies have demonstrated that androgens have a suppressive effect on immune function after trauma with a 2-fold decrease in proinflammatory cytokine release in splenic and peritoneal macrophages after a laparotomy and hemorrhagic shock.37–39 Testosterone specifically has also been demonstrated to reduce Toll-like receptor 4 (TLR4) expression on macrophages which is known to bind “danger signals” and stimulate inflammation in vivo and in vitro.40 This is the first study to examine the potential association of oxandrolone therapy after burn injury with the serious complication of HO in humans. In this retrospective study, oxandrolone was not associated with an increased marginal risk of HO in burned subjects already at high risk for HO.
It is important to investigate potential adverse effects of medications that are used in humans, especially those that are standard of care. As seen with estrogen used in hormone replacement therapy, research into the mechanisms of these drugs can find areas of inefficacy or of increased risk to patients.41 For this reason, all potential risks of widely prescribed drugs, such as oxandrolone, should be investigated to mitigate harm and improve quality of care. While there are data in animal studies that suggest testosterone in general may be related to HO formation, those data discuss the severity of HO rather than the formation of HO in general.22 As this is a retrospective review of data, we cannot speak to the severity of the HO that was formed by those who took oxandrolone vs those who did not take it. Future prospective studies should compare outcomes in those who develop HO and the severity of HO developed as related to administration of oxandrolone. There are two scales for measuring the severity of HO, the Brooker Classification System,42 a scale that has been previously validated in patients who developed HO following hip arthroplasty, and the Hastings and Graham Classification System,43 which specifically addressed HO at the elbow.
Prior research has shown that administration of oxandrolone for 2 years to severe burn children is correlated with increased bone mineral density (BMD).44 Considering the importance of androgen in the male skeleton,45 we wanted to understand if administration of oxandrolone is affecting bone mass. To answer this, we have analyzed the cortical bone mass and trabecular bone volume BV/TV had a trend toward oxandrolone group (P = .0515). The increase in BMD observed in the patient study and the well-established actions of androgen on the trabecular bone volume in mice were not recapitulated, which can be explained by either the duration of the study, dosing of the oxandrolone, or limited number of mice in the study. The weight gain observed in the oxandrolone-treated patients was also not mimicked in the mice.21 Finally, Reeves et al44 have observed that administration of oxandrolone for 2 years did not have an effect on the liver function, which is in agreement with our study (Supplementary Figure 4).
This study has several limitations. The retrospective nature of this study does not allow it to assess causality. The control group was mainly historical, as oxandrolone was introduced to the center in 2005 and became part of the universal standard of care by 2008; thus, there could have been new therapies that mitigated increased risk of HO in the population treated with oxandrolone that masked any potential association. We have no information on whether oxandrolone made HO more severe. The study does not address the group of patients who are at low risk for HO development with HO risk scores less than 7. Given the low frequency of HO at sites other than the elbow in our study population, we have insufficient evidence to determine whether HO was formed at sites such as the amputation site or the knee were in any way related to oxandrolone administration. We did not address a possible association of oxandrolone with HO in children, as no prospective risk factor scoring system that exists for children. Finally, we considered HO to be present if it was diagnosed on discharge, and it is possible that HO could have developed after discharge.
For our animal studies, there are several experimental parameters that would benefit from further investigation. First, we only treated male mice. Additionally, treatment length was shorter than in human subjects; however, prior work indicates this length is sufficient to develop HO in the mouse HO model.33,45 Though we felt that the dose used was clinically relevant, pharmacologic metabolism and bioavailability is likely different between humans and mice. Because we did not observe any differences between groups, administering more oxandrolone to mice might be still considered within acceptable range of oxandrolone dosing for mice.46 Even though direct comparison is difficult, the relative dosage administered to these animals (11.81 mg/kg per week, average body weight 25.4 g), is still more than administered to patients (140 mg per week/~88.8 kg ~1.58 mg/kg per week). Additionally, the frequency of treatment was different in mice compared to patients. Further studies in mice might allow us to elucidate the mechanism of action of oxandrolone and whether the actions are sex-specific. Finally, while these study results suggest a lack of association between oxandrolone and HO in order to definitively assess for a causal relationship between postburn oxandrolone administration and HO development, a prospective, controlled, clinical trial would be needed.
HO remains a serious but relatively rare burn complication. We found no evidence that oxandrolone administration during the acute burn hospitalization put patients at increased risk for HO development, even though there was a trend in mice toward increased bone formation with oxandrolone. However, given the limitations of retrospective data, the animal model, severity of disability in those with HO, and the widespread usage of oxandrolone, the authors propose for future studies to collect prospective data to more robustly examine this issue.
Supplementary Material
Funding: B.L. is funded by the National Institutes of Health R01GM123069, the National Institute of General Medical Sciences K08GM109105, and the American College of Surgeons Clowes Award. M.S. is funded by the Plastic Surgery Foundation National Endowment Award and C.H. is funded by the Howard Hughes Medical Institute Medical Student Scholar Award. C.T. was supported by the Shriners Hospitals for Children Helen Lemieux Scholarship. J.S., L.S., R.G. and C.R. were supported in part by National Institute on Disability, Independent Living and Rehabilitation Research (NIDILRR #90DPBU0001), G.G. and C.R. were supported in part by Shriners Hospitals for Children, Grant #72000 and the Fraser Fund of the Massachusetts General Hospital.
Conflict of interest statement. None declared.
REFERENCES
- 1. Hart DW, Wolf SE, Ramzy PI et al. Anabolic effects of oxandrolone after severe burn. Ann Surg 2001;233:556–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Herndon DN, Voigt CD, Capek KD et al. Reversal of growth arrest with the combined administration of oxandrolone and propranolol in severely burned children. Ann Surg 2016;264:421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jeschke MG, Finnerty CC, Suman OE, Kulp G, Mlcak RP, Herndon DN. The effect of oxandrolone on the endocrinologic, inflammatory, and hypermetabolic responses during the acute phase postburn. Ann Surg 2007;246:351–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miller JT, Btaiche IF. Oxandrolone treatment in adults with severe thermal injury. Pharmacotherapy 2009;29:213–26. [DOI] [PubMed] [Google Scholar]
- 5. Pham TN, Klein MB, Gibran NS et al. Impact of oxandrolone treatment on acute outcomes after severe burn injury. J Burn Care Res 2008;29:902–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wolf SE, Edelman LS, Kemalyan N et al. Effects of oxandrolone on outcome measures in the severely burned: a multicenter prospective randomized double-blind trial. J Burn Care Res 2006;27:131–41. [DOI] [PubMed] [Google Scholar]
- 7. Demling RH. Comparison of the anabolic effects and complications of human growth hormone and the testosterone analog, oxandrolone, after severe burn injury. Burns 1999;25:215–21. [DOI] [PubMed] [Google Scholar]
- 8. Chen HC, Yang JY, Chuang SS, Huang CY, Yang SY. Heterotopic ossification in burns: our experience and literature reviews. Burns 2009;35:857–62. [DOI] [PubMed] [Google Scholar]
- 9. Maender C, Sahajpal D, Wright TW. Treatment of heterotopic ossification of the elbow following burn injury: recommendations for surgical excision and perioperative prophylaxis using radiation therapy. J Shoulder Elbow Surg 2010;19:1269–75. [DOI] [PubMed] [Google Scholar]
- 10. Pakos EE, Ioannidis JP. Radiotherapy vs. nonsteroidal anti-inflammatory drugs for the prevention of heterotopic ossification after major hip procedures: a meta-analysis of randomized trials. Int J Radiat Oncol Biol Phys 2004;60:888–95. [DOI] [PubMed] [Google Scholar]
- 11. Burd TA, Lowry KJ, Anglen JO. Indomethacin compared with localized irradiation for the prevention of heterotopic ossification following surgical treatment of acetabular fractures. J Bone Joint Surg Am 2001;83A:1783–8. [DOI] [PubMed] [Google Scholar]
- 12. Ranganathan K, Loder S, Agarwal S et al. Heterotopic ossification: basic-science principles and clinical correlates. J Bone Joint Surg Am 2015;97:1101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hamid N, Ashraf N, Bosse MJ et al. Radiation therapy for heterotopic ossification prophylaxis acutely after elbow trauma: a prospective randomized study. J Bone Joint Surg Am 2010;92:2032–8. [DOI] [PubMed] [Google Scholar]
- 14. Holavanahalli RK, Helm PA, Parry IS, Dolezal CA, Greenhalgh DG. Select practices in management and rehabilitation of burns: a survey report. J Burn Care Res 2011;32:210–23. [DOI] [PubMed] [Google Scholar]
- 15. Schneider JC, Simko LC, Goldstein R et al. Predicting heterotopic ossification early after burn injuries: a risk scoring system. Ann Surg 2017;266:179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Manalagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 2000;21:115–37. [DOI] [PubMed] [Google Scholar]
- 17. Carson JA, Manolagas SC. Effects of sex steroids on bones and muscles: similarities, parallels, and putative interactions in health and disease. Bone 2015;80:67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Manolagas SC, O’Brien CA, Almeida M. The role of estrogen and androgen receptors in bone health and disease. Nat Rev Endocrinol 2013;9:699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jilka RL, Hangoc G, Girasole G et al. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science 1992;257:88–91. [DOI] [PubMed] [Google Scholar]
- 20. Bi LX, Wiren KM, Zhang XW et al. The effect of oxandrolone treatment on human osteoblastic cells. J Burns Wounds 2007;6:e4. [PMC free article] [PubMed] [Google Scholar]
- 21. Li H, Guo Y, Yang Z, Roy M, Guo Q. The efficacy and safety of oxandrolone treatment for patients with severe burns: a systematic review and meta-analysis. Burns 2016;42:717–27. [DOI] [PubMed] [Google Scholar]
- 22. Callewaert F, Boonen S, Vanderschueren D. Sex steroids and the male skeleton: a tale of two hormones. Trends Endocrinol Metab 2010;21:89–95. [DOI] [PubMed] [Google Scholar]
- 23. Chamouni A, Oury F. Reciprocal interaction between bone and gonads. Arch Biochem Biophys 2014;561:147–53. [DOI] [PubMed] [Google Scholar]
- 24. Syed F, Khosla S. Mechanisms of sex steroid effects on bone. Biochem Biophys Res Commun 2005;328:688–96. [DOI] [PubMed] [Google Scholar]
- 25. Gill RK, Turner RT, Wronski TJ, Bell NH. Orchiectomy markedly reduces the concentration of the three isoforms of transforming growth factor beta in rat bone, and reduction is prevented by testosterone. Endocrinology 1998;139:546–50. [DOI] [PubMed] [Google Scholar]
- 26. Suero EM, Meindl R, Schildhauer TA, Citak M. Clinical prediction rule for heterotopic ossification of the hip in patients with spinal cord injury. Spine 2018;43:1572–8. [DOI] [PubMed] [Google Scholar]
- 27. Kim JY, Rhee YG. The prevalence and morphometric analysis of ossified superior transverse scapular ligaments in patients with rotator cuff tears. J Shoulder Elbow Surg 2018;27:1044–50. [DOI] [PubMed] [Google Scholar]
- 28. Ranganathan K, Peterson J, Agarwal S et al. Role of gender in burn-induced heterotopic ossification and mesenchymal cell osteogenic differentiation. Plast Reconstr Surg 2015;135:1631–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc 1979;74:829–36. [Google Scholar]
- 30. Agarwal S, Loder SJ, Sorkin M et al. Analysis of bone-cartilage-stromal progenitor populations in trauma induced and genetic models of heterotopic ossification. Stem Cells 2016;34:1692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. The Jackson Laboratory. Jax Mice and Services. Body Weight Information for C57BL/6J (000664) Accessed 18 Apr. 2019; available from https://www.jax.org/jax-mice-and-services/strain-data-sheet-pages/body-weight-chart-000664.
- 32. Fryar CD, Gu Q, Ogden CL, Flegal KM. Anthropometric reference data for children and adults: United States, 2011–2014. Vital Health Stat 2016;39:1–46. [PubMed] [Google Scholar]
- 33. Peterson JR, Okagbare PI, De La Rosa S et al. Early detection of burn induced heterotopic ossification using transcutaneous Raman spectroscopy. Bone 2013;54:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Agarwal S, Loder SJ, Breuler C et al. Strategic targeting of multiple BMP receptors prevents trauma-induced heterotopic ossification. Mol Ther 2017;25:1974–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Almeida M, Han L, Martin-Millan M et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem 2007;282:27285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Forsberg JA, Potter BK, Polfer EM, Safford SD, Elster EA. Do inflammatory markers portend heterotopic ossification and wound failure in combat wounds? Clin Orthop Relat Res 2014;472:2845–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wichmann MW, Zellweger R, DeMaso CM, Ayala A, Chaudry IH. Mechanism of immunosuppression in males following trauma-hemorrhage. Critical role of testosterone. Arch Surg 1996;131:1186–92. [DOI] [PubMed] [Google Scholar]
- 38. Angele MK, Knöferl MW, Schwacha MG et al. Sex steroids regulate pro- and anti-inflammatory cytokine release by macrophages after trauma-hemorrhage. Am J Physiol 1999;277(1 Pt 1):C35–42. [DOI] [PubMed] [Google Scholar]
- 39. Angele MK, Schwacha MG, Ayala A, Chaudry IH. Effect of gender and sex hormones on immune responses following shock. Shock 2000;14:81–90. [DOI] [PubMed] [Google Scholar]
- 40. Rettew JA, Huet-Hudson YM, Marriott I. Testosterone reduces macrophage expression in the mouse of toll-like receptor 4, a trigger for inflammation and innate immunity. Biol Reprod 2008;78:432–7. [DOI] [PubMed] [Google Scholar]
- 41. Torre DS, Biserni A, Rando G et al. The conundrum of estrogen receptor oscillatory activity in the search for an appropriate hormone replacement therapy. Endocrinology 2011;152:2256–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wright JG, Moran E, Bogoch E. Reliability and validity of the grading of heterotopic ossification. J Arthroplasty 1994;9:549–53. [DOI] [PubMed] [Google Scholar]
- 43. Hastings H 2nd, Graham TJ. The classification and treatment of heterotopic ossification about the elbow and forearm. Hand Clin 1994;10:417–37. [PubMed] [Google Scholar]
- 44. Reeves PT, Herndon DN, Tanksley JD et al. Five-year outcomes after long-term oxandrolone administration in severely burned children: a randomized clinical trial. Shock 2016;45:367–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Almeida M, Laurent MR, Dubois V et al. Estrogens and androgens in skeletal physiology and pathophysiology. Physiol Rev 2017;97: 135–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Peterson JR, De La Rosa S, Eboda O et al. Treatment of heterotopic ossification through remote ATP hydrolysis. Sci Transl Med 2014;6:255ra132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.