Abstract
Background
Benzathine benzylpenicillin G (BPG) is recommended as secondary prophylaxis to prevent recurrence of acute rheumatic fever and subsequent rheumatic heart disease (RHD). Following intramuscular injection, BPG is hydrolysed to benzylpenicillin. Little is known of the pharmacokinetics of benzylpenicillin following BPG in populations at risk of RHD.
Methods
We conducted a longitudinal pharmacokinetic study of children and adolescents receiving secondary prophylaxis throughout six monthly cycles of BPG. Dried blood spot samples were assayed with LC-MS/MS. Benzylpenicillin concentrations were analysed using non-linear mixed-effects modelling with subsequent simulations based on published BMI-for-age and weight-for-age data.
Results
Eighteen participants contributed 256 concentrations for analysis. None had benzylpenicillin concentrations >0.02 mg/L for the full time between doses. The median duration above this target was 9.8 days for those with a lower BMI (<25 kg/m2), who also had lower weights, and 0 days for those with a higher BMI (≥25 kg/m2). Although fat-free mass was a key determinant of benzylpenicillin exposure after a standard dose of BPG, having a higher BMI influenced absorption and almost doubled (increase of 86%) the observed t½.
Conclusions
Few children and adolescents receiving BPG as secondary prophylaxis will achieve concentrations >0.02 mg/L for the majority of the time between injections. The discordance of this observation with reported efficacy of BPG to prevent rheumatic fever implies a major knowledge gap relating to pharmacokinetic/pharmacodynamic relationships between benzylpenicillin exposure and clinical outcomes.
Introduction
Acute rheumatic fever (ARF) is an autoimmune condition caused by untreated group A Streptococcus (GAS) infection of the upper respiratory tract, and possibly skin, that can lead to rheumatic heart disease (RHD).1–3 The global prevalence of RHD is estimated to be 34 million people, with 319 400 deaths per year.4 Australian Aboriginal and Torres Strait Islanders,5 Māori and Pacific children have some of the highest rates in the world.6
Australian guidelines recommend that intramuscular injections of 1.2 million IU (MIU; 900 mg) of benzathine benzylpenicillin (BPG) should be administered every 4 weeks (or 3 weekly in selected cases) as secondary prophylaxis for patients ≥20 kg.7 After intramuscular injection, BPG is hydrolysed to benzylpenicillin and absorbed from the depot site into the plasma.
To prevent GAS infections using secondary prophylaxis, it is widely accepted that a plasma benzylpenicillin concentration above the laboratory-derived MIC of 0.02 mg/L is required for most of the time between intramuscular injections.8 Despite this assumption, there are no convincing data demonstrating a quantitative, inverse relationship between exposure to benzylpenicillin and either GAS infection or subsequent ARF episodes. Furthermore, there are limited data relating to BPG pharmacokinetics in populations at highest risk of ARF.9 Most current dosing regimens are underpinned by data from studies conducted in healthy male military recruits or from children more than 50 years ago.10–14 Extrapolating more recent population pharmacokinetic models, also performed in military recruits,15 to patients with ARF/RHD may also not be appropriate owing to differences in age, body composition and disease effects.
The development of pharmacokinetic/pharmacodynamic models of benzylpenicillin after BPG injection are important steps towards understanding how benzylpenicillin exposure relates to GAS colonization, infection, ARF and subsequent RHD. With accompanying simulations, these models can be applicable to wider populations and used to inform decisions on optimal dosing regimens for BPG and the development of newer longer-acting penicillin preparations. A population pharmacokinetic model could also inform personalized, adaptive dosing regimens for patients currently receiving secondary prophylaxis.
We conducted a longitudinal, prospective population pharmacokinetic study of children and adolescents with RHD receiving BPG for secondary prophylaxis. To facilitate sampling in a community setting, benzylpenicillin assays were measured from dried blood spot (DBS) samples collected from finger pricks.16
Methods
Ethics
This study was approved by the Western Australian Child and Adolescent Health Services Human Research Ethics Committee (20160604EP, RGS0000002547) and the Western Australian Aboriginal Human Ethics Committee (709). The study was registered with the Australia and New Zealand Clinical Trials Registry (ACTRN12618001288213).
Clinical study procedures
The study was conducted between March 2017 and November 2017. Participants were identified through the Princess Margaret Hospital ambulatory care service, which provides outpatient care to metropolitan Perth, Western Australia. Children and adolescents <18 years old receiving regular BPG as secondary prophylaxis were eligible. Written consent/assent was obtained from participants and parents/guardians prior to study commencement.
Baseline characteristics including age, ethnicity, gender, weight, height, haemoglobin (HemoCue® Hb201; Angelholm, Sweden) and the presence of comorbidities were recorded. The date of diagnosis of ARF/RHD and the number of recurrent ARF episodes were also recorded. A value for the haematocrit was derived from the haemoglobin concentration.17 Venesection was not performed and creatinine was not measured. However, all participants were part of a long-term ambulatory care programme and none had known established renal disease.
Over a period of six monthly cycles, a DBS sample and a throat swab were collected prior to administration of BPG to measure trough benzylpenicillin levels. Participants were contacted on day 21 of each cycle. If present, a symptomatic sore throat triggered a home visit and an additional DBS sample and throat swab were collected, which were included in the analysis. Participants were also encouraged to contact the study nurse if they developed a sore throat throughout the study, triggering DBS sample and throat swab collection. Treatment for the sore throat was determined by the treating clinician. Given the reported minimal rates of impetigo in this urban cohort, we did not formally collect data relating to skin sores.
BPG is available in Australia as Bicillin® L-A [PfizerTM, Australia; 2.3 mL containing 900 mg (1.2 MIU) of BPG]. Trained nurses administered BPG injections to the upper outer quadrant, alternating each cycle. No specific assessment was undertaken to determine whether the injection had been given intramuscularly as planned.
Intensive sampling was performed during two of the six monthly BPG cycles with scheduled DBS sampling on days 1, 3, 6, 12 and 21 following BPG. It was expected that school-aged children might miss some appointments. Any missed intensive timepoints were collected in ‘non-intensive’ months and collated to ensure there were two complete DBS sets per participant over the 6 month collection period.
At each timepoint, five spots were collected onto filter paper cards (Whatman 903 Protein Saver™ Cards, GE Healthcare Australia Pty Ltd, Parramatta, NSW, Australia). Samples were stored in a portable car-refrigerator (Waeco, TC-14FL) owing to high ambient temperatures (up to 45°C) for transport and stored at <10°C for at least 3 h to ensure adequate drying, before being stored in a −80°C freezer until the DBS could be analysed (see Supplementary data available at JAC Online, including Figure S1).
Measuring penicillin from DBS
The concentration of benzylpenicillin in DBS samples was measured using a validated LC-MS/MS assay,16 with minor modification (see Supplementary data). The lower limit of quantification was 0.0025 mg/L and the limit of detection was 0.001 mg/L.
Pharmacokinetic modelling and simulations
Loge plasma concentration–time datasets for benzylpenicillin were analysed by non-linear mixed-effects modelling using NONMEM (v 7.2.0, ICON Development Solutions, Ellicott City, MD, USA) with an Intel Visual FORTRAN 10.0 compiler. The Laplacian with interaction (LAPLACIAN with INTER) estimation method was used (see Supplementary data).
Once a final population pharmacokinetic model was established, simulations were performed using WHO Growth Reference Data,18,19 which provide weight-for-age and BMI-for-age distributions. Separate simulations containing 500 male and 500 female children for each year of age from 5 to 19 were performed for those with BMI <25 kg/m2 and for those with BMI ≥25 kg/m2. The BPG doses for the simulation were based on the current Australian RHD guidelines7 with a lower dose of 450 mg (0.6 MIU) for children with weight <20 kg. A plasma benzylpenicillin concentration was simulated every 6 h, between doses of a 28 day dosing period at steady-state. For each simulated child, Cmin, Cmax, Tmax and time >0.02 mg/L were determined. Results were depicted according to weight to represent time above this concentration with the current recommended dosing regimens. Fat-free mass was estimated from weight and BMI from a published model in children.20
Results
Twenty-two eligible participants were enrolled. Four subsequently withdrew without contributing samples for pharmacokinetic analysis: two inpatients were discharged back to a remote area; one did not return from a remote community after school holidays; and one withdrew. Eighteen participants provided data for analysis with 16 (89%) full datasets over six monthly injection cycles. All participants received the full dose (900 mg) of BPG. The clinical characteristics are summarized in Table 1.
Table 1.
Baseline characteristics of children administered BPG for RHD prophylaxis; N = 18
| Age (years), median (range) | 14.1 (7.9–17.7) |
| Male, n (%) | 8 (44) |
| Weight (kg), median (range) | 62.9 (29.9–149) |
| Height (m), median (range) | 1.61 (1.36–2.05) |
| BMI (kg/m2), median (range) | 23.6 (16.2–44.4) |
| BMI ≥25 kg/m2, n (%) | 8 (44) |
| Haemoglobin (g/L), median (range) | 127 (100–156) |
| Ethnicity, n | |
| Aboriginal | 14 |
| Māori | 2 |
| Samoan | 2 |
There were 256 individual plasma benzylpenicillin concentrations included in the pharmacokinetic analysis. Twenty-five (9.7%) benzylpenicillin concentrations were below the limit of quantification (BLQ). These timepoints were retained and the likelihood of each being BLQ was estimated using methods described elsewhere.21
Initial analysis using standard compartmental modelling with various absorption models resulted in estimates of elimination t½ that were much longer than previously reported for benzylpenicillin. Therefore, the elimination rate constant was fixed with allometric scaling with an exponential of −¼ (equivalent to an exponential of ¾ for CL and 1 for V) based on previously published data in children receiving intravenous benzylpenicillin.22,23 Multiple sequential stages of absorption were assessed to describe the time–concentration profile with the final model including two absorption rate constants, ka-1 and ka-2, which were parameterized in terms of their respective t½ values (t½, abs-1 and t½, abs-2, respectively). First-order absorption for both these stages performed better than models with zero-order process. The addition of peripheral compartment(s) did not improve the model. The final model structure is shown in Figure 1.
Figure 1.
Structure of the final pharmacokinetic model. kel, elimination rate constant.
The interindividual variability was 78%, 63% and 26% for t½, abs-1, t½, abs-2 and V, respectively. The interoccasion variability for the second, slower absorption parameter (t½, abs-2) was 30%. A full covariance matrix model was used. After inclusion of covariate effects, there was close inverse correlation between the two absorption parameters and the correlation coefficient (r) was fixed to −1. Fat-free mass was the best size parameter for allometric scaling on V. Although many body composition covariates were correlated with ka-2, BMI as a categorical variable, with a threshold of ≥25 kg/m2, resulted in the best fit and was associated with an 86.5% increase in t½, abs-2 (Table 2). No other significant covariate relationships were identified.
Table 2.
Pharmacokinetic parameters for children administered BPG for RHD prophylaxis
| BMI <25 kg/m2 (n = 10), median (IQR) (range) | BMI ≥25 kg/m2 (n = 8), median (IQR) (range) | |
|---|---|---|
| t ½, abs-1 (days) | 0.35 (0.29–1.60) (0.13–1.95) | 0.30 (0.26–0.63) (0.17–2.13) |
| t ½, abs-2 (days) | 9.8 (4.7–12.8) (3.1–26.5) | 20.3 (13.4–23.8) (4.7–38.4) |
| C min (μg/L) | 5.64 (1.31–9.76) (0.43–11.9) | 7.15 (6.27–9.11) (0.01–17.0) |
| C max (μg/L) | 34.8 (29.1–52.4) (21.6–68.4) | 19.8 (17.1–22.1) (8.99–38.1) |
| T max (h) | 45.6 (36.3–72.0) (18.7–102) | 43.0 (39.1–59.3) (23.3–156) |
| Time >0.02 mg/L (days) | 9.75 (7.75–12.0) (3.50–18.5) | 0 (0–4.31) (0–23.3) |
| Time >0.02 mg/L (%) | 35 (27–42) (12–67) | 0 (0–15) (0–80) |
| Time >0.01 mg/L (days) | 19.0 (15.3–26.5) (12.3–32.4) | 18.5 (15.8–25.2) (0–37.26) |
| Time >0.01 mg/L (%) | 65 (53–95) (29–100) | 69 (55–91) (0–100) |
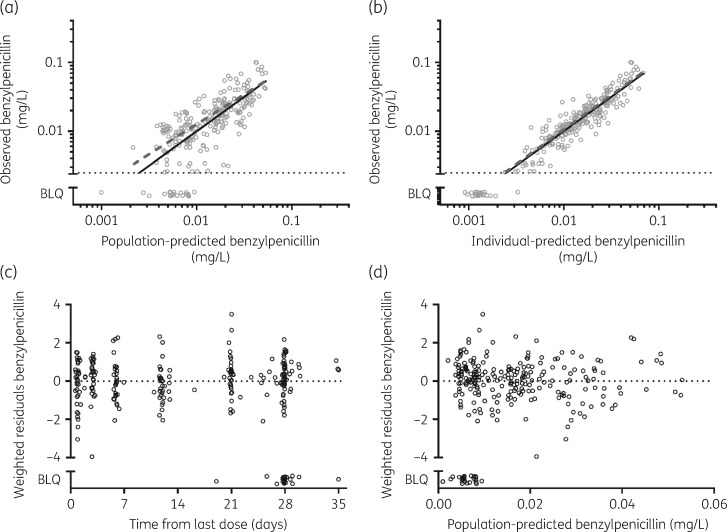
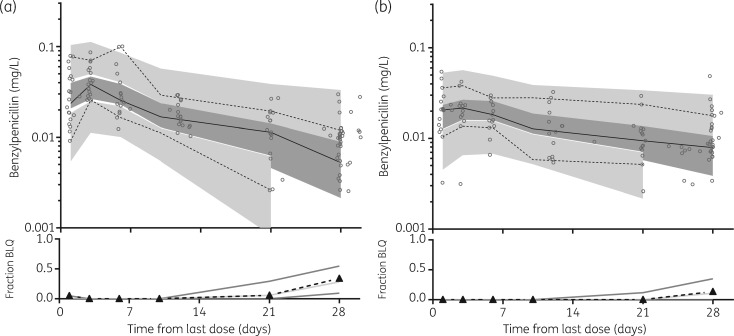
The final model parameter estimates and bootstrap results are summarized in Table 3. Bias was less than 2% for all fixed model parameters and less than 7% for random model parameters. Goodness-of-fit plots (Figure 2) and visual predictive check (VPC) plots stratified for BMI (Figure 3) are shown.
Table 3.
Final population pharmacokinetic estimates and bootstrap results for benzylpenicillin after administration of monthly injections of BPG in children and adolescents
| Parameter | Mean | Bootstrap, median (95% CI) |
|---|---|---|
| Objective function value | −265.078 | −300.214 (−458.616 to −161.877) |
| Structural model parameters | ||
| k el (h−1·70 kg−1) | 1.32 | fixed |
| V (L·70 kg−1) | 72.2 | 72.0 (64.0–84.2) |
| t ½, abs-1 (days) | 0.455 | 0.461 (0.174–0.948) |
| t ½, abs-2 (days) | 8.88 | 8.79 (5.71–12.5) |
| increase in t½, abs-2 with BMI ≥25 kg/m2 (%) | 86.5 | 86.8 (33.4–198) |
| Variable model parameters (shrinkage%) | ||
| IIV in V | 26 (9) | 24 (10–37) |
| IIV in t½, abs-1 | 78 (12) | 75 (40–107) |
| IIV in t½, abs-2 | 63 (12) | 62 (32–85) |
| IOV in t½, abs-2 | 30 (46) | 31 (20–48) |
| r (t½, abs-1, t½, abs-2) | −1 | fixed |
| r (t½, abs-2, V) | −0.746 | −0.808 (−1.00 to −0.316) |
| RV (%) | 35 (13) | 34 (30–38) |
k el, elimination rate constant; t½, abs, absorption t½; IIV, interindividual variability; IOV, interoccasion variability; RV, residual variability. IIV, IOV and RV are presented as 100% × √variability estimate.
Figure 2.
Diagnostic plots of the population pharmacokinetic model. (a) Observed versus population-predicted plasma concentrations. (b) Observed versus individual-predicted plasma concentrations. (c) Weighted residuals versus time. (d) Weighted residuals versus population-predicted concentrations. The continuous lines are lines of identity. BLQ data are included in each plot.
Figure 3.
Prediction-corrected VPCs for plasma benzylpenicillin concentrations (mg/L on log10 scale) for children with a lower BMI (<25 mg/kg2; a) and a higher BMI (≥25 mg/kg2; b). Observed 50th (continuous line) and 10th and 90th (dotted lines) percentiles within their simulated 95% CI (dark grey shading represents the 95% CI for the observed; light grey areas represent the 95% CI for the 10%–90% percentiles) are shown; data points are indicated by circles. Fraction BLQ (triangles with dashed black line) is also demonstrated with the simulated median (light grey line) and 95% CI (darker grey lines).
Simulations
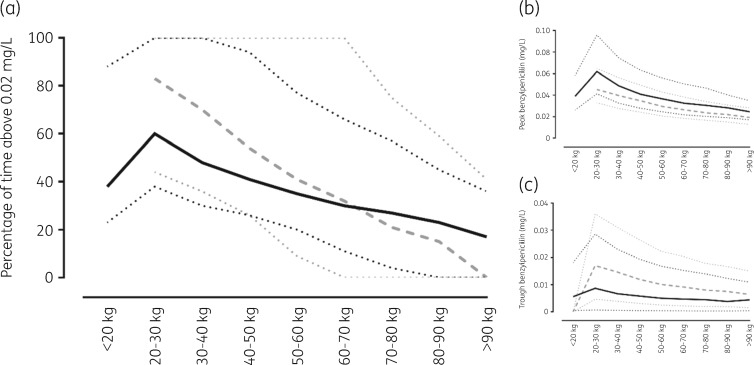
Separate simulations for children with lower and higher BMIs (<25 kg/m2 and ≥25 kg/m2, respectively) are summarized as median and 90% prediction intervals according to weight (Figure 4). Consistent with a 450 mg dose, children with a lower BMI and weighing <20 kg had lower predicted Cmin, Cmax and time >0.02 mg/L than those weighing 20–40 kg who received 900 mg. These parameters decreased as weight increased, as would be expected with a lower mg/kg dose.
Figure 4.
Summary of simulations of 1000 children with a lower BMI [<25 mg/kg2; continuous black line (median) and dotted black lines (90% prediction intervals)] and a higher BMI [≥25 mg/kg2; dashed grey line (median) and dotted grey lines (90% prediction intervals)] with equal numbers of gender. (a) Percentage of time >0.02 mg/L. (b) Peak concentrations. (c) Trough concentrations.
Discussion
In this cohort of predominantly urban Aboriginal or Torres Strait Islander children and adolescents receiving regular BPG, none had benzylpenicillin concentrations >0.02 mg/L for the full time period between injections. The median observed duration above this target was 9.8 days for those with a lower BMI and 0 days for those who had a higher BMI. The results for the children with a lower BMI in this study accord with a recent pharmacokinetic study of BPG in healthy adult male army recruits, for whom the median duration >0.02 mg/L was 9 days; the mean weight in that cohort was 77 kg (range 50–109 kg). One of the strengths of the present study was that it was undertaken in an at-risk population with a wide range of weights (30–149 kg) and body composition (BMI 16.2–44.4 kg/m2).
Fat-free mass and BMI were the key determinants of the benzylpenicillin exposure profiles during each monthly intramuscular injection. Fat-free mass correlated best with V. Owing to the standard current recommended dose of 900 mg per month for all patients ≥20 kg,7 as weight increased, the administered mg/kg dose decreased. This accounts for the generally short duration of benzylpenicillin concentrations >0.02 mg/L for all patients as weight increased beyond 40–50 kg.
A BMI of ≥25 kg/m2 was the only other significant covariate in the model and provides a novel mechanism to account for the observed patient data. For participants of equal weight, having a higher BMI nearly doubled the t½, abs-2, the slowest process in the model, which then determined the observed terminal t½. For the 8 participants in the present study with a higher BMI, the median t½, abs-2 was 20 days, compared with 10 days for the 10 participants with a lower BMI. The net effect of delayed absorption is to ‘smooth’ or flatten out the time–concentration profile of benzylpenicillin observed in the blood.
Our model differs from other population pharmacokinetic models of BPG. Although the most recent study of army recruits demonstrated a similar duration of median plasma concentrations >0.02 mg/L,15 the model applied an elimination t½ from the central compartment of 6 h to explain the observed data. This estimate is not consistent with published data about benzylpenicillin CL from the central compartment of 20–60 min following intravenous benzylpenicillin.24 The ideal structural basis for a population pharmacokinetic model of BPG should be informed by a biologically sound hypothesis. In this case, the absorption characteristics of penicillin from the BPG depot are much more important than CL from the central compartment (i.e. plasma). Owing to the rapid CL relative to absorption, our approach was to fix this parameter for benzylpenicillin. Once accounted for in this way, it was evident that two sequential absorption phases might reflect a series of separate processes. The prepared injection is a suspension of BPG crystals in a water-based matrix. To be measured as benzylpenicillin in the blood, the crystals first need to dissolve and then BPG needs to be hydrolysed to its constituent benzathine and penicillin moieties within the depot site—potentially corresponding to the two phases of absorption in the present model.
One possible explanation for the observation of delayed absorption of benzylpenicillin in children with a higher BMI is inadvertent injection into the subcutaneous or adipose tissues, rather than intramuscular as intended. Although we did not have the opportunity to assess the actual site of injection in the current patient cohort, there is circumstantial evidence to suggest this may be the case in those with increasing BMI. Radiological imaging studies that investigate the site of planned intramuscular injections into the upper outer quadrant of the buttock estimate that in adults more than 85% of males and 95% of females receive injections outside the gluteal muscle.25 Adipose calcification was also seen in that study, suggesting that previous ‘intramuscular’ injections were possibly intra-adipose or subcutaneous.25 In another study of participants with a BMI of 25–29.9 kg/m2, only 33% received their injections intramuscularly, and when BMI was ≥30 kg/m2, no participants received their injections into the muscle.26 Animal models of penicillin administration also support the explanation of delayed absorption of benzylpenicillin for subcutaneous injections.27 If the target of secondary prophylaxis is to maintain prolonged, low-level benzylpenicillin concentrations, these kinetics may favour subcutaneous, or intralipomatous, rather than intramuscular injection, something yet to be formally assessed in humans receiving BPG.
This study extends knowledge about the disposition of BPG and provides additional justification for reconsidering weight-based dosage regimens and the development of reformulated agents. The need for a reformulated product has been suggested by a panel of RHD experts. From a pharmacokinetic perspective, the ideal characteristics for this product would include subcutaneous administration and the ability to be dosed at more than a 6 weekly interval.28 The results of the present study further justify a formal comparative study of subcutaneous versus intramuscular injection of BPG, including assessment of pharmacokinetics in addition to safety and tolerability. There is evidence that for some medicines subcutaneous administration is tolerated better than intramuscular administration and is often preferred by patients.29,30 Taken together, these findings could facilitate changes to administration guidelines with the existing drug formulation. But even with a doubling of the observed apparent terminal t½, much higher doses would need to be given to achieve current targets and a 6 weekly dosing interval would still not be possible.
It is difficult to reconcile the observed pharmacokinetic profiles following BPG injection with the widely accepted concept that benzylpenicillin concentrations need to be above 0.02 mg/L for all, or most, of the period between BPG injections to prevent GAS acquisition and subsequent ARF. A target concentration of 0.02 mg/L is based on standardized susceptibility breakpoints that are determined from the 90th percentile of MICs within a population of GAS isolates. It should be noted that MIC values for individual bacterial isolates are based on the concentration required to prevent bacterial growth in a static in vitro environment and may not necessarily be the same concentration required to prevent pharyngeal acquisition and colonization of GAS. Despite this, the evidence from prior studies suggests that BPG at the current dosing is effective in preventing sore throats for some, but not all, of the interval between injections and has efficacy in preventing ARF episodes.31
At present there are no convincing data demonstrating a quantitative, inverse relationship linking exposure to benzylpenicillin with either GAS infection or subsequent ARF episodes. Many well-characterized GAS isolates have MICs <0.02 mg/L, so it is plausible that such high concentrations may not be necessary in every situation. It is also possible that concentrations lower than the MIC may be adequate to prevent colonization with new strains of GAS. If subsequent pharmacokinetic/pharmacodynamic and human challenge models do indeed show that, for example, concentrations ∼0.01 mg/L are a better target threshold for protection, the results from the current study will inform further studies to optimize the dosing of BPG and reformulation efforts.
Defining exposure–response relationships, determining the impact of intra-adipose injection, revising weight-based BPG dosing and redeveloping long-acting penicillin formulations should be key elements of the research agenda for secondary prophylaxis.
Several limitations exist in this observational study. One challenge to performing pharmacokinetic studies in vulnerable populations such as patients with RHD is that there may be logistical, ethical and cultural barriers to frequent venesection. As in this study, using a DBS assay overcomes this challenge but does have some limitations. Some DBS assays are vulnerable to systematic bias when there are a wide range of haematocrits.32 With this particular assay, and with a narrow range of haemoglobin measurements seen amongst the participants of this study, a significant haematocrit effect is unlikely. Secondly, as we only collected DBS samples, we did not have creatinine measurements available to measure renal function. Whilst this would ordinarily be an expected component of pharmacokinetic studies, there was no suspicion of renal dysfunction in these children. Thirdly, all participants weighed >20 kg, therefore simulation values <20 kg were unable to be verified with DBS samples in this cohort. Finally, in keeping with current practice, we were unable to use real-time imaging to confirm intramuscular rather than subcutaneous injection of BPG.
Conclusions
Most children and adolescents receiving BPG as secondary prophylaxis will achieve concentrations >0.02 mg/L for only a small proportion of the period between injections. The discordance of this observation with the reported efficacy of BPG to prevent ARF implies a major knowledge gap relating to the pharmacokinetic/pharmacodynamic relationship between exposure to benzylpenicillin and clinical outcomes. Defining this relationship should be considered a critical component of future research into optimizing secondary prophylaxis and penicillin reformulation activities. Delayed absorption in participants with a higher BMI raises the possibility of subcutaneous (including intralipomatous) rather than intramuscular injection and provides the justification to consider a definitive study comparing these different routes of administration.
Supplementary Material
Acknowledgements
We thank Mara West, Isabelle Adams and Glenn Pearson of the Kulunga Aboriginal Research Development Unit and the Aboriginal Research Projects Forum for their expert cultural guidance. We would like to acknowledge the ambulatory care nursing staff of Princess Margaret Hospital for their role in this study and Dr Brioni Moore for her expertise. We thank the Aboriginal, Torres Strait Islander, Māori and Samoan participants and families for taking part in this study. Without their contribution, this study would not have been possible.
Funding
This work was supported by Commonwealth funding from the Australian Tropical Medical Commercialisation program (grant number ATMC50298), Wesfarmers Centre of Vaccines and Infectious Diseases and Novartis Institutes for BioMedical Research. A. C. B. is supported by a National Health and Medical Research Council Early Career Fellowship (grant number 1088735).
Transparency declarations
None to declare.
References
- 1. Carapetis JR, McDonald M, Wilson NJ.. Acute rheumatic fever. Lancet 2005; 366: 155–68. [DOI] [PubMed] [Google Scholar]
- 2. Parks T, Smeesters PR, Steer AC.. Streptococcal skin infection and rheumatic heart disease. Curr Opin Infect Dis 2012; 25: 145–53. [DOI] [PubMed] [Google Scholar]
- 3. O'Sullivan L, Moreland NJ, Webb RH. et al. Acute rheumatic fever after Group A Streptococcus pyoderma and Group G Streptococcus pharyngitis. Pediatr Infect Dis J 2017; 36: 692–4. [DOI] [PubMed] [Google Scholar]
- 4. Watkins DA, Johnson CO, Colquhoun SM. et al. Global, regional, and national burden of rheumatic heart disease, 1990-2015. N Engl J Med 2017; 377: 713–22. [DOI] [PubMed] [Google Scholar]
- 5. Roberts KV, Maguire GP, Brown A. et al. Rheumatic heart disease in Indigenous children in northern Australia: differences in prevalence and the challenges of screening. Med J Aust 2015; 203: 221.e1–7. [DOI] [PubMed] [Google Scholar]
- 6. de Dassel JL, Ralph AP, Carapetis JR.. Controlling acute rheumatic fever and rheumatic heart disease in developing countries: are we getting closer? Curr Opin Pediatr 2015; 27: 116–23. [DOI] [PubMed] [Google Scholar]
- 7.RHDAustralia, National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand. Australian Guideline for Prevention, Diagnosis and Management of Acute Rheumatic Fever and Rheumatic Heart Disease. 2nd Edition. Darwin, Australia: Menzies School of Health Research, 2012.
- 8. Wyber R. Global Status of BPG Report https://rhdaction.org/sites/default/files/RHD%20Action_Global%20Status%20of%20BPG%20Report_Online%20Version.pdf.
- 9. Currie BJ, Burt T, Kaplan EL.. Penicillin concentrations after increased doses of benzathine penicillin G for prevention of secondary rheumatic fever. Antimicrob Agents Chemother 1994; 38: 1203–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davis PS, Copeman WS.. Rheumatic diseases. Br J Clin Pract 1957; 11: 936–9. [PubMed] [Google Scholar]
- 11. Stollerman GH, Rusoff JH.. Prophylaxis against group A streptococcal infections in rheumatic fever patients: use of new repository penicillin preparation. JAMA 1952; 150: 1571–5. [DOI] [PubMed] [Google Scholar]
- 12. Denny FW, Wannamaker LW, Brink WR. et al. Prevention of rheumatic fever: treatment of the preceding streptococcic infection. JAMA 1950; 143: 151–3. [DOI] [PubMed] [Google Scholar]
- 13. Feinstein AR, Wood HF, Epstein JA. et al. A controlled study of three methods of prophylaxis against streptococcal infection in a population of rheumatic children—results of the first three years of the study, including methods for evaluating the maintenance of oral prophylaxis. N Engl J Med 1959; 260: 697–702. [DOI] [PubMed] [Google Scholar]
- 14. Feinstein AR, Spagnuolo M, Jonas S. et al. Prophylaxis of recurrent rheumatic fever. Therapeutic-continuous oral penicillin vs monthly injections. JAMA 1968; 206: 565–8. [PubMed] [Google Scholar]
- 15. Neely M, Kaplan EL, Blumer JL. et al. A population pharmacokinetic modeling approach shows that serum penicillin G concentrations are below inhibitory concentrations by two weeks after benzathine penicillin G injection in the majority of young adults. Antimicrob Agents Chemother 2014; 58: 6735–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Page-Sharp M, Coward J, Moore BR. et al. Penicillin dried blood spot assay for use in patients receiving intramuscular benzathine penicillin G and other penicillin preparations to prevent rheumatic fever. Antimicrob Agents Chemother 2017; 61: e00252-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee SJ, Stepniewska K, Anstey N. et al. The relationship between the haemoglobin concentration and the haematocrit in Plasmodium falciparum malaria. Malar J 2008; 7: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CDC National Center for Health Statistics. CDC Growth Charts http://www.cdc.gov/growthcharts/. [DOI] [PubMed]
- 19.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards http://www.who.int/childgrowth/.
- 20. Anderson BJ, Holford NH.. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet 2009; 24: 25–36. [DOI] [PubMed] [Google Scholar]
- 21. Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn 2001; 28: 481–504. [DOI] [PubMed] [Google Scholar]
- 22. Bolme P, Eriksson M, Paalzow L. et al. Malnutrition and pharmacokinetics of penicillin in Ethiopian children. Pharmacol Toxicol 1995; 76: 259–62. [DOI] [PubMed] [Google Scholar]
- 23. Buchanan N, Robinson R, Koornhof HJ. et al. Penicillin pharmacokinetics in kwashiorkor. Am J Clin Nutr 1979; 32: 2233–6. [DOI] [PubMed] [Google Scholar]
- 24. Kampmann J, Hansen JM, Siersboek-Nielsen K. et al. Effect of some drugs on penicillin half-life in blood. Clin Pharmacol Ther 1972; 13: 516–9. [DOI] [PubMed] [Google Scholar]
- 25. Cockshott WP, Thompson GT, Howlett LJ. et al. Intramuscular or intralipomatous injections? N Engl J Med 1982; 307: 356–8. [DOI] [PubMed] [Google Scholar]
- 26. Chan VO, Colville J, Persaud T. et al. Intramuscular injections into the buttocks: are they truly intramuscular? Eur J Radiol 2006; 58: 480–4. [DOI] [PubMed] [Google Scholar]
- 27. Ranheim B, Ween H, Egeli AK. et al. Benzathine penicillin G and procaine penicillin G in piglets: comparison of intramuscular and subcutaneous injection. Vet Res Commun 2002; 26: 459–65. [DOI] [PubMed] [Google Scholar]
- 28. Wyber R, Boyd BJ, Colquhoun S. et al. Preliminary consultation on preferred product characteristics of benzathine penicillin G for secondary prophylaxis of rheumatic fever. Drug Deliv Transl Res 2016; 6: 572–8. [DOI] [PubMed] [Google Scholar]
- 29. Brooks PJ, Spruill WJ, Parish RC. et al. Pharmacokinetics of methotrexate administered by intramuscular and subcutaneous injections in patients with rheumatoid arthritis. Arthritis Rheum 1990; 33: 91–4. [DOI] [PubMed] [Google Scholar]
- 30. Jin JF, Zhu LL, Chen M. et al. The optimal choice of medication administration route regarding intravenous, intramuscular, and subcutaneous injection. Patient Prefer Adherence 2015; 9: 923–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lue HC, Wu MH, Wang JK. et al. Three- versus four-week administration of benzathine penicillin G: effects on incidence of streptococcal infections and recurrences of rheumatic fever. Pediatrics 1996; 97: 984–8. [PubMed] [Google Scholar]
- 32. Mukap M, Sprod C, Tefuarani N. et al. Validation of a dried blood spot ceftriaxone assay in Papua New Guinean children with severe bacterial infections. Antimicrob Agents Chemother 2018; 62: e00940-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.