Abstract
Objective
To determine if there was a significant increase in Endocrine consultations postinitiation of the more stringent 2015 guidelines for persistent neonatal hypoglycemia.
Methods
A retrospective chart review was conducted using data from November 2011 to October 2016. All infants with persistent hypoglycemia past 72 hours of life were included. Data included age, critical sample values, anthropometric measures, and maternal health. Descriptive statistical analyses were performed as was an interrupted time series analysis assuming a Poisson distribution.
Results
Fifty-eight infants were evaluated. Postintervention, there was a significant increase in the number of consults (P<0.03, 95% confidence interval [CI]: 1.14 to 8.93). Most infants with hypoglycemia persisting >72 hours were hypoglycemic shortly after birth. Half had intrauterine growth restriction; 75% were male. The median age for investigation was 8.3 days. Hyperinsulinism was the most common etiology (52/58 infants); diazoxide treatment was utilized in roughly half (29/52 to 56%) with a median duration of treatment for 91 days. The phenotype of the infants and duration of diazoxide pre- and post-Pediatric Endocrine Society protocol did not differ; two infants on diazoxide developed pulmonary hypertension. Mothers were typically of lower socioeconomic status.
Conclusion
Not surprisingly, there was significant increase in the number of infants with persistent hypoglycemia using the 2015 guidelines. Prolonged hyperinsulinism was the major cause; medical management was typically sufficient and typically well tolerated. Care to reduce adverse effects of diazoxide is advised. We postulate that infants diagnosed using the more stringent 2015 guidelines have real disease based on the protracted medical management required.
Keywords: Diazoxide, Fetal growth retardation, Hyperinsulinism, Hypoglycemia, Neonatal
In the first 24 to 72 hours of life, neonates undergo a transition from intrauterine to extrauterine life when their plasma glucose levels are usually low (1,2). Typically, after 48 hours, the mean plasma glucose rises to 3.9 mmol/L, similar to older children. Euglycemia is important as significant neonatal hypoglycemia may be associated with poor neurodevelopmental outcomes (2–4).
The Pediatric Endocrine Society (PES) released a position statement in 2015 that addressed persistent neonatal hypoglycemia beyond age 48 hours; it suggested raising the glucose target to at least 3.3 mmol/L for high-risk infants without a suspected congenital hypoglycemia disorder (2). Based on these recommendations, the Sections of Pediatric Endocrinology and Neonatology, Children’s Hospital HSC Winnipeg, revised their standardized protocol for persistent neonatal hypoglycemia in November 2015. Hypoglycemia is now defined as 2 consecutive whole blood glucose by glucometer (point-of-care) or 1 plasma glucose <3.3 mmol/L. Hypoglycemia prompts a standardized critical sample and a consult to paediatric endocrinology. Metabolics is consulted if the blood gas is abnormal. Prior to the revised 2015 protocol, a glucose of <2.6 mmol/L triggered a critical sample.
Currently, there are no studies assessing the impact of the more stringent 2015 guidelines. Our primary objective was to determine if there is a statistically significant increase in the number of consults to paediatric endocrinology after the implementation of the 2015 guidelines. Our secondary objective was to compare the phenotype of infants and their mothers before and after the 2015 guidelines. Lastly, we compared parental socioeconomic status (SES) using a validated area-based index (5).
METHODS
We conducted a retrospective chart review to determine the number of consults to endocrinology for inpatient infants with hypoglycemia. Consults originated from both the neonatal intensive care units and intermediate care nurseries at two tertiary care centres. These centres are the only level III nurseries in the province and deliver roughly two of three (11,000 of 17,000/year) of all births in Manitoba and our catchment area (Ontario, Nunavut, and Saskatchewan). Consults from November 2011 to October 2016 were identified using an endocrine database; there were no exclusion criteria.
Chart data extracted included: gestational age, age, date of consult, anthropometric data, sex of infant, postal code, APGAR score, asphyxiation, method of delivery, maternal history of smoking in pregnancy or diabetes (gestational or pre-existing), consanguinity, genetic syndromes, age of hypoglycemia, plasma laboratory glucose or whole blood (glucometer) concentrations, symptoms, and dextrose requirements PO and IV (mg/kg/minute). World Health Organization (WHO) length, weight, and head circumference z-scores were calculated to two decimal places using the igrowup R macros provided by the WHO for ages ≤5 years (6). Extracted data also included management, duration of medications, effective dose, and adverse effects.
Plasma glucose, cortisol, insulin, and beta-hydroxybuterate were analyzed (Roche Cobas, 8000, Laval, Canada). Growth hormone was assayed (Beckman Coulter DX1 800, Indianapolis, IN, USA); blood gas and lactate were analyzed (GEM premier 4000, Instrumentation Laboratories, Bedford, MA, USA). Point-of-care glucometers measuring whole blood glucose were manufactured by Nova Biomedical (Waltham, MA, USA).
Means or percentages with 95% confidence intervals (CI) or medians with ranges were calculated. Frequency of consults was plotted by year. Interrupted time series analysis assuming a Poisson distribution was used for primary objective analysis. For our secondary objectives, groups were stratified as either pre- or postintervention (intervention - November 2015). Data were compared using nonparametric testing (Rank-Sum or Fisher’s Exact) due to non-normal distribution or small cell size. Data were analyzed using STATA12 (College Station, TX, USA). The level of significance was P<0.05.
Individual six-digit postal codes were used to identify geographic location and area-based SES. Census dissemination areas (DAs) are an administrative unit provided by Statistics Canada, each containing 400 to 700 individuals. Individual postal codes were mapped to DAs using the postal code conversion file provided by Statistics Canada (Postal CodeOMConversion File (PCCF), Reference Guide, 2013. Statistics Canada Catalogue no. 92-154-G). The Raymond-Pampalon deprivation indices were assigned to each DA based on data from the 2006 census (5). Within each DA, the material deprivation quintile is a composite score based on household income, unemployment, and high school completion rates. Social deprivation quintiles are based on the proportion of single parent families; adults who are separated, divorced or widowed; and adults who are living alone. Fifty-six postal codes were available for geomapping, while 51 were matched for deprivation indices. We did not delete children from analyses whose residences could not be geomapped. Chi-squared tests were used to test the null hypothesis of homogeneity in the distribution of deprivation quintiles.
Sample size
Pre-November 2015, on average 4 ± 0.8 SD endocrine consults were performed per yearly quarter over a period of 12 quarters. We required 3.7 quarters to see a 50% increase in the number of consults (i.e., 6) using an alpha of 0.05 and 80% power.
Ethics
Approval was obtained from the University of Manitoba Health Ethics Research Board.
RESULTS
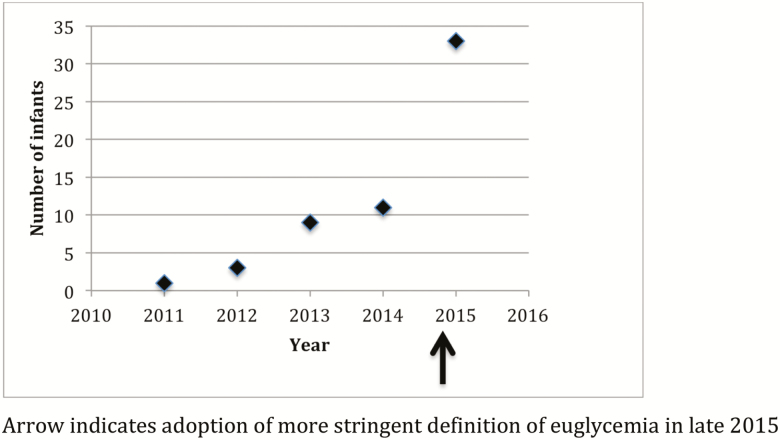
Fifty-eight infants were included in our study. Postintervention, there was a significant increase in the number of consults (P<0.03, 95% CI: 1.14 to 8.93) (Figure 1). Over half (33/58; 56.9%) of all consults occurred after the new protocol. Prior, there had only been a small increase from 2011 to early 2015 (Figure 1).
Figure 1.
Number of infants consulted for hypoglycemia per year; arrow indicates adoption of a more stringent definition of euglycemia in late 2015. On the x-axis, each year denotes a 12-month period starting in November until the following October (e.g., 2011 represents data from November 2011 to October 2012).
For all, median gestational age was 37.0 weeks while median age for consultation was 8.3 days. The majority of infants were male (75.9%), tended to be leaner (total weight z-score −0.8) with smaller head circumferences (total z-score −0.6); approximately half were intrauterine growth restriction (49%; 95% CI: 36.4 to 63.7%). Median APGAR scores were 8 and 9, at 1 and 5 minutes, respectively. There were no significant differences in our infants between groups across all variables (Tables 1–3), except they were shorter postintervention (length z-score −0.8 versus 0.8 [pre]; P<0.05).
Table 1.
Characteristics of infants with hypoglycemia: Pre- and post-November 2015
| Characteristic | Total | Pre | Post |
|---|---|---|---|
| Gestational age, weeks, median (range) (58:25/33) | 37.0 (24.1–42.0) | 37.5(31.0–42.0) | 36.6 (24.1–41.9) |
| Sex n (%; 95% CI) | |||
| Male | 44 (75.9; 62.8, 86.1) | 16 (64.0; 42.5, 82.0) | 28 (84.8; 68.1, 94.9) |
| Female | 14 (24.1; 13.9, 37.2) | 9 (36.0; 18.0, 57.4) | 5 (15.2; 5.1, 31.9) |
| (58:25/33) | |||
|
Age at consult, days, median (range) (58:25/33) |
8.3 (0–37) | 7.4 (0–37) | 8.9 (1–29) |
|
Weight, z-score, median (range) (57:25/32) |
−0.8 (−3.6–3.0) | −0.6 (−3.1–3.0) | −1.0 (−3.6–1.9) |
|
Length*, z-score, median (range) (40:17/23) |
−0.1 (−10.2–4.2) | 0.8 (−1.2–4.2)* | −0.8 (−10.2–2.7) |
|
Head circumference, z-score, median (range) (40:17/23) |
−0.6 (−8.2–3.1) | −0.5 (−8.2–3.1) | −0.7 (−2.7–0.8) |
|
Asphyxia
n (%; 95% CI) (50:23/27) |
12 (24; 13.8, 38.3) | 5 (21.8; 4.4, 43.7) | 7 (25.9; 11.1, 46.2) |
|
IUGR
n (%; 95% CI) (57:25/32) |
28 (49.1; 35.6, 62.7) | 12 (48.0; 27.8, 68.7) | 16 (50.0; 31.98, 68.1) |
CI Confidence interval; IUGR Intrauterine growth restriction.
*P<0.05, (Total n:pre/post).
Table 2.
Details of infants with persistent hypoglycaemia
| Characteristic | Total | Pre | Post |
|---|---|---|---|
| Age when first hypoglycemia noted (days), median (range)(53:25/28) | 0.9 (0–28) | 1.4 (0–28) | 0.8 (0–7) |
| Number of infants with hypoglycemic at <72 h of age n (%; 95% CI) (55:25/30) |
51 (92.7; 82.5,98.3) | 23 (92.0; 74.0, 99.0) | 28 (93.3; 77.9, 99.2) |
| Whole blood sugar <72 h of age, median (range) mmol/L (50:24/26) |
1.6 (0.5–2.9) | 1.4 (0.2–2.9) | 1.7 (0.3–2.9) |
| Number of infants with hypoglycemia event >72 h of age n (%; 95% CI) (58:25/33) |
53 (91.4; 81.0, 97.1) | 22 (88.0; 66.8, 97.5) | 31 (93.9; 79.8, 99.3) |
| Value critical whole blood sugar >72 h of age, median (range)(53:21/32) mmol/L |
2.3 (1.4–3.2) | 2.1 (1.4–2.9) | 2.4 (1.4–3.2) |
| Critical lab values, median (range) | |||
| Plasma glucose (55:24/31) mmol/L |
2.2 (1.3–3.5) | 2.0 (1.3–32.7) | 2.6 (1.6–3.5) |
| Growth hormone (50:24/26) µg/L |
12.3 (0.2–31.3) | 12.3 (0.2–28.4) | 12.4 (1.6–31.3) |
| Insulin (48:22/26) pmol/L |
50.5 (2–487) | 51.6 (5–288) | 49.5 (2–487) |
| Cortisol (57:24/33) mmol/L |
316 (15–1588) | 356 (97–761) | 287 (15–1588) |
| Beta-hydroxybuterate (n=39:13/29) mmol/L |
0.09 (0.07–0.3) | 0.07 (0.07–0.3) | 0.1 (0.07–0.3) |
| Mg/kg/min carbohydrate requirement, median (range) (54:22/32) |
11.1 (5–20.6) | 12.1 (5.6–20.6) | 10.8 (5.2–18.7) |
| Days of life when diazoxide initiated median (range) (29:12/17) |
9 (2–76) | 17.1 (3–76) | 9.5 (2–23) |
| Days treated with diazoxide median (range) (29:12/17) |
91 (1–229) | 96.6 (1–229) | 87.8 (3–247) |
| Etiology of hypoglycaemia | |||
| Hyperinsulinemia | 52 | 23 | 29 |
| Metabolic | 2 | 1 | 1 |
| Panhypopituitarism | 1 | 0 | 1 |
| Unknown/rapidly resolving | 3 | 1 | 2 |
| (58:25/33) |
(Total n:pre/post).
Table 3.
Maternal characteristics
| Maternal characteristics | Total | Pre | Post |
|---|---|---|---|
| Age, years, median (range) (48:22/26) | 27.5 (17–42) | 27.4 (18–42) | 28.3 (17–42) |
|
Gravida, median (range) (50:22/28) |
3 (1–10) | 2 (1–9) | 3 (1–10) |
|
Hypertension n (%; 95% CI) (50:22/28) |
13 (26.0; 15.3, 40.7) | 5 (22.7; 7.8, 45.4) | 8 (28.6; 13.2, 48.7) |
|
Diabetes (Type 1, 2, gestational) n (%; 95% CI) (51:23/28) |
9 (17.6; 8.4,30.9) | 4 (17.4; 5.0, 38.8) | 5 (17.9; 6.1, 36.9) |
|
Smoking
n (%; 95% CI) (39:16/23) |
14 (35.9; 21.7, 53.2) | 3 (18.8; 4.1, 45.6) | 11 (47.8; 26.8, 69.4) |
|
Delivery Method
n (%; 95% CI) (51:22/29) Caesarian section |
30 (58.8; 44.3, 72.0) | 14 (63.6; 40.7, 82.8) | 16 (55.2; 35.7, 73.6) |
| Vaginal | 21 (41.2; 27.6, 55.8) | 8 (36.4; 17.2, 59.7) | 13 (44.9; 26.4, 64.3) |
(Total n:pre/post).
Over 90% of all infants presented with early hypoglycemia (<72 hours), which persisted (Table 2). The median critical plasma glucose was 2.2 mmol/L, with no significant difference pre or post (2.0 mmol/L pre versus 2.6 mmol/L post, P=0.4). The median insulin concentration was inappropriately elevated at 50.5 pmol/L with typically robust growth hormone, cortisol, and suppressed beta-hydroxybutyrate concentrations. Infants had high glucose requirements (median 11.1 mg/kg/minute) to maintain glucometer readings ≥ 3.3mmol/L. Only 8 of 58 (13.8%) were symptomatic at presentation (1 seizure, 6 jittery, and 1 lethargic).
Ninety per cent (52/58) of the infants were diagnosed with hyperinsulinemic hypoglycemia; 50 were diagnosed based on laboratory data and two based on clinical index of suspicion (transient high-glucose demands). One infant was diagnosed with panhypopituitarism, one infant with carnitine palmitoyltransferase 1A deficiency, one with an unknown metabolic disorder, and three had an unknown etiology of their rapidly resolving hypoglycemia. Most (50/58: 86%) required IV dextrose and 29 of 58 (50%) were treated with diaxozide (Table 2). One infant was treated with octreotide for hypoglycemia associated with Beckwith-Wiedemann syndrome; she was resistant to diazoxide. Of those prescribed diaxozide, median duration of treatment was 91 days with a mean dose requirement of ~7.5 mg/kg/day. There was no statistical difference in the absolute number of infants prescribed diaxozide pre- and postintervention or in the duration of therapy (pre 96.6 days versus post 87.8 days, P=0.3). Two infants developed pulmonary hypertension with diazoxide; this was self-limiting. No infants required surgical intervention.
Thirteen infants had plasma glucose concentrations between 2.6 and 3.2 mmol/L at the time of consultation after November 2015; this represents 40% of infants with hypoglycemia as per the new protocol. These infants would not have met the previously defined diagnosis of hypoglycemia. Twelve of 13 had hyperinsulinism with glucose requirements identical to the median for the whole 58 infants at 11 mg/kg/minute. Interestingly, 3 of 12 (25%) had glucose requirements < 8−10 mg/kg/minute; this was not statistically different from 10 of 54 (19%) using < 8−10 mg/kg/minute in the whole cohort. Nine of twelve infants who would not have been diagnosed with hypoglycemia (i.e., in November 2015 using the older protocol) required treatment with diazoxide. Two infants had this medication halted shortly after initiation in hospital while the median duration was 79 days for the remaining seven.
Median maternal age was 27.5 years (Table 3); there were no significant differences in the maternal characteristics between groups. Just over half (58.8%) of women delivered via caesarian section. One-third of the mothers reported smoking during pregnancy; one-sixth had diabetes mellitus.
Geomapping revealed that fifty percent (28/56) of cases were from rural or Northern communities. When assessing area-based deprivation indices, 53% (27/51) fell in the highest quintile of material deprivation (P<0.01). There was no difference in social deprivation quintiles among the cases (P=0.6).
DISCUSSION
Our study identified an expected significant increase in the number of endocrine consults after the implementation of a more stringent standardized protocol in November 2015 (2). While this increase in temporal trend is not surprising, the characteristics of the infants reflect that we are now detecting true disease that would have not likely been picked up using our older protocol. Although endocrine consultation may have been more systematic post-2015 adding to this increase, diazoxide management has always been under the purview of our service. Of note, both groups had similar critical plasma glucoses and duration of diazoxide treatment.
Our two cohorts of infants did not demonstrate significant differences in their clinical characteristics. The majority (52/58) had hyperinsulinism with increased glucose consumption, low beta-hydroxybutyrate, prolonged hypoglycaemia, and exclusion of any pituitary deficiencies (7–9). Given that relatively short duration diazoxide therapy (3−4 months) was effective for most and no surgical intervention was required, those treated for hyperinsulinism likely met the criteria for an entity called ‘prolonged neonatal hyperinsulism’ (7,10). Moreover, many of our infants had risk factors for ‘prolonged neonatal hyperinsulinism’ which included early preterm birth, male sex, intrauterine growth restriction, or birth asphyxia (7,11–13). Infants with this disorder were first described in 1984; however, it was Hoe et al. in 2006, who created this diagnostic category (7). It describes a self-limited condition of insulin dysregulation with hypoglycemia lasting typically weeks to months; the etiology is unknown (7). The hypoglycemia is usually responsive to diazoxide and rarely requiring pancreatectomy (7). Additionally, infants were not known to have congenital hyperinsulinism, the most common cause of persistent hypoglycemia. This is usually due to mutations in genes controlling insulin regulation (e.g., ATP- sensitive potassium channels mutations). Children with congenital hyperinsulinsim tend to have more severe hypoglycemia that is refractory to medical treatment and often requires surgical intervention (8,14,15).
There is still much that is unknown regarding neonatal hypoglycemia especially after 48 hours of life. Although there is abundant literature examining the impact of hypoglycemia and neurodevelopmental outcomes (3,16–18), there is no consensus as to what blood glucose level or duration of hypoglycemia is harmful to the neonatal brain.
By adopting the recommendations from the PES, the absolute number of children being treated with diazoxide increased, though the proportion of those needing treatment did not differ between the groups. We estimated that roughly 2.9 per 10,000 live births annually were diagnosed with hyperinsulinism requiring diazoxide in Manitoba. While diaxozide appears to be the treatment of choice for hyperinsulinemic hypoglycemia, it is not without adverse effects including hypertrichosis, electrolyte disturbances, and fluid retention (19). An infrequent serious adverse effect is pulmonary hypertension; the FDA recently released a warning in July 2015 (20). There have been several publications reporting isolated cases (19,21,22). A literature review did not show any consistency with regards to length of treatment and timing of this adverse effect. In our population, two infants developed pulmonary hypertension within several days of initiating diazoxide giving a rate of 6.8% (2/29). It may be hard to generalize this to other populations given our small numbers. Additionally, an echocardiogram done postdiaxozide showed an atrial septal defect, patent ductus stenosis, and mild right ventricular hypertrophy in one of the neonates. It is unclear whether these defects were enough to predispose to pulmonary hypertension and may confound the calculation of rates of pulmonary hypertension.
When searching for clues to the etiology of the hypoglycemia, we evaluated maternal characteristics. Only 17% of mothers had diabetes; our provincial rate for gestational diabetes mellitus is much lower at 4.1% (23). In our centres, Neonatology will manage the infants with transient hyperinsulinism born to mothers with diabetes mellitus without involving Endocrinology because of its self-limiting nature; this is a longstanding policy and did not impact our 2015 protocol. Over half of the infants identified were delivered via caesarian section; this is much higher compared to our provincial rate of roughly 20% (23). Delivery via caesarian section is often due to fetal distress, a known association with neonatal hypoglycemia (24). Additionally, 35% of mothers smoked compared to the provincial rate of 21% (25). These findings suggest that risk factors for hypoglycemia may be modifiable (diabetes mellitus and smoking) and that these were fetuses in distress (high caesarian rate).
Much of the current literature does not comment on the maternal SES with infants with hypoglycemia. Our finding of striking material disparity among these mothers appears novel. Lower SES is associated with increased small for gestational age (SGA) infants; this has been noted in our centre as well as in other jurisdictions (23,26). Roughly 50% of the infants in both of our cohorts were considered SGA, which is much higher than our provincial rate of 7.3% (23). It is well known SGA infants are at increased risk of hypoglycemia. Maternal SES disparity and high rate of SGA leads us to wonder if perhaps low SES is the common underlying factor that predisposes our infants to prolonged hypoglycemia. Poverty leads to poor nutrition, poor prenatal care, and overall suboptimal health (27). This likely warrants additional study.
Our study resulted in important local practice changes. First, due to the known cardiac issues associated with diaxozide, all infants must have an echocardiogram prior to its use to exclude any pre-existing cardiopulmonary defects that may predispose to potential adverse effects. While our study only had two infants develop pulmonary hypertension secondary to diaxozide, it is a significant disease with morbidity risks that is important to prevent or detect early. Second, our centre will now typically wait a week using IV and/or nutritional support prior to initiating diaxozide. This is to ensure that the hypoglycemia is not transient and that the child truly requires diazoxide.
The strengths of work includes the fact that we are the lone paediatric endocrine referral centre serving our catchment area and that protocols across two large tertiary care hospitals have been synchronized. We were sufficiently powered to explore our primary objective. These strengths may also be considered as limitations in that our experience may not be generalizable. The study was not conducted prospectively; data from chart reviews may be missing.
In conclusion, we saw an anticipated increase in the number of consults. The overall range of etiologies was similar using the new criteria compared to the old; prolonged hyperinsulinism controlled with medical management was the major cause of hypoglycemia. Infants tolerated diazoxide well and those treated using the new guidelines had a comparable duration of treatment and critical laboratory glucose concentrations. The length of diazoxide treatment was relatively prolonged, therefore likely indicating true disease. We postulate that neonates diagnosed using the 2015 guidelines have real disease and comparable infants were likely missed before 2015.
Funding: There was internal funding for this project.
Potential Conflicts of Interest: All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Stanley CA, Rozance PJ, Thornton PS, et al. Re-evaluating “transitional neonatal hypoglycemia”: Mechanism and implications for management. J Pediatr 2015;166(6):1520–5.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thornton PS, Stanley CA, De Leon DD, et al. ; Pediatric Endocrine Society Recommendations from the pediatric endocrine society for evaluation and management of persistent hypoglycemia in neonates, infants, and children. J Pediatr 2015;167(2):238–45. [DOI] [PubMed] [Google Scholar]
- 3. Goode RH, Rettiganti M, Li J, et al. Developmental outcomes of preterm infants with neonatal hypoglycemia. Pediatrics. 2016;138(6):e20161424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adamkin DH. Neonatal hypoglycemia. Semin Fetal Neonatal Med 2017;22(1):36–41. [DOI] [PubMed] [Google Scholar]
- 5. Pampalon R, Hamel D, Gamache P, Raymond G. A deprivation index for health planning in Canada. Chronic Dis Can 2009;29(4):178–91. [PubMed] [Google Scholar]
- 6. Calculator: WHO igrowup Z-scores [Internet] (cited July 24, 2018). Available from: https://apps.cpeg-gcep.net/igrowup_cpeg/ (Accessed March 21, 2018).
- 7. Hoe FM, Thornton PS, Wanner LA, Steinkrauss L, Simmons RA, Stanley CA. Clinical features and insulin regulation in infants with a syndrome of prolonged neonatal hyperinsulinism. J Pediatr 2006;148(2):207–12. [DOI] [PubMed] [Google Scholar]
- 8. Stanley CA. Hyperinsulinism in infants and children. Pediatr Clin North Am 1997;44(2):363–74. [DOI] [PubMed] [Google Scholar]
- 9. Finegold DN, Stanley CA, Baker L. Glycemic response to glucagon during fasting hypoglycemia: An aid in the diagnosis of hyperinsulinism. J Pediatr 1980;96(2):257–9. [DOI] [PubMed] [Google Scholar]
- 10. Collins JE, Leonard JV, Teale D, et al. Hyperinsulinaemic hypoglycaemia in small for dates babies. Arch Dis Child 1990;65(10):1118–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kochar IS, Hussain K. From hyperinsulinaemic hypoglycaemia to ketotic hypoglycaemia: The range of glucose abnormalities in patients born with intrauterine growth retardation. Eur J Pediatr 2007;166(10):1003–7. [DOI] [PubMed] [Google Scholar]
- 12. Lubchenco LO, Bard H. Incidence of hypoglycemia in newborn infants classified by birth weight and gestational age. Pediatrics 1971;47(5):831–8. [PubMed] [Google Scholar]
- 13. Simchen MJ, Weisz B, Zilberberg E, et al. Male disadvantage for neonatal complications of term infants, especially in small-for-gestational age neonates. J Matern Fetal Neonatal Med 2014;27(8):839–43. [DOI] [PubMed] [Google Scholar]
- 14. De Leon DD, Stanley CA. Congenital hypoglycemia disorders: New aspects of etiology, diagnosis, treatment and outcomes: Highlights of the Proceedings of the Congenital Hypoglycemia Disorders Symposium, Philadelphia April 2016. Pediatr Diabetes 2017;18(1):3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stanley CA. Advances in diagnosis and treatment of hyperinsulinism in infants and children. J Clin Endocrinol Metab 2002;87(11):4857–9. [DOI] [PubMed] [Google Scholar]
- 16. Boluyt N, van Kempen A, Offringa M. Neurodevelopment after neonatal hypoglycemia: A systematic review and design of an optimal future study. Pediatrics 2006;117(6):2231–43. [DOI] [PubMed] [Google Scholar]
- 17. McKinlay CJ, Alsweiler JM, Ansell JM, et al. ; CHYLD Study Group Neonatal glycemia and neurodevelopmental outcomes at 2 years. N Engl J Med 2015;373(16):1507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cornblath M, Reisner SH. Blood glucose in the neonate and its clinical significance. N Engl J Med 1965;273(7):378–81. [DOI] [PubMed] [Google Scholar]
- 19. Timlin MR, Black AB, Delaney HM, Matos RI, Percival CS. Development of pulmonary hypertension during treatment with diazoxide: A case series and literature review. Pediatr Cardiol 2017;38(6):1247–50. [DOI] [PubMed] [Google Scholar]
- 20. Safety alerts for human medical products: FDA warns about a serious lung condition in infants and newborns treated with Proglycem (diazoxide) [Internet] Available from: https://www.fda.gov/Drugs/DrugSafety/ucm454833.htm (Accessed July 24, 2018).
- 21. Abu-Osba YK, Manasra KB, Mathew PM. Complications of diazoxide treatment in persistent neonatal hyperinsulinism. Arch Dis Child 1989;64(10):1496–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Silvani P, Camporesi A, Mandelli A, Wolfler A, Salvo I. A case of severe diazoxide toxicity. Paediatr Anaesth 2004;14(7):607–9. [DOI] [PubMed] [Google Scholar]
- 23. Heaman M, Kingson D, Helewa M, Brownell M, Derksen S, Bogdanovic B.. Perinatal Services and Outcomes in Manitoba. Winnipeg, Manitoba. Manitoba Center for Health Policy, 2012. [Google Scholar]
- 24. Ogunyemi D, Friedman P, Betcher K, et al. Obstetrical correlates and perinatal consequences of neonatal hypoglycemia in term infants. J Matern Fetal Neonatal Med 2017;30(11):1372–7. [DOI] [PubMed] [Google Scholar]
- 25. Millar WJ, Hill G. Pregnancy and smoking. Health Rep 2004;15(4):53–6. [PubMed] [Google Scholar]
- 26. Hasmasanu MG, Bolboaca SD, Baizat MI, Drugan TC, Zaharie GC. Neonatal short-term outcomes in infants with intrauterine growth restriction. Saudi Med J 2015;36(8):947–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Poverty, Income Inequality, and Health in Canada.pdf [Internet] D. Raphael, 2002. (cited December, 15 2017). Available from: http://www.socialjustice.org/uploads/pubs/PovertyIncomeInequalityandHealthinCanada.pdf (Accessed December 15, 2017).