Abstract
M281 is a fully human, anti‐neonatal Fc receptor (FcRn) antibody that inhibits FcRn‐mediated immunoglobulin G (IgG) recycling to decrease pathogenic IgG while preserving IgG production. A randomized, double‐blind, placebo‐controlled, first‐in‐human study with 50 normal healthy volunteers was designed to probe safety and the physiological maximum for reduction of IgG. Intravenous infusion of single ascending doses up to 60 mg/kg induced dose‐dependent serum IgG reductions, which were similar across all IgG subclasses. Multiple weekly doses of 15 or 30 mg/kg achieved mean IgG reductions of ≈85% from baseline and maintained IgG reductions ≥75% from baseline for up to 24 days. M281 was well tolerated, with no serious or severe adverse events (AEs), few moderate AEs, and a low incidence of infection‐related AEs similar to placebo treatment. The tolerability and consistency of M281 pharmacokinetics and pharmacodynamics support further evaluation of M281 in diseases mediated by pathogenic IgG.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑Anti‐neonatal Fc receptor (FcRn) therapeutic agents that block immunoglobulin G (IgG)–FcRn interaction, inhibit FcRn‐mediated recycling, and decrease serum IgG are in early development and have the potential to treat diseases induced by pathogenic IgG antibodies.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑This study provides proof of mechanism for M281, a novel anti‐FcRn blocking monoclonal antibody, and provides, for the first time, robust data relating pharmacokinetics, target saturation (receptor occupancy), and IgG decrease and recovery, as well as data on initial safety and tolerability for this agent.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑A consistent and close relationship is observed between FcRn receptor occupancy, serum IgG reduction, and M281 pharmacokinetics. This, together with initial safety and tolerability data, supports further clinical evaluation of M281 in autoimmune and alloimmune diseases driven by pathogenic IgG autoantibodies.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑An understanding of the anti‐FcRn mechanism and dose‐pharmacokinetics‐pharmacodynamics relationship will enable clinical studies in diseases induced by pathogenic IgG, in which efficacy may require different levels and duration of IgG decrease.
Pathogenic immunoglobulin G (IgG) antibodies mediate tissue destruction or dysfunction through direct or complement‐mediated mechanisms in several autoimmune and alloimmune diseases.1, 2 Although current therapies, such as glucocorticoids and immunosuppressive drugs, may be effective in reducing pathogenic IgG antibody production, they are broadly immunosuppressive and frequently limited by toxicities. In addition, inadequate response or relapse complicates management of several autoimmune and alloimmune diseases.3, 4 B‐cell–depleting therapies decrease antibody production and are effective in some patient populations, but they may increase the risk for serious infections and can require several months of treatment to achieve a maximal response.5, 6, 7, 8, 9 Treatments such as intravenous immunoglobulin, plasmapheresis, and immunoadsorption can reduce IgG to 50% and 95% below baseline, respectively, and produce clinical responses within days to weeks, typically with ≈50% serum IgG reduction.10, 11, 12 However, these regimens are difficult to sustain chronically.10, 11, 13 Recently, novel therapeutics targeting the neonatal Fc receptor (FcRn) aim to more specifically and rapidly decrease pathogenic IgG through decreasing IgG half‐life (t 1/2), thereby inducing IgG clearance (CL) similar to plasmapheresis or immunoadsorption, but with greater potential for long‐term maintenance treatment and disease control.14, 15
FcRn is the endosomal IgG transporter responsible for the long t 1/2 of IgG.16 Residing primarily in early endosomes, FcRn in the reticuloendothelial system serves to salvage IgG, internalized by nonspecific pinocytosis or Fcγ receptor interactions, from lysosomal catabolism and facilitates trafficking of bound IgG to the cell surface for release back into circulation.16 This pH‐sensitive process relies on the increased affinity of IgG binding to FcRn at endosomal pH (≈6.0) and the low affinity of this interaction at extracellular pH (≈7.5). FcRn also transports IgG across several tissue barriers, and in the setting of pregnancy, FcRn is required for transplacental transfer of IgG from mother to fetus.16 Results from preclinical studies demonstrate that blockade of FcRn–IgG binding can prevent or ameliorate pathogenic antibody‐induced disease in a variety of autoimmune and fetal–neonatal alloimmune disease models, including collagen‐induced arthritis,17 idiopathic thrombocytopenic purpura,18 immune complex–mediated glomerular disease,19 experimental autoimmune myasthenia gravis,20 fetal–neonatal alloimmune thrombocytopenia,21 and antiplatelet antibody‐induced placenta disruption and miscarriage.22
The key components of IgG metabolism—FcRn‐mediated recycling, plasma cell/plasmablast‐derived synthesis, endothelial/reticuloendothelial catabolism, and, in some pathological conditions, renal/gastrointestinal elimination—have been well described in human and nonhuman species in a variety of healthy and disease settings.23, 24 Specific blockade of FcRn recycling is expected to reduce serum and tissue levels of all IgGs, including pathogenic IgG, without affecting synthesis, elimination, or catabolism.25, 26 On loss of IgG recycling, the normal equilibrium between synthesis, recycling, catabolism, and elimination, which maintains each individual's baseline serum IgG concentration, is disrupted and IgG decreases. However, the IgG decline is expected to reach a lower equilibrium set point because of the known decrease in the IgG fractional catabolic rate with decreasing serum IgG concentrations, the insensitivity of IgG synthesis to changes in serum IgG levels, and unchanged elimination rates.24, 27 The anticipated lower equilibrium set point may be near 80–90% below baseline, as seen in humans or mice lacking FcRn.26, 28
M281 is a high‐affinity, fully human, effectorless monoclonal IgG1 anti‐FcRn antibody that binds with picomolar affinity to FcRn at both endosomal pH 6.0 (K d (dissociation constant), 29 pM) and extracellular pH 7.6 (K d, 44 pM), allowing occupancy of FcRn throughout the recycling pathway (Table S1 ). M281 blocks binding of IgG to FcRn at nanomolar concentrations (IC50 (half maximal inhibitory concentration), 3 nM or 0.4 μg/mL) (Figure S1 ). We evaluated the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of M281 in healthy volunteers in a first‐in‐human, two‐part, ascending‐dose study. This study was designed to assess the potential of M281 for modulating FcRn blockade to achieve the full range of IgG reduction with M281 and the relationship between dose, PK, FcRn binding, and serum IgG and IgG subclasses.
Results
First‐in‐human study
A total of 160 subjects were screened; 50 subjects enrolled and completed the study. A total of 28 men and 22 women between 20 and 55 years of age, who had a body mass index (BMI) between 19.2 and 29.7 kg/m2, participated (Table 1). Age, weight, BMI, and race were similar between part 1, single ascending dose (SAD), and part 2, multiple dose (MD). In SAD, 24 subjects received M281 and 10 subjects received placebo; all 34 subjects completed per protocol (Table 1). In MD, 12 subjects received up to 4 weekly doses of 30 mg/kg (n = 6) in the first cohort and 15 mg/kg (n = 6) in the second cohort, and four subjects received up to 4 weekly doses of placebo. Two subjects received two of four doses planned, and four subjects received three of four doses planned; 10 subjects received all four planned doses.
Table 1.
Subject demographics and baseline characteristics
| Sex, % | Race ethnicity, % | ||||
|---|---|---|---|---|---|
| Dose | Patients, n | Age, mean (SD), y | Female/male | BMI, mean (SD), kg/m2 | W/B/A/M |
| Single ascending dose | |||||
| Placebo | 10 | 36 (12) | 30:70 | 25.4 (3.0) | 90:0:0:10 |
| 0.3 mg/kg | 3 | 34 (17) | 0:100 | 22.1 (2.0) | 100:0:0:0 |
| 3 mg/kg | 3 | 23 (4) | 33:67 | 24.0 (1.4) | 100:0:0:0 |
| 10 mg/kg | 6 | 33 (16) | 33:67 | 25.7 (2.6) | 100:0:0:0 |
| 30 mg/kg | 6 | 29 (13) | 67:33 | 23.1 (3.1) | 67:0:33:0 |
| 60 mg/kg | 6 | 42 (12) | 50:50 | 24.5 (3.1) | 83:0:17:0 |
| Total | 34 | 34 (13) | 38:62 | 24.5 (2.9) | 88:0:9:3 |
| Multiple ascending dose | |||||
| Placebo | 4 | 32 (14) | 50:50 | 22.7 (2.8) | 75:25:0:0 |
| 30 mg/kg | 6 | 37 (13) | 67:33 | 24.4 (3.8) | 83:17:0:0 |
| 15 mg/kg | 6 | 38 (12) | 50:50 | 26.0 (3.4) | 50:33:0:17 |
| Total | 16 | 36 (12) | 56:44 | 24.6 (3.5) | 69:25:0:6 |
A, Asian; B, black; BMI, body mass index; M, mixed; W, white.
Primary PK and PD
SAD study.
M281 exhibited consistent and dose‐dependent PK. M281 serum concentrations increased proportionally to dose, and area under the concentration–time curve for time 0 to infinity (AUC0‐inf) increased greater than dose proportionally across all SAD cohorts (Table 2). All single‐dose groups showed a consistent time to maximum serum concentration (T max) of 2 hours after start of the 2‐hour infusion. Serum terminal t 1/2 and exposure of M281 increased with dose. The dependence of increasing t 1/2, decreasing overall CL, and greater than proportional increase in AUC on increasing dose is consistent with target‐mediated disposition that results in more rapid CL at concentrations <10 μg/mL. AUC, CL, and t 1/2 were unaffected by body weight and sex.
Table 2.
PK parameters of M281 after single‐dose administration
| PK parameters | M281 single dose, mean (SD), mg/kg | ||||
|---|---|---|---|---|---|
| 0.3 (N = 3) | 3 (N = 3) | 10 (N = 6) | 30 (N = 6) | 60 (N = 6) | |
| C max, μg/mL | 0.446 (0.017) | 53.8 (0.73) | 206 (23.6) | 600 (50.1) | 1,320 (152) |
| T max, houra | 2.00 (2.00–2.02) | 2.03 (2.02–2.08) | 2.07 (2.00–12.03) | 2.00 (2.00–12.03) | 2.06 (2.00–2.13) |
| t 1/2, hour | NC | 7.82 (3.52) | 24.4 (6.32) | 30.7 (3.90) | 33.7 (10.3) |
| AUC0‐inf, hour•μg/mL | NC | 945 (122) | 9,757 (1,346) | 39,213 (7,351) | 115,288 (22,528) |
| CL, L/hour | NC | 0.241 (0.036) | 0.089 (0.017) | 0.052 (0.010) | 0.039 (0.009) |
| V z, L | NC | 2.62 (0.846) | 3.09 (0.715) | 2.31 (0.614) | 1.87 (0.671) |
AUC0‐inf, area under the concentration–time curve for time 0 to infinity; CL, clearance; C max, maximum serum concentration; NC, not calculated because of insufficient data; PK, pharmacokinetic; t 1/2, terminal half‐life; T max, time to C max; V z, volume of distribution.
Data are shown as median (range).
Single doses of M281 resulted in rapid FcRn saturation, as measured by receptor occupancy (RO) using flow cytometry. The percentage of occupied receptors reached ≈50% transiently at 2 hours after M281 administration at 0.3 mg/kg (maximum serum concentration (C max), 0.4 μg/mL), but nearly all (>90%) receptors were occupied within 2 hours at doses of 3 mg/kg (C max, 53.8 μg/mL) or greater, suggesting rapid saturation of FcRn at low serum drug concentrations (Figure 1 a). The duration of complete receptor saturation increased with increasing dose, and the loss of FcRn saturation occurred rapidly, associated with plasma drug levels <10 μg/mL.
Figure 1.
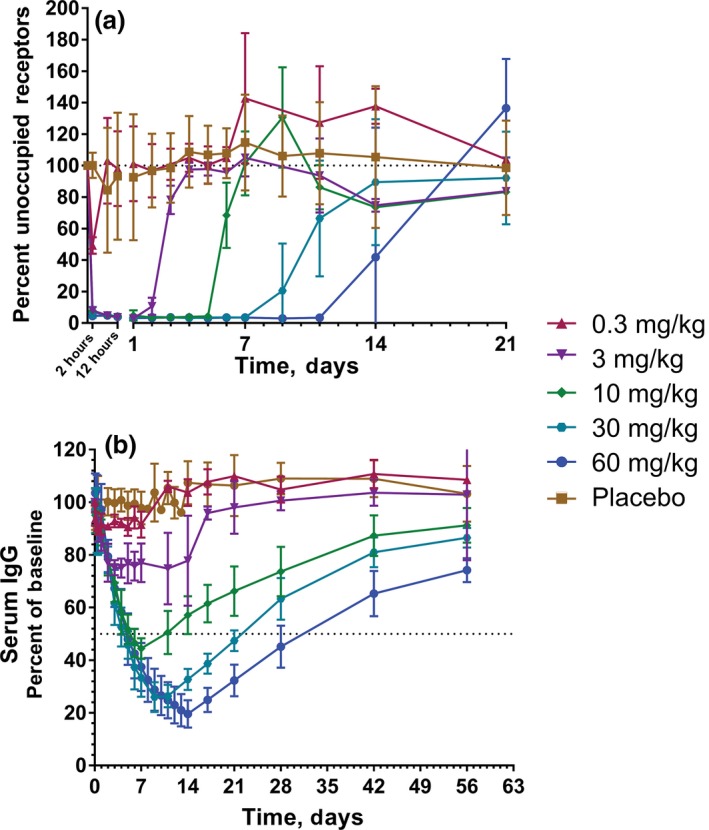
Single ascending dose receptor occupancy and serum IgG. Values are shown as mean (SD). (a) M281 receptor occupancy, as measured by percentage unoccupied FcRn compared with baseline in monocytes. (b) Serum immunoglobulin G (IgG) percentage relative to baseline. M281‐treated cohorts are represented by n = 3 (0.3 or 3 mg/kg), n = 6 (30 mg/kg), and n = 5 (60 mg/kg). Placebo controls from each single‐dose cohort were combined (n = 10).
In addition, serum IgG decreased within a day of M281 administration, and the rate of decrease was consistent across doses and individual subjects (Figure 1 b). The depth of IgG decrease was more extensive with increasing dose as the downward trajectory was maintained longer with each ascending dose level (Figure 1; Table S2 ). A mean decrease in serum IgG of 25%, 55%, 74%, and 80% from baseline was observed at doses of 3, 10, 30, and 60 mg/kg, respectively, suggesting serum IgG decrease may be nearing an asymptote at 60 mg/kg. The recovery of IgG initiated on loss of RO and returned toward baseline levels in an asymptotic manner, reaching within 20–25% of baseline levels within 56 days. Single doses of M281 at 30 or 60 mg/kg maintained serum IgG at 50% of baseline or below for 18 and 27 days, respectively.
Multiple‐dose study.
PK assessed at first C max and each trough indicated MD PK was similar to SAD PK at comparable doses showing minimal accumulation. Trough levels with 30 mg/kg weekly were maintained near 100–200 μg/mL but were mostly <10 μg/mL with 15 mg/kg weekly. At 30 mg/kg, full RO was achieved at 2 hours after the first dose and maintained throughout the dosing period, whereas some subjects in the 15 mg/kg MD group lost full RO at the first trough (Figure 2 a). Consistent with the findings in SAD, loss of RO occurs between 9–10 days after the last M281 30 mg/kg dose and between 7–9 days after the last 15 mg/kg dose, regardless of the number of doses given (Figure 2 a). IgG decreases initiated within 1 day after M281 administration and decreased by 14 days after initiation of weekly dosing to an asymptote ≈85% below baseline. Decreased IgG was maintained for 16 days at 15 mg/kg or 24 days at 30 mg/kg (Figure 2 b, Table S2 ). As in SAD, loss of RO after multiple doses was followed within a day by an increase in serum IgG, and both PD events corresponded to the period when drug levels decreased below 10 μg/mL.
Figure 2.
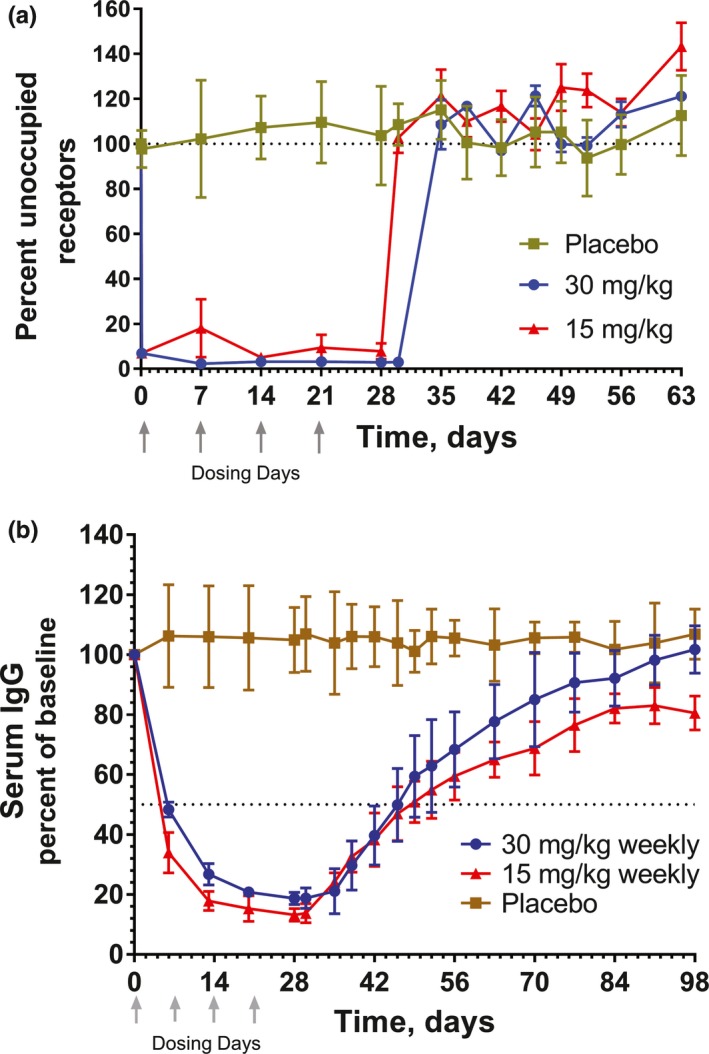
Multiple‐dose receptor occupancy and serum IgG after 4 once weekly doses of M281 of 15 or 30 mg/kg. Values are shown as mean (SD). (a) M281 receptor occupancy, as measured by percentage unoccupied FcRn compared with baseline in monocytes. (b) Serum immunoglobulin G (IgG) percentage relative to baseline. Each M281‐treated cohort is represented by n = 3. Placebo controls from each multiple‐dose cohort were combined (n = 4).
Exploratory PD
Each IgG subclass exhibited similar decreases in serum levels, an observation that was highly consistent among subjects and reached a maximum reduction within 7–14 days of ≈75–90% below baseline (Figure 3; Table S2 ). Recoveries to baseline were initiated ≈1 week after the last dose for subjects receiving two, three, or four consecutive doses, and neared baseline levels within 30–80 days of the last dose (Figure 3). In contrast to IgG, no effect of M281 was observed on immunoglobulin M (IgM), immunoglobulin A (IgA), and immunoglobulin E (IgE) or in a panel of inflammatory cytokines assessed at T max and every 3–7 days in SAD cohorts and weekly in MD cohorts.
Figure 3.
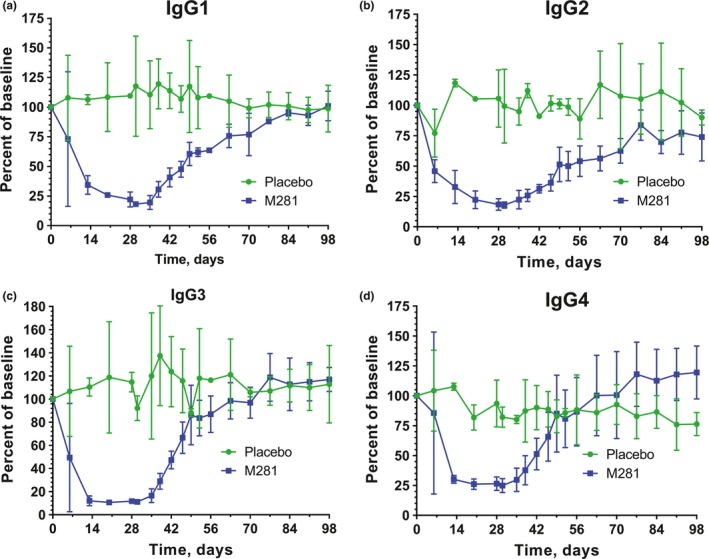
Serum immunoglobulin G subclasses after four once weekly doses of M281 (30 mg/kg). Values are shown as mean (SD). IgG1 (a), IgG2 (b), IgG3 (c), and IgG4 (d) percentage relative to baseline. Each M281‐treated cohort is represented by n = 3, and placebo controls from the same cohort are represented by n = 2.
Safety
Overall, M281 administration was well tolerated in all cohorts evaluated. The incidence of treatment‐emergent adverse events (TEAEs) considered related to study drug was similar between M281‐ and placebo‐treated subjects in both SAD (Table 3) and MD (Table 4 ). TEAEs related to study drug constituted 15% of TEAEs in M281 subjects and 10% of TEAEs in placebo subjects in SAD and 41% of TEAEs in M281 subjects and 45% of TEAEs in placebo subjects in MD. All TEAEs were transient, and all subjects recovered without treatment, with one–two doses of paracetamol, or, in one case, ibuprofen.
Table 3.
Single ascending dose: treatment‐emergent adverse events related to study drug
| Preferred term | Patients, n (%)a | ||||||
|---|---|---|---|---|---|---|---|
| Placebo (N = 10) | M281, mg/kg | ||||||
| 0.3 (N = 3) | 3 (N = 3) | 10 (N = 6) | 30 (N = 6) | 60 (N = 6) | Total (N = 24) | ||
| Total | 2 (20) | 0 | 0 | 1 (16.7) | 2 (33.3) | 3 (50) | 6 (25) |
| Asthenia | 1 (10) | 0 | 0 | 0 | 0 | 1 (16.7) | 1 (4.2) |
| Infusion site erythema | 0 | 0 | 0 | 1 (16.7) | 0 | 0 | 1 (4.2) |
| Fatigue | 1 (10) | 0 | 0 | 0 | 0 | 0 | 0 |
| Headache | 0 | 0 | 0 | 0 | 1 (16.7) | 1 (16.7) | 2 (8.3) |
| Influenza | 0 | 0 | 0 | 0 | 0 | 1 (16.7) | 1 (4.2) |
| Cough | 0 | 0 | 0 | 0 | 1 (16.7) | 0 | 1 (4.2) |
| Oropharyngeal pain | 0 | 0 | 0 | 0 | 1 (16.7) | 0 | 1 (4.2) |
| Eczema | 0 | 0 | 0 | 0 | 1 (16.7) | 0 | 1 (4.2) |
All treatment‐emergent adverse events with a reasonable possibility to be related to study drug were mild except for those indicated by b.
Event considered moderate in intensity.
Table 4.
Multiple ascending dose: treatment‐emergent adverse events related to study drug
| Parameter | Patients, n (%)a | |||
|---|---|---|---|---|
| Placebo (N = 4) | M281, mg/kg | |||
| 30 (N = 6) | 15 (N = 6) | Total (N = 12) | ||
| Total | 3 (75.0) | 4 (66.7) | 4 (66.7) | 8 (66.7) |
| Blood creatine phosphokinase increased | 0 | 0 | 3 (50) | 3 (25) |
| Alanine aminotransferase increased | 0 | 0 | 1 (16.7) | 1 (8.3) |
| Influenza‐like illness | 0 | 0 | 1 (16.7)b | 1 (8.3) |
| Infusion site erythema | 0 | 1 (16.7) | 0 | 1 (8.3) |
| Fatigue | 1 (25) | 0 | 0 | 0 |
| Injection site pain | 1 (25) | 0 | 0 | 0 |
| Viral upper respiratory tract infection | 1 (25)b | 1 (16.7) | 1 (16.7) | 2 (16.7) |
| Gastroenteritis | 0 | 0 | 1 (16.7)b | 1 (8.3) |
| Back pain | 0 | 0 | 1 (16.7) | 1 (8.3) |
| Myalgia | 1 (25) | 1 (16.7) | 0 | 1 (8.3) |
| Headache | 3 (75)c | 1 (16.7) | 0 | 1 (8.3) |
| Tremor | 0 | 1 (16.7) | 0 | 1 (8.3) |
| Nausea | 0 | 1 (16.7) | 0 | 1 (8.3) |
| Oropharyngeal pain | 1 (25) | 0 | 1 (16.7) | 1 (8.3) |
aAll treatment‐emergent adverse events with a reasonable possibility to be related to study drug were mild except for those indicated by b and c.
bEvent considered moderate in intensity.
cTwo of these 3 events were considered to be moderate in intensity.
Similar to placebo, most TEAEs in the M281‐treated subjects (94% SAD, 92% MD) were mild. Moderate TEAEs were transient and were reported in 4 of 36 (11%) of M281‐treated and 3 of 14 (21%) of placebo‐treated subjects. No severe TEAEs, serious AEs, deaths, significant infusion site reactions, or systemic allergic responses were noted.
The incidence of infection was of interest given that serum IgG concentrations were low with M281 treatment. The incidence of TEAEs in the category of infections and infestations was similar between M281‐ and placebo‐treated groups, with 12.5% in M281‐treated subjects vs. 20% in placebo‐treated subjects in SAD cohorts and 41.7% in M281‐treated subjects vs. 50% in placebo‐treated subjects in MD cohorts. Infection‐related TEAEs with possible relation to treatment were also similar in M281‐treated subjects compared with placebo‐treated subjects, with TEAEs occurring in 4.2% of M281‐treated subjects compared with 0% of placebo‐treated subjects in SAD and in 16.7% of M281‐treated subjects compared with 25% of placebo‐treated subjects in MD.
Antidrug antibodies (ADAs) were low or absent across most SAD and MD subjects. ADAs were detected in 12% (4/34, of all M281‐treated subjects; two at 10 mg/kg and one each at 30 and 60 mg/kg) of SAD subjects; no subjects were positive for neutralizing antibody. ADAs were detected in 31% (5/16, four in M281‐treated subjects and one in a placebo‐treated subject) of the MD subjects, all of whom were in the 15 mg/kg cohort and with low titer (at maximum, 1:4, 1:32, 1:64, or 1:128). The four M281‐treated MD subjects were neutralizing antibody positive. No determination of the effect of ADA or neutralizing antibodies on M281 PK could be made because ADAs that developed after M281 had decreased below the level of quantification. ADA and neutralizing antibody development and the effect on PK after long‐term dosing in patient populations remain to be determined.
Because high‐dose SAD cohorts and the first MD cohort, 30 mg/kg weekly, were expected to achieve maximal projected IgG decreases, a stopping rule was in place based on low total IgG and two common immunization titers, which led to discontinuation of three asymptomatic subjects in the 30 mg/kg weekly MD cohort after two (n = 2) or three (n = 1) doses. Because no M281‐related AEs were observed and IgG recovery occurred, this stopping rule was removed for the second 15 mg/kg cohort in whom decreases below the original stopping criteria occurred in three of six subjects and in whom safety concerns did not develop. Three other subjects in the MD 15 mg/kg group discontinued treatment after three doses as a precaution after they developed transient asymptomatic elevations in creatine phosphokinase, with values up to 1,534, 2,452, and 6,748 U/L, respectively, for each subject; one of these subjects experienced transiently elevated alanine aminotransferase (119 U/L). These abnormal laboratory values were assessed as mild AEs on the day before the fourth dose and determined on follow‐up not to be of cardiac origin or associated with myoglobinuria, recoverable within 1–2 weeks, and preceded by exercise in one case. No other clinically significant changes or trends were observed in laboratory parameters, with the exception of asymptomatic, transient reductions in total protein and albumin in the 60 mg/kg single‐dose group and the 30 and 15 mg/kg MD groups (Table S3 ). These were observed without proteinuria or changes in serum creatinine or electrolytes. No treatment‐related changes were noted in hematological parameters, including white blood cell and lymphocyte counts, CH50, C3, C4, interferon‐γ, tumor necrosis factor‐α, interleukin (IL)‐2, or IL‐6.
Discussion
This first‐in‐human study provides initial safety and tolerability data for M281 and demonstrates proof of mechanism by achieving the maximal anticipated decrease in serum IgG. M281 was safe and well tolerated; it demonstrated a rapid onset of action and the potential for durable maintenance of a range of decreased serum IgG levels at or below efficacious levels obtained by plasmapheresis or immunoadsorption for up to 27 days after a single dose or for longer with MDs. In patients whose disease is mediated by pathogenic IgG antibodies, M281 would be expected to lower the circulating levels of such antibodies and could potentially provide clinical benefit across multiple rare and severe pathogenic antibody‐driven diseases.
This study demonstrates proof of mechanism that M281 can fully saturate FcRn, resulting in the pharmacological consequence of reduction in serum IgG levels. This finding is also supported by the close PK–PD relationship observed in this study. M281 achieves FcRn RO rapidly on intravenous infusion, consistent with its high‐affinity binding of FcRn, its rapid cellular uptake, and binding to intracellular FcRn, as established in cellular and animal studies. Serum IgG decreased rapidly on achieving complete RO, consistent with the blockade of IgG binding to FcRn and the enhanced IgG catabolism that results. Serum levels of IgG started to return toward baseline within a day or two after loss of complete RO. This occurred as M281 plasma concentrations reached near or below 10 μg/mL, consistent with in vitro concentrations required for inhibition of IgG binding to FcRn. The maximal decrease in IgG plateaued to ≈85% of baseline, consistent with a full blockade of FcRn and similar to that observed in individuals lacking FcRn in whom serum IgG concentrations are ≈80–90% below baseline.26, 28
The results also support the initial safety and tolerability of M281, despite sustaining IgG at the physiological maximum level of reduction. Consistent with nonclinical studies, TEAEs in subjects who received M281 were generally mild, and all TEAEs were monitorable and transient and resolved with minimal or no treatment. The most frequently reported treatment‐related TEAE in subjects receiving M281 was headache, which occurred at a low frequency, similar to that of placebo. There were no severe TEAEs, serious AEs, or deaths during the study.
Given the 85% reduction in serum IgG, 50% of individuals in the normal range at baseline experienced a decrease of IgG to levels <200 mg/dL over the two highest SAD cohorts and both MD cohorts. AEs related to infections were of special interest, and although this small study cannot fully assess infection risk, the absence of severe or serious infections and comparable incidence of infection compared with placebo, along with previous findings with anti‐FcRn agents in humans and nonhuman primates,29, 30 is reassuring. It is important to highlight that M281 is not a broadly immunosuppressive agent. Mechanistically, FcRn blockade specifically decreases IgG t 1/2, but does not affect IgG production or the metabolism of other immunoglobulins. M281 was also shown to lower total IgG, IgG subclasses, and antigen‐specific IgG titers, without affecting IgM, IgA, or IgE. The lowering of circulating and systemic IgG by therapies such as plasmapheresis and, more similar to M281, intensive immunoadsorption without intravenous immunoglobulin supplementation have not been associated with infection.11, 12 Clinical conditions of low IgG due to IgG clearance rather than low IgG production, such as protein‐losing enteropathy, post‐Fontan hypogammaglobulinemia, myotonic dystrophy, and familial hypercatabolic hyperproteinemia, are not associated with increased infection.26, 31, 32 Unlike individuals with common variable immune deficiency and X‐linked agammaglobulinemia, which are broadly immunosuppressed conditions, many individuals with more selective immunodeficiencies, such as transient hypogammaglobulinemia of infancy and selective IgG subclass deficiency, do not display increased infection risk.33, 34
Three instances of asymptomatic creatine phosphokinase elevations and one instance of concomitant alanine aminotransferase elevation were noted in the 15 mg/kg MD cohort, but not in the 30 mg/kg MD cohort; these elevated enzyme levels were associated with exercise or increased activity in at least one case. Notably, creatine phosphokinase elevations are often seen in healthy individuals and in phase I studies,35, 36, 37, 38 and no M281‐related AEs, including these noted herein, were observed in short‐ or long‐term cynomolgus monkey toxicology evaluations (data not shown).
The limitations of this study include its small number of subjects, short treatment duration, and assessment of healthy volunteers who have no significant comedications or comorbidities and do not fully reflect patients with autoimmune or alloimmune disease. However, the results do provide a preliminary tolerability and safety profile and substantial PK–PD data to support dose selection and study design in phase II evaluation of M281 in autoimmune or alloimmune diseases, in which lowering of pathogenic IgG or inhibiting pathogenic IgG transplacental transport is anticipated to be of benefit.
Methods
Participants
Healthy men and women volunteers, aged 18–55 years, with a BMI of 18–30 kg/m2 with baseline IgG concentrations between 680–1,650 mg/dL at screening and antibody titers for tetanus toxoid or Haemophilus influenzae (≥0.01 IU/mL and ≥0.15 μg/mL, respectively) were eligible to participate. Women of childbearing potential and nonsterile men agreed to use two adequate methods of contraception from 30 days before dosing until the final follow‐up visit. Subjects with any vaccination or planned vaccination, significant whole blood loss, or those who donated blood within 1 month before dosing or donated plasma within 14 days of dosing were excluded.
Study design
This was a two‐part, phase I, single‐center, randomized, double‐blind, placebo‐controlled, two‐arm, parallel‐group study (NCT02828046) conducted between May 9, 2016, and August 8, 2017, at PRA Health Sciences (Groningen, The Netherlands). Randomization occurred after a 4‐week enrollment period. In the SAD group, subjects were randomized into five groups receiving 0.3, 3, 10, 30, and 60 mg/kg. At dose cohorts of 0.3 and 3 mg/kg, five subjects were randomized 3:2, M281/placebo. At all subsequent doses, eight subjects were randomized 6:2, M281/placebo. In the MD group, subjects were randomized 6:2, M281/placebo, and received 30 mg/kg M281 in the first cohort and 15 mg/kg M281 in the second cohort once weekly or placebo for 4 weeks. M281 or placebo (saline) was administered as an intravenous infusion over 2 hours. A safety review committee reviewed all safety and PK data, and an ethics committee granted approval before dose escalations between cohorts during SAD and initiation of MD. Subjects, investigators, and site personnel remained blinded to treatment throughout the study, with the exception of the pharmacy staff who prepared infusions but who were not involved with assessments and an appointed, unblinded staff member who reviewed IgG, tetanus, and H. influenzae antibody titers to ensure safety.
Ethics
Informed consent was obtained from all subjects. The study was conducted in accordance with the principles of the Declaration of Helsinki. The study was conducted in compliance with the International Conference on Harmonisation E6 Guideline for Good Clinical Practice, and it was compliant with the European Union Clinical Trial Directive: Directive 2001/20/EC. The original and amended clinical study protocol (EudraCT number 2016‐000986‐22) and informed consent forms were reviewed and approved by the Independent Ethics Committee of the foundation Evaluation of Ethics in Biomedical Research (Assen, The Netherlands). All subjects were informed verbally and in writing of the objectives, procedures, and risks of study participation.
Safety assessment
Safety and tolerability were assessed through vital sign measurements, pulse oximetry measurements, 12‐lead electrocardiograms, clinical laboratory tests, targeted physical examinations, body weight, infusion reaction evaluations, immunogenicity evaluations, reporting of AEs, and any other parameter that was relevant for safety assessment. Pretreatment patient medical histories were obtained at screening. AEs were defined using the Medical Dictionary for Regulatory Activities (Version 19.0). Subjects were followed up for up to 12 weeks in the SAD study and up to 14 weeks in the MD study. Infusion reactions and administration site AEs were carefully monitored. In the event of any infusion or local site reaction, blood was drawn to measure histamine, anaphylatoxin, immunoglobulin E, and tryptase levels; these levels were then compared against baseline levels. Any occurrence of a moderate or severe infusion reaction or severe local site reaction prompted a safety review committee meeting to determine whether dosing should continue.
Based conservatively on a general threshold in primary immunodeficiencies,39 total serum IgG < 200 mg/dL as well as antibody titers for tetanus and/or H. influenzae below standard protective levels were considered safety markers for potential stopping criteria. IgG levels had to be ≥ 200 mg/dL on day 8 for the subject to be discharged from the clinic. If a subject developed a moderate or severe infection, grade 3 hypoalbuminemia, or grade 2 hypoalbuminemia with clinically significant edema, the study drug was to be discontinued.
PK and PD assays
Blood samples for PK analysis were obtained at baseline and at 0.5, 1, 2, 8, and 12 hours and days 1–4, 7, 9, 11, 14, 17, 21, and 28 after administration of study drug. Free serum M281 concentrations were measured by a contract laboratory (Charles River Laboratories, Reno, Nevada) using a validated enzyme‐linked immunosorbent assay with a lower limit of quantification of 0.15 μg/mL and an upper limit of quantification of 1.25 μg/mL. The following PK parameters were estimated using noncompartmental analysis: C max, T max, t 1/2, AUC for time 0 to infinity, CL, and volume of distribution.
The primary PD parameters were serum levels of total IgG and RO on circulating monocytes. Blood samples to assess serum total IgG were obtained at baseline and after administration of study drug at indicated timepoints. Blood samples for RO assessment were obtained at baseline and after administration of study drug at indicated timepoints. Exploratory PD assessments evaluated IgG subclasses (IgG1, IgG2, IgG3, and IgG4), tetanus or H. influenzae titers, IgM, IgA, IgE, CH50, C3, C4, and a panel of cytokines (interferon‐γ, tumor necrosis factor‐α, IL‐2, and IL‐6) using clinically validated immunoassays. ADAs to M281 and neutralizing antibodies were also assessed.
RO was assessed by an ex vivo fit‐for‐purpose assay on monocytes. Lysed whole blood cells from subjects treated with or without M281 were incubated with fluorescently labeled antibodies to CD45, CD3, CD20, CD14, and CD66 as immunophenotyping markers and then fixed and permeabilized before incubation with fluorescently labeled M281. Fluorescence‐activated, cell‐sorting evaluation of the geometric mean fluorescence index for M281 label at baseline for each subject constituted an assessment of 100% unoccupied receptors. Saturated RO was defined as > 90% occupied receptors (expressed as < 10% of fluorescent M281 cell labeling compared with each subject's baseline value).
Statistics
All statistical analysis and reporting were done using SAS for Windows version 9.4 (SAS Institute, Cary, NC). PK parameters were estimated using noncompartmental methods with Phoenix WinNonlin version 6.3 (Certara, Princeton, NJ) with the model for intravascular infusion (model 202). Below quantifiable limit (BQL) values occurring at the beginning of the profile or occurring after the first quantifiable point were considered missing. Values that were embedded between BQLs or quantifiable values occurring after two or more BQLs were recorded as missing at the discretion of the pharmacokineticist. Actual sampling times, rather than scheduled sampling times, were used in all individual‐subject PK parameter computations.
Descriptive statistics (number of subjects, mean, and SD) were used to summarize PK and PD parameters. For PD parameters, arithmetic mean change from baseline, maximum percentage reduction expressed as percentage change from baseline, and time of maximum reduction were calculated. RO is expressed as percentage unoccupied receptors.
Funding
This study was funded by Momenta Pharmaceuticals.
Conflict of Interest
L.E.L., L.M., J.D., A.M.M., and S.A. are full‐time employees of Momenta Pharmaceuticals and may own stock or stock options. J.L.H. was an employee of Momenta Pharmaceuticals when the study was designed and conducted and subsequently a paid consultant of Momenta Pharmaceuticals and may own stock or stock options of Momenta Pharmaceuticals. N.A.C. was a full‐time employee of Momenta Pharmaceuticals during the execution of the study and subsequently a paid consultant of Momenta Pharmaceuticals, and owned stock, restricted stock, and stock options in Momenta Pharmaceuticals during manuscript preparation. R.G.T., T.B., and M.P.v.I. are full‐time employees of PRA Health Sciences and may own stock or stock options in PRA Health Sciences. D.J.N. was principal of Drug Development Consulting, currently is a full‐time employee of Certara Strategic Consulting and is a paid consultant of Momenta Pharmaceuticals. J.B.S. is principal at Streisand Biomedical Consulting and a paid consultant of Momenta Pharmaceuticals.
Author Contributions
L.E.L. wrote the article; L.E.L., M.P.v.I., J.L.H., D.J.N., and N.A.C. designed the research; R.G.T., T.B., J.L.H., L.M., and J.D. performed research; L.E.L., R.G.T., T.B., M.P.v.I., D.J.N., J.L.H., N.A.C., J.D., A.M.M., J.B.S., and S.A. analyzed data; L.E.L. and L.M. contributed new reagents/analytical tools.
Data Sharing Statement
Momenta Pharmaceuticals is committed to responsible sharing of data from clinical trials we sponsor. This includes summary data and anonymized individual patient data, as well as other information (e.g., protocols and clinical study reports). Requests from any qualified researchers who engage in rigorous, independent scientific research will be considered if the trials are not part of an ongoing or planned regulatory submission (this includes requests for data on unlicensed products and indications). Data will be provided after review and approval of a research proposal or statistical analysis plan, confirmation that the requested data can be shared under applicable privacy laws, and execution of a data sharing agreement. Data requests can be submitted at any time, and the data will be accessible for 12 months, with possible extensions considered. Requests can be sent to medinfo@momentapharma.com.
Supporting information
Figure S1. Inhibition of IgG binding to FcRn by M281.
Table S1. Binding affinity of M281 to human FcRn at pH 7.6 and 6.0.
Table S2. E max and T max for total IgG and IgG subclass by dose group.
Table S3. Mean (SD) serum albumin and total serum protein in subjects (n = 3) receiving 4 consecutive M281 weekly doses.
Acknowledgments
The authors thank the volunteers who participated in this study. Appreciation is also extended to Josh Apgar (Applied Biomath, LLC) for initial modeling for phase I dose projection, Agnes Costello for helpful discussions during manuscript preparation and for reviewing the manuscript for scientific accuracy, Bill Denney (Human Predictions, LLC) for helpful discussions and analysis of pharmacokinetics/pharmacodynamics during manuscript preparation, Shiyin Foo for contributions in medical monitoring and safety, Jim Jin for statistical input and analysis, Chelsea Toner for clinical operational support, and Jacqueline Wildeman for providing input on pharmacokinetic parameters and biostatistical programming. The authors also thank Lamara D. Shrode, PhD, ISMPP CMPP, of JB Ashtin, who provided editorial support during the preparation of the manuscript. Editorial support was funded by Momenta Pharmaceuticals. This study was sponsored by Momenta Pharmaceuticals (Cambridge, MA). Momenta Pharmaceuticals contributed to the study design, research, data interpretation and the writing, review, and approval of the manuscript.
References
- 1. Eggert, M. , Zettl, U.K. & Neeck, G. Autoantibodies in autoimmune diseases. Curr. Pharm. Des. 16, 1634–1643 (2010). [DOI] [PubMed] [Google Scholar]
- 2. Suurmond, J. & Diamond, B. Autoantibodies in systemic autoimmune diseases: specificity and pathogenicity. J. Clin. Invest. 125, 2194–2202 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Giacomelli, R. et al International consensus: what else can we do to improve diagnosis and therapeutic strategies in patients affected by autoimmune rheumatic diseases (rheumatoid arthritis, spondyloarthritides, systemic sclerosis, systemic lupus erythematosus, antiphospholipid syndrome and Sjogren's syndrome)? The unmet needs and the clinical grey zone in autoimmune disease management. Autoimmun. Rev. 16, 911–924 (2017). [DOI] [PubMed] [Google Scholar]
- 4. Winthrop, K.L. et al The unmet need in rheumatology: reports from the targeted therapies meeting 2017. Clin. Immunol. 186, 87–93 (2018). [DOI] [PubMed] [Google Scholar]
- 5. Anderson, D. , Phan, C. , Johnston, W.S. & Siddiqi, Z.A. Rituximab in refractory myasthenia gravis: a prospective, open‐label study with long‐term follow‐up. Ann. Clin. Transl. Neurol. 3, 552–555 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kelly, K. , Gleeson, M. & Murphy, P.T. Slow responses to standard dose rituximab in immune thrombocytopenic purpura. Haematologica 94, 443–444 (2009); author reply 444‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Randall, K.L. Rituximab in autoimmune diseases. Aust. Prescr. 39, 131–134 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rutherford, A.I. , Patarata, E. , Subesinghe, S. , Hyrich, K.L. & Galloway, J.B. Opportunistic infections in rheumatoid arthritis patients exposed to biologic therapy: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatology (Oxford) 57, 997–1001 (2018). [DOI] [PubMed] [Google Scholar]
- 9. Tony, H.P. et al Safety and clinical outcomes of rituximab therapy in patients with different autoimmune diseases: experience from a national registry (GRAID). Arthritis Res. Ther. 13, R75 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu, J.F. et al Comparing the autoantibody levels and clinical efficacy of double filtration plasmapheresis, immunoadsorption, and intravenous immunoglobulin for the treatment of late‐onset myasthenia gravis. Ther. Apher. Dial. 14, 153–160 (2010). [DOI] [PubMed] [Google Scholar]
- 11. Schmaldienst, S. et al Intravenous immunoglobulin application following immunoadsorption: benefit or risk in patients with autoimmune diseases? Rheumatology (Oxford) 40, 513–521 (2001). [DOI] [PubMed] [Google Scholar]
- 12. Stummvoll, G.H. , Schmaldienst, S. , Smolen, J.S. , Derfler, K. & Biesenbach, P. Lupus nephritis: prolonged immunoadsorption (IAS) reduces proteinuria and stabilizes global disease activity. Nephrol. Dial. Transplant. 27, 618–626 (2012). [DOI] [PubMed] [Google Scholar]
- 13. Stummvoll, G.H. et al IgG immunoadsorption reduces systemic lupus erythematosus activity and proteinuria: a long term observational study. Ann. Rheum. Dis. 64, 1015–1021 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sesarman, A. , Vidarsson, G. & Sitaru, C. The neonatal Fc receptor as therapeutic target in IgG‐mediated autoimmune diseases. Cell. Mol. Life Sci. 67, 2533–2550 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sockolosky, J.T. & Szoka, F.C. The neonatal Fc receptor, FcRn, as a target for drug delivery and therapy. Adv. Drug Deliv. Rev. 91, 109–124 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roopenian, D.C. & Akilesh, S. FcRn: the neonatal Fc receptor comes of age. Nat. Rev. Immunol. 7, 715–725 (2007). [DOI] [PubMed] [Google Scholar]
- 17. Patel, D.A. , Puig‐Canto, A. , Challa, D.K. , Perez Montoyo, H. , Ober, R.J. & Ward, E.S. Neonatal Fc receptor blockade by Fc engineering ameliorates arthritis in a murine model. J. Immunol. 187, 1015–1022 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ling, L.E. et al M281: a therapeutic FcRn blocking antibody for rapid clearance of IgG and IgG autoantibodies in immune cytopenias and other autoimmune diseases. Blood 126, 3472 (2015). [Google Scholar]
- 19. Olaru, F. et al Neonatal Fc receptor promotes immune complex‐mediated glomerular disease. J. Am. Soc. Nephrol. 25, 918–925 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu, L. et al Amelioration of experimental autoimmune myasthenia gravis in rats by neonatal FcR blockade. J. Immunol. 178, 5390–5398 (2007). [DOI] [PubMed] [Google Scholar]
- 21. Chen, P. et al Animal model of fetal and neonatal immune thrombocytopenia: role of neonatal Fc receptor in the pathogenesis and therapy. Blood 116, 3660–3668 (2010). [DOI] [PubMed] [Google Scholar]
- 22. Li, C. et al The maternal immune response to fetal platelet GPIbalpha causes frequent miscarriage in mice that can be prevented by intravenous IgG and anti‐FcRn therapies. J. Clin. Invest. 121, 4537–4547 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lobo, E.D. , Hansen, R.J. & Balthasar, J.P. Antibody pharmacokinetics and pharmacodynamics. J. Pharm. Sci. 93, 2645–2668 (2004). [DOI] [PubMed] [Google Scholar]
- 24. Waldmann, T.A. & Strober, W. Metabolism of immunoglobulins. Prog. Allergy 13, 1–110 (1969). [DOI] [PubMed] [Google Scholar]
- 25. Nixon, A.E. et al Fully human monoclonal antibody inhibitors of the neonatal Fc receptor reduce circulating IgG in non‐human primates. Front Immunol. 6, 176 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Waldmann, T.A. & Terry, W.D. Familial hypercatabolic hypoproteinemia: a disorder of endogenous catabolism of albumin and immunoglobulin. J. Clin. Invest. 86, 2093–2098 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kendrick, F. et al Analysis of a compartmental model of endogenous immunoglobulin G metabolism with application to multiple myeloma. Front. Physiol. 8, 149 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stein, C. et al Clinical chemistry of human FcRn transgenic mice. Mamm. Genome 23, 259–269 (2012). [DOI] [PubMed] [Google Scholar]
- 29. Kiessling, P. et al The FcRn inhibitor rozanolixizumab reduces human serum IgG concentration: a randomized phase 1 study. Sci. Transl. Med. 9, eaan1208 (2017). [DOI] [PubMed] [Google Scholar]
- 30. Robak, T. et al Phase II, multiple‐dose study of anti‐FcRn antibody, rozanolixizumab (UCB7665), in patients with primary immune thrombocytopenia: interim analysis. Blood 130, 15 (2017). [Google Scholar]
- 31. Garcia‐Lloret, M. , McGhee, S. & Chatila, T.A. Immunoglobulin replacement therapy in children. Immunol. Allergy Clin. North Am. 28, 833–849, ix (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morsheimer, M.M. et al Risk factors and clinical significance of lymphopenia in survivors of the fontan procedure for single‐ventricle congenital cardiac disease. J. Allergy Clin. Immunol. Pract. 4, 491–496 (2016). [DOI] [PubMed] [Google Scholar]
- 33. Stiehm, E.R. The four most common pediatric immunodeficiencies. J. Immunotoxicol. 5, 227–234 (2008). [DOI] [PubMed] [Google Scholar]
- 34. Whelan, M.A. , Hwan, W.H. , Beausoleil, J. , Hauck, W.W. & McGeady, S.J. Infants presenting with recurrent infections and low immunoglobulins: characteristics and analysis of normalization. J. Clin. Immunol. 26, 7–11 (2006). [DOI] [PubMed] [Google Scholar]
- 35. Baird, M.F. , Graham, S.M. , Baker, J.S. & Bickerstaff, G.F. Creatine‐kinase‐ and exercise‐related muscle damage implications for muscle performance and recovery. J. Nutr. Metab. 2012, 960363 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moghadam‐Kia, S. , Oddis, C.V. & Aggarwal, R. Approach to asymptomatic creatine kinase elevation. Cleve. Clin. J. Med. 83, 37–42 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pettersson, J. et al Muscular exercise can cause highly pathological liver function tests in healthy men. Br. J. Clin. Pharmacol. 65, 253–259 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Young, T.C. et al A systematic review and pooled analysis of select safety parameters among normal healthy volunteers taking placebo in phase 1 clinical trials. J. Clin. Pharmacol. 57, 1079–1087 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Agarwal, S. & Cunningham‐Rundles, C. Assessment and clinical interpretation of reduced IgG values. Ann. Allergy Asthma Immunol. 99, 281–283 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Inhibition of IgG binding to FcRn by M281.
Table S1. Binding affinity of M281 to human FcRn at pH 7.6 and 6.0.
Table S2. E max and T max for total IgG and IgG subclass by dose group.
Table S3. Mean (SD) serum albumin and total serum protein in subjects (n = 3) receiving 4 consecutive M281 weekly doses.


