Abstract
Finasteride 1 mg is considered to be the standard treatment method for male androgenetic alopecia (AGA). However, there have only been a few studies investigating its long‐term efficacy. Moreover, its effect on various types of AGA remains unknown. In this study, the authors investigated the 5‐year efficacy of finasteride 1 mg in Korean men with AGA and analyzed the changes in hair growth according to the distribution of hair loss. The medical records of male AGA patients who were treated with oral finasteride for a period of at least 5 years at two university hospitals were retrospectively reviewed. Patients' photographs were evaluated using the basic and specific (BASP) classification and investigator's global assessment. Of the total 126 patients, 108 (85.7%) showed improvement after 5 years of treatment. According to the BASP classification, hair loss of the anterior hair line (basic type), vertex (V type), and frontal area (F type) was improved in 44.4%, 89.7% and 61.2% of patients, respectively. The V type showed a more rapid and steady improvement compared with the other types. Progression of alopecia after peak improvement was seen in 10.3% of cases of the V type, 16.2% of the F type and 0% of the basic type. In conclusion, finasteride 1 mg showed a sustainable effect for at least 5 years in Korean male AGA patients. The exact time points showing signs of first clinical improvement and sustainability were different depending on the type of alopecia.
Keywords: androgenetic alopecia, basic and specific classification, finasteride, long‐term efficacy, type 2‐selective 5α‐reductase
Introduction
Androgenetic alopecia (AGA) is the most common type of baldness, characterized as a patterned, progressive hair loss of the scalp. Traditionally, various classification methods have been proposed to describe the pattern and severity of AGA, including Hamilton, Norwood–Hamilton and Olsen classifications. Recently, Lee et al.1 devised a new method – the basic and specific (BASP) classification (Fig. 1) – that can describe the variable clinical patterns of AGA. This classification provides a precise description of female pattern hair loss and may be applicable to both male and female patients.2, 3 The BASP classification was demonstrated to be useful in describing the exact amount and distribution of hair loss in those with patterned hair loss;1 therefore, it has been utilized in previous studies to investigate the association between the distribution of hair loss and familial factors.4, 5
Figure 1.
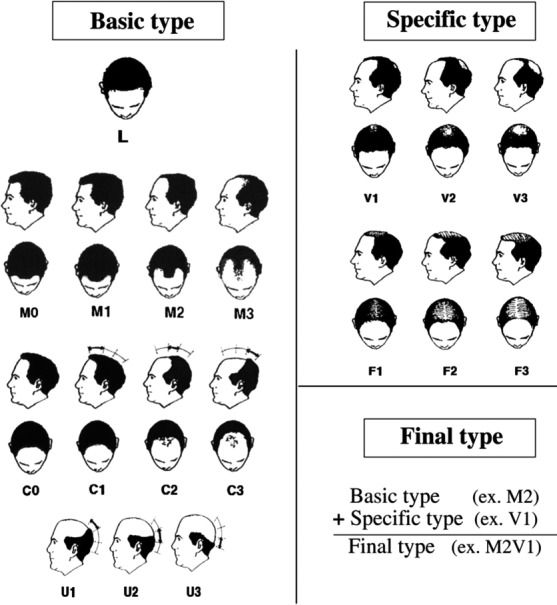
The basic and specific classification system (cited from Lee et al.).1
Finasteride 1 mg is an approved treatment for male AGA patients aged 18–40 years. It selectively inhibits type 2‐selective 5α‐reductase, which converts testosterone to dihydrotestosterone, which is known to be the main pathogenetic factor of AGA.6, 7 Although finasteride has been used widely to treat male AGA in clinical settings, to the best of our knowledge, its efficacy or safety has not been fully studied in the Korean population to date. Furthermore, there are only a few clinical studies investigating its long‐term (>5 year) efficacy,8, 9, 10 and none – to the best of our knowledge – evaluating the treatment outcome using the BASP classification. In this study, we investigated the 5‐year efficacy of finasteride 1 mg in Korean men with AGA using the BASP classification and analyzed the changes in hair growth after finasteride 1 mg treatment according to the distribution of hair loss.
Methods
Patients
This study was approved by the institutional review board of Seoul National University Bundang Hospital and Pusan National University Hospital. We identified the electronic medical records of male patients with AGA who were treated in a tertiary or a secondary hospital with oral finasteride for at least 5 years between May 2004 and August 2017. Their medical records and clinical photographs were reviewed. The inclusion criteria were: (i) patients who started the treatment of finasteride at the age of 18–40 years; (ii) those who were treated only with finasteride (1 mg/day); (iii) those who visited regularly (3 months ± 1 month, 6 months ± 1 month, 1 year ± 2 months, 2 years ± 3 months, 3 years ± 3 months and 5 years ± 3 months after starting to take finasteride); and (iv) those who exhibited good compliance (drug off period <10% of total treatment period).
Assessment
Clinical photographs and progression notes of patients' medical records were reviewed. Clinical assessments were performed by four dermatologists using clinical photographs with a standardized vertex and frontal view. Evaluation points were: baseline, 3 and 6 months, and 1, 2, 3 and 5 years after starting the finasteride treatment.
First, all patients' photographs were evaluated using the BASP classification (Fig. 1). The basic types represent the shape of the anterior hairline, and the specific types represent the density of hair on distinct areas (frontal [F] and vertex [V]). The basic types were divided into four groups (L, M, C and U), and the specific types into two groups (F and V). The final type was decided by a combination of assigned BASP types.1 When there was any decrease in the grades of the basic type (ex. M3 → M2) or the specific type (ex. V3 → V1) compared with the baseline, it was regarded as an improvement in the BASP classification. Moreover, to evaluate the changes of global hair loss after treatment, the investigator's global assessment (IGA) using a 7‐point scale was performed. The 7‐point scale was as follows: “greatly improved” (score of +3); “moderately improved” (+2); “slightly improved” (+1); “unchanged” (0); “slightly aggravated” (−1); “moderately aggravated” (−2); and “greatly aggravated” (−3).11, 12 The presence or absence of adverse reactions, types of adverse reactions and treatment for adverse reactions were also evaluated by reviewing the patients' medical records.
Statistical analysis
The χ2‐test was used to compare the outcomes of 5‐year finasteride treatment between the three types of BASP classification. In case there was a significant difference, pair‐wise comparisons using the χ2‐test with post‐hoc Bonferroni correction were performed. All analyses were performed using SPSS version 20 for Windows (IBM, Chicago, IL, USA). P‐values of less than 0.05 were considered statistically significant.
Results
Patient characteristics
A total of 166 AGA patients were treated with finasteride (1 mg/day) for more than 5 years in two different hospitals. Among them, 126 patients were included in this study. The other 40 patients were excluded due to low compliance (n = 21) and insufficient medical records (n = 19). The baseline characteristics of patients are summarized in Table 1. According to the BASP classification, 84.9% of patients had basic M type. Among those with a specific type, 46.0% of the patients had the V type and 29.4% had the F type.
Table 1.
Baseline characteristics of patients with androgenetic alopecia (n = 126)
| Age, year, mean ± SD | 43.9 ± 10.8 |
| Age started taking finasteride, year, mean ± SD | 34.1 ± 10.5 |
| BASP classification before the treatment | |
| Basic type | |
| L type, n (%) | 8 (6.3) |
| M type | |
| M0, n (%) | 10 (7.9) |
| M1, n (%) | 48 (38.1) |
| M2, n (%) | 41 (32.5) |
| M3, n (%) | 8 (6.3) |
| Total, n (%) | 107 (84.9) |
| C type | |
| C0, n (%) | 1 (0.8) |
| C1, n (%) | 8 (6.3) |
| C2, n (%) | 1 (0.8) |
| C3, n (%) | 1 (0.8) |
| Total, n (%) | 11 (8.7) |
| U type | 0 (0) |
| Specific type | |
| V type, n (%) | |
| V1, n (%) | 19 (15.1) |
| V2, n (%) | 23 (18.2) |
| V3, n (%) | 16 (12.7) |
| Total, n (%) | 58 (46.0) |
| F type, n (%) | |
| F1, n (%) | 15 (11.9) |
| F2, n (%) | 15 (11.9) |
| F3, n (%) | 7 (5.6) |
| Total, n (%) | 37 (29.4) |
BASP, basic and specific classification; SD, standard deviation.
Efficacy evaluation
Based on the IGA score, after 5 years of treatment, proportions of patients with improvement (IGA score, ≥1) or prevention of disease progression (IGA score, ≥0) were 85.7% (108/126) or 98.4% (124/126), respectively (Fig. 2). The mean IGA score was 0.60 ± 0.59, 1.24 ± 0.72, 1.46 ± 0.83, 1.55 ± 0.83, 1.51 ± 0.87 and 1.48 ± 0.98 after 3 months, 6 months, and 1, 2, 3 and 5 years, respectively (Fig. 3). The mean IGA scores after 6 months, 1 year and 2 years were significantly increased compared with the previous scores (P = 0.000 after 6 months and 1 year, P = 0.014 after 2 year). On the other hand, the mean IGA scores after 3 and 5 years were not significantly different from that of after 2 years (P = 0.277, P = 0.105)
Figure 2.
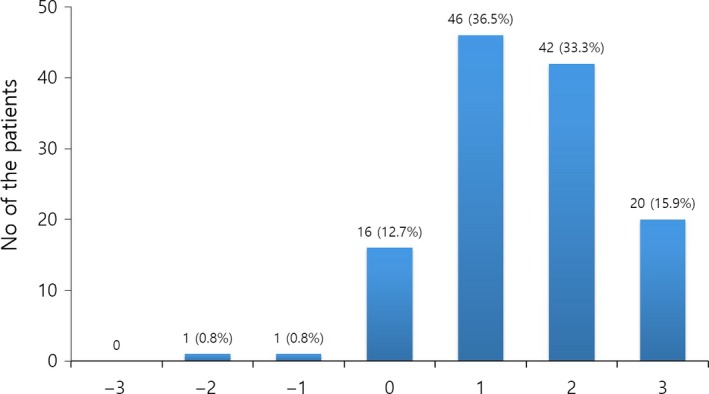
Distribution of patients with respect to the investigator's global assessment score after 5 years of finasteride treatment.
Figure 3.
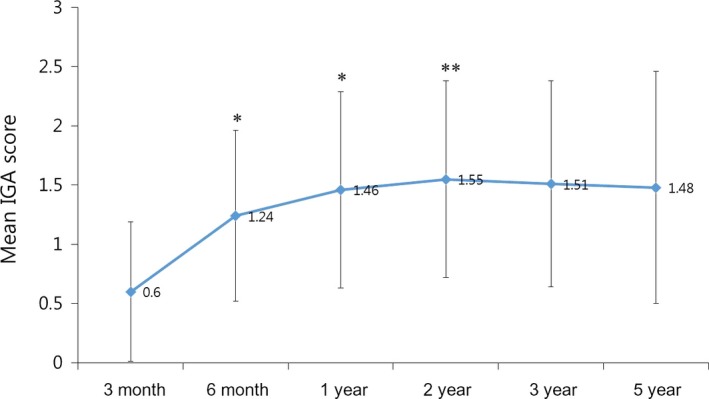
Change of investigator's global assessment (IGA) score during the 5‐year treatment period. *P < 0.01 compared with previous observational time point. **P < 0.05 compared with previous observational time point.
Figure 4 shows clinical improvement in the BASP classification according to the treatment duration. After 5 years of treatment, 44.4% (48/108) of the basic type showed clinical improvement. Among the specific types, 89.7% (52/58) of the V type and 61.2% (23/37) of the F type showed improvement after 5 years. The proportion of the cases that showed improvement of the V type was significantly higher than that of the F type (P = 0.000) and basic type (P = 0.000). In addition, the V type showed a rapid (~26% of the patients improved within 3 months) and steady (~10% of the patients improved after 2 years) improvement.
Figure 4.
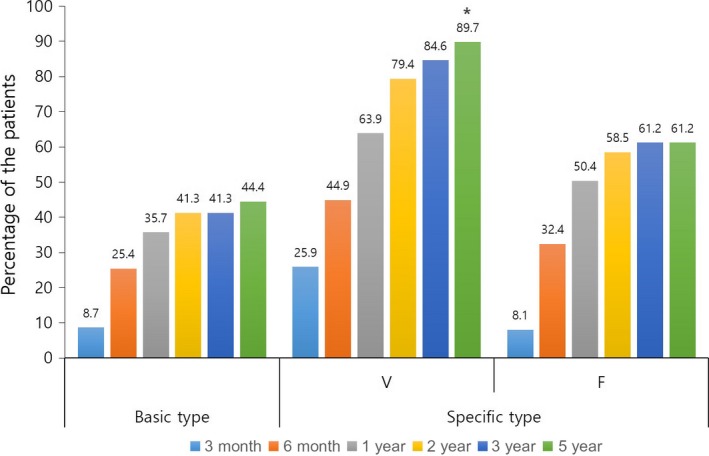
Ratio of patients who showed clinical improvement according to treatment duration and basic and specific classification. *P < 0.01 compared with basic and F type.
Progression after peak efficacy
Based on the IGA score, 26 (20.6%) patients experienced progression of alopecia after peak improvement during the treatment period. According to the BASP classification, the proportion of these patients was significantly different between the three types (Fig. 5, P = 0.000). Six patients in the V type (10.3%) experienced progression after improvement; one patient (1.7%) after 2 years, two patients (3.4%) after 3 years and three patients (5.2%) after 5 years of starting the treatment. Six patients of the F type (16.2%) experienced progression of alopecia after initial improvement; one patient (2.7%) after 2 years, two patients (5.4%) after 3 years and three patients (8.1%) after 5 years of starting the treatment. Conversely, there was no case showing the progression of alopecia after peak improvement in the basic type.
Figure 5.
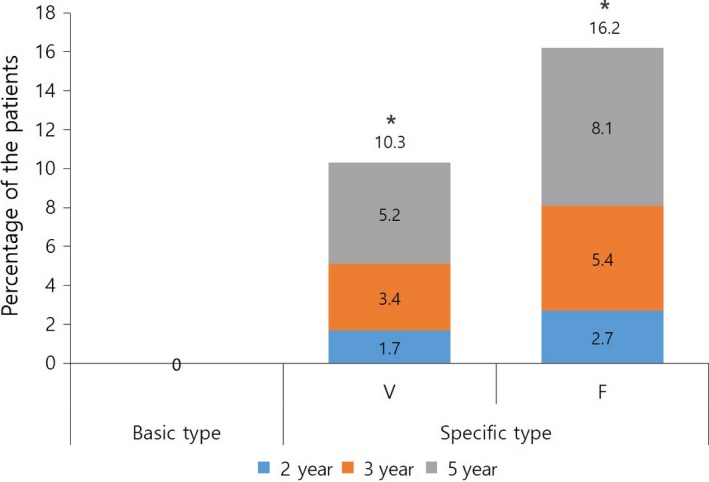
Ratio of patients who showed progression of alopecia after showing peak efficacy. *P < 0.01 compared with basic and F type.
Adverse events
According to the review of patients' medical records, 12 patients (9.5%) experienced adverse events during the treatment period. Among them, 10 patients (7.9%) reported sexually related symptoms: four (3.1%) with decreased libido, three (2.4%) with erectile dysfunction and three (2.4%) with decreased semen volume or watery semen. Additionally, liver function abnormality (n = 1, 0.8%), gastrointestinal trouble (n = 1, 0.8%) and gynecomastia (n = 1, 0.8%) were also reported. Six cases (4.8%) developed adverse events within the first 6 months of the treatment period. Most of the adverse events were mild and subsided spontaneously without treatment. One case with abnormality of liver function and two cases with sexual dysfunction stopped the treatment temporally (1–3 months).
Discussion
In the present study, the proportion of patients showing improvement – including no disease progression – was 98.4% after 5‐year treatment of finasteride. This score is similar with the scores in previous studies by Rossi et al. (93.0%)9 and Yoshitake et al. (100%). This suggests that long‐term use of finasteride can, at least, prevent the progression of AGA in most cases. AGA is a progressive disease. In a previous randomized, placebo‐controlled trial, it was shown that AGA deteriorates gradually throughout a 5‐year period if not treated.8 Thus, disease management may be just as important in the treatment of AGA. The proportion of patients who showed improvement in the present study was 85.7%, which is higher than that of Rossi et al. (38.6%)9 and lower than that of Yoshitake et al. (99.4%).10 This discrepancy may be due to the difference in the study design. The present study is a retrospective study, while the other studies were prospective and open label. There might have been some selection bias in this study, leading to overestimation of the treatment efficacy. However, the response rate in this study was rather lower than that in the Japanese study. Little is known about this discrepancy. Yoshitake et al.10 suggested that it may be partially due to the characteristics of Asian hair –lower hair density, greater hair diameter and greater contrast with the black color of the hair shaft – compared with Caucasian hair, which may increase the likelihood of being able to notice minor and slight changes. In addition, drug efficacy may be impacted by racial differences. In a previous study, Choi et al.13 demonstrated a difference in hair steroid levels between Koreans and Caucasians, leading to potential differences in drug response. Further investigations regarding aspects of race and drug response are necessary.
In this study, the pattern changes of alopecia in the long‐term treatment were evaluated using the BASP classification. The proportion of cases that showed clinical improvement after 5 years of treatment was highest of the V type (89.8%), followed by the F type (61.2%) and the basic type (44.5%) (both P = 0.000, Fig. 3). Most patients of the basic type and F type experienced improvement somewhere between 3 months and 1 year after starting the treatment. Conversely, a considerable portion of patients (27.3%) of the V type showed improvement even within the first 3 months. These findings indicate that the V type responds better and more rapidly to finasteride compared with the other types. Furthermore, a considerable portion of patients (25.9%) of the V type showed clinical improvement after 1 year. This means that approximately 70% of patients who do not show any improvement after the first year of finasteride treatment of the V type can be improved with continued medication. Meanwhile, by using the BASP classification, we could investigate the efficacy of finasteride for female pattern hair loss in male patients. In previous studies, female pattern was observed in 11.1–37% of Korean males with AGA; this is much higher than that observed in Caucasian males.1, 14 Although it has been reported that finasteride showed greater improvement in the vertex region than in the frontal region after long‐term treatment,9 little is known about the female pattern. In the present study, the treatment response of patients of the F type was significantly lower than those of the V type after 5 years of finasteride treatment (61.2% vs 89.7%).
Based on the IGA score change, the degree of improvement was most obvious between 3 and 6 months of treatment (Fig. 3). The peak efficacy was observed after 2 years, followed by a very slight decrease from 3 to 5 years, which was statistically insignificant. In a previous study, improvement reached a peak at 1–2 years of treatment with finasteride, followed by a slight decrease from 3 to 5 years of treatment based on global photographic assessment scores as well as the mean hair count change.8 We explained that this apparent peaking effect may be due to the effects of finasteride on the hair growth cycle. The initiation of finasteride treatment rapidly increases the number of anagen‐phase hairs, and these affected hairs are driven to a cycle in a synchronous manner.15 After a somewhat similar anagen phase, these hairs would enter the telogen phase, followed by subsequent shedding, in a partially synchronized fashion, and this would produce a slight decline in the peak efficacy. In the present study, there were some differences in sustainability between the patterns of alopecia. The proportion of patients who showed a decline from the peak efficacy was highest in the F type (16.2%), followed by the V type (10.3%), and there was no such case in the basic type (Fig. 5). These findings cannot be explained by decreased pharmacological efficacy because there was no progression in the frontal hairline, or senescent change because our patients were generally younger (<40 years old). On the other hand, it can be explained by the effects of finasteride on the hair growth cycle. Shedding of hairs that go in to the telogen phase in a semi‐synchronized fashion may be a characteristic of telogen effluvium, showing diffuse hair loss with hairline preservation.
The major limitation of this study was its retrospective nature; we only included patients who were treated for more than 5 years with finasteride. This might have led to selection bias in our study. In this setting, it may not be possible to precisely estimate patients' adherence to drugs: the efficacy can be overestimated and adverse events can be underestimated. A future, prospective randomized controlled trial is necessary to confirm our results. Nevertheless, our efficacy data regarding the treatment response was similar or rather slightly lower than that of the Japanese study, whereby the race of the two populations are expected to be most similar.10 Moreover, the percentage of patients who experienced drug‐related adverse reactions in our study was similar or somewhat higher than in previous studies,8, 9 even though cases that interrupted the treatment due to adverse reaction were not included in our study. Our work is noteworthy in that we analyzed the long‐term efficacy of finasteride according to the type of alopecia using the BASP classification. Particularly, we investigated the female pattern hair loss in male patients. This has gained less attention thus far and is thought to occupy a much higher proportion of the Asian population than the Caucasian population. Meanwhile, we were unable to analyze the overall hair changes in patients who had combined types of alopecia. Although the outcome evaluation using BASP classification may be beneficial with respect to its ability to analyze changes in hair growth according to each type of hair loss, it may be inappropriate for the analysis of overall hair changes in combined types. Developing a grading system that can evaluate the overall alopecia status in BASP classification would be beneficial.
In conclusion, finasteride 1 mg showed a sustainable effect for at least 5 years in Korean male AGA patients. The exact time points showing signs of first clinical improvement and sustainability were different depending on the type of alopecia. We believe this data will be helpful to clinicians in treating AGA patients.
Conflict of Interest:
None declared.
References
- 1. Lee WS, Ro BI, Hong SP et al A new classification of pattern hair loss that is universal for men and women: basic and specific (BASP) classification. J Am Acad Dermatol 2007; 57: 37–46. [DOI] [PubMed] [Google Scholar]
- 2. Sehgal VN, Kak R, Aggarwal A, Srivastava G, Rajput P. Male pattern androgenetic alopecia in an Indian context: a perspective study. J Eur Acad Dermatol Venereol 2007; 21: 473–479. [DOI] [PubMed] [Google Scholar]
- 3. Su LH, Chen TH. Association of androgenetic alopecia with smoking and its prevalence among Asian men: a community‐based survey. Arch Dermatol 2007; 143: 1401–1406. [DOI] [PubMed] [Google Scholar]
- 4. Lee WS, Oh Y, Ji JH et al Analysis of familial factors using the basic and specific (BASP) classification in Korean patients with androgenetic alopecia. J Am Acad Dermatol 2011; 65: 40–47. [DOI] [PubMed] [Google Scholar]
- 5. Park SY, Oh SS, Lee WS. Relationship between androgenetic alopecia and cardiovascular risk factors according to BASP classification in Koreans. J Dermatol 2016; 43: 1293–1300. [DOI] [PubMed] [Google Scholar]
- 6. Imperato‐McGinley J, Guerrero L, Gautier T, Peterson RE. Steroid 5alpha‐reductase deficiency in man: an inherited form of male pseudohermaphroditism. Science 1974; 186: 1213–1215. [DOI] [PubMed] [Google Scholar]
- 7. Drake L, Hordinsky M, Fiedler V et al The effects of finasteride on scalp skin and serum androgen levels in men with androgenetic alopecia. J Am Acad Dermatol 1999; 41: 550–554. [PubMed] [Google Scholar]
- 8. Finasteride Male Pattern Hair Loss Study Group . Long‐term (5‐year) multinational experience with finasteride 1 mg in the treatment of men with androgenetic alopecia. Eur J Dermatol 2002;12:38–49. [PubMed] [Google Scholar]
- 9. Rossi A, Cantisani C, Scarno M, Trucchia A, Fortuna MC, Calvieri S. Finasteride, 1 mg daily administration on male androgenetic alopecia in different age groups: 10‐year follow‐up. Dermatol Ther 2011; 24: 455–461. [DOI] [PubMed] [Google Scholar]
- 10. Yoshitake T, Takeda A, Ohki K et al Five‐year efficacy of finasteride in 801 Japanese men with androgenetic alopecia. J Dermatol 2015; 42: 735–738. [DOI] [PubMed] [Google Scholar]
- 11. Kaufman KD, Olsen EA, Whiting D et al Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol 1998; 39: 578–589. [DOI] [PubMed] [Google Scholar]
- 12. Jung JY, Yeon JH, Choi JW et al Effect of dutasteride 0.5 mg/d in men with androgenetic alopecia recalcitrant to finasteride. Int J Dermatol 2014; 53: 1351–1357. [DOI] [PubMed] [Google Scholar]
- 13. Choi MH, Kim SJ, Lew BL, Sim WY, Chung BC. Hair steroid profiling reveals racial differences in male pattern baldness between Korean and Caucasian populations. J Invest Dermatol 2013; 133: 822–824. [DOI] [PubMed] [Google Scholar]
- 14. Paik JH, Yoon JB, Sim WY, Kim BS, Kim NI. The prevalence and types of androgenetic alopecia in Korean men and women. Br J Dermatol 2001; 145: 95–99. [DOI] [PubMed] [Google Scholar]
- 15. Van Neste D, Fuh V, Sanchez‐Pedreno P et al Finasteride increases anagen hair in men with androgenetic alopecia. Br J Dermatol 2000; 143: 804–810. [DOI] [PubMed] [Google Scholar]


