Abstract
Myelin, the multilayered membrane surrounding many axons in the nervous system, increases the speed by which electrical signals travel along axons and facilitates neuronal communication between distant regions of the nervous system. However, how neuronal signals influence the myelinating process in the CNS is still largely unclear. Recent studies have significantly advanced this understanding, identifying important roles for neuronal activity in controlling oligodendrocyte development and their capacity of producing myelin in both developing and mature CNS. Here, we review these recent advances, and discuss potential mechanisms underpinning activity‐dependent myelination and how remyelination may be stimulated via manipulating axonal activity, raising new questions for future research.
![]()
Keywords: CNS, myelination, neuronal activity, neurotransmission, oligodendrocyte, OPC
Abbreviations used
- AMPAR
AMPA receptors
- BDNF
brain‐derived neurotrophic fator
- CCP
caudal cerebellar peduncle
- CNS
central nervous system
- EdU
ethynyl‐2′‐deoxyuridine
- FA
fractional anisotropy
- KKDR
delayed outward‐rectifying potassium channels
- NMDAR
NMDA receptors
- NRGs
neuregulin
- OL
oligodendrocytes
- OPCs
oligodendrocyte precursor cells
- P35
postnatal day 35
- PDGF
platelet‐derived growth factor
- PDGFR
platelet‐derived growth factor receptor
- PNS
peripheral nervous system
- TTX
tetrodotoxin
The evolutionary importance of myelin is that it provides the structural basis for saltatory action potential propagation by restricting action potentials to short unmyelinated axonal segments (the nodes of Ranvier), thus allowing nerve conduction 20–100 faster than nonmyelinated axons of the same diameter—without occupying much space (Nave and Werner 2014). This has effectively enhanced the nervous system compactness, permitting the fast and efficient processing of complex information. It is also increasingly apparent that myelin confers trophic and metabolic support to the axons that it ensheaths (Lappe‐Siefke et al. 2003; Simons and Nave 2015; Yin et al. 2006). The CNS myelination comprises a series of complex cellular events including proliferation and migration of oligodendrocyte precursor cells (OPCs), differentiation of OPCs into mature myelinating oligodendrocytes, axonal ensheathment and node formation (Fig. 1). This programme is tightly controlled by a number of intrinsic factors (Barres and Raff 1999; Baumann and Pham‐Dinh 2001; Emery 2010; Mitew et al. 2014; Nave and Werner 2014) that are responsive to a range of extracellular cues, including signalling molecules released by neurons or glial cells such as growth factors and neurotransmitters and molecules expressed on the surface of these cells and axons (Mitew et al. 2014; Nave and Werner 2014) (Fig. 1).
Figure 1.
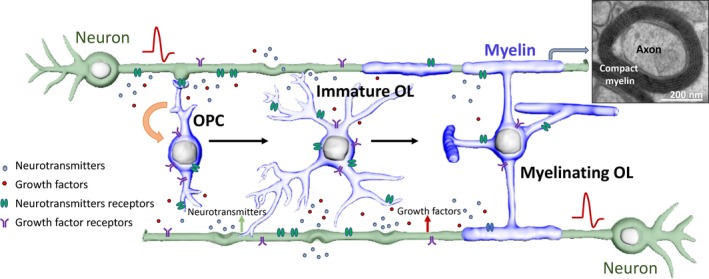
Schematic showing oligodendroglial lineage development and de novo myelination. During development, subventricular cells in the CNS (brain and spinal cord) give rise to committed oligodendrocyte precursor cells (OPCs), which can proliferate and/or then terminally differentiate into post‐mitotic immature oligodendrocytes (OL). In response to the appropriate extracellular cues, these immature OL can further mature and become myelinating oligodendrocytes, ensheathing receptive axons and forming compact myelin. Action potential firing by active neurons results in the release of neurotransmitters (such as glutamate, GABA, ATP or acetylcholine) and/or growth factors (such as platelet‐derived growth factor, brain‐derived neurotrophic factor or neuregulin) via synaptic and non‐synaptic mechanisms, and exert multifaceted influence upon both oligodendroglial lineage development and axonal ensheathment. This process is regulated not only at the level of oligodendroglial proliferation and differentiation but also at the level of individual axons.
A potential explanation for the long‐lasting controversy over whether neuronal activity regulates myelination (Barres and Raff 1993; Barres et al. 1992; Demerens et al. 1996; Gautier et al. 2015; Gibson et al. 2014; Hines et al. 2015; Hughes et al. 2018; Liu et al. 2012, 2016; Makinodan et al. 2012; Mensch et al. 2015; Mitew et al. 2018) or not (Barres and Raff 1999; Bechler et al. 2015; Lee et al. 2012, 2013; Li et al. 2014), is that two distinct modes of myelination both exist: one that is independent of axonal activity and one that depends. Although there is no clear definition of the two modes of myelination, the activity‐dependent myelination is considered as a myelinating process dependent on and/or regulated by electrical activity and molecular cues such as growth factors, neurotransmitters or other molecules whose expression or release is regulated by axonal electrical activity (Barres and Raff 1993; Barres et al. 1992; Demerens et al. 1996; Gautier et al. 2015; Gibson et al. 2014; Hines et al. 2015; Hughes et al. 2018; Liu et al. 2012, 2016; Makinodan et al. 2012; Mensch et al. 2015; Mitew et al. 2018), while the activity‐independent myelination is an oligodendrocyte‐driven process independent of axonal electrical activity (Barres and Raff 1999; Bechler et al. 2015; Lee et al. 2012, 2013; Li et al. 2014). Cultured oligodendrocytes can survive, proliferate and differentiate in the absence of axons (Barres et al. 1993b; Chan et al. 2004; Watkins et al. 2008; Xiao et al. 2010), and produce myelin membrane around ‘electrically silent’ nanofibres (Bechler et al. 2015; Lee et al. 2012, 2013; Li et al. 2014), suggesting a neuronal activity‐independent programme that drives oligodendroglial lineage progression and the initiation of myelination. On the other hand, increasing studies have revealed important roles for activity‐dependent myelination, demonstrating that oligodendrocyte development and their capacity of producing myelin is a dynamic process driven by neural activity and experience (Barres and Raff 1993; Barres et al. 1992; Demerens et al. 1996; Gautier et al. 2015; Gibson et al. 2014; Hines et al. 2015; Hughes et al. 2018; Liu et al. 2012, 2016; Makinodan et al. 2012; Mensch et al. 2015; Mitew et al. 2018; Wake et al. 2011, 2015). CNS myelination is a lifelong process that peaks during development and continues into adulthood (Baumann and Pham‐Dinh 2001; Hill et al. 2018; Hughes et al. 2018; Young et al. 2013). There is clear evidence for ongoing addition of oligodendrocytes throughout life, at least in rodents (Hill et al. 2018; Hughes et al. 2018; Young et al. 2013). Thus, a more complete understanding of activity‐dependent myelination will provide new insights into the mechanisms that govern nervous system plasticity and aid the development of new therapies that directly target myelin repair after a demyelinating insult. Below, we will perform a systematic review on recent research focusing on activity‐dependent myelination during development and adulthood as well as after injury.
Activity‐dependent myelination during development
While Río‐Hortega initially descried oligodendrocyte morphologies, in 1901, Flechsig put forward a fundamental law of myelogenesis – before the identification of oligodendrocytes, which states ‘that the myelinization of nerve fibers in the developing brain follows a definite chronologic sequence such that those fibers belonging to particular functional systems mature at the same time’ (Flechsig 1901). But this did not go unchallenged, and a contemporary scholar argued that myelination depended on the size of nerve calibre (Vogt and Vogt 1908). However, it was not until 1960s when Gyllensten and Malmfors (1963) first introduced the intriguing idea that neuronal activity could influence the behaviour of oligodendroglial lineage cells, that a biological evidence was provided. This study demonstrated that dark rearing could inhibit developmental myelination in the optic nerve (Gyllensten and Malmfors 1963), which was later supported by a similar study showing premature eye‐opening could accelerate it (Tauber et al. 1980). These studies were also challenged by number of similar studies arguing that axonal electrical activity did not regulate CNS myelination (Colello and Pott 1997; Colello et al. 1995; Shrager and Novakovic 1995). This debate is still ongoing, but evidence for this exciting notion, that axonal electrical activity can regulate myelination has now been significantly advanced by a number of recent works investigating multiple aspects of the myelinating process using sophisticated genetic and powerful imaging tools. Here we discuss some key aspects of the activity‐dependent myelinating process during development, particularly on OPC proliferation, survival, differentiation and myelin ensheathment.
Neuronal activity regulates oligodendrocyte proliferation
Under normal homeostatic conditions, new myelinating oligodendrocytes can be generated from OPC proliferation (Young et al. 2013), or from a pool of constantly differentiating pre‐myelinating oligodendrocytes (Xiao et al. 2016). In the 1990s, the optic nerve was used as a model to study activity‐dependent effects on OPC proliferation and the number of oligodendroglial lineage cells (Barres and Raff 1993). When the developing optic nerves was transected just behind the eyeball, the number of mitotic OPCs dropped by 90% in 4 days, suggesting OPC division was largely regulated by the axons (Barres and Raff 1993). Concordant with this finding, silencing the axonal electrical activity via an intraocular injection of tetrodotoxin (TTX), a toxin that blocks voltage‐gated sodium channels and action potentials, also reduced the number of dividing OPCs by 80% (Barres and Raff 1993). This early study provided the compelling experimental evidence that axonal electrical activity stimulates OPC proliferation in the optic nerve during development, indicating a mechanism by which electrical activity in neighbouring axons could control local OPC proliferation.
More recent studies using a range of techniques have confirmed that manipulating neuronal activity alters oligodendrogenesis in multiple CNS regions during development (Gibson et al. 2014; Mangin et al. 2012; Mitew et al. 2018; Venkatesh et al. 2015). By far the most common way to identify the OPC proliferation in response to altered neural activity has been the use of thymidine analogues such as 5‐ethynyl‐2′‐deoxyuridine (EdU). Thymidine analogues are incorporated during DNA synthesis and have the advantage of permanently marking dividing cells and their progeny (Nowakowski et al. 1989). Gibson et al. (2014) adopted an optogenetic approach to stimulate neuronal electrical activity in the premotor cortex of postnatal day 35 mice, OPCs in both the premotor cortex and the subcortical white matter tracts displayed a marked proliferative response. In this experiment, EdU was given at the beginning of the 30‐min stimulation period to label dividing OPCs in these mice which were subsequently sacrificed 3 h after stimulation and displayed an approximately fourfold increase EdU+ OPCs, indicating a rapid proliferative response (Gibson et al. 2014). This hyperproliferative response to increased neuronal activity was specific to OPCs as other glial cells such as the microglial number (predominantly localized to the superficial cortex) remained intact (Gibson et al. 2014). Consistent with these findings, another recent study employed a pharmacogenetic approach and found that 1‐week simulation of a subset of somatosensory cortex neurons during early postnatal weeks significantly increased the number of proliferating OPCs in the corpus callosum of both juvenile and adult mice (Mitew et al. 2018). These studies not only provide further evidence supporting activity‐dependent OPC proliferation but indicate that enhancing synchronized neural activity even in a relatively small number of axons can lead to significant OPC production and/or survival. Interestingly, the same laboratory also found that attenuating neuronal activity of a subset of callosal axons exerted no effect upon OPC proliferation although it was sufficient to suppress the extent of myelination of the silenced axons (Mitew et al. 2018).
It is difficult to determine whether neuronal activity directly drives OPC proliferation in vivo or whether an altered proliferation is partially secondary to increased differentiation. It has recently been shown that providing mice with a complex motor learning task (running on a complex wheel with variably spaced rungs) has led to a rapid increase in oligodendrocyte production (McKenzie et al. 2014; Xiao et al. 2016). This increased oligodendrogenesis could be partially because of accelerated cell differentiation. It has long been known that OPC proliferation involves growth factors such as platelet‐derived growth factor (PDGF) AA, which increases OPC proliferation in vitro and in vivo. Exogenous PDGF AA was able to rescue the reduced OPC proliferation as a result of blocked neuronal activity (Barres and Raff 1993), supporting activity‐dependent proliferation. PDGF AA signalling has been shown to up‐regulate the expression of delayed outward‐rectifying potassium channels (K KDR) that are exclusively expressed in OPCs (Barres et al. 1990; Chittajallu et al. 2005; Larson et al. 2016) and these potassium channels are closed linked to cell cycle regulation and OPC proliferation (Barres et al. 1990; Chittajallu et al. 2002, 2005), whereas glutamate released from active neurons, acting on α‐amino‐3‐hydroxy‐5‐methylisoxazole‐4‐propionate (AMPA)/kainate receptors, inhibits K KDR channels, thus reducing proliferation (Borges et al. 1994; Chittajallu et al. 2005). It is possible that active axons somehow release signals that stimulate OPC proliferation and then these newly generated OPCs are to differentiate and specifically myelinate the active axons that have simulated their production.
Neurons form structural and functional synaptic connections with OPCs (Fig. 2a). OPCs can receive synaptic input mediated by neurotransmitters such as glutamate and GABA (Bergles et al. 2000; Clarke et al. 2012; Ge et al. 2009; Karadottir et al. 2005, 2008; Kolodziejczyk et al. 2010; Kukley et al. 2007; Lin and Bergles 2004; Osterstock et al. 2018; Ziskin et al. 2007; Zonouzi et al. 2015), suggesting that these neurotransmitters may also control oligodendrocyte development and myelination. Both glutamate and GABA receptors are present throughout the oligodendroglial cell lineage (Hamilton et al. 2017; Spitzer et al. 2016), suggesting that these cells are well equipped to sense neuronal activity. Glutamate is the most common excitatory neurotransmitter in the CNS and can be released from axons via synaptic and non‐synaptic, vesicular and non‐vesicular based mechanisms (Spitzer et al. 2016; Wake et al. 2015). Analysis of cultured OPCs has shown that activating glutamatergic signalling via AMPA/kainate receptors inhibits OPC proliferation (Gallo et al. 1996). Concordant with this in vitro finding, ex vivo studies using cultured brain slices found similar results (Fannon et al. 2015; Yuan et al. 1998). Similarly, early sensory deprivation via removing whiskers in mice at birth (possibly resulting in a loss of sensory input to the barrel cortex) increased the proliferation of NG2+ OPCs at P6, suggesting that glutamatergic signalling from thalamocortical axons acted to inhibit proliferation in vivo (Mangin et al. 2012). On the other hand, the role of GABA, the most common inhibitory neurotransmitter in the CNS, on OPC proliferation is less clear. GABA is excitatory in OPCs (as OPCs have high intracellular chloride concentration) but has been shown to exert little effect on OPC development in vitro (Gallo et al. 1996; Yuan et al. 1998). However, a reduction in GABAA receptor‐mediated signalling to OPCs increased their proliferation during hypoxia (Zonouzi et al. 2015). Furthermore, a myelination assay using organotypic cortical slices has shown that endogenous GABA release decreased OPC proliferation (Hamilton et al. 2017), indicating an inhibitory effect. The contradicting influence that glutamate and GABA exert upon OPCs is interesting and may relate to the neuronal firing pattern that governs the release of neurotransmitters. The fact that OPCs have synapses and express an array of neurotransmitter receptors makes them equipped to interpret and differentially respond to distinct activity patterns, as both the firing rate of the neuron and the kinetics of receptors activated will determine different downstream signalling and potential outcome (Karadottir and Kuo 2018).
Figure 2.
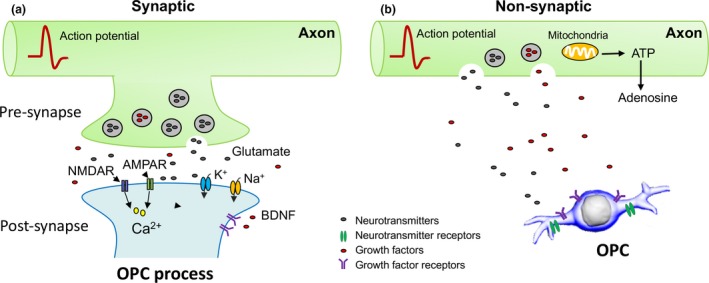
Neuronal depolarization traverses action potentials along axons, resulting in an activity‐dependent vesicular release and activity‐dependent non‐vesicular release. (a) This enhances the release of neurotransmitters such as glutamate and the fusion of synaptic vesicles into the periaxonal space, which subsequently activates neurotransmitter receptors such as glutamate receptors [α‐amino‐3‐hydroxy‐5‐methylisoxazole‐4‐propionate receptors (AMPARs) and NMDA receptors (NMDARs)] expressed on the processes of oligodendrocyte precursor cells (OPCs) or oligodendrocytes, promoting influx of Ca2+ into the cytoplasm. (b) Alternatively, active axons can also signal OPCs via non‐synaptic vascular release of growth factors [e.g. platelet‐derived growth factor (PDGF) AA and neurotrophins] and neurotransmitters (e.g. glutamate, GABA or ATP). OPCs express not only ion channels including glutamate‐activated ion channels, the sodium and potassium channels, but also receptors of growth factors. These cellular properties make OPCs equipped to respond to neuronal activity.
Neuronal activity enhances oligodendrocyte survival
A few studies have demonstrated that the axon is vital to oligodendrocyte survival during development in vitro and in vivo (Barres et al. 1992, 1993a, 1994). It is possible that the aforementioned activity‐dependent proliferative effect results from increased cell survival. Indeed, axonal electrical activity controls the production and/or release of trophic factors such as PDGF AA, which ultimately controls the number of oligodendroglial lineage cells that are produced locally (Barres and Raff 1993; Barres et al. 1992, 1993a). This activity‐dependent survival effect is further supported by another in vitro study in which electrical stimulation to mixed cortical cultures promoted oligodendroglial survival, an effect blocked by TTX (Gary et al. 2012). Interestingly, electrical stimulation failed to alter cell survival in the absence of neurons in vitro (Gary et al. 2012), suggesting that a neuron–OPC interaction is critical to this activity‐dependent survival effect. Oligodendroglial lineage cells including OPCs and differentiated post‐mitotic oligodendrocytes can survival in vitro in the absence of neurons through experimentally providing exogenous growth factors such as PDGF AA, neurotrophins (e.g. Neurotrophin‐3), insulin‐like growth factors or ciliary neurotrophic factor (Barres et al. 1993b), and these factors are readily produced by axons as well as other cellular sources. Neuronal activity can regulate oligodendroglial survival via vesicular (Fig. 2) and non‐vesicular release of growth factors.
Furthermore, synaptic transmission from neurons to OPCs is another potential mechanism that regulates OPC survival. Inhibiting AMPA receptors via deleting GluA2 and GluA3 in early lineage‐stage oligodendroglial cells over the course of early postnatal development in vivo exerted no influence upon OPC proliferation as oligodendroglial cells number were unchanged, but led to reduced survival of pre‐myelinating oligodendrocytes in the subcortical white matter (Kougioumtzidou et al. 2017). In triple knockouts in which all three subunits GluA2/3/4 were knocked out in OPCs, this shortfall persisted into adulthood, which has ultimately resulted in ~ 20% fewer myelin (Kougioumtzidou et al. 2017), suggesting a pro‐survival role of glutamatergic signalling towards OPCs without altering proliferation and subsequent differentiation, and that activity‐dependent oligodendroglial survival and proliferation are mediated by different mechanisms. On the other hand, GABAergic signalling has been reported to increase oligodendroglial death (Hamilton et al. 2017). These studies raise a few interesting questions as to how neuronal activity control oligodendrocyte survival via axoglial synapses and homeostatic regulation of neurotransmission, and whether this is a stage specific influence during CNS development.
Perhaps unsurprisingly, oligodendrocyte survival rates are regionally different. Genetically labelling oligodendrocytes and tracking their subsequent survival has shown that the half‐life (t1/2) of myelinating oligodendrocytes in the optic nerve is likely to be approximately 2 years which contrast with being over 10 years in the corpus callosum (Tripathi et al. 2017). Although this study labelled oligodendrocytes in the young adult mice, it raises an interesting question as to whether axons in these white matter tracts differentially influence oligodendrocyte survival and whether axonal activity of different neural circuits exerts regionally dependent survival effects. In the optic nerve (mouse), most of the axons are myelinated by 4 months of age (Dangata and Kaufman 1997), whereas, in the corpus callosum, there are approximately 30% of axons being myelinated by 6 months of age (Robain and Mandel 1974; Sturrock 1980). Thus, the degree of oligodendrocyte survival may be dependent upon the developmental stage of axons available for myelination and the trophic factors released from these axons in response to altered neural activity. The number of unmyelinated axons (axons available for myelination) may provide a positive feedback signal to enhance the survival of local oligodendroglial cells, whereas the number of myelinated axons may send a negative feedback signal to inhibit the survival (Ueda et al. 1999). Neuron‐OPC synaptic contacts (at least the glutamatergic synapses) appear to be present predominantly on unmyelinated axons or unmyelinated axonal segments (Kukley et al. 2007; Tomassy et al. 2014; Ziskin et al. 2007), providing structural evidence to support the hypothesis that the number of myelinated axons somehow dictates the production/survival of oligodendroglial cells.
Neuronal activity potentiates oligodendrocyte differentiation
During early postnatal development, the brain is particularly responsive to activity‐dependent stimulation. It would be logical to speculate that oligodendrocyte adjusts its differentiation according to neuronal development, and in turn axon‐derived signals play important roles in potentiating the transformation of OPCs into post‐mitotic mature oligodendrocytes ready to produce myelin. However, unlike in the peripheral nervous system, in which neuregulin‐1 on the axonal membrane determines the myelinating fate of Schwann cells (Birchmeier and Nave 2008; Nave and Salzer 2006; Taveggia et al. 2005), an equivalent instructive axonal signal that triggers oligodendrocyte differentiation towards myelination has yet been identified in the CNS. Indeed, in the developing CNS, there appears to be a series of neuronal signals that are inhibitory to the differentiation of OPCs towards mature myelinating cells (Emery 2010). Therefore, the question remains whether the neuron plays an active role in promoting oligodendrocyte differentiation.
Recent studies have demonstrated that enhanced neuronal activity increases not only normal oligodendrocyte production but also differentiation during development (Gibson et al. 2014; Mitew et al. 2018). Optogenetic stimulation of the premotor cortex enhances oligodendrogenesis in both deep cortical layers and subcortical white matter regions. A rapid response of oligodendroglial proliferation was observed within 3 h of stimulation, and 4 weeks after a 7‐day stimulation paradigm a subset of oligodendrocytes (equating to approximately 20% of the total initial spike) integrated into mature cells (Gibson et al. 2014). Concordant with this finding, a 7‐day pharmacogenomic activation of a subset of cortical somatosensory neurons almost doubled the production of differentiated oligodendrocytes in the underlying white matter tract corpus callosum, resulting in a 20% increase in the total density of mature oligodendrocytes (Mitew et al. 2018). While neuronal activity‐induced oligodendrocyte differentiation could be secondary to a proliferative response of OPCs, it is plausible that neuronal activity promotes OPCs (newly generated and/or residential) to directly differentiate into post‐mitotic oligodendrocyte without prior cell division. This is supported by two recent studies in which providing mice with a complex motor learning task (complex wheel running) rapidly increased the generation of pre‐myelinating (Enpp6+) oligodendrocytes (McKenzie et al. 2014; Xiao et al. 2016) and only a small proportion of OPC differentiation is directly preceded by proliferation (Xiao et al. 2016). Through genetically tracing newly differentiated oligodendrocyte, Mitew et al. (2018) have shown that enhanced neuronal activity resulted in an approximately twofold increase in the rate of oligodendrocyte differentiation, an effect possibly incorporating the contribution of both OPC proliferation as well as direct differentiation of these cells into mature myelinating oligodendrocyte. Thus, these findings collectively indicate that stimulating neuronal activity could directly potentiate the differentiation of newly generated OPCs without prior cell proliferation to mature myelinating oligodendrocytes.
The importance of neuronal activity in ensuring normal oligodendrocyte maturation has also been demonstrated by situations of neuronal inhibition. Socially isolating mice upon weening resulted in irreversible deficits to late‐stage oligodendroglial generation and subsequent myelination of the prefrontal cortex (Liu et al. 2012, 2016; Makinodan et al. 2012). In this study, oligodendrocytes proliferate normally, however failed to develop morphological complexity and made approximately half the normal number of processes and internodes. A similar deficit was induced in the somatosensory cortex upon sensory deprivation via whisker removal (Hill et al. 2014), which resulted in a reduced density of mature cells and an increased density of apoptotic progenitors. Somewhat in contrast to this, or a demonstration of differential white matter versus grey matter oligodendroglial regulation, blocking visual input to the optic nerve upon eye‐opening resulted in excessive oligodendrocyte maturation (Etxeberria et al. 2016) and abnormal myelination pattern as evident by increased density of paranodes with shorter internodes (Etxeberria et al. 2016), in line with the hypothesis that activity fine‐tunes myelination for precise neurotransmission or may reflect that OPCs differentiation depends on certain frequency of firing or firing rate, as Schwann cells differentiation and myelination in culture can either be reduced or enhanced with different stimulation frequencies (Stevens et al. 1998).
A few recent studies have attempted to elucidate potential mechanisms underpinning the activity‐dependent effects on oligodendroglial differentiation. It is known that OPCs respond to glutamate through AMPA/Kainate and NMDA receptors, with receptor density being down‐regulated during oligodendrocyte differentiation (Kukley et al. 2010). The neuronal action potential‐stimulated release of vesicular glutamate onto OPCs has been shown to induce glial excitatory post‐synaptic currents mediated via AMPA/Kainate receptors (Nagy et al. 2017). This study also suggests that the specific pattern of neuronal activity could differentially regulate glutamate release, which in turn could differentially influence the post‐synaptic current within OPCs, further supporting the hypothesis that neuronal activity fine‐tunes myelination for precise neurotransmission. Furthermore, new evidences have revealed that neuron–OPC interactions are more complex than expected, and there exit non‐synaptic mechanisms of communication between the two cell types. The activation of non‐synaptic receptors by ambient neurotransmitters or local spillover and the ability of OPCs to sense neuronal activity through a potassium channel suggest that distinct modes of communication mediate different functions of OPCs (Goebbels et al. 2017; Karadottir and Kuo 2018; Maldonado and Angulo 2015; Spitzer et al. 2016). Alternatively, axons can release factors to instruct OPC to exit the cell cycle (Stevens et al. 2002). Increasing axonal activity leads to the release of ATP, which is then converted to adenosine that inhibits OPC proliferation and promotes oligodendrocyte differentiation (Stevens et al. 2002). In culture, ATP released by axons can indirectly signal to oligodendrocytes via stimulating adjacent astrocytes to release the promyelination cytokine such as LIF (Ishibashi et al. 2006). In addition, contact‐mediated replacement may be another indirect mechanism that explains increased proliferation to replace cells from those in an existing pool that differentiate into new oligodendrocytes to meet acute demand (Hughes et al. 2013; Xiao et al. 2016). Importantly, a contact‐mediated mechanism may also explain why increased OPC proliferation may occur to maintain density in conditions where sensory deprivation (presumably decreased activity) leads to excessive death of immature oligodendrocytes (Hill et al. 2014).
Neuronal activity drives the extent of myelin formation
One oligodendrocyte is able to generate approximately 50 myelinating processes with intermodal lengths ranging between 20 and 200 μm (Hildebrand et al. 1993; Matthews and Duncan 1971). The roles of neuronal activity in driving the late stages of myelination such as the initiation of myelination and axonal ensheathment by myelin membranes have been demonstrated both in vitro and in vivo. One study in the late 1990s has shown that blocking axonal activity in the myelinating co‐cultures via TTX significantly reduced the formation of myelinated axonal segments, demonstrating that diminishing neural activity reduces the myelinating capacity of oligodendrocytes in vitro (Demerens et al. 1996). This is also supported in vivo, where intraocular injections of TTX in the developing optic nerve prior to the onset of myelination significantly reduced the number of myelinating, but not pre‐myelinating, MBP+ mature oligodendrocytes (Demerens et al. 1996). This is further supported by Bruce Trapp and colleagues, who found oligodendrocytes can still survive following axotomy of the neonatal optic nerve, however they only produce fewer and shorter processes, and ultimately fail to form myelin in the transected nerves (Ueda et al. 1999). Results of these studies together suggest that the presence of viable axonal signals not only initiates but enhances the myelinating capacity of oligodendrocytes without influencing the total number of cells, indicating a powerful role of neuronally derived signals in determining the extent of myelin formation by oligodendrocytes ensheathing the axon.
Recently a few elegant studies have made powerful use of the real‐time imaging in the developing zebrafish model to further interrogate the effects of axonal activity on the myelinating capacity of oligodendrocytes (Czopka et al. 2013; Hines et al. 2015; Mensch et al. 2015), in particular the number and length of myelin sheaths surrounding the axon (Baraban et al. 2018; Hines et al. 2015; Koudelka et al. 2016; Mensch et al. 2015). The study by David Lyons's laboratory (Mensch et al. 2015) has found that synaptic vesicle release significantly influenced the number of myelin sheaths formed by individual oligodendrocytes within a short period of formation (only a few hours in the zebrafish; Czopka et al. 2013). Furthermore, Bruce Appel's laboratory has demonstrated when single axons were silenced oligodendrocytes preferentially ensheathed neighbouring axons (Hines et al. 2015), suggesting an axonal selection in oligodendrocyte myelination. Concordant with these findings, analysis of the mammalian brain has demonstrated that electrically stimulated axons were ‘favourably’ myelinated by the newly generated oligodendrocytes in vivo (Mitew et al. 2018) and in vitro (Wake et al. 2015). Furthermore, nascent myelin sheaths formed on electrically silenced axons were shorter in length than controls, suggesting that axonal activity regulates the longitudinal growth of myelin sheath (Hines et al. 2015). What is more interesting, however, is that axonal ‘competition’ may exits in surrounding axons as silencing a single axon, but not all axons, impedes nascent myelin formation (Hines et al. 2015). Together, these studies indicate that neuronal activity is likely to not only influence axon selection for myelination but also powerfully control the extent of myelination by individual oligodendrocytes ensheathing the axon.
The above animal‐based work is also supported by human studies that early life experience can affect myelin microstructure and associated functions. Using diffusion tensor neuroimaging (a measure of water diffusivity in diffusion‐weighted imaging that correlates with myelination, fiber density, and axonal diameter), it has been shown that the extent of piano practice during development (childhood and adolescence) exert positive influence upon myelinating tracts of multiple brain regions such as subcortical fibres in the frontal lobe and the corpus callosum (Bengtsson et al. 2005). In addition, myelination of the pyramidal tract was found to be more structured in pianists than in non‐musicians (Bengtsson et al. 2005). Conversely, a longitudinal study of over 260 families suggests that early childhood traumatic experience such as abuse or neglect may exert detrimental influence upon cognitive function later in life (Egeland et al. 1983). Similarly, children aged between 8 and 10 years who were poor readers display hypomyelination of cerebral white matter (left anterior centrum semiovale) compared to good readers, however, this white matter structure difference can be partially rescued after remediation in reading skills (Keller and Just 2009). Thus, these findings suggest that experience associated with critical developmental periods may exert dramatic influence upon the plasticity of human myelinating tracts and associated functions, and that myelination is a dynamic process driven by experience.
Then, the questions remain how neuronal activity controls the dynamic process of myelinogenesis. Several recent studies have suggested vesicular release as one key mechanism through which neuronal activity regulates the extent of myelination (Fig. 2) (Hines et al. 2015; Koudelka et al. 2016; Mensch et al. 2015; Wake et al. 2011, 2015). Action potential firing by electrically active axons in culture could result in glutamate release from synaptic vesicles, promoting early events that regulate myelin formation such as local myelin protein synthesis (Wake et al. 2011), proposing a scenario that neuronal activation traverses action potentials along axons, resulting in an activity‐dependent vesicular (synaptic or non‐synaptic) release of neurotransmitters such as glutamate which subsequently activates their receptors such as AMPA receptors and NMDARs expressed on the processes of OPCs, promoting influx of Ca2+ into the cytoplasm and initiating intracellular pathways of myelinogenesis (Fig. 2). Indeed, the intracellular Ca2+ has recently been shown to mediate the dynamics of myelin formation such as elongation in response to neuronal activity (Baraban et al. 2018; Krasnow et al. 2018). Electrical stimulation of axons rapidly increases Ca2+ in OPCs processes in culture, which is inhibited by blocking vesicular release using botulinum A (Wake et al. 2015). In vivo live imaging in zebrafish has shown that local Ca2+ signalling within oligodendrocytes influences their capacity of myelin elongation, and that higher frequency Ca2+ transient activity in myelin sheaths precedes faster elongation (Baraban et al. 2018). Complementary to this finding, a study has demonstrated that neuronal activity evokes Ca2+ transients within the developing oligodendrocytes and myelin, and that Ca2+ transients correlates with myelin sheath lengthening (Krasnow et al. 2018). Myelin sheath elongation occurs rapidly (around 1 h) after Ca2+ elevation within myelinating oligodendrocytes, and likewise its shortening is associated with a low frequency of Ca2+ transients (Krasnow et al. 2018). Thus, the aforementioned studies together indicate a neurotransmission pathway from axons to OPCs to induce local Ca2+ signalling, underpinning the activity‐dependent myelinogenesis. In the context of ischaemia, NMDA glutamate receptors has been shown to drive Ca2+ increase in myelin (Micu et al. 2006), suggesting axo‐myelinic neurotransmission could be another potential new mode of cell signalling that regulates activity‐induced myelination in the CNS (Micu et al. 2018).
Myelin plasticity in the adult CNS
The early intriguing idea that neuronal activity could influence the behaviour of oligodendroglial lineage cells during early development (Barres and Raff 1993; Gyllensten and Malmfors 1963; Tauber et al. 1980) was further stoked by fascinating studies associating changes myelin microstructure with experience in the adult. For example, social isolation of young adult mice resulted in myelin thinning in the prefrontal cortex, a region important for social function (Liu et al. 2012; Makinodan et al. 2012). Humans who learn a new complex motor tasks exhibited alterations to MRI‐based measures of myelin microstructure in regions involved in hand‐eye coordination (Scholz et al. 2009). Furthermore, after repeated training in a complex body balance task for several weeks, adult human participants exhibited increased myelin microstructure in the frontal subcortical white matter, assessed by fractional anisotropy (Taubert et al. 2010). It is noteworthy that this complex training also led to a volume increase in multiple grey matter regions such as the prefrontal cortex (Taubert et al. 2010), and there is a continuous increase in these white and grey matter changes during a 6‐week training period, indicating a dose‐responsive plasticity effect (Taubert et al. 2010). Thus, both rodent and human studies suggest that environmental alterations and experience influences myelin plasticity in white and grey matters of the adult CNS.
The experimental tools of modern neuroscience have enabled the generation of direct evidence for neuronal activity regulation of oligodendroglial lineage cell function in adult CNS. Optogenetic stimulation of excitatory projection neurons in the premotor cortex demonstrates that neuronal activity elicits rapid, robust and circuit‐specific OPC proliferation, promotes oligodendrogenesis and increases myelin sheath thickness within the projections of the premotor circuit. Neuronal activity was found in this study to regulate oligodendrogenesis similarly in juvenile and in adult mice (Gibson et al. 2014). Neuronal activity‐regulated oligodendrogenesis was associated with improved motor function, and oligodendrogenesis was necessary for the observed functional improvement (Gibson et al. 2014). Similarly, chemogenetic stimulation or inhibition of somatosensory cortex activity regulates somatosensory projection neuron myelination (Mitew et al. 2018). Chemogenetic strategies for regulating parvalbumin‐positive interneuron activity in the medial prefrontal cortex similarly demonstrated that inhibitory interneuron myelination is regulated by activity in adulthood (Stedehouder et al. 2018). Topographical analysis revealed that myelination of axons after stimulation is accompanied by higher branch orders paralleled by an increase in axonal arborization (Stedehouder et al. 2018), suggesting axonal structure may also underpin activity‐dependent myelin plasticity. Nevertheless, finding of this study adds a layer of complexity to that ways that myelin alterations could influence neural circuit function. The proliferative response of OPCs to neuronal stimulation appears to be more protracted in the adult mice than in juvenile mice, suggesting that there is an additional homeostatic regulation of OPC behaviour in response to neuronal activity in the adult CNS (Mitew et al. 2018).
To what extent myelin plasticity contributes to forms of learning in the adult CNS is largely unknown. An important set of experiments by the Richardson lab has provided the first evidence that adaptive oligodendrogenesis contributes to cognitive function (McKenzie et al. 2014). Training mice to perform a complex motor task was found to elicit OPC proliferation and oligodendrogenesis (McKenzie et al. 2014) similar to that observed with the abovementioned optogenetic stimulation of premotor cortex (Gibson et al. 2014), a region involved in motor planning. New oligodendrocyte production proved to be necessary for acquisition of the complex motor task (Gibson et al. 2014; Xiao et al. 2016). These experiments illustrate a role for activity‐regulated oligodendrogenesis in motor learning and underscore the possible role that activity‐regulated changes in myelin‐forming cells could play in other forms of learning, indicating that ongoing myelination regulates aspects of learning in the adult CNS. We still have limited knowledge of how new oligodendrocytes integrate into the pre‐existing myelinated circuits, and whether learning complex skills require new circuits brought to play.
How might changes to myelin microstructure, and resultant alterations in conduction velocity, alter neural circuit function and influence behaviour? Much remains to be learned about how myelin plasticity may alter neural circuit dynamics, and different rules may be operant in different circuits. For example, myelin plasticity‐regulated changes in spike time arrival could influence spike‐time‐dependent synaptic plasticity, while in other circuits the first impulses to arrive may be selected to exert dominant influence over downstream neuronal activity. This is a fertile area for further research by computational neurobiologists.
A number of mechanistic and conceptual questions remain, including the molecular mechanisms that mediate activity‐regulated communication between neurons and oligodendroglial lineage cells and the manner in which newly generated oligodendrocytes contribute to myelination. Do the new oligodendrocytes replace older internodes, wrap previously unmyelinated axons or wrap unmyelinated regions on axons exhibiting a variable internode length and pattern (Tomassy et al. 2014)? An obvious question is what the cellular source of new myelin is in the adult CNS. In the adult CNS, there is an ongoing OPC differentiation into myelinating oligodendrocytes (Hill et al. 2018; Hughes et al. 2018; Young et al. 2013), indicating that adult myelination/maintenance could be generated by newly differentiated oligodendrocytes from the existing pool. However, it is unknown whether these newly differentiated cells exhibit sufficient plasticity to respond to neuronal activity and generate new myelin segments (myelin remodelling) and/or replace existing ones (myelin turnover). The degree to which this ongoing differentiation and myelin formation is activity dependent is unknown. A recent series of studies indicate that oligodendrocytes are generated throughout life (Young et al. 2013) and are very stable in the healthy brain of mice until late adulthood (Hill et al. 2018; Hughes et al. 2018; Tripathi et al. 2017). This stability of oligodendrocytes throughout life argues against a cellular turnover mechanism of new myelin incorporation, although replacement or remodelling of individual internodes remains a possibility concordant with the co‐existence of older and newer oligodendrocytes. Two‐photon imaging through a chronic cranial window reveals that newly generated oligodendrocytes myelinate previously unmyelinated axonal territory in the superficial somatosensory cortex, and that this increases with sensory stimulation (Hughes et al. 2018). Thus, regions of the nervous system that exhibit incomplete myelination well into adulthood, such as the cerebral cortex and intercortical association fibres, accumulate increasing amounts of myelin throughout adulthood, at least in the healthy brain and in the absence of sensory deprivation. Whether adult myelin plasticity similarly exists in regions that are more completely myelinated during development, such as the optic nerve and the spinal cord, remains to be determined.
Activity‐dependent remyelination after injury
Myelin regeneration or remyelination is the endogenous regenerative mechanism by which myelin is restored to demyelinated axons, resulting in the alleviation of neurological symptoms that are associated with many neurological disorders, in particular demyelinating diseases such as multiple sclerosis (MS). The degree of myelin repair within MS lesions is variable; generally MS lesions remyelinate relatively efficiently early on in the disease; however, at later stages many lesions remain chronically demyelinated (Irvine and Blakemore 2008). Following a demyelinating insult, endogenous adult OPCs respond to injury and migrate into the lesion where they proliferate to repopulate the lesion and differentiate into myelinating oligodendrocytes (Franklin and Ffrench‐Constant 2008). However, this initial remyelinating process ultimately fails, mainly because of ‘stalled’ OPC differentiation, leaving sustained clinical symptoms (Franklin et al. 2012). Indeed, Gautier et al. (2015) found an increase in the number of OPCs accompanied by reduced differentiation in the induced rodent myelin lesion (Gautier et al. 2015), a scenario that resembles that of chronically demyelinated lesions in MS where there is a differentiation block (Wolswijk 1998, 2000). Persistent demyelination followed by failure of remyelination ultimately results in axonal damage and neuronal loss, leading to progressive clinical disabilities (Irvine and Blakemore 2008). Thus, understanding the regenerative process, and the regulation of OPC differentiation, is of great therapeutic potential, and as currently, no remyelinating therapies are available for demyelinating diseases.
The fact that neuronal activity regulates myelinogenesis in the mature CNS suggests the possibility that remyelination might also be activity dependent. However, that would require that demyelinated axons retain their capacity to conduct action potentials. While the axonal conduction block has been reported after myelin injury (McDonald and Sears 1969; Pender 1988; Smith et al. 1979), it is clear that not all demyelinated axons become electrically silent in lesions and that some axons can in fact conduct following demyelination (Felts et al. 1997), or become spontaneously active within myelin lesions (Smith and McDonald 1982). Furthermore, it has been argued that, in some demyelinated MS lesions, the demyelinated axons are capable of conducting action potentials (Ghatak et al. 1974; Phadke and Best 1983). These findings are supported by the analyses of rodent models of central demyelination in which axon integrity is preserved and demyelinated axons are able to conduct action potentials, albeit at an expected reduced velocity (Etxeberria et al. 2010; Gautier et al. 2015; Sahel et al. 2015). Thus, the human and rodent studies collectively suggest that demyelination itself does not necessarily result in a blockage of nerve conduction and that there may exist a time window when axons are still healthy and electrically active in myelin lesions. Axonal conduction along demyelinated axons is likely made possible by a switch in voltage‐gated Na+ channel subunit expression and distribution, from focal to diffuse, as has been observed in experimental allergic encephalomyelitis and MS (Craner et al. 2003, 2004; Moll et al. 1991)
Recent evidence has shown that OPCs within the myelin lesions express glutamate receptors and receive synaptic inputs (Etxeberria et al. 2010; Gautier et al. 2015; Sahel et al. 2015). Thus, like in development, OPCs within the myelin lesions are capable of sensing neuronal activity. Whether these inputs are from demyelinated axon or previously unmyelinated axons that synapse with these OPCs in lesions can only be tested in a white matter tract where all axons are myelinated in the adult. There are a few white matter tracts that fulfil these criteria, as a mixture of myelinated and unmyelinated axons are found in most white matter areas such as the caudal cerebellar peduncle. When inducing demyelination in the caudal cerebellar peduncle, synaptic input is detected in OPCs, indicating that indeed demyelinated axons generate de novo synapses with OPCs (Gautier et al. 2015). Furthermore, demyelinated axons up‐regulate presynaptic proteins in both the animal model of demyelination and human MS lesions (Etxeberria et al. 2010; Gautier et al. 2015). The OPCs recruited to demyelinating lesions sense glutamate release primarily by AMPA/kainate receptors at the time of highest proliferation and, while at a later stage just prior to differentiation, these cells start to sense glutamate release and also via NMDA receptors (Gautier et al. 2015). Neuronal activity, vesicular release of glutamate, AMPA/kainate or NMDA receptors seems to play important roles in promoting remyelination, as when any of these mechanisms are inhibited locally within demyelinating lesions, remyelination is severely perturbed (Gautier et al. 2015; Li et al. 2013; Lundgaard et al. 2013). A functional communication between axons and OPCs is potentially required for a successful remyelination.
The time course of glutamate receptors (AMPA and NMDA) expression in OPCs in demyelinated lesions is in line with their roles during developmental myelination: AMPA receptors are seemingly important in the early phase of OPC proliferation, survival and initial differentiation, whilst NMDA receptors are expressed at a late stage of oligodendroglial lineage progression and required for terminal differentiation and axonal ensheathment by myelin membranes (Gautier et al. 2015; Li et al. 2013; Lundgaard et al. 2013). Furthermore, the effects of NMDA receptor activation on remyelination appear to be mediated via the Akt/mTOR pathway (Li et al. 2013; Lundgaard et al. 2013) and as in development (Mensch et al. 2015) it is predominantly the remyelination of small diameter axons that depends on neuronal activity and glutamate. Larger calibre axons still become myelinated when vesicular release of neurotransmitters is blocked (Mensch et al. 2015) and are also remyelinated even when activity or synaptic signalling are inhibited (Gautier et al. 2015). Therefore, neuronal activity is likely to regulate remyelination by via a glutamate‐mediated signalling between axons and OPCs. Targeting of this mechanism may be developed into new remyelinating strategies and, in fact, drug discovery studies have now identified several compounds that promote OPC differentiation and remyelination which modulate neurotransmitter signalling in OPCs (Abiraman et al. 2015; Deshmukh et al. 2013; Mei et al. 2014, 2016; Najm et al. 2015).
A switch between activity‐independent and activity‐dependent myelination?
While neuronal activity clearly influences oligodendroglial lineage progression and their capacity of myelination, myelination can also occur independent of axonal activity, at least in vitro (Bechler et al. 2015; Lee et al. 2012, 2013; Li et al. 2014). Oligodendroglial cells can survive, proliferate and differentiate in vitro in the absence of neurons (Barres et al. 1993b). Recently, oligodendrocyte myelination including oligodendroglial‐axon contact, myelin wrapping and compaction have also been achieved in vitro using electrically silent artificial nanofibers (Bechler et al. 2015; Lee et al. 2012, 2013), suggesting that there exit intrinsic oligodendrocyte‐driven mechanisms that regulate fibre‐glial contact and myelinogenesis. Although there is currently no direct evidence demonstrating that axonal calibre alone dictates the myelinating fate of central neurons, genetically increasing the axonal diameter of the cerebellar parallel fibres, and concomitantly change a number of important signalling molecules, induces myelination on these normally unmyelinated axons (Goebbels et al. 2017). Analysis of myelinating cultures using engineered nanofibers have shown the size of fibre diameter exerts a direct impact upon the number of fibres being ensheathed by oligodendrocytes and their subsequent myelinogenesis (Lee et al. 2012), suggesting that oligodendrocytes display some level of sensitivity to the biophysical properties of fibres themselves and that this is a myelinating progress dependent upon fibre calibre but not axonal electrical activity. This is further supported by Bechler et al. (2015) that, in the absence of axonal molecules, oligodendrocytes that originate from different CNS regions exhibit diverse capacities in myelin sheath elongation with spinal cord oligodendrocytes generating longer myelin sheaths than the cortical ones. Thus, oligodendrocytes have a remarkable intrinsic capacity to initiate and generate myelin independent of axonal electrical activity, although biophysical properties of fibres such as axonal calibre still influence the extent of myelinogenesis.
A prevailing hypothesis is that two modes of myelination (activity‐dependent and activity‐independent) may exist simultaneously in vivo (Koudelka et al. 2016) and in vitro (Li et al. 2013; Lundgaard et al. 2013). When levels of the growth factors such as neuregulin (NRGs) or brain‐derived neurotrophic factor (BDNF) are elevated, presumably by release from active neurons (Esper and Loeb 2009; Greenberg et al. 2009; Matsuda et al. 2009), OPCs switch to myelinating mode via an activity‐dependent mechanism (Lundgaard et al. 2013), probably because NMDAR activation increases the energy supply to developing oligodendrocytes (Krasnow and Attwell 2016; Saab et al. 2016). However, the relative importance of each myelination mode is still unclear. Indeed, BDNF itself has long been known to regulate activity‐dependent neuronal functions such as synaptic plasticity (Huang and Reichardt 2001; Lu 2003). Neuronal activity can trigger BDNF release from axons and dendrites (Matsuda et al. 2009). BDNF itself plays a critical role in the myelinating process, in particular myelin formation and axonal ensheathment, presumably via signalling to the TrkB/Erk signalling pathway (Ishii et al. 2012, 2013, 2014; Nicholson et al. 2018; Wong et al. 2013; Xiao et al. 2010, 2012), suggesting it as a candidate mediating activity‐dependent myelin formation. In addition, NRGs are predominantly expressed by neurons during CNS development. Interestingly, blocking NRG1 signalling in oligodendrocyte lineage cells via ablating its receptor ErbB3 exerts litter effect upon myelination during normal development (Brinkmann et al. 2008), but significantly disrupts experience‐induced myelination (Makinodan et al. 2012). That said, forced over‐expression of NRG‐1 is able to enhance axonal ensheathement (Brinkmann et al. 2008), leaving the possibility that NRG signalling plays at least some roles within the CNS. Conceivably activity‐independent and ‐dependent myelination may occur within the same or different neural circuits. The relative roles of the two modes of myelination are still largely unknown. It is possible that an activity‐independent intrinsic pathway may pre‐establish an initial pattern of oligodendrocyte myelination which is then controlled and modified by activity‐dependent cues to meet neural circuit development and functions. The activity‐dependent myelination might have evolved in order to accelerate the myelinating process along the ‘correctly’ firing active axons during periods of learning, and thus may be important to fine‐tuned neuronal circuits.
Conclusion and future perspectives
In summary, myelination can proceed via axonal‐dependent and ‐independent mechanisms. Neuronal activity clearly exerts multifaceted influence upon the myelinating process, ranging from oligodendroglial cells proliferation/survival, differentiation through to myelin formation. It is unclear which stage(s) of the myelinating process are most susceptible to neuronal activity or experience. The sequential cellular events of CNS myelination could be regulated via activity‐dependent synaptic and non‐synaptic vesicular release as well as non‐vesicular release. Synaptic contacts between the processes of neurons and oligodendrocytes may regulate vesicle release functions and that such synapses may control the activity‐dependent myelinating process including oligodendroglial cells proliferation, the stabilization of axon–oligodendrocyte contacts or their subsequent myelin sheath elongation. It remains unclear whether this functional synapse only occurs between electrically ‘active’ axons and the processes of oligodendrocytes. Alternatively, activity‐regulated axonal release could locally influence individual oligodendrocytes in a non‐synaptic manner, regulating their lineage progression and overall myelinating capacity such as myelinate axons that are previously unmyelinated or incompletely myelinated. In the CNS, activity‐dependent myelination occurs not only during normal development when myelination is most active but also into late adulthood when the myelinating rate slows down. It is unknown whether similar or differential mechanisms underpins activity‐dependent myelination during development, in adult and after injury. The technological advance in neuroscience and the ability to precisely manipulate neuronal activity will allow future studies to address the above questions, which will significantly advance our understanding in the axoglial signals that regulate myelination, providing new insights into neural plasticity and functions in both health and diseases.
Acknowledgments and conflict of interest disclosure
We thank all grant sponsors: Australian Research Council Discovery Project to J.X (#DP18010239); Australian National Health & Medical Research Council and Multiple Sclerosis Research Australia postdoctoral fellowship to D.G. (#APP1111041); the Australian Postgraduate Scholarship and the Melbourne Neuroscience Institute (University of Melbourne) STRAPA Scholarship to M.N. We give special thanks to Dr Ragnhildur Thóra Káradóttir from University of Cambridge, Dr Michelle Monje from Stanford University School of Medicine and Dr Ben Emery from Oregon Heal and Science University for their critical comments and reading of the manuscript. The authors declare that there is no conflict of interest regarding this research.
References
- Abiraman K., Pol S. U., O'Bara M. A. et al (2015) Anti‐muscarinic adjunct therapy accelerates functional human oligodendrocyte repair. J. Neurosci. 35, 3676–3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraban M., Koudelka S. and Lyons D. A. (2018) Ca (2+) activity signatures of myelin sheath formation and growth in vivo. Nat. Neurosci. 21, 19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres B. A. and Raff M. C. (1993) Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature 361, 258–260. [DOI] [PubMed] [Google Scholar]
- Barres B. A. and Raff M. C. (1999) Axonal control of oligodendrocyte development. J. Cell Biol. 147, 1123–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres B. A., Koroshetz W. J., Swartz K. J., Chun L. L. and Corey D. P. (1990) Ion channel expression by white matter glia: the O‐2A glial progenitor cell. Neuron 4, 507–524. [DOI] [PubMed] [Google Scholar]
- Barres B. A., Hart I. K., Coles H. S., Burne J. F., Voyvodic J. T., Richardson W. D. and Raff M. C. (1992) Cell death and control of cell survival in the oligodendrocyte lineage. Cell 70, 31–46. [DOI] [PubMed] [Google Scholar]
- Barres B. A., Jacobson M. D., Schmid R., Sendtner M. and Raff M. C. (1993a) Does oligodendrocyte survival depend on axons? Curr. Biol. 3, 489–497. [DOI] [PubMed] [Google Scholar]
- Barres B. A., Schmid R., Sendnter M. and Raff M. C. (1993b) Multiple extracellular signals are required for long‐term oligodendrocyte survival. Development 118, 283–295. [DOI] [PubMed] [Google Scholar]
- Barres B. A., Raff M. C., Gaese F., Bartke I., Dechant G. and Barde Y. A. (1994) A crucial role for neurotrophin‐3 in oligodendrocyte development. Nature 367, 371–375. [DOI] [PubMed] [Google Scholar]
- Baumann N. and Pham‐Dinh D. (2001) Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol. Rev. 81, 871–927. [DOI] [PubMed] [Google Scholar]
- Bechler M. E., Byrne L. and Ffrench‐Constant C. (2015) CNS myelin sheath lengths are an intrinsic property of oligodendrocytes. Curr. Biol. 25, 2411–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson S. L., Nagy Z., Skare S., Forsman L., Forssberg H. and Ullen F. (2005) Extensive piano practicing has regionally specific effects on white matter development. Nat. Neurosci. 8, 1148–1150. [DOI] [PubMed] [Google Scholar]
- Bergles D. E., Roberts J. D., Somogyi P. and Jahr C. E. (2000) Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 405, 187–191. [DOI] [PubMed] [Google Scholar]
- Birchmeier C. and Nave K. A. (2008) Neuregulin‐1, a key axonal signal that drives Schwann cell growth and differentiation. Glia 56, 1491–1497. [DOI] [PubMed] [Google Scholar]
- Borges K., Ohlemeyer C., Trotter J. and Kettenmann H. (1994) AMPA/kainate receptor activation in murine oligodendrocyte precursor cells leads to activation of a cation conductance, calcium influx and blockade of delayed rectifying K+ channels. Neuroscience 63, 135–149. [DOI] [PubMed] [Google Scholar]
- Brinkmann B. G., Agarwal A., Sereda M. W. et al (2008) Neuregulin‐1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron 59, 581–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J. R., Watkins T. A., Cosgaya J. M., Zhang C., Chen L., Reichardt L. F., Shooter E. M. and Barres B. A. (2004) NGF controls axonal receptivity to myelination by Schwann cells or oligodendrocytes. Neuron 43, 183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittajallu R., Chen Y., Wang H., Yuan X., Ghiani C. A., Heckman T., McBain C. J. and Gallo V. (2002) Regulation of Kv1 subunit expression in oligodendrocyte progenitor cells and their role in G1/S phase progression of the cell cycle. Proc. Natl Acad. Sci. USA 99, 2350–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittajallu R., Aguirre A. A. and Gallo V. (2005) Downregulation of platelet‐derived growth factor‐alpha receptor‐mediated tyrosine kinase activity as a cellular mechanism for K+‐channel regulation during oligodendrocyte development in situ. J. Neurosci. 25, 8601–8610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L. E., Young K. M., Hamilton N. B., Li H., Richardson W. D. and Attwell D. (2012) Properties and fate of oligodendrocyte progenitor cells in the corpus callosum, motor cortex, and piriform cortex of the mouse. J. Neurosci. 32, 8173–8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colello R. J. and Pott U. (1997) Signals that initiate myelination in the developing mammalian nervous system. Mol. Neurobiol. 15, 83–100. [DOI] [PubMed] [Google Scholar]
- Colello R. J., Devey L. R., Imperato E. and Pott U. (1995) The chronology of oligodendrocyte differentiation in the rat optic nerve: evidence for a signaling step initiating myelination in the CNS. J. Neurosci. 15, 7665–7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craner M. J., Lo A. C., Black J. A. and Waxman S. G. (2003) Abnormal sodium channel distribution in optic nerve axons in a model of inflammatory demyelination. Brain 126, 1552–1561. [DOI] [PubMed] [Google Scholar]
- Craner M. J., Hains B. C., Lo A. C., Black J. A. and Waxman S. G. (2004) Co‐localization of sodium channel Nav1.6 and the sodium‐calcium exchanger at sites of axonal injury in the spinal cord in EAE. Brain 127, 294–303. [DOI] [PubMed] [Google Scholar]
- Czopka T., Ffrench‐Constant C. and Lyons D. A. (2013) Individual oligodendrocytes have only a few hours in which to generate new myelin sheaths in vivo. Dev. Cell 25, 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangata Y. Y. and Kaufman M. H. (1997) Myelinogenesis in the optic nerve of (C57BL x CBA) F1 hybrid mice: a morphometric analysis. Eur. J. Morphol. 35, 3–17. [DOI] [PubMed] [Google Scholar]
- Demerens C., Stankoff B., Logak M., Anglade P., Allinquant B., Couraud F., Zalc B. and Lubetzki C. (1996) Induction of myelination in the central nervous system by electrical activity. Proc. Natl Acad. Sci. USA 93, 9887–9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh V. A., Tardif V., Lyssiotis C. A. et al (2013) A regenerative approach to the treatment of multiple sclerosis. Nature 502, 327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeland B., Sroufe L. A. and Erickson M. (1983) The developmental consequence of different patterns of maltreatment. Child Abuse Negl. 7, 459–469. [DOI] [PubMed] [Google Scholar]
- Emery B. (2010) Regulation of oligodendrocyte differentiation and myelination. Science 330, 779–782. [DOI] [PubMed] [Google Scholar]
- Esper R. M. and Loeb J. A. (2009) Neurotrophins induce neuregulin release through protein kinase Cdelta activation. J. Biol. Chem. 284, 26251–26260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etxeberria A., Mangin J. M., Aguirre A. and Gallo V. (2010) Adult‐born SVZ progenitors receive transient synapses during remyelination in corpus callosum. Nat. Neurosci. 13, 287–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etxeberria A., Hokanson K. C., Dao D. Q., Mayoral S. R., Mei F., Redmond S. A., Ullian E. M. and Chan J. R. (2016) Dynamic modulation of myelination in response to visual stimuli alters optic nerve conduction velocity. J. Neurosci. 36, 6937–6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fannon J., Tarmier W. and Fulton D. (2015) Neuronal activity and AMPA‐type glutamate receptor activation regulates the morphological development of oligodendrocyte precursor cells. Glia 63, 1021–1035. [DOI] [PubMed] [Google Scholar]
- Felts P. A., Baker T. A. and Smith K. J. (1997) Conduction in segmentally demyelinated mammalian central axons. J. Neurosci. 17, 7267–7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flechsig P. (1901) Developmental (myelogenetic) localisation of the cerebral cortex in the human subject. Lancet 158, 4. [Google Scholar]
- Franklin R. J. and Ffrench‐Constant C. (2008) Remyelination in the CNS: from biology to therapy. Nat. Rev. Neurosci. 9, 839–855. [DOI] [PubMed] [Google Scholar]
- Franklin R. J., ffrench‐Constant C., Edgar J. M. and Smith K. J. (2012) Neuroprotection and repair in multiple sclerosis. Nat. Rev. Neurol. 8, 624–634. [DOI] [PubMed] [Google Scholar]
- Gallo V., Zhou J. M., McBain C. J., Wright P., Knutson P. L. and Armstrong R. C. (1996) Oligodendrocyte progenitor cell proliferation and lineage progression are regulated by glutamate receptor‐mediated K+ channel block. J. Neurosci. 16, 2659–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary D. S., Malone M., Capestany P., Houdayer T. and McDonald J. W. (2012) Electrical stimulation promotes the survival of oligodendrocytes in mixed cortical cultures. J. Neurosci. Res. 90, 72–83. [DOI] [PubMed] [Google Scholar]
- Gautier H. O., Evans K. A., Volbracht K. et al (2015) Neuronal activity regulates remyelination via glutamate signalling to oligodendrocyte progenitors. Nat. Commun. 6, 8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge W. P., Zhou W., Luo Q., Jan L. Y. and Jan Y. N. (2009) Dividing glial cells maintain differentiated properties including complex morphology and functional synapses. Proc. Natl Acad. Sci. USA 106, 328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghatak N. R., Hirano A., Lijtmaer H. and Zimmerman H. M. (1974) Asymptomatic demyelinated plaque in the spinal cord. Arch. Neurol. 30, 484–486. [DOI] [PubMed] [Google Scholar]
- Gibson E. M., Purger D., Mount C. W. et al (2014) Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344, 1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebbels S., Wieser G. L., Pieper A. et al (2017) A neuronal PI(3,4,5)P3‐dependent program of oligodendrocyte precursor recruitment and myelination. Nat. Neurosci. 20, 10–15. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Xu B., Lu B. and Hempstead B. L. (2009) New insights in the biology of BDNF synthesis and release: implications in CNS function. J. Neurosci. 29, 12764–12767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllensten L. and Malmfors T. (1963) Myelinization of the optic nerve and its dependence on visual function–a quantitative investigation in mice. J. Embryol. Exp. Morphol. 11, 255–266. [PubMed] [Google Scholar]
- Hamilton N. B., Clarke L. E., Arancibia‐Carcamo I. L. et al (2017) Endogenous GABA controls oligodendrocyte lineage cell number, myelination, and CNS internode length. Glia 65, 309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand C., Remahl S., Persson H. and Bjartmar C. (1993) Myelinated nerve fibres in the CNS. Prog. Neurobiol. 40, 319–384. [DOI] [PubMed] [Google Scholar]
- Hill R. A., Patel K. D., Goncalves C. M., Grutzendler J. and Nishiyama A. (2014) Modulation of oligodendrocyte generation during a critical temporal window after NG2 cell division. Nat. Neurosci. 17, 1518–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R. A., Li A. M. and Grutzendler J. (2018) Lifelong cortical myelin plasticity and age‐related degeneration in the live mammalian brain. Nat. Neurosci. 21, 683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines J. H., Ravanelli A. M., Schwindt R., Scott E. K. and Appel B. (2015) Neuronal activity biases axon selection for myelination in vivo. Nat. Neurosci. 18, 683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. J. and Reichardt L. F. (2001) Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 24, 677–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes E. G., Kang S. H., Fukaya M. and Bergles D. E. (2013) Oligodendrocyte progenitors balance growth with self‐repulsion to achieve homeostasis in the adult brain. Nat. Neurosci. 16, 668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes E. G., Orthmann‐Murphy J. L., Langseth A. J. and Bergles D. E. (2018) Myelin remodeling through experience‐dependent oligodendrogenesis in the adult somatosensory cortex. Nat. Neurosci. 21, 696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine K. A. and Blakemore W. F. (2008) Remyelination protects axons from demyelination‐associated axon degeneration. Brain 131, 1464–1477. [DOI] [PubMed] [Google Scholar]
- Ishibashi T., Dakin K. A., Stevens B., Lee P. R., Kozlov S. V., Stewart C. L. and Fields R. D. (2006) Astrocytes promote myelination in response to electrical impulses. Neuron 49, 823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii A., Fyffe‐Maricich S. L., Furusho M., Miller R. H. and Bansal R. (2012) ERK1/ERK2 MAPK signaling is required to increase myelin thickness independent of oligodendrocyte differentiation and initiation of myelination. J. Neurosci. 32, 8855–8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii A., Furusho M. and Bansal R. (2013) Sustained activation of ERK1/2 MAPK in oligodendrocytes and schwann cells enhances myelin growth and stimulates oligodendrocyte progenitor expansion. J. Neurosci. 33, 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii A., Furusho M., Dupree J. L. and Bansal R. (2014) Role of ERK1/2 MAPK signaling in the maintenance of myelin and axonal integrity in the adult CNS. J. Neurosci. 34, 16031–16045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadottir R. T. and Kuo C. T. (2018) Neuronal activity‐dependent control of postnatal neurogenesis and gliogenesis. Annu. Rev. Neurosci. 41, 139–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadottir R., Cavelier P., Bergersen L. H. and Attwell D. (2005) NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature 438, 1162–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadottir R., Hamilton N. B., Bakiri Y. and Attwell D. (2008) Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat. Neurosci. 11, 450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller T. A. and Just M. A. (2009) Altering cortical connectivity: remediation‐induced changes in the white matter of poor readers. Neuron 64, 624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziejczyk K., Saab A. S., Nave K. A. and Attwell D. (2010) Why do oligodendrocyte lineage cells express glutamate receptors? F1000 Biol. Rep., 2 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koudelka S., Voas M. G., Almeida R. G., Baraban M., Soetaert J., Meyer M. P., Talbot W. S. and Lyons D. A. (2016) Individual neuronal subtypes exhibit diversity in CNS myelination mediated by synaptic vesicle release. Curr. Biol. 26, 1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kougioumtzidou E., Shimizu T., Hamilton N. B., Tohyama K., Sprengel R., Monyer H., Attwell D. and Richardson W. D. (2017) Signalling through AMPA receptors on oligodendrocyte precursors promotes myelination by enhancing oligodendrocyte survival. Elife 6, pii: e28080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnow A. M. and Attwell D. (2016) NMDA receptors: power switches for oligodendrocytes. Neuron 91, 3–5. [DOI] [PubMed] [Google Scholar]
- Krasnow A. M., Ford M. C., Valdivia L. E., Wilson S. W. and Attwell D. (2018) Regulation of developing myelin sheath elongation by oligodendrocyte calcium transients in vivo. Nat. Neurosci. 21, 24–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukley M., Capetillo‐Zarate E. and Dietrich D. (2007) Vesicular glutamate release from axons in white matter. Nat. Neurosci. 10, 311–320. [DOI] [PubMed] [Google Scholar]
- Kukley M., Nishiyama A. and Dietrich D. (2010) The fate of synaptic input to NG2 glial cells: neurons specifically downregulate transmitter release onto differentiating oligodendroglial cells. J. Neurosci. 30, 8320–8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappe‐Siefke C., Goebbels S., Gravel M., Nicksch E., Lee J., Braun P. E., Griffiths I. R. and Nave K. A. (2003) Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat. Genet. 33, 366–374. [DOI] [PubMed] [Google Scholar]
- Larson V. A., Zhang Y. and Bergles D. E. (2016) Electrophysiological properties of NG2(+) cells: matching physiological studies with gene expression profiles. Brain Res. 1638, 138–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Leach M. K., Redmond S. A., Chong S. Y., Mellon S. H., Tuck S. J., Feng Z. Q., Corey J. M. and Chan J. R. (2012) A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat. Methods 9, 917–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Chong S. Y., Tuck S. J., Corey J. M. and Chan J. R. (2013) A rapid and reproducible assay for modeling myelination by oligodendrocytes using engineered nanofibers. Nat. Protoc. 8, 771–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Xiao L., Liu X., Yang W., Shen W., Hu C., Yang G. and He C. (2013) A functional role of NMDA receptor in regulating the differentiation of oligodendrocyte precursor cells and remyelination. Glia 61, 732–749. [DOI] [PubMed] [Google Scholar]
- Li Y., Ceylan M., Shrestha B., Wang H., Lu Q. R., Asmatulu R. and Yao L. (2014) Nanofibers support oligodendrocyte precursor cell growth and function as a neuron‐free model for myelination study. Biomacromol 15, 319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. C. and Bergles D. E. (2004) Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat. Neurosci. 7, 24–32. [DOI] [PubMed] [Google Scholar]
- Liu J., Dietz K., DeLoyht J. M. et al (2012) Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat. Neurosci. 15, 1621–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Dupree J. L., Gacias M., Frawley R., Sikder T., Naik P. and Casaccia P. (2016) Clemastine enhances myelination in the prefrontal cortex and rescues behavioral changes in socially isolated mice. J. Neurosci. 36, 957–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B. (2003) BDNF and activity‐dependent synaptic modulation. Learn. Mem. 10, 86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgaard I., Luzhynskaya A., Stockley J. H. et al (2013) Neuregulin and BDNF induce a switch to NMDA receptor‐dependent myelination by oligodendrocytes. PLoS Biol. 11, e1001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinodan M., Rosen K. M., Ito S. and Corfas G. (2012) A critical period for social experience‐dependent oligodendrocyte maturation and myelination. Science 337, 1357–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado P. P. and Angulo M. C. (2015) Multiple modes of communication between neurons and oligodendrocyte precursor cells. Neuroscientist 21, 266–276. [DOI] [PubMed] [Google Scholar]
- Mangin J. M., Li P., Scafidi J. and Gallo V. (2012) Experience‐dependent regulation of NG2 progenitors in the developing barrel cortex. Nat. Neurosci. 15, 1192–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda N., Lu H., Fukata Y., Noritake J., Gao H., Mukherjee S., Nemoto T., Fukata M. and Poo M. M. (2009) Differential activity‐dependent secretion of brain‐derived neurotrophic factor from axon and dendrite. J. Neurosci. 29, 14185–14198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews M. A. and Duncan D. (1971) A quantitative study of morphological changes accompanying the initiation and progress of myelin production in the dorsal funiculus of the rat spinal cord. J. Comp. Neurol. 142, 1–22. [DOI] [PubMed] [Google Scholar]
- McDonald W. I. and Sears T. A. (1969) Effect of demyelination on conduction in the central nervous system. Nature 221, 182–183. [DOI] [PubMed] [Google Scholar]
- McKenzie I. A., Ohayon D., Li H., de Faria J. P., Emery B., Tohyama K. and Richardson W. D. (2014) Motor skill learning requires active central myelination. Science 346, 318–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei F., Fancy S. P. J., Shen Y. A. et al (2014) Micropillar arrays as a high‐throughput screening platform for therapeutics in multiple sclerosis. Nat. Med. 20, 954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei F., Mayoral S. R., Nobuta H. et al (2016) Identification of the kappa‐opioid receptor as a therapeutic target for oligodendrocyte remyelination. J. Neurosci. 36, 7925–7935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensch S., Baraban M., Almeida R., Czopka T., Ausborn J., El Manira A. and Lyons D. A. (2015) Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat. Neurosci. 18, 628–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micu I., Jiang Q., Coderre E. et al (2006) NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature 439, 988–992. [DOI] [PubMed] [Google Scholar]
- Micu I., Plemel J. R., Caprariello A. V., Nave K. A. and Stys P. K. (2018) Axo‐myelinic neurotransmission: a novel mode of cell signalling in the central nervous system. Nat. Rev. Neurosci. 19, 49–58. [DOI] [PubMed] [Google Scholar]
- Mitew S., Hay C. M., Peckham H., Xiao J., Koenning M. and Emery B. (2014) Mechanisms regulating the development of oligodendrocytes and central nervous system myelin. Neuroscience 276, 29–47. [DOI] [PubMed] [Google Scholar]
- Mitew S., Gobius I., Fenlon L. R. et al (2018) Pharmacogenetic stimulation of neuronal activity increases myelination in an axon‐specific manner. Nat. Commun. 9, 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll C., Mourre C., Lazdunski M. and Ulrich J. (1991) Increase of sodium channels in demyelinated lesions of multiple sclerosis. Brain Res. 556, 311–316. [DOI] [PubMed] [Google Scholar]
- Nagy B., Hovhannisyan A., Barzan R., Chen T. J. and Kukley M. (2017) Different patterns of neuronal activity trigger distinct responses of oligodendrocyte precursor cells in the corpus callosum. PLoS Biol. 15, e2001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najm F. J., Madhavan M., Zaremba A. et al (2015) Drug‐based modulation of endogenous stem cells promotes functional remyelination in vivo. Nature 522, 216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave K. A. and Salzer J. L. (2006) Axonal regulation of myelination by neuregulin 1. Curr. Opin. Neurobiol. 16, 492–500. [DOI] [PubMed] [Google Scholar]
- Nave K. A. and Werner H. B. (2014) Myelination of the nervous system: mechanisms and functions. Annu. Rev. Cell Dev. Biol. 30, 503–533. [DOI] [PubMed] [Google Scholar]
- Nicholson M., Wood R. J., Fletcher J. L., van den Buuse M., Murray S. S. and Xiao J. (2018) BDNF haploinsufficiency exerts a transient and regionally different influence upon oligodendroglial lineage cells during postnatal development. Mol. Cell Neurosci. 90, 12–21. [DOI] [PubMed] [Google Scholar]
- Nowakowski R. S., Lewin S. B. and Miller M. W. (1989) Bromodeoxyuridine immunohistochemical determination of the lengths of the cell cycle and the DNA‐synthetic phase for an anatomically defined population. J. Neurocytol. 18, 311–318. [DOI] [PubMed] [Google Scholar]
- Osterstock G., Le Bras B., Arulkandarajah K. H., Le Corronc H., Czarnecki A., Mouffle C., Bullier E., Legendre P. and Mangin J. M. (2018) Axoglial synapses are formed onto pioneer oligodendrocyte precursor cells at the onset of spinal cord gliogenesis. Glia. 2018 Mar 30. doi: 10.1002/glia.23331. [DOI] [PubMed] [Google Scholar]
- Pender M. P. (1988) The pathophysiology of acute experimental allergic encephalomyelitis induced by whole spinal cord in the Lewis rat. J. Neurol. Sci. 84, 209–222. [DOI] [PubMed] [Google Scholar]
- Phadke J. G. and Best P. V. (1983) Atypical and clinically silent multiple sclerosis: a report of 12 cases discovered unexpectedly at necropsy. J. Neurol. Neurosurg. Psychiatry 46, 414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robain O. and Mandel P. (1974) Quantitative study of myelination and axonal growth in corpus callosum and posterior columns of spinal cord in the Jimpy mouse (author's transl). Acta Neuropathol. 29, 293–309. [DOI] [PubMed] [Google Scholar]
- Saab A. S., Tzvetavona I. D., Trevisiol A. et al (2016) Oligodendroglial NMDA receptors regulate glucose import and axonal energy metabolism. Neuron 91, 119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahel A., Ortiz F. C., Kerninon C., Maldonado P. P., Angulo M. C. and Nait‐Oumesmar B. (2015) Alteration of synaptic connectivity of oligodendrocyte precursor cells following demyelination. Front. Cell. Neurosci. 9, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J., Klein M. C., Behrens T. E. and Johansen‐Berg H. (2009) Training induces changes in white‐matter architecture. Nat. Neurosci. 12, 1370–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrager P. and Novakovic S. D. (1995) Control of myelination, axonal growth, and synapse formation in spinal cord explants by ion channels and electrical activity. Brain Res. Dev. Brain Res. 88, 68–78. [DOI] [PubMed] [Google Scholar]
- Simons M. and Nave K. A. (2015) Oligodendrocytes: myelination and axonal support. Cold Spring Harb. Perspect. Biol. 8, a020479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. J. and McDonald W. I. (1982) Spontaneous and evoked electrical discharges from a central demyelinating lesion. J. Neurol. Sci. 55, 39–47. [DOI] [PubMed] [Google Scholar]
- Smith K. J., Blakemore W. F. and McDonald W. I. (1979) Central remyelination restores secure conduction. Nature 280, 395–396. [DOI] [PubMed] [Google Scholar]
- Spitzer S., Volbracht K., Lundgaard I. and Karadottir R. T. (2016) Glutamate signalling: a multifaceted modulator of oligodendrocyte lineage cells in health and disease. Neuropharmacology 110, 574–585. [DOI] [PubMed] [Google Scholar]
- Stedehouder J., Brizee D., Shpak G. and Kushner S. A. (2018) Activity‐dependent myelination of parvalbumin interneurons mediated by axonal morphological plasticity. J. Neurosci. 38, 3631–3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B., Tanner S. and Fields R. D. (1998) Control of myelination by specific patterns of neural impulses. J. Neurosci. 18, 9303–9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B., Porta S., Haak L. L., Gallo V. and Fields R. D. (2002) Adenosine: a neuron‐glial transmitter promoting myelination in the CNS in response to action potentials. Neuron 36, 855–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturrock R. R. (1980) Myelination of the mouse corpus callosum. Neuropathol. Appl. Neurobiol. 6, 415–420. [DOI] [PubMed] [Google Scholar]
- Tauber H., Waehneldt T. V. and Neuhoff V. (1980) Myelination in rabbit optic nerves is accelerated by artificial eye opening. Neurosci. Lett. 16, 235–238. [DOI] [PubMed] [Google Scholar]
- Taubert M., Draganski B., Anwander A., Muller K., Horstmann A., Villringer A. and Ragert P. (2010) Dynamic properties of human brain structure: learning‐related changes in cortical areas and associated fiber connections. J. Neurosci. 30, 11670–11677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taveggia C., Zanazzi G., Petrylak A. et al (2005) Neuregulin‐1 type III determines the ensheathment fate of axons. Neuron 47, 681–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassy G. S., Berger D. R., Chen H. H., Kasthuri N., Hayworth K. J., Vercelli A., Seung H. S., Lichtman J. W. and Arlotta P. (2014) Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science 344, 319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi R. B., Jackiewicz M., McKenzie I. A., Kougioumtzidou E., Grist M. and Richardson W. D. (2017) Remarkable stability of myelinating oligodendrocytes in mice. Cell Rep. 21, 316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H., Levine J. M., Miller R. H. and Trapp B. D. (1999) Rat optic nerve oligodendrocytes develop in the absence of viable retinal ganglion cell axons. J. Cell Biol. 146, 1365–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh H. S., Johung T. B., Caretti V. et al (2015) Neuronal activity promotes glioma growth through neuroligin‐3 secretion. Cell 161, 803–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt C. and Vogt O. (1908) Die myelo‐architektonischen Rindenfelder des Menschen. Neurol. Centralbl. 27, 137. [Google Scholar]
- Wake H., Lee P. R. and Fields R. D. (2011) Control of local protein synthesis and initial events in myelination by action potentials. Science 333, 1647–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H., Ortiz F. C., Woo D. H., Lee P. R., Angulo M. C. and Fields R. D. (2015) Nonsynaptic junctions on myelinating glia promote preferential myelination of electrically active axons. Nat. Commun. 6, 7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins T. A., Emery B., Mulinyawe S. and Barres B. A. (2008) Distinct stages of myelination regulated by gamma‐secretase and astrocytes in a rapidly myelinating CNS coculture system. Neuron 60, 555–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolswijk G. (1998) Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J. Neurosci. 18, 601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolswijk G. (2000) Oligodendrocyte survival, loss and birth in lesions of chronic‐stage multiple sclerosis. Brain 123(Pt 1), 105–115. [DOI] [PubMed] [Google Scholar]
- Wong A. W., Xiao J., Kemper D., Kilpatrick T. J. and Murray S. S. (2013) Oligodendroglial expression of TrkB independently regulates myelination and progenitor cell proliferation. J. Neurosci. 33, 4947–4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J., Wong A. W., Willingham M. M., van den Buuse M., Kilpatrick T. J. and Murray S. S. (2010) Brain‐derived neurotrophic factor promotes central nervous system myelination via a direct effect upon oligodendrocytes. Neurosignals 18, 186–202. [DOI] [PubMed] [Google Scholar]
- Xiao J., Ferner A. H., Wong A. W., Denham M., Kilpatrick T. J. and Murray S. S. (2012) Extracellular signal‐regulated kinase 1/2 signaling promotes oligodendrocyte myelination in vitro. J. Neurochem. 122, 1167–1180. [DOI] [PubMed] [Google Scholar]
- Xiao L., Ohayon D., McKenzie I. A., Sinclair‐Wilson A., Wright J. L., Fudge A. D., Emery B., Li H. and Richardson W. D. (2016) Rapid production of new oligodendrocytes is required in the earliest stages of motor‐skill learning. Nat. Neurosci. 19, 1210–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X., Baek R. C., Kirschner D. A., Peterson A., Fujii Y., Nave K. A., Macklin W. B. and Trapp B. D. (2006) Evolution of a neuroprotective function of central nervous system myelin. J. Cell Biol. 172, 469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K. M., Psachoulia K., Tripathi R. B., Dunn S. J., Cossell L., Attwell D., Tohyama K. and Richardson W. D. (2013) Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron 77, 873–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X., Eisen A. M., McBain C. J. and Gallo V. (1998) A role for glutamate and its receptors in the regulation of oligodendrocyte development in cerebellar tissue slices. Development 125, 2901–2914. [DOI] [PubMed] [Google Scholar]
- Ziskin J. L., Nishiyama A., Rubio M., Fukaya M. and Bergles D. E. (2007) Vesicular release of glutamate from unmyelinated axons in white matter. Nat. Neurosci. 10, 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonouzi M., Scafidi J., Li P. et al (2015) GABAergic regulation of cerebellar NG2 cell development is altered in perinatal white matter injury. Nat. Neurosci. 18, 674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]


