Abstract
Aim
To investigate the effects of the sodium‐glucose co‐transporter‐2 inhibitor empagliflozin on myocardial ketone body utilization in diabetic, obese rats with spontaneously hypertensive heart failure (SHHF), after 6 months of treatment.
Materials and Methods
Myocardial ketone body utilization was measured in vivo real time using a novel ketone probe (hyperpolarized [3‐13C]acetoacetate) and magnetic resonance spectroscopy (MRS). Myocardial glucose utilization and cardiac function were also determined in vivo using hyperpolarized [1‐13C]pyruvate MRS and magnetic resonance imaging (MRI), respectively. Myocardial fatty acid uptake and liver ketogenesis were assessed via protein expression.
Results
At baseline, myocardial ketone and glucose utilization were both higher in SHHF compared with control rats. Six months of empagliflozin treatment in SHHF rats was associated with less obesity, lower blood pressure, reduced blood glucose and insulin levels, and increased fasting blood β‐hydroxybutyrate levels, as expected. Contrary to the hypothesis, myocardial ketone body utilization was lower in empagliflozin‐treated SHHF rats, while glucose utilization and cardiac function were unaltered and hepatic congestion was reduced, compared with vehicle‐treated SHHF rats.
Conclusions
In diabetic hypertensive heart disease, empagliflozin reduces afterload without altering myocardial function and glucose utilization in the face of falling blood glucose levels, but does not enhance myocardial ketone utilization despite increased circulating levels.
Keywords: animal pharmacology, cardiovascular disease, diabetes complications, empagliflozin, experimental pharmacology, glucose metabolism
1. INTRODUCTION
Diabetes is a major risk factor for cardiovascular disease.1 Despite the vast availability of antidiabetic drugs and their efficacy in glycaemic control, rates of cardiovascular hospitalization or mortality associated with diabetes remain high.2 The EMPA‐REG OUTCOME trial showed remarkable benefits of the sodium‐glucose co‐transporter‐2 (SGLT2) inhibitor empagliflozin in reducing heart failure hospitalization and cardiovascular mortality in patients with type 2 diabetes3; however, the mechanisms of the cardioprotective effects of empagliflozin are yet to be fully elucidated.
Alterations in myocardial substrate metabolism have been implicated in the development of diabetic cardiomyopathy and heart failure.4 In the healthy heart, fatty acids (FAs; 60%‐70%) and glucose (30%‐40%) substrates provide most of the energy requirement, while in the diabetic heart, the substrate balance shifts towards more exclusive use of FAs, which is associated with reduced cardiac efficiency.4, 5 In addition to FAs and glucose, ketone bodies and amino acids may play an important role, although collectively, they normally contribute to only ~10% of total energy production.6 It has recently been shown that the failing heart relies on ketone bodies as a fuel.7, 8 More interestingly, circulating ketone bodies (ie, β‐hydroxybutyrate [β‐OHB]) have been shown to be increased by empagliflozin,9 leading to the hypothesis that an increase in ketone body utilization in the heart may explain the cardioprotective effects of empagliflozin, by improving cardiac efficiency.10, 11 This hypothesis relies, however, on indirect evidence from circulating ketone bodies, and direct evidence of myocardial ketone body utilization in response to SGLT inhibitors has not been available to date.
Techniques to measure myocardial ketone body utilization in vivo are challenging and not widely available. We have recently developed a novel technique to determine myocardial ketone body utilization in vivo in animals using hyperpolarized [3‐13C]acetoacetate. In the body, ketone bodies are available in the form of β‐OHB and acetoacetate. Prior to its use in the heart, β‐OHB is first converted into acetoacetate. Using 13C magnetic resonance spectroscopy (MRS), the conversion of [3‐13C]acetoacetate into its metabolic products, ie, [1‐13C]acetylcarnitine and [5‐13C]glutamate, can be followed in vivo in real time.
In the present study, we applied our novel hyperpolarized 13C MRS to investigate the effects of empagliflozin on myocardial ketone body utilization in vivo in diabetic obese rats with spontaneously hypertensive heart failure (SHHF), reminiscent of the patient population studies in the EMPA‐REG OUTCOME trial. Furthermore, we comprehensively determined the effects of empagliflozin on in vivo myocardial glucose utilization using hyperpolarized [1‐13C]pyruvate MRS, myocardial FA uptake and liver ketogenesis via expression of proteins, myocardial structure via histology, and myocardial function via magnetic resonance imaging (MRI).
2. MATERIALS AND METHODS
2.1. Animals
Male obese SHHF rats bred by Charles River (Kingston, New York) were purchased via iDNA (Singapore). Male Sprague–Dawley (SpD) rats, which served as lean controls, were purchased from InVivos (Singapore). The rats were housed in a 12‐hour:12‐hour dark: light cycle, with ad libitum access to food (Rat/Mouse Diet #1324 mod., Altromin, Lage, Germany) and water. Starting from 10 weeks of age, a subset of SHHF rats (SHHF‐EMPA; n = 13) were given empagliflozin (25 mg/kg body weight) dissolved in 0.5% hydroxyethylcellulose (Sigma‐Aldrich/Merck, St Louis, Missouri) by oral gavage daily for ~6 months (25‐27 weeks). Another SHHF subset (SHHF‐VEH; n = 8) and the lean SpD control (SpD‐VEH; n = 14) were given vehicle (ie, 0.5% hydroxyethylcellulose). The rats underwent in vivo 13C MRS and MRI to assess myocardial ketone body and glucose utilization, and cardiac function, respectively. Blood pressure was also measured. At the end of the study, organ and blood samples were collected. All procedures involving animals were approved by A*STAR Institutional Animal Care and Use Committee (#171212).
2.2. In vivo hyperpolarized 13C MRS and MRI
13C MRS was performed at a 9.4 T magnetic resonance (MR) scanner (Bruker, Ettlingen, Germany) under isoflurane anaesthesia. A cardiac‐triggered 13C MR pulse‐acquire spectroscopy was first initiated, followed immediately by injection of hyperpolarized [3‐13C]acetoacetate (0.240 mmol/kg body weight) or [1‐13C]pyruvate (0.5 mmol/kg body weight), via tail vein at a rate of 6 mL/min. Cinematic FLASH MRI was used to assess cardiac function. The details of hyperpolarized substrate preparation, 13C MRS and cinematic MRI are provided in File S1.
2.3. Blood pressure measurements
Systolic and diastolic blood pressure were measured using a near‐infrared blood pressure system (CODA; Kent‐Scientific, Torrington, Connecticut).
2.4. Ex vivo blood and tissue analysis
Blood glucose and ketone body levels were determined in a drop of blood using meters. Serum acetoacetate, free fatty acids (FFAs), triglycerides, brain natriuretic peptide (BNP), atrial natriuretic peptide (ANP), insulin and glucagon were determined using assay kits. Expression of cardiac CPT1B and liver HMGCS2 was determined via Western blotting. Fibrosis was assessed via Sirius Red histology. Details of blood analysis, Western blotting and histology are provided in File S1.
2.5. Statistical analysis
All statistical analysis was performed with the Graphpad Prism software package (GraphPad Software, San Diego, California). Statistical analysis was performed using one‐way analysis of variance (ANOVA) using Holm‐Sidak's post hoc test. For data acquired at more than one time point, statistics were analysed using ANOVA repeated measures. If the interaction term between time and groups was significant, Holm‐Sidak's multiple comparisons were performed. Data were presented as means ± SD. The significance level was set at P < 0.05.
3. RESULTS
3.1. Empagliflozin attenuated weight gain and high blood pressure in obese SHHF rats
At baseline, body weight was similar in all groups (P > 0.99). Over the study period, SHHF‐VEH rats gained weight rapidly compared with SpD‐VEH rats (Figure S1, File S1). At the end of the study period, the body weight of SHHF‐VEH rats was 31% higher than that of SpD‐VEH rats (P < 0.001), while the body weight of SHHF‐EMPA rats was 13% higher than that of SpD‐VEH rats (P < 0.001) and 14% lower compared with that of SHHF‐VEH rats (P < 0.001; Table 1).
Table 1.
Body weight, blood pressure, blood characteristics and organ weight
| Baseline | ∼6 months of treatment | |||||
|---|---|---|---|---|---|---|
| SpD‐VEH | SHHF‐VEH | SHHF‐EMPA | SpD‐VEH | SHHF‐VEH | SHHF‐EMPA | |
| Body weight, g | 340.7 ± 23.6 | 338.7 ± 23.1 | 340.0 ± 18.7 | 542.2 ± 60.2† | 709.5 ± 84.2*, † | 610.7 ± 47.9*, #, † |
| Systolic blood pressure, mm Hg | ‐ | ‐ | ‐ | 128.7 ± 7.8 | 166.8 ± 21.3* | 146.6 ± 11.5*, # |
| Diastolic blood pressure, mm Hg | ‐ | ‐ | ‐ | 90.3 ± 8.7 | 112.0 ± 18.4* | 103.4 ± 12.6 |
| Blood glucose, mmol/L | 8.9 ± 1.6 | 13.3 ± 4.7* | 16.1 ± 5.3* | 8.1 ± 1.6 | 10.5 ± 3.8 | 8.9 ± 2.9† |
| Blood β‐OHB, mmol/L | 0.4 ± 0.1 | 0.5 ± 0.1ξ | 0.5 ± 0.1ξ | 0.4 ± 0.1 | 0.5 ± 0.1ξ | 0.5 ± 0.1ξ |
| Blood β‐OHB: fasted, mmol/L | 1.0 ± 0.2 | 1.2 ± 0.5 | 1.0 ± 0.3 | 0.9 ± 0.1 | 1.0 ± 0.4 | 1.3 ± 0.4*, † |
| Serum AcAc, mmol/L | ‐ | ‐ | ‐ | 0.19 ± 0.03 | 0.14 ± 0.04* | 0.14 ± 0.05* |
| Serum TG, mmol/L | ‐ | ‐ | ‐ | 1.5 ± 0.9 | 6.2 ± 1.6* | 5.4 ± 2.1* |
| Serum FFAs, μmol/L | ‐ | ‐ | ‐ | 42.7 ± 11.4 | 43.3 ± 12.2 | 68.3 ± 30.1*, # |
| Serum insulin, μg/L | ‐ | ‐ | ‐ | 1.5 ± 1.3 | 117.1 ± 98.6* | 36.5 ± 32.8# |
| Serum glucagon, pmol/L | ‐ | ‐ | ‐ | 39.4 ± 12.8 | 18.3 ± 8.9* | 10.7 ± 3.2* |
| Serum insulin/glucagon ratio | ‐ | ‐ | ‐ | 0.04 ± 0.04 | 9.5 ± 5.4* | 3.8 ± 3.5*, # |
| Serum ANP, pg/mL | ‐ | ‐ | ‐ | 162.4 ± 90.5 | 509.1 ± 279.8* | 335.4 ± 99.3 |
| Serum BNP, pg/mL | ‐ | ‐ | ‐ | 158.0 ± 68.6 | 274.8 ± 85.5* | 202.1 ± 57.3 |
| Heart weight/tibia length, mg/mm | ‐ | ‐ | ‐ | 35.4 ± 3.5 | 54.8 ± 19.6* | 49.7 ± 6.8* |
| LV weight/tibia length, mg/mm | ‐ | ‐ | ‐ | 21.8 ± 2.7 | 32.0 ± 7.9* | 30.1 ± 2.4* |
| Septum weight/tibia length, mg/mm | ‐ | ‐ | ‐ | 7.0 ± 1.4 | 11.0 ± 1.4* | 10.0 ± 1.0* |
| Liver weight/tibia length, mg/mm | ‐ | ‐ | ‐ | 39.3 ± 5.0 | 79.3 ± 8.6* | 67.2 ± 7.3*, # |
| Lungs weight/tibia length, wet mg/mm | ‐ | ‐ | ‐ | 42.8 ± 5.9 | 51.1 ± 13.5 | 51.0 ± 10.9 |
| Lungs, wet/dry ratio | ‐ | ‐ | ‐ | 6.1 ± 0.9 | 6.4 ± 1.1 | 6.7 ± 1.7 |
| Tibia length, mm | ‐ | ‐ | ‐ | 45.3 ± 1.8 | 37.6 ± 1.8* | 37.4 ± 1.4* |
Abbreviations: β‐OHB, β‐hydroxyxbutyrate; AcAc, acetoacetate; ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; FFAs, free fatty acids; LV, left ventricular; SHHF‐VEH, rats with spontaneously hypertensive heart failure given vehicle; SpD‐VEH, SpD rats given vehicle; SHHF‐EMPA, rats with spontaneously hypertensive heart failure given empagliflozin; TG, triglycerides.
All data are at non‐fasting condition unless indicated. Data are means ± SD (SpD‐VEH n = 9‐13, SHHF‐VEH n = 7‐8, SHHF‐EMPA n = 9‐12; except for systolic and diastolic blood pressure SpD‐VEH n = 6, SHHF‐VEH n = 4, SHHF‐EMPA n = 5).
P < 0.05 vs. SpD‐VEH.;
P < 0.05 vs. SHHF‐VEH.
P < 0.05 vs. baseline for the same group.
P < 0.05 vs. SpD‐VEH independent of time.
At ~6 months of treatment, systolic blood pressure was 30% higher in SHHF‐VEH rats compared with SpD‐VEH rats (P < 0.001). Systolic blood pressure was also higher in SHHF‐EMPA than in SpD‐VEH rats (P = 0.049); however, this was 12% lower than in SHHF‐VEH rats (P = 0.047; Table 1). Diastolic blood pressure was 24% higher in SHHF‐VEH rats (P = 0.024) than in SpD‐VEH rats, while it was not different between SHHF‐EMPA and SHHF‐VEH rats (P = 0.34) and between SHHF‐EMPA and SpD‐VEH rats (P = 0.12; Table 1).
3.2. Empagliflozin modulated circulating substrate availability
At baseline, insulin‐induced blood glucose lowering was less in SHHF rats than in SpD rats, which indicates that obese SHHF rats were less insulin‐sensitive compared with SpD rats (Figure S2, File S1). Baseline blood glucose levels were higher in the SHHF‐VEH and SHHF‐EMPA groups compared with the SpD‐VEH group (P = 0.030 and P < 0.001, respectively; Table 1). At the end of treatment, blood glucose levels of SHHF‐VEH rats did not change compared with baseline (P = 0.29), while the levels in SHHF‐EMPA rats were reduced by 45% compared with baseline (P < 0.001; Table 1). Serum insulin levels were 78‐fold higher in SHHF‐VEH compared with SpD‐VEH rats (P < 0.001), while the levels were 69% lower in SHHF‐EMPA compared with SHHF‐VEH rats (P = 0.007). Serum FFA levels were 58% higher in SHHF‐EMPA than in SHHF‐VEH rats (P = 0.035), while serum glucagon and triglyceride levels were not different from those in SHHF‐VEH rats (P = 0.11 and P = 0.30, respectively). Fasting blood β‐OHB levels were 49% higher in SHHF‐EMPA than in SpD‐VEH rats (P = 0.027), and 36% higher compared with baseline values (P = 0.01). At fed state, blood β‐OHB and serum acetoacetate levels were similar between SHHF‐EMPA and SHHF‐VEH rats (P = 0.82 and P = 0.96, respectively).
3.3. Empagliflozin lowered in vivo myocardial ketone body utilization in obese SHHF rats
Using 13C MRS and hyperpolarized [3‐13C]acetoacetate, we investigated myocardial ketone body utilization in vivo. Upon uptake into the heart, acetoaocetate is metabolized into acetoacetyl‐CoA by a rate‐limiting enzyme, succinyl‐CoA‐3‐ketoacid‐CoA transferase (SCOT). Acetoacetyl‐CoA is then converted into acetyl‐CoA, which can be converted into acetylcarnitine, or utilized further in the tricarboxylic acid (TCA) cycle (Figure 1A). The production of [1‐13C]acetylcarnitine, [5‐13C]citrate and [5‐13C]glutamate upon injection of hyperpolarized [3‐13C]acetoacetate can be quantified from the 13C MR spectra (Figure 1B,C).
Figure 1.
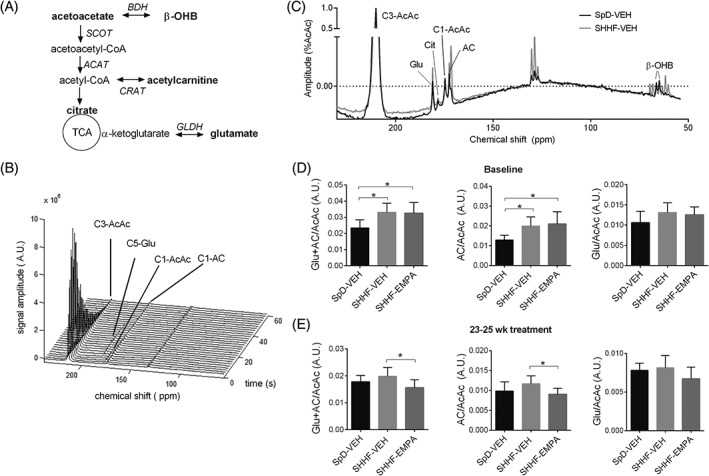
Myocardial acetoacetate metabolism as studied using hyperpolarized [3‐13C]acetoacetate. A, Acetoacetate metabolic pathway (metabolites detected by magnetic resonance are indicated in bold). B, Cardiac 13C spectra upon administration of hyperpolarized [3‐13C] acetoacetate over 60 seconds (data from rats with spontaneously hypertensive heart failure given vehicle [SHHF‐VEH] at baseline). C, Representative summed spectra of SpD rats given vehicle (SpD‐VEH) and SHHF‐VEH rats at baseline, and quantification of the total production of [5‐13C]glutamate + [1‐13C]acetylcarnitine, [1‐13C]acetylcarnitine, and [5‐13C]glutamate upon [3‐13C]acetoacetate injection, at D, baseline and E, 23 to 25 weeks after treatment. Data are means ± SD (SpD‐VEH, n = 8‐10; SHHF‐VEH, n = 7‐9; rats with spontaneously hypertensive heart failure given empagliflozin [SHHF‐EMPA], n = 9‐12). *P < 0.05. AC, acetylcarnitine; AcAc, acetoacetate; ACAT, acetoacetyl‐CoA thiolase; BDH, β‐hydroxybutyrate dehydrogenase; CRAT, carnitine acetyltransferase; GLDH, glutamate dehydrogenase; glu, glutamate; SCOT, succinyl‐CoA‐3‐ketoacid‐CoA transferase; SHHF, spontaneously hypertensive heart failure; TCA, tricarboxylic acid
At baseline, total production of [1‐13C]acetylcarnitine + [5‐13C]glutamate was higher in SHHF‐VEH rats (40% higher; P = 0.004) and SHHF‐EMPA rats (39% higher; P = 0.004) compared with that in SpD‐VEH rats (Figure 1D), which indicates higher myocardial ketone body utilization in obese SHHF rats. [5‐13C]Glutamate production was not significantly different among any of the groups (ANOVA P = 0.075), while [1‐13C]acetylcarnitine production was higher in SHHF‐VEH (55% higher; P = 0.007) and SHHF‐EMPA rats (63% higher; P = 0.002) than in SpD‐VEH rats (Figure 1D). This suggests that the higher myocardial ketone body utilization in obese SHHF rats was accounted for by a higher conversion into acetylcarnitine, rather than being used in the TCA cycle for oxidation.
At 23 to 25 weeks of treatment, the total production of [1‐13C]acetylcarnitine + [5‐13C]glutamate was 21% lower in SHHF‐EMPA compared with SHHF‐VEH rats (P = 0.030; Figure 1E). Similarly, [1‐13C]acetylcarnitine production in SHHF‐EMPA rats was 23% lower than that in SHHF‐VEH rats (P = 0.037; Figure 1E), while [5‐13C]glutamate production was not different among any of the groups (ANOVA P = 0.10; Figure 1E).
3.4. Empagliflozin did not alter in vivo myocardial glucose utilization in obese SHHF rats
To investigate whether empagliflozin also affected the metabolism of glucose‐derived substrates, we assessed myocardial pyruvate utilization in vivo using hyperpolarized [1‐13C]pyruvate. We quantified the production of [13C]bicarbonate, [1‐13C]lactate and [1‐13C]alanine upon [1‐13C]pyruvate injection (Figure 2A), as observed in the 13C MR spectra (Figure 2B,C).
Figure 2.
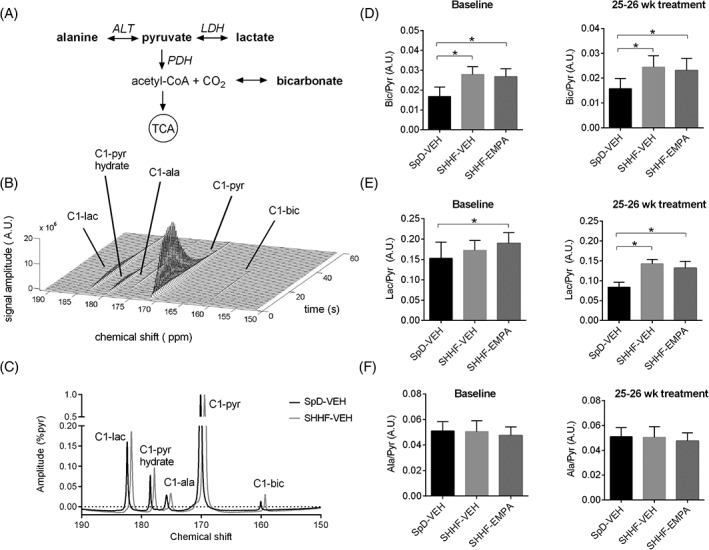
Myocardial pyruvate metabolism as studied using hyperpolarized [1‐13C]pyruvate. A, Pyruvate metabolic pathway (metabolites detected by magnetic resonance are indicated in bold). B, Cardiac 13C MR spectra on administration of hyperpolarized [1‐13C] pyruvate over 60 seconds (data from SpD rats given vehicle [SpD‐VEH] at baseline). C, Representative summed spectra of SpD‐VEH and rats with spontaneously hypertensive heart failure given vehicle (SHHF‐VEH) at baseline, and quantification of the production of D, [13C] bicarbonate, E, [1‐13C]lactate, and F, [1‐13C]alanine upon [1‐13C]pyruvate administration, at baseline and at 25 to 26 weeks after treatment. Data are means ± SD (SpD‐VEH, n = 9‐10; SHHF‐VEH, n = 7‐9; rats with spontaneously hypertensive heart failure given empagliflozin [SHHF‐EMPA], n = 7‐13). *P < 0.05. ala, alanine; ALT, alanine aminotransferase; bic, bicarbonate; lac, lactate; LDH, lactate dehydrogenase; pyr, pyruvate; PDH, pyruvate dehydrogenase
At baseline, [13C]bicarbonate production was higher in SHHF‐VEH rats (66% higher; P < 0.001) and SHHF EMPA rats (59% higher; P < 0.001; Figure 2D) compared with that in SpD‐VEH rats, which suggests higher pyruvate dehydrogenase (PDH) flux in obese SHHF rats. The higher [13C]bicarbonate production was also maintained at 23 to 25 weeks after treatment (SHHF‐VEH vs. SpD‐VEH rats: 55% higher; P = 0.002; SHHF‐EMPA vs. SpD‐VEH rats: 46% higher; P = 0.004), and was not different between SHHF‐VEH and SHHF‐EMPA rats (P = 0.57; Figure 2D). At 23 to 25 weeks after treatment, [1‐13C]lactate production, indicating glycolytic rates, was also higher in SHHF‐VEH rats (71% higher; P < 0.001) and SHHF‐EMPA rats (58% higher; P < 0.001) compared with SpD‐VEH rats, while this was not different between SHHF‐VEH and SHHF‐EMPA rats (P = 0.16; Figure 2E). [1‐13C]Alanine production was not different among groups (ANOVA P = 0.18; Figure 2F). Taken together, this result suggests that myocardial pyruvate utilization was higher in SHHF rats, and was not affected by EMPA treatment.
3.5. Empagliflozin did not affect mitochondrial FA uptake in obese SHHF rats
To obtain insight into the FA metabolic pathway, we measured protein expression of CPT1B, the protein involved in long‐chain FA transport into mitochondria. CPT1B expression was higher in SHHF‐VEH (P = 0.012) and SHHF‐EMPA rats (P < 0.001) compared with SpD‐VEH rats, and was not different between SHHF‐VEH and SHHF‐EMPA rats (P = 0.14; Table 2 and Figure S3A, File S1).
Table 2.
Protein expression and histology
| SpD‐VEH | SHHF‐VEH | SHHF‐EMPA | |
|---|---|---|---|
| Protein expression | |||
| Cardiac CPT1B | 1.00 ± 0.35 | 1.68 ± 0.53* | 2.04 ± 0.57* |
| Liver HMGCS2 | 1.00 ± 0.12 | 0.85 ± 0.14* | 0.79 ± 0.09* |
| Histology | |||
| Sirius Red‐positive area, % | 3.0 ± 0.7 | 8.8 ± 4.2* | 10.2 ± 0.03* |
Abbreviations: CPT1B, carnitine palmitoyoltransferase 1B; HMGCS2, 3‐hydroxy‐3‐methylglutaryl‐CoA synthase 2; SHHF‐VEH, rats with spontaneously hypertensive heart failure given vehicle; SpD‐VEH, SpD rats given vehicle; SHHF‐EMPA, rats with spontaneously hypertensive heart failure given empagliflozin.
Protein expression is normalized to GAPDH. Representative immunoblots are provided in Figure S3, File S1. Representative Sirius Red‐stained sections are provided in Figure S4. Data are means ± SD (SpD‐VEH, n = 12‐13; SHHF‐VEH, n = 7‐8; SHHF‐EMPA, n = 10‐11).
P < 0.05 vs. SpD‐VEH.
3.6. Empagliflozin did not change liver ketogenesis in obese SHHF rats
We also measured the expression of 3‐hydroxyl‐3‐methylglutaryl‐CoA synthase 2 (HMGCS2), the protein involved in the liver metabolism of acetoacetyl‐CoA and acetyl‐CoA into 3‐hydroxyl‐3‐methylglutaryl‐CoA. In the next step, 3‐hydroxyxl‐3‐methylglutaryl‐CoA is metabolized into acetoacetate. HMGCS2 expression was lower in SHHF‐VEH (P = 0.012) and SHHF‐EMPA rats (P < 0.001) than in SpD‐VEH rats, while it was similar in SHHF‐VEH and SHHF‐EMPA rats (P = 0.27; Table 2 and Figure S3B, File S1).
3.7. Empagliflozin reduced hepatic congestion but did not have measurable effects on left ventricular hypertrophy or fibrosis in obese SHHF rats
To determine whether changes in myocardial substrate metabolism induced by empagliflozin were manifested in improvement in cardiac hypertrophy and function, we performed cinematic MRI at baseline, and after 5 to 8 weeks and 24 to 26 weeks of treatment (Figure 3A). At baseline, left ventricular mass (LVM)/tibia length (TL) was similar in all groups (Figure 3B); however, at 5 weeks of study period, LVM/TL in SHHF‐VEH rats was 35% higher compared with that in SpD‐VEH rats (P < 0.001), which was further increased to 63% at 24 to 26 weeks of study period (P < 0.001 vs. SpD‐VEH rats), indicating progressive left ventricular (LV) hypertrophy. Ejection fraction (EF), as a measure of cardiac systolic function, was similar in all groups at baseline and at 5 weeks of study period (Figure 3C), and remained normal (>60%) in all groups throughout the study. Similarly, end diastolic volume and stroke volume were comparable in all groups at all time points (general group effects P = 0.79 and P = 0.41, respectively; Figure 3D,E). The MRI findings were corroborated by higher heart, LV, septal mass indexed to TL at the time the rats were killed in SHHF‐VEH compared with SpD‐VEH rats (Table 1). The LV hypertrophy in SHHF‐VEH rats was associated with increased natriuretic peptides (ie, ANP, P < 0.001 and BNP, P = 0.005), evidence of hepatic congestion (higher liver weight/TL; P < 0.001) and a trend towards pulmonary congestion (numerically higher wet lung weight/TL although not achieving statistical significance, ANOVA P = 0.11; Table 1), thus indicating that the SHHF‐VEH rats had evidence of early heart failure with preserved EF. Furthermore, SHHF‐VEH had more cardiac fibrosis by picrosirius red staining (P < 0.001) compared with SpD‐VEH rats (Table 2 and Figure S4, File S1).
Figure 3.
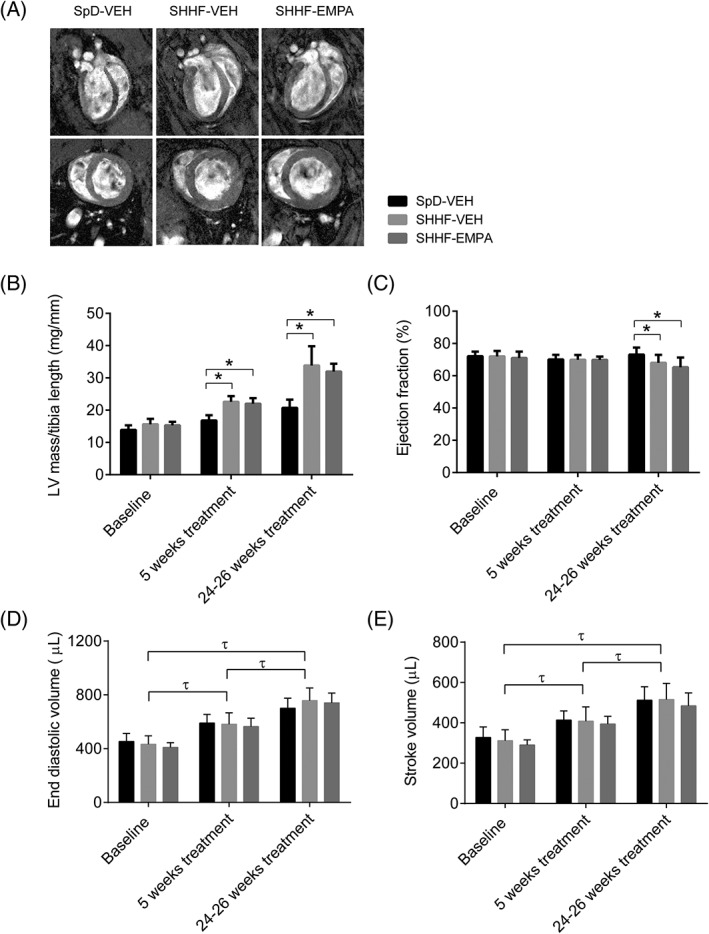
Left ventricular (LV) hypertrophy and function as measured using cinematic MRI. A, Representative images of four‐chamber long axis, two‐chamber long axis, and short axis of the heart at 24 to 26 weeks of study period. B, LV mass/tibia length, and C, ejection fraction (EF), D, end diastolic volume (EDV), and E, stroke volume (SV). Data are means ± SD (SpD rats given vehicle [SpD‐VEH], n = 9; rats with spontaneously hypertensive heart failure given vehicle [SHHF‐VEH], n = 8; rats with spontaneously hypertensive heart failure given empagliflozin [SHHF‐EMPA], n = 10). *P < 0.05. τGeneral time effect independent of group, P < 0.05
After treatment with empagliflozin, LVM/TL remained higher in SHHF‐EMPA compared with SpD‐VEH rats at 5 weeks (30% higher; P < 0.001) and 24 to 26 weeks of study period (54% higher; P < 0.001), respectively. The LVM/TL in SHHF‐EMPA rats was not different from that in SHHF‐VEH rats (P = 0.64 at 5 weeks and P = 0.13 at 24 to 26 weeks of study period). Ex vivo heart, LV and septum weight normalized to TL was higher in SHHF‐EMPA rats (P < 0.001 for all variables) compared with SpD‐VEH rats and not different between SHHF‐EMPA and SHHF‐VEH rats (P = 0.72, P = 0.63, P = 0.11, respectively; Table 1). Of note, liver weight/TL was lower in SHHF‐EMPA than in SHHF‐VEH rats (P < 0.001), indicating improvement in hepatic congestion. Consistently, ANP and BNP values trended lower in SHHF‐EMPA compared with SHHF‐VEH rats, although this difference did not achieve statistical significance (P = 0.051 and P = 0.10, respectively). However, we did not detect statistically significant differences in wet lung weight/TL among groups (Table 1; ANOVA P = 0.11), suggesting that none of the groups had overt decompensated heart failure with pulmonary oedema. Furthermore, cardiac fibrosis was not different between SHHF‐VEH and SHHF‐EMPA rats (P = 0.33; Table 2 and Figure S4, File S1).
4. DISCUSSION
This study is the first to demonstrate the effects of empagliflozin on myocardial ketone body utilization in vivo. Using novel 13C MRS and hyperpolarized [3‐13C]acetoacetate, we were able to measure myocardial ketone body utilization in vivo real time in diabetic hypertensive rats with mild heart failure and preserved EF. Six months of empagliflozin treatment in SHHF rats was associated with less obesity, lower blood pressure, reduced blood glucose and insulin levels, and increased circulating FFAs and fasting blood β‐hydroxybutyrate levels. Despite increased circulating ketone bodies, empagliflozin lowered myocardial ketone body utilization in SHHF rats and reduced hepatic congestion, while not affecting myocardial PDH flux and cardiac function. There was no change in myocardial CPT1B or liver HMGCS2 expression. Empagliflozin did not affect LV hypertrophy or fibrosis in SHHF rats within the study period.
The most striking finding of the present study is that myocardial ketone body utilization in SHHF‐EMPA rats was lower, not higher, compared with SHHF‐VEH rats. It has been hypothesized in the “thrifty substrate” fuel hypothesis,10 that cardioprotective effects of empagliflozin may be associated with increased circulating ketone bodies and the subsequent shift towards ketone body use in the heart.10, 11 As ketone bodies are a more oxygen‐efficient fuel, the increase in ketone body utilization is thought to increase cardiac efficiency10, 11. However, to date, it has not been clear what causes the increase in circulating β‐OHB upon empagliflozin treatment. Ferrannini et al10 proposed that glucose removal and a reduction in insulin/glucagon ratio by empagliflozin increased FA oxidation in the liver, and subsequently, ketogenesis, which results in an increase in circulating β‐OHB levels. By contrast, Lopaschuk and Verma12 argued that the increase in circulating β‐OHB levels may rather be attributable to a reduction in myocardial ketone body utilization, because increased ketone body use may potentially be maladaptive. In turn, these authors hypothesized that the increase in ketone body levels may inhibit histone deacetylase and increase anti‐hypertrophic transcription.12
The fuel hypothesis was based on increased circulating ketone bodies as an enhanced source for higher myocardial ketone utilization with SGLT2 inhibitors; however, the increased circulating ketone body levels may be a result of changes in myocardial substrate utilization, rather than the cause. Accordingly, potential explanations for the increase in circulating β‐OHB with empagliflozin include increased liver production of ketone bodies, reduced myocardial utilization of ketone bodies, or both. Our findings suggest that in diabetic hypertensive heart disease, increased circulating β‐OHB with empagliflozin is predominantly related to reduced myocardial ketone body utilization rather than increased liver ketogenesis. While serum FFA levels were higher in SHHF‐EMPA rats, this was not accompanied by an increase in liver HMGCS2 expression, which suggests unaltered liver ketogenesis in SHHF‐EMPA rats. It cannot be excluded, however, that the unaltered HMGCS2 expression in the SHHF‐EMPA rats may be related to the early diabetic stage of the obese SHHF rats, as shown by a mild hyperglycaemia and a 78‐fold increase in insulin levels. It is possible that, in the setting of advanced diabetes, further removal of blood glucose and further lowering in insulin/glucagon ratio may further stimulate an increase in liver ketogenesis. In humans, the effect of empagliflozin on circulating β‐OHB levels was more pronounced in people with diabetes than in those with impaired glucose tolerance or without diabetes.9, 13 Nevertheless, it is tempting to speculate that, even in conditions in which liver ketogenesis contributes to the increase in circulating β‐OHB upon empagliflozin treatment, myocardial ketone body utilization may still be reduced instead of being increased. Wentz et al14 previously demonstrated that in a sustained ketosis environment, the heart adapts by limiting its ketone body utilization.
While ketone body oxidation has been demonstrated to be increased in the failing heart,7, 8 there is no consensus on whether this is adaptive or maladaptive.12, 15, 16 An increase in ketone body oxidation may lead to an increase in mitochondrial protein acetylation because of an increase in ketone body‐derived acetyl‐CoA,17 and a reduction in mitochondrial oxidative phosphorylation because of decreased anaplerosis.18 The suppression of glucose oxidation because of increased ketone body use may result in reduced cardiac efficiency.12 Similarly, ketone bodies suppress FA use,19 which may not be preferable as FAs produce more energy compared with ketone bodies for the same amount of carbon molecules. It is interesting to note that it has previously been shown that the heart adapted to a ketosis environment not only by lowering ketone body use, but also by diminishing the ability of ketone bodies to suppress FA oxidation,14 which suggests the importance of FA oxidation for ATP production. Previous studies have also shown that FAs may be important in maintaining cardiac function under stressed conditions.20, 21, 22 In the present study, while myocardial ketone body utilization was lower in SHHF‐EMPA rats, we did not observe changes in PDH flux in vivo. We did not measure myocardial FA utilization rate; however, CPT1B protein expression was similar in the hearts of SHHF‐EMPA and SHHF‐VEH rats, which suggests unaltered long‐chain FA uptake into the mitochondria. Moving forward, we aim to develop hyperpolarized 13C‐FAs to investigate changes in FA oxidation.
The effects of empagliflozin on myocardial ketone body utilization were accompanied by a lower systolic blood pressure in SHHF‐EMPA rats, which is in agreement with observations in patients with diabetes and hypertension.23, 24 Lower blood pressure decreases cardiac afterload and oxygen consumption (MVO2), which may be related to the reduced need for alternative substrate utilization by the heart. Of note, empagliflozin treatment was associated with improvement in hepatic congestion with a trend towards reduction in natriuretic peptides in SHHF rats, although we were unable to detect differences in LV hypertrophy or fibrosis. Even without invoking a direct myocardial effect of empagliflozin, a reduction in LV afterload may have been expected to be associated with reduction in LV hypertrophy and fibrosis. Potential explanations include the baseline early diabetic status, mild cardiomyopathy, modest reduction in blood pressure, limited duration of therapy, or small sample size. The effects of empagliflozin on cardiac hypertrophy and function in animal models indeed varied. Empagliflozin has been shown to reduce LV mass in SHR rats,25 but not in obese ob/ob or diabetic db/db mice.26, 27 In these mice, empagliflozin improved diastolic function, without affecting systolic function,26, 27 which was accompanied by a reduction in fibrosis.27 Data on cardiac structure and function were not available in the EMPA‐REG OUTCOME trial, although in a follow‐up study involving 10 patients with diabetes, empagliflozin as an add‐on treatment improved LVM index and diastolic function.28 Finally, it is possible that the beneficial effects of empagliflozin on cardiac function would have been more evident under stress conditions, for example, during dobutamine challenge26 or myocardial infarction.29
Obese SHHF rats had a cardiac metabolic profile consistent with LV hypertrophy with preserved EF. Particularly, myocardial glucose utilization was increased, as shown in pressure‐overloaded mice with normal EF.21, 30 A reduction in EF in obese SHHF rats, potentially at an older age,31, 32 may be accompanied by a further increase in myocardial glucose utilization, as myocardial glucose uptake has been shown to negatively correlate with EF.21 Myocardial ketone body utilization may also be further increased, as it was shown to be upregulated in advanced heart failure.7, 8 In this case, it remains to be seen whether empagliflozin would improve EF, and whether this is achieved via normalization of myocardial glucose and/or ketone body utilization.
In conclusion, the present study contributes to the elucidation of myocardial ketone body utilization with empagliflozin treatment. In diabetic hypertensive heart disease, empagliflozin reduces afterload without affecting myocardial function and glucose utilization in the face of falling blood glucose levels, but does not enhance myocardial ketone utilization despite increased circulating levels. Our results further suggest that the increase in circulating ketone bodies upon empagliflozin treatment is related to a reduction in myocardial ketone body use in vivo while liver ketogenesis is not altered. For future research, it will be of interest to determine whether empagliflozin normalizes myocardial substrate utilization in an overtly decompensated failing heart, and whether this translates to an improved prognosis.
CONFLICTS OF INTEREST
None declared.
Author contributions
D.A. and P.T.H.L. designed the study. D.A., X.Q.T., C.C.W., W.X.C., J.L. and P.T.H.L. performed the experiments. D.A. and P.T.H.L. analysed the data. D.A. wrote and C.S.P.L. and P.T.H.L. revised the manuscript.
Supporting information
File S1. Supplementary materials and methods.
Figure S1. Body weight during 25 to 27 weeks of study period. Data are mean ± SEM (SD‐VEH N = 14, SHHF‐VEH N = 8, SHHF‐EMPA N = 12). General time effect independent of group was P < 0.001. Group effect independent of time: *P < 0.05 vs. SD‐VEH, # P < 0.05 vs SHHF‐VEH.
Figure S2. Changes in blood glucose levels (relative to the baseline value) during 120 minutes upon intraperitoneal insulin administration. Data are means ± SD (n = 3 per group). *P < 0.05 vs SD.
Figure S3. Representative immunoblots of (A) cardiac CPT1B and (B) HMGCS2. CPT1B: carnitine palmitoyltransferase 1B, HMGCS2: 3‐hydroxy‐3‐methylglutaryl‐CoA synthase 2. GAPDH: Glyceraldehyde 3‐phosphate dehydrogenase.
Figure S4. Representative Sirius Red‐stained heart sections. Collagen appear red, cytoplasm appears yellow. White bars indicate 100 μm.
ACKNOWLEDGMENTS
This study was supported by an intramural funding (Asian neTwork for Translational Research and Cardiovascular Trials, ATTRaCT) from A*STAR Biomedical Research Council.
Abdurrachim D, Teo XQ, Woo CC, et al. Empagliflozin reduces myocardial ketone utilization while preserving glucose utilization in diabetic hypertensive heart disease: A hyperpolarized 13C magnetic resonance spectroscopy study. Diabetes Obes Metab. 2019;21:357–365. 10.1111/dom.13536
Funding information This study was supported by an intramural funding (Asian neTwork for Translational Research and Cardiovascular Trials, ATTRaCT) from A*STAR Biomedical Research Council.; A*STAR Biomedical Research Council, Grant Award Number: ATTRaCT
REFERENCES
- 1. Kannel WB, McGee DL. Diabetes and cardiovascular risk factors: the Framingham study. Circulation. 1979;59(1):8‐13. [DOI] [PubMed] [Google Scholar]
- 2. Smith RJ, Goldfine AB, Hiatt WR. Evaluating the cardiovascular safety of new medications for type 2 diabetes: time to reassess? Diabetes Care. 2016;39(5):738‐742. 10.2337/dc15-2237. [DOI] [PubMed] [Google Scholar]
- 3. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 4. Lopaschuk GD, Ussher JR, Folmes CDL, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90(1):207‐258. 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 5. Glatz JFC, Bonen A, Ouwens DM, Luiken JJFP. Regulation of sarcolemmal transport of substrates in the healthy and diseased heart. Cardiovasc Drugs Ther. 2006;20(6):471‐476. 10.1007/s10557-006-0582-8. [DOI] [PubMed] [Google Scholar]
- 6. Lopaschuk GD, Ussher JR. Evolving concepts of myocardial energy metabolism: more than just fats and carbohydrates. Circ Res. 2016;119(11):1173‐1176. 10.1161/CIRCRESAHA.116.310078. [DOI] [PubMed] [Google Scholar]
- 7. Aubert G, Martin OJ, Horton JL, et al. The failing heart relies on ketone bodies as a fuel. Circulation. 2016;133(8):698‐705. 10.1161/CIRCULATIONAHA.115.017355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bedi KC, Snyder NW, Brandimarto J, et al. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation. 2016;133(8):706‐716. 10.1161/CIRCULATIONAHA.115.017545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferrannini E, Baldi S, Frascerra S, et al. Shift to fatty substrate utilization in response to sodium–glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. 2016;65(5):1190‐1195. [DOI] [PubMed] [Google Scholar]
- 10. Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA‐REG OUTCOME trial: a thrifty substrate hypothesis. Diabetes Care. 2016;39(7):1108‐1114. 10.2337/dc16-0330. [DOI] [PubMed] [Google Scholar]
- 11. Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA‐REG OUTCOME study? A unifying hypothesis. Diabetes Care. 2016;39(7):1115‐1122. 10.2337/dc16-0542. [DOI] [PubMed] [Google Scholar]
- 12. Lopaschuk GD, Verma S. Empagliflozin's fuel hypothesis: not so soon. Cell Metab. 2016;24(2):200‐202. 10.1016/j.cmet.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 13. Al Jobori H, Daniele G, Adams J, et al. Determinants of the increase in ketone concentration during SGLT2 inhibition in NGT, IFG and T2DM patients. Diabetes Obes Metab. 2017;19(6):809‐813. 10.1111/dom.12881. [DOI] [PubMed] [Google Scholar]
- 14. Wentz AE, D'Avignon DA, Weber ML, et al. Adaptation of myocardial substrate metabolism to a ketogenic nutrient environment. J Biol Chem. 2010;285(32):24447‐24456. 10.1074/jbc.M110.100651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kolwicz SC, Airhart S, Tian R. Ketones step to the plate: a game changer for metabolic remodeling in heart failure? Circulation. 2016;133(8):689‐691. 10.1161/CIRCULATIONAHA.116.021230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taegtmeyer H. Failing heart and starving brain: ketone bodies to the rescue. Circulation. 2016;134(4):265‐266. 10.1161/CIRCULATIONAHA.116.022141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Horton JL, Martin OJ, Lai L, et al. Mitochondrial protein hyperacetylation in the failing heart. JCI Insight. 2016;1(2):1‐14. 10.1172/jci.insight.84897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Russell RR, Taegtmeyer H. Changes in citric acid cycle flux and anaplerosis antedate the functional decline in isolated rat hearts utilizing acetoacetate. J Clin Invest. 1991;87(2):384‐390. 10.1172/JCI115008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stanley WC, Meadows SR, Kivilo KM, Roth BA, Lopaschuk GD. Beta‐hydroxybutyrate inhibits myocardial fatty acid oxidation in vivo independent of changes in malonyl‐CoA content. Am J Physiol Heart Circ Physiol. 2003;285(4):H1626‐H1631. 10.1152/ajpheart.00332.2003. [DOI] [PubMed] [Google Scholar]
- 20. Kolwicz SCJ, Olson DP, Marney LC, Garcia‐Menendez L, Synovec RE, Tian R. Cardiac‐specific deletion of acetyl CoA carboxylase 2 prevents metabolic remodeling during pressure‐overload hypertrophy. Circ Res. 2012;111(6):728‐738. 10.1161/CIRCRESAHA.112.268128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abdurrachim D, Nabben M, Hoerr V, et al. Diabetic db/db mice do not develop heart failure upon pressure overload: a longitudinal in vivo PET, MRI, and MRS study on cardiac metabolic, structural, and functional adaptations. Cardiovasc Res. 2017;113(10):1148‐1160. 10.1093/cvr/cvx100. [DOI] [PubMed] [Google Scholar]
- 22. Abdurrachim D, Luiken JJFP, Nicolay K, Glatz JFC, Prompers JJ, Nabben M. Good and bad consequences of altered fatty acid metabolism in heart failure: evidence from mouse models. Cardiovasc Res. 2015;106(2):194‐205. 10.1093/cvr/cvv105. [DOI] [PubMed] [Google Scholar]
- 23. Chilton R, Tikkanen I, Cannon CP, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17(12):1180‐1193. 10.1111/dom.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tikkanen I, Narko K, Zeller C, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38(3):420‐428. 10.2337/dc14-1096. [DOI] [PubMed] [Google Scholar]
- 25. Kusaka H, Koibuchi N, Hasegawa Y, Ogawa H, Kim‐Mitsuyama S. Empagliflozin lessened cardiac injury and reduced visceral adipocyte hypertrophy in prediabetic rats with metabolic syndrome. Cardiovasc Diabetol. 2016;15(1):157 10.1186/s12933-016-0473-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hammoudi N, Jeong D, Singh R, et al. Empagliflozin improves left ventricular diastolic dysfunction in a genetic model of type 2 diabetes. Cardiovasc Drugs Ther. 2017;31(3):233‐246. 10.1007/s10557-017-6734-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Habibi J, Aroor AR, Sowers JR, et al. Sodium glucose transporter 2 (SGLT2) inhibition with empagliflozin improves cardiac diastolic function in a female rodent model of diabetes. Cardiovasc Diabetol. 2017;16(1):9 10.1186/s12933-016-0489-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Verma S, Garg A, Yan AT, et al. Effect of empagliflozin on left ventricular mass and diastolic function in individuals with diabetes: an important clue to the EMPA‐REG OUTCOME trial? Diabetes Care. 2016;39(12):e212 LP‐e213. [DOI] [PubMed] [Google Scholar]
- 29. Andreadou I, Efentakis P, Balafas E, et al. Empagliflozin limits myocardial infarction in vivo and cell death in vitro: role of STAT3, mitochondria, and redox aspects. Front Physiol. 2017;8:1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhong M, Alonso CE, Taegtmeyer H, Kundu BK. Quantitative PET imaging detects early metabolic remodeling in a mouse model of pressure‐overload left ventricular hypertrophy in vivo. J Nucl Med. 2013;54(4):609‐615. 10.2967/jnumed.112.108092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heyen JRR, Blasi ER, Nikula K, et al. Structural, functional, and molecular characterization of the SHHF model of heart failure. Am J Physiol Heart Circ Physiol. 2002;283(5):H1775‐H1784. 10.1152/ajpheart.00305.2002. [DOI] [PubMed] [Google Scholar]
- 32. Youcef G, Olivier A, L'Huillier CPJ, et al. Simultaneous characterization of metabolic, cardiac, vascular and renal phenotypes of lean and obese SHHF rats. PLoS One. 2014;9(5):e96452. 10.1371/journal.pone.0096452. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1. Supplementary materials and methods.
Figure S1. Body weight during 25 to 27 weeks of study period. Data are mean ± SEM (SD‐VEH N = 14, SHHF‐VEH N = 8, SHHF‐EMPA N = 12). General time effect independent of group was P < 0.001. Group effect independent of time: *P < 0.05 vs. SD‐VEH, # P < 0.05 vs SHHF‐VEH.
Figure S2. Changes in blood glucose levels (relative to the baseline value) during 120 minutes upon intraperitoneal insulin administration. Data are means ± SD (n = 3 per group). *P < 0.05 vs SD.
Figure S3. Representative immunoblots of (A) cardiac CPT1B and (B) HMGCS2. CPT1B: carnitine palmitoyltransferase 1B, HMGCS2: 3‐hydroxy‐3‐methylglutaryl‐CoA synthase 2. GAPDH: Glyceraldehyde 3‐phosphate dehydrogenase.
Figure S4. Representative Sirius Red‐stained heart sections. Collagen appear red, cytoplasm appears yellow. White bars indicate 100 μm.


