Abstract
Aims
To compare the time spent in specified glycaemic ranges in people with type 1 diabetes (T1D) during 5 consecutive days of moderate‐intensity exercise while on either 100% or 75% of their usual insulin degludec (IDeg) dose.
Materials and Methods
Nine participants with T1D (four women, mean age 32.1 ± 9.0 years, body mass index 25.5 ± 3.8 kg/m2, glycated haemoglobin 55 ± 7 mmol/mol (7.2% ± 0.6%) on IDeg were enrolled in the trial. Three days before the first exercise period, participants were randomized to either 100% or 75% of their usual IDeg dose. Participants exercised on a cycle ergometer for 55 minutes at a moderate intensity for 5 consecutive days. After a 4‐week wash‐out period, participants performed the last exercise period for 5 consecutive days with the alternate IDeg dose. Time spent in specified glycaemic ranges, area under the curve and numbers of hypoglycaemic events were compared for the 5 days on each treatment allocation using a paired Students' t test, Wilcoxon matched‐pairs signed‐rank test and two‐way ANOVA.
Results
Time spent in euglycaemia over 5 days was greater for the 75% IDeg dose versus the 100% IDeg dose (4008 ± 938 minutes vs. 3566 ± 856 minutes; P = 0.04). Numbers of hypoglycaemic events (P = 0.91) and time spent in hypoglycaemia (P = 0.07) or hyperglycaemia (P = 0.38) was similar for both dosing schemes.
Conclusions
A 25% reduction in usual IDeg dose around regular exercise led to more time spent in euglycaemia, with small effects on time spent in hypo‐ and hyperglycaemia.
Keywords: exercise intervention, insulin therapy, type 1 diabetes
1. INTRODUCTION
Insulin degludec (IDeg) is associated with improved glycaemic control (glycated haemoglobin [HbA1c] concentration), lower risk of hypoglycaemia and less glycaemic variability compared with insulin glargine (IGlar) because of its favourable pharmacokinetic and pharmacodynamic properties.1, 2, 3 The risk of exercise‐induced hypoglycaemia during exercise and for the subsequent 24 hours is similar with IDeg and IGlar, despite different pharmacodynamic profiles and duration of action.4
Various therapy strategies have been suggested to reduce the risk of exercise‐induced hypoglycaemia, namely, prandial bolus insulin dose reductions, additional carbohydrate consumption and/or basal insulin reductions.5 Therapy adjustment is mainly based on acute exercise‐specific factors, such as the intensity, duration and type of exercise; however, other factors, such as circulating insulin concentrations, hepatic and skeletal muscle glycogen storage and counterregulatory hormone concentrations need to be considered when performing exercise. Patients on multiple daily injections are advised to reduce pre‐ and post‐exercise prandial bolus insulin dose by 25% for low‐intensity, 50% for moderate‐intensity and 75% for high‐intensity exercise performed for 30 to 45 minutes.6 Blood glucose levels between 7 mmol/L (126 mg/dL) and 10 mmol/L (180 mg/dL) should be targeted by administering additional carbohydrates depending on the volume of exercise (eg, 15‐20 g/h).6
An unaltered usual dose of IDeg can be employed safely as a background insulin if patients wish to engage in low‐, moderate‐ and high‐intensity exercise when applying bolus insulin dose reductions.7, 8 The risk of hypoglycaemia of an acute bout of exercise may persist for at least 24 hours, driven partly by circulating insulin levels and increased tissue insulin sensitivity.5 Although basal rate reductions are recommended in patients running on continuous subcutaneous insulin infusion, limited data exist on basal insulin dose reductions for multiple dose insulin injections.5, 9 Campbell et al10 reported that reducing the basal insulin dose combined with reduced prandial bolus insulin dose protects the individual from hypoglycaemia for 24 hours after moderate‐intensity exercise. Importantly, these adjustments were not associated with significant increases in time spent in hyperglycaemia (67 ± 16 minutes) or hypoglycaemia (5 ± 6 minutes) within the 24 hours post‐exercise period.
The vast majority of research investigating the safety of basal insulins assessed only an acute bout of exercise.4, 8, 11 In line with international guidelines, individuals with type 1 diabetes (T1D) are recommended to exercise regularly as part of their usual care.9 This raises the question of whether regular exercise exposes the patient to a greater risk of hypoglycaemia as a result of the additive insulin‐sensitizing effects of these repeated exercise bouts.
Regular exercise is a cornerstone of good diabetes management and more people with T1D are channelling their motivation to exercise into attempting sport events such as marathon running, long distance cycling or triathlons.12, 13 This commitment is accompanied by regular training for many months, with acute exercise sessions often performed over consecutive days. The same is also true for people with T1D performing a week of irregular exercise, such as a skiing week or an activity holiday.14 Considering the long‐lasting effect of IDeg,1, 2, 3 rapid changes in the circulating IDeg concentrations for isolated exercise bouts are not possible; however, there is no research in the literature that has explored the impact of IDeg dose reduction on glycaemia when people with T1D are regularly exercising. Interestingly, despite this lack of research, a recent review did not recommend IDeg dose reductions around regular exercise because this might compromise overall glycaemic control.5 Nocturnal hypoglycaemia, a potentially life‐threatening event,15, 16 occurs more frequently in people with T1D after performing exercise compared with when daily exercise was not performed because of the extended effect of exercise.17 The incidence of developing post‐exercise hypoglycaemia after moderate‐intensity exercise has been reported to be up to 66% in individuals with T1D across studies.18, 19 So far, no research has investigated the effect of basal insulin dose reductions on day‐ and night‐time glycaemia when patients are exercising regularly.
In the present study, we compared the time spent in specified glycaemic ranges during 5 consecutive days of continuous moderate‐intensity exercise, stratified for time of day as well as for first and last period within the 5 days, either treated with 100% or 75% of their usual IDeg dose in people with T1D.
2. MATERIAL AND METHODS
2.1. Study procedures
This single‐centre, randomized, open‐label, two‐period, crossover trial was performed in line with the Declaration of Helsinki and Good Clinical Practice and was approved by the ethics committee of the Medical University of Graz (29‐334 ex 16/17) and local health authority (EudraCT number: 2017‐000922‐37). The trial was registered at the German Clinical Trials Register (DRKS.de; DRKS00013477).
2.2. Screening visit
Adults (aged 18‐65 years, both inclusive) with T1D (diagnosed > 12 months) eligible for this study had a body mass index of 18.5 to 32 kg/m2, an HbA1c concentration of 42 to 86 mmol/mol (6%‐10%), a fasting C‐peptide level of < 0.25 nmol/L and were on multiple daily injections. Participants were exercising >150 min/wk with moderate intensity or > 75 min/wk with high intensity. People with diabetes‐related complications (micro‐ and macrovascular), women of childbearing potential, who were pregnant, breastfeeding, intending to become pregnant or not using adequate contraceptive methods, people with illness or disease that might confound the study results, people receiving drugs that might interfere with insulin action, people with suspected allergy to the trial products or addiction to alcohol or drugs, and people with mental incapacity were excluded.
After assessment of eligibility, participants filled in the International Physical Activity Questionnaire to assess physical activity (MET min/wk). They then performed an incremental cardiopulmonary exercise test on a cycle ergometer until maximal volitional exhaustion, defined as detected oxygen uptake (VO2) plateau, a maximum lactate concentration > 10 mmol/L, a respiratory exchange ratio (RER) > 1.1 and inability to maintain a cadence ≥ 50 rpm for 5 seconds. Pulmonary gas exchange variables were measured continuously during the incremental cardiopulmonary exercise testing by breath‐by‐breath measurement, and were averaged over 5 seconds (METAMAX® 3B; Cortex Biophysik GmbH, Leipzig, Germany). Heart rate was measured continuously via chest belt telemetry and was also averaged over 5 seconds (s810i, Polar Electro, Espoo, Finland). A 12‐lead ECG and blood pressure measurement were obtained for cardiovascular monitoring (ZAN 600; nSpire Health, Oberthulba, Germany). Capillary blood lactate and glucose concentration were taken from the earlobe at rest, at the end of the warm‐up phase, after each exercise step until maximum voluntary exhaustion, at the end of the active recovery and finally, at the end of the passive recovery (Biosen S‐line, EKF Diagnostics, Barleben, Germany).8 Additionally, blood glucose obtained from fingertip was measured via blood glucometer (FreeStyle Libre, Maidenhead, UK) every 2 minutes to minimize the risk of hypoglycaemia.
At the beginning of the incremental cardiopulmonary exercise test, participants sat on the cycle ergometer for 3 minutes (0 W) before they started the warm‐up period of 3 minutes by pedalling at a workload of 20 W. Then, the workload increased by 10, 15 or 20 W every minute, depending on their expected functional capacity to reach a total test duration of 8 to 12 minutes. Finally, a 3‐minute active recovery at 20 W followed by 3 minutes' passive recovery (0 W) were conducted. The first and second lactate points (LTP1 and LTP2) as well as the maximum power output (Pmax) were determined to prescribe the exercise intensity for the upcoming two exercise periods.20, 21, 22 As participants were already on IDeg (Tresiba; Novo Nordisk A/S, Bagsvaerd, Denmark), a stable basal insulin therapy was defined as a pre‐breakfast self‐measured blood glucose concentration of 4 to 7 mmol/L (72‐126 mg/dL) over 6 days.
Additionally, participants received an unblinded intermittently viewed continuous glucose monitoring (CGM) system (FreeStyle Libre) and were trained on how to use the system. Participants inserted and wore the sensor on the back of the upper arm without any over‐bandage for 14 days. If the sensor expired or fell off, participants immediately inserted a new sensor on the back of the alternate upper arm. Within the first 24‐hour calibration period, participants performed at least five additional blood glucose measurements via glucometer because of the system's inaccuracy during this run‐in period. Additionally, participants changed the sensor earlier if the sensor would expire within the 5‐day exercise period. Data were then exported at the end of each dosing scheme and 15‐minute averaged data were used for the analyses. Seven participants were already using the intermittently viewed CGM system prior to the start of the study.
2.3. Trial visits
Three days before the first exercise period, participants were randomized to either 100% or 75% of their usual IDeg dose, injected once‐daily in the evening. Randomization was performed using the web‐based randomization tool “Randomiser for Clinical Trials: http://www.randomizer.at/” provided by the Institute of Medical Informatics, Statistics and Documentation, Medical University of Graz. Then participants exercised on a cycle ergometer for 55 minutes at a moderate intensity (midpoint between LTP1 and LTP2 [63% ± 7% VO2max]) for 5 consecutive days in the evening (at 5:00 pm or 6:30 pm) at the clinical research facility. In total, 90 days of continuous interstitial glucose measurements were available (nine participants performed 10 exercise sessions; five for each of the two dosing schemes). Participants were instructed to avoid additional structured exercise during the days of exercise procedures.
Prior to the exercise periods, participants consumed on average 3.4 ± 0.3 g carbohydrates per body weight (kg) per day. Participants were encouraged to consume 5 to 7 g carbohydrates per body weight (kg) per day, beginning 1 day before the 5‐day exercise period, and to replicate their diet for both dosing schemes.23 All participants were treated with IDeg as a basal insulin and insulin lispro (Eli Lilly, Indianapolis, Indiana; n = 3) or insulin aspart (Novo Nordisk; n = 6) as a bolus insulin. Prandial bolus insulin dose, correction bolus insulin doses, basal insulin doses, prandial carbohydrates and correction carbohydrates were documented in a standardized diary, beginning 1 day before the first exercise session. Participants were counting carbohydrates prior to the start of the trial and this was maintained during the run of the trial. They were trained and experienced in carbohydrate‐counting, with annual refreshers. At each visit, diaries were reviewed by the study team and a dietician was consulted if necessary. Prior to the incremental cardiopulmonary exercise testing and the 5‐day exercise period, participants were told to inject the last bolus insulin and consume the last pre‐exercise carbohydrate‐rich meal at least 2 hours before exercise to minimize the impact of bolus insulin/meal‐induced blood glucose variability. If blood glucose concentration was <7 mmol/L (126 mg/dL) 15 minutes before the exercise, 15 to 30 g carbohydrates were given to the participants (fruit juice or glucose gel); in contrast, when blood glucose concentration was >10 mmol/L (180 mg/dL) carbohydrate administration was delayed until reaching 7 mmol/L (126 mg/dL).23 Administration of carbohydrates was repeated if blood glucose concentration did not rise above 7 mmol/L (126 mg/dL) within 10 minutes; however, when blood glucose concentration reached a hypoglycaemic threshold of 3.9 mmol/L (70 mg/dL), exercise testing was discontinued until reaching 7 mmol/L (126 mg/dL). During the continuous moderate‐intensity exercise sessions, pulmonary gas exchange variables and heart rate were collected continuously, and capillary blood samples were taken from the ear lobe at rest, at the end of the warm‐up period, every 7 minutes during the target workload, and at the end of the active and passive recovery periods, to determine lactate concentrations. Additionally, glucose concentrations were measured via blood glucose from fingertip and via interstitial glucose from the intermittently viewed CGM system every 7 minutes. Venous blood samples were taken from the antecubital vein immediately before (M1, reference value) and immediately after the first exercise session (M2), 23 hours after the first exercise session (M3), immediately before (M4) and immediately after the last exercise session (M5) as well as 23 hours after the last exercise session (M6) to determine plasma insulin levels by chemiluminescence on an ADVIA Centaur system (Siemens Healthcare Diagnostics, Eschborn, Germany).
2.4. Statistical analyses
The sample size was estimated using the calculator freely available at http://www.sample-size.net, applying a paired Student's t test. For the sample size estimation, we assumed a mean time in euglycaemia in the full basal insulin dosing scheme (100% IDeg) of 643 ± 54 min/d.10 Assuming a moderate and clinically relevant improvement of 10% of mean time in euglycaemia in the reduced dosing scheme (75% IDeg), 10 participants were required for the study to detect this difference, with an α value of 0.05 and a power of 0.90. Data for time spent in euglycaemia (4.0‐9.9 mmol/L [71‐179 mg/dL]), hypoglycaemia (≤3.9 mmol/L [≤70 mg/dL]), serious hypoglycaemia (≤2.9 mmol/L [≤53 mg/dL]), hyperglycaemia (≥10 mmol/L, [≥180 mg/dL]) and serious hyperglycaemia (≥16.7 mmol/L [≥300 mg/dL]) were analysed for the period from post‐first‐exercise session until 24 hours after the last exercise session for comparing both dosing schemes (100% IDeg vs. 75% IDeg) excluding the exercise sessions.24 For all glycaemic ranges the area under the curve (AUC; eg, AUC euglycaemia = excluding hypo‐ and hyperglycaemia) was calculated using the trapezoid method (sum of trapezoids and compared between the dosing schemes. Additionally, glycaemic variability, defined as coefficient of variation in glycaemic values, numbers of hypoglycaemic episodes (interstitial glucose ≤ 3.9 mmol/L [70 mg/dL] for ≥15 minutes), dose of preprandial and correction bolus insulin doses as well as prandial and hypoglycaemic correction carbohydrates were compared between the dosing schemes. Interstitial glucose data obtained from the intermittently viewed CGM system were averaged over 15 minutes. Data were tested for distribution using the Shapiro–Wilk normality test. Comparisons between dosing schemes were performed via paired Student's t test, Wilcoxon matched‐pairs signed‐rank test and two‐way ANOVA with Bonferroni's multiple comparisons test (P < 0.05). Data were analysed with SPSS (version 22; SPSS Inc, Chicago, Illinois) and Graphpad Prism (version 7; San Diego, California).
3. RESULTS
Out of 11 patients screened, 10 were eligible and randomized in the study. Nine participants completed both trial arms as one patient withdrew after the first exercise period for personal reasons. Data for this participant were not used in the analysis. All included participants were already on IDeg at least 3 months prior to the start of the study. Accompanying home physical activity did not differ between the two dosing schemes as shown by the median (interquartile range) MET of 2499 (2109‐7284) min/wk versus 2958 (1358‐8360) min/wk (P = 0.84) in the 100% IDeg and 75% IDeg periods, respectively. The median (interquartile range) amount of accompanying physical exercise, as documented by the participants, was also not significantly different between the two dosing schemes: 100% IDeg: 30 (8‐54) min/wk versus 75% IDeg: 20 (3‐35) min/wk (P = 0.34). Participants consumed comparable amounts of carbohydrates at their last pre‐exercise meals on each of the 5 exercise days (P = 0.18). The mean carbohydrate amount at their last pre‐exercise meals was also comparable between the dosing schemes (100% IDeg 62 ± 6 g vs. 75% IDeg 64 ± 15 g, respectively; P = 0.83). Absolute recorded time periods with available glucose readings were similar in both dosing schemes (P = 0.15), hence we used calculations for absolute and relative time spent in glycaemic ranges for our analyses.
3.1. Participant characteristics
The four women and five men completing the present study had a mean ± SD age of 32.1 ± 9.0 years, a mean ± SD body mass index of 25.5 ± 3.8 kg/m2, a mean ± SD (min.– max.) HbA1c of 55 ± 7 (43–62) mmol/mol (7.2 ± 0.6 [6.1‐7.8]%), a mean ± SD (min.– max.) diabetes duration of 19 ± 10.9 (6‐42) years and a mean ± SD total daily insulin dose of 35 ± 13 IU. The mean ± SD (min.– max.) maximum oxygen uptake (VO2max) was 39 ± 12 (22‐64) mL/kg/min, maximum heart rate was 182 ± 13 beats/min, maximum RER was 1.23 ± 0.09, maximum lactate concentration was 11.8 ± 2.2 mmol/L, power at LTP1 (PLTP1) 57 ± 21 W, power output at LTP2 (PLTP2) 147 ± 47 W and maximum power output (Pmax) 227 ± 77 W. All participants achieved the predefined criteria for maximal exhaustion.
3.2. Physiological and performance data
The 100% IDeg dose corresponded to a mean ± SD total daily basal insulin dose of 19 ± 4 IU while for 75% IDeg it was 14 ± 3 IU. The mean physiological characteristics over the 5 exercising days were similar during the 100% IDeg and 75% IDeg dosing periods (Table 1).
Table 1.
Exercise physiological data comparing 100% insulin degludec (IDeg) and 75% IDeg dosing
| 100%IDeg | 75%IDeg | P | |
|---|---|---|---|
| VO2 of VO2max, % | 62 ± 6 | 64 ± 7 | 0.14 |
| VO2, mL/kg/min | 23 ± 6 | 25 ± 6 | 0.56 |
| Heart rate, beats/min | 132 ± 12 | 135 ± 11 | 0.06 |
| RER | 0.97 ± 0.06 | 0.96 ± 0.07 | 0.08 |
| Lactate, mmol/L | 2.1 ± 0.1 | 2.2 ± 0.2 | 0.78 |
Abbreviations: IDeg, insulin degludec; RER, respiratory exchange ratio; VO2, oxygen uptake; VO2 of VO2max, oxygen uptake presented as percentage of maximum oxygen uptake.
Values are given as mean ± SD and mean difference.
3.3. Glycaemic responses during the 5‐day exercise period
Glycaemic data were compared from immediately post‐first exercise session until 24 hours post‐last exercise session for each dosing period. The 75% IDeg dose resulted in a significant increase in time spent in euglycaemia (P = 0.04). No significant differences were found for time spent in hypo‐ and hyperglycaemic ranges, hypoglycaemic episodes, glycaemic variation, carbohydrate consumption or insulin administration, as shown in Table 2 (P > 0.05).
Table 2.
Comparison of the entire 5 days of exercise in the 100% insulin degludec (IDeg) and 75% IDeg dosing period for glycaemic ranges, area under the curve for hyperglycaemic, euglycaemic, hypoglycaemic ranges, hypoglycaemic events, coefficient of variation for glycaemia, carbohydrate consumption and insulin administration
| Overall | 100% IDeg | 75% IDeg | P |
|---|---|---|---|
| Timeeuglycaemia, min | 3566 ± 856 | 4008 ± 938 | 0.04 |
| Timeeuglycaemia, % | 57 ± 14 | 62 ± 15 | 0.16 |
| Timehypoglycaemia, min | 240 (128‐465) | 270 (90‐683) | 0.07 |
| Timehypoglycaemia, % | 3.6 (1.9‐7.9) | 4.0 (1.3‐10) | 0.10 |
| Timeserious hypoglycaemia, min | 45 (0‐105) | 30 (0‐233) | 0.15 |
| Timeserious hypoglycaemia, % | 0.7 (0‐1.7) | 0.4 (0‐3.5) | 0.30 |
| Timehyperglycaemia, min | 2440 ± 1094 | 2187 ± 1046 | 0.38 |
| Timehyperglycaemia, % | 38 ± 16 | 33 ± 16 | 0.23 |
| Timeserious hyperglycaemia, min | 195 (0‐420) | 165 (23‐405) | 0.36 |
| Timeserious hyperglycaemia, % | 2.8 (0‐7.3) | 2.7 (0.4‐5.9) | 0.64 |
| AUCeuglycaemia | 11 310 ± 3190 | 12 772 ± 3214 | 0.08 |
| AUChypoglycaemia | 121 ± 106 | 153 ± 138 | 0.09 |
| AUCserious hypoglycaemia | 107 (0–248) | 65 (0–600) | 0.30 |
| AUChyperglycaemia | 5893 ± 3092 | 5332 ± 3024 | 0.52 |
| AUCserious hyperglycaemia | 374 (0‐1259) | 192 (7‐846) | 0.84 |
| Hypoglycaemic events per participant over 5 days, n | 4.7 ± 2.9 | 4.8 ± 3.4 | 0.91 |
| CVglycaemia, % | 39 ± 7 | 40 ± 7 | 0.57 |
| Prandial insulin used, IU | 73 ± 40 | 72 ± 32 | 0.89 |
| Correction insulin used, IU | 17 ± 10 | 20 ± 10 | 0.31 |
| Prandial carbohydrates, g | 648 ± 115 | 739 ± 237 | 0.10 |
| Correction carbohydrates, g | 259 ± 114 | 219 ± 112 | 0.17 |
Abbreviations: AUC, area under the curve; CV, coefficient of variation; IDeg, insulin degludec.
3.4. Glycaemia at daytime versus night‐time during the 5‐day exercise period
While there was a numerical trend for more time spent in euglycaemia for the 75% IDeg dosing period during night‐time, it did not reach statistical significance (P = 0.059; Table 3):
Table 3.
Comparison of 100% insulin degludec (IDeg) and 75% IDeg dosing for glycaemic ranges, hypoglycaemic events, coefficient of variation and for glycaemia, based on time of day (night‐time or daytime)
| 100% IDeg | 75% IDeg | P | |
|---|---|---|---|
| Night‐time (12:00 am to 6:00 am) | |||
| Timeeuglycaemia, min | 695 ± 228 | 947 ± 350 | 0.059 |
| Timeeuglycaemia (%) | 43 ± 12 | 54 ± 20 | 0.10 |
| Timehypoglycaemia, min | 105 (8‐300) | 195 (0‐330) | 0.39 |
| Timehypoglycaemia (%) | 6 (0.5‐21) | 12 (0‐18) | 0.64 |
| Timehyperglycaemia, min | 755 ± 397 | 582 ± 379 | 0.23 |
| Timehyperglycaemia (%) | 45 ± 23 | 33 ± 22 | 0.17 |
| Hypoglycaemic events (n) (per participant over 5 days) | 1 (1‐3) | 1.7 ± 1.5 | 0.99 |
| CVglycaemia (%) | 39 ± 13 | 40 ± 11 | 0.86 |
| Daytime (6:00 am–12:00 am) | |||
| Timeeuglycaemia, min | 2732 ± 686 | 2739 ± 1342 | 0.49 |
| Timeeuglycaemia,% | 60 ± 14 | 57 ± 24 | 0.31 |
| Timehypoglycaemia, min | 165 (53‐210) | 105 (38‐405) | 0.82 |
| Timehypoglycaemia, % | 3 (1.5‐5.5) | 2 (1.5‐9) | 0.93 |
| Timehyperglycaemia, min | 1677 ± 789 | 1598 ± 799 | 0.71 |
| Timehyperglycaemia, % | 36 ± 16 | 38 ± 25 | 0.75 |
| Hypoglycaemic events (n) | 4 ± 2.5 | 2 (1.5–6) | 0.44 |
| CVglycaemia, % | 38 ± 6 | 38 ± 7 | 0.95 |
Abbreviations: CV, coefficient of variation; IDeg, insulin degludec.
Values are given as mean ± SD or median (25‐75 percentile).
3.5. Glycaemia during the first and last phase of the exercise period
No significant differences for glycaemic ranges were found comparing the 100% IDeg and 75% IDeg period when analysing data within the first 48 hours and last 48 hours of the 5‐day exercise intervention (P > 0.05; Table 4).
Table 4.
Comparison of 100% insulin degludec (IDeg) and 75%IDeg for glycaemic ranges, hypoglycaemic events, coefficient of variation (CV) and for glycaemia based on the first (first 48 h) and last exercise phase (last 48 h)
| 100% IDeg | 75% IDeg | P | |
|---|---|---|---|
| First phase (first 48 h) | |||
| Timeeuglycaemia, min | 1560 (1343‐2880) | 1738 ± 680 | 0.15 |
| Timeeuglycaemia, % | 59 (48‐76) | 63 ± 11 | 0.28 |
| Timehypoglycaemia, min | 90 (23‐390) | 60 (23‐428) | 0.99 |
| Timehypoglycaemia, % | 4 (1‐8) | 2 (1.5‐15) | 0.87 |
| Timehyperglycaemia, min | 953 ± 533 | 1003 ± 248 | 0.74 |
| Timehyperglycaemia, % | 35 ± 20 | 34 ± 18 | 0.66 |
| Hypoglycaemic events, n (per participant per 5 days) | 2.1 ± 1.3 | 2 (1‐3.5) | 0.67 |
| CVglycaemia, % | 37 ± 8 | 36 ± 6 | 0.69 |
| Last phase (last 48 h) | |||
| Timeeuglycaemia, min | 1497 ± 338 | 1653 ± 479 | 0.20 |
| Timeeuglycaemia, % | 55 ± 13 | 62 ± 16 | 0.16 |
| Timehypoglycaemia, min | 105 (30‐248) | 75 (15‐188) | 0.71 |
| Timehypoglycaemia, % | 4 (1‐10) | 3 (0.5‐7) | 0.71 |
| Timehyperglycaemia, min | 1055 ± 502 | 783 ± 39 | 0.13 |
| Timehyperglycaemia, % | 38 ± 18 | 30 ± 15 | 0.17 |
| Hypoglycaemic events (n) | 2.3 ± 1.6 | 1.9 ± 1.5 | 0.46 |
| CVglycaemia, % | 38 ± 8 | 41 ± 11 | 0.53 |
Abbreviations: CV, coefficient of variation; IDeg, insulin degludec.
Values are given as mean ± SD or median (25‐75 percentile).
3.6. Plasma insulin levels
No significant differences were found for plasma insulin concentrations in the two dosing schemes (P = 0.34). Significant differences were found within dosing schemes in plasma insulin levels over the time course (basal insulin dose × time) with a drop during the exercise and a rise over the next 23 hours (P = 0.002; Figure 1).
Figure 1.
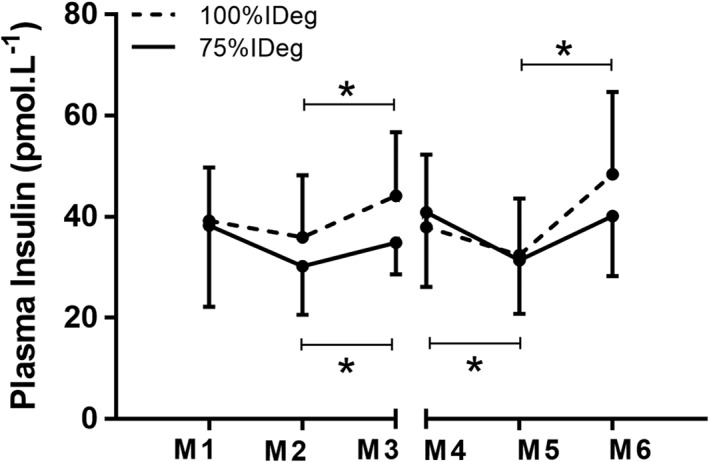
Plasma insulin for 100% insulin degludec (IDeg; dotted line) and 75% IDeg (full line) for immediately before (M1) and immediately after the first exercise session (M2), 23 hours after the first exercise session (M3), immediately before (M4) and immediately after the last exercise session (M5) as well as 23 hours after the last exercise session (M6). *Indicates significant differences over time for 100% IDeg and 75% IDeg. Values are given as mean ± SD
4. DISCUSSION
The present study showed that a reduction in IDeg dose in people with T1D while performing regular exercise over 5 consecutive days improves time spent in euglycaemia significantly. Numerically, albeit not statistically significantly, 75% IDeg reduced time spent in hyperglycaemia. Interestingly, time spent in hypoglycaemia (3.9 mmol/L [70 mg/dL]) was numerically increased, while time spent below 3 mmol/L (54 mg/dL) was numerically lower under the 75% dosing scheme.
Although participants administered similar amounts of prandial insulin for both dosing schemes, the prandial carbohydrate intake was non‐significantly but numerically higher for the 75% IDeg dosing scheme while the correction carbohydrate intake was numerically higher during the 100% IDeg dosing scheme. It could be speculated that participants were more hesitant about having additional correction carbohydrates as they knew they were on the lower basal insulin dose; however, given these inconclusive data, the small proportion of time spent in hypoglycaemia and the fact that none of these findings were statistically significant, future, larger studies are needed to clarify the impact of various IDeg doses on hypoglycaemia.
Importantly, the reductions in IDeg dose did not alter bolus insulin therapy while improving time spent in euglycaemia, which support the ease of use in regularly exercising people with T1D. A non‐significant difference was observed for heart rate (−3 ± 0.4 b/min) and RER (−0.01 ± 0.03) when comparing the two dosing schemes, which was considered biological variability.
The effects of regular exercise training on glycaemic control are equivocal.25 Exercise may lead to a glycaemic benefit in young people with T1D when undertaken for longer periods, but this was not found in adults with T1D. A potential reason for this finding may be a mandatory increased carbohydrate intake to prevent exercise‐induced hypoglycaemia leading to a mismatch of carbohydrate to insulin ratio. Reducing the basal insulin dose by 25% might support the beneficial effects of regular exercise on glycaemic control in individuals with T1D if additional carbohydrate intake is reduced.
Regular exercise increases insulin sensitivity in both people with type 1 and those with type 2 diabetes.26, 27 The physiological underpinning mainly lies in the transcription of glucose transport proteins (glucose transporter type 4), its movement and insertion into membranes, facilitating insulin‐independent intramuscular glucose uptake.28 While blood glucose homeostasis in healthy individuals is regulated via increasing counterregulatory hormones and its effect on hepatic glycogen depletion, glucagon fails to increase in people with T1D and thus to prevent exercise‐induced hypoglycaemia.7 Furthermore, circulating exogenous insulin levels persist on the basis of its duration of action,29 which increases the risk of short‐ and long‐term exercise‐induced hypoglycaemia.
Interestingly, neither the time of day (daytime or night‐time) nor the phase within the 5 exercise days (first 48 hours or last 48 hours) differed significantly when comparing glycaemic ranges, AUC, hypoglycaemic events or glycaemic variably for 100% IDeg versus 75% IDeg. As participants were trained in how to prevent nocturnal hypoglycaemia, this potentially had an influence on our results. Training was needed for safety reasons as participants used an intermittently viewed CGM system instead of real‐time CGM, which does not offer an automatic hypoglycaemia alarm.
The coefficient of variation in glycaemia was similar when comparing dosing schemes for the 5‐day exercise period, time of day and phase within the 5 days (first and last 48 hours), which showed that IDeg dose reductions around regular exercise were not accompanied by large glucose fluctuations. Free plasma insulin levels were similar between 100% IDeg and 75% IDeg, with numerically slightly higher levels while on 100% IDeg. Similarly to the authors of previous studies, we observed a drop in plasma insulin levels during exercise.6, 30 Most bolus insulins show their peak action ~2 hours after injection, followed by a decrease in circulating insulin levels through attenuation of bolus insulin.31, 32 In the present study, exercise was performed at least 2 hours after bolus insulin injection, which might explain the decrease in plasma insulin levels during exercise, as bolus insulin action mitigates.
While the present study shows that the time spent in euglycaemia during multiple moderate exercise sessions is increased with the 75% IDeg dosing scheme, we have to clearly acknowledge that a number of findings (eg, time spent in hypoglycaemia, prandial carbohydrate intake) were approaching but not reaching statistical significance and should be interpreted with caution. Larger exercise trials in people with T1D are urgently needed to clarify dose adjustments around exercise sessions of various types and volumes.
Despite the fact that regular IGlar is associated with more hypoglycaemic episodes than basal insulin detemir (IDet; Levemir, Novo Nordisk) during and after exercise,11 a 20% reduction in both IGlar and IDet protected against hypoglycaemia for 24 hours after evening exercise.10 The extended duration of action of IDeg could lead to the misinterpretation that patients lose flexibility in dose adjustments around exercise.5 This might hold true for isolated, unregular exercise bouts, but the present study demonstrates that for participants with T1D performing exercise over 5 consecutive days, IDeg can be used but the dose should be reduced by 25%, as has been observed for other long‐acting insulin analogues.
CONFLICTS OF INTEREST
O.M. has received lecture fees from Medtronic, travel grants from Novo Nordisk A/S and research grants from Sêr Cymru II COFUND fellowship/European Union and Novo Nordisk A/S. M.L.E. has received a KESS2/European Social Fund scholarship. R.M.B. reports having received honoraria, travel and educational grant support from, Boehringer‐Ingelheim, Eli Lilly and Company, Novo Nordisk and Sanofi‐Aventis. G.K. has received lecture fees from Novo Nordisk, AstraZeneca, Bristol‐Myers Squibb, Roche Diagnostics, Novartis, MSD and Eli Lilly. H.S. has received honoraria, travel support or unrestricted research grants by Amgen, Astra Zeneca, Boehringer‐Ingelheim, Eli Lilly, MSD, Novo Nordisk and Sanofi‐Aventis. The remaining authors have no relevant conflict of interest to disclose.
Author contributions
O.M., M.L.E., G.K., H.S. and P.H. designed and performed the study, interpreted data and contributed to discussions. O.M., R.M.B., H.S. and M.L.E. drafted the manuscript and performed statistical analysis. P.H., H.S., A.M., P.B., C.S., P.P., H.K. and P.D. performed the study. All authors critically revised the article and approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors would like to thank the study participants for their excellent adherence to the study protocol. We would like to thank Faisal Aziz for his assistance with the statistical analyses.
Moser O, Eckstein ML, Mueller A, et al. Reduction in insulin degludec dosing for multiple exercise sessions improves time spent in euglycaemia in people with type 1 diabetes: A randomized crossover trial. Diabetes Obes Metab. 2019;21:349–356. 10.1111/dom.13534
Funding information This study was supported by an unrestricted grant from Novo Nordisk, Austria.
REFERENCES
- 1. Bode BW, Buse JB, Fisher M, et al. Insulin degludec improves glycaemic control with lower nocturnal hypoglycaemia risk than insulin glargine in basal‐bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN(®) Basal‐Bolus type 1): 2‐year results of a randomized clinical trial. Diabet Med. 2013;30(11):1293‐1297. 10.1111/dme.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heise T, Hermanski L, Nosek L, Feldman A, Rasmussen S, Haahr H. Insulin degludec: four times lower pharmacodynamic variability than insulin glargine under steady‐state conditions in type 1 diabetes. Diabetes Obes Metab. 2012;14:1‐6. 10.1111/j.1463-1326.2012.01627.x. [DOI] [PubMed] [Google Scholar]
- 3. Vora J, Cariou B, Evans M, et al. Clinical use of insulin degludec. Diabetes Res Clin Pract. 2015;109(1):19‐31. 10.1016/j.diabres.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 4. Heise T, Bain SC, Bracken RM, et al. Similar risk of exercise‐related hypoglycaemia for insulin degludec to that for insulin glargine in patients with type 1 diabetes: a randomized cross‐over trial. Diabetes Obes Metab. 2016;18(2):196‐199. 10.1111/dom.12588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Riddell MC, Gallen IW, Smart CE, et al. Exercise management in type 1 diabetes: a consensus statement. Lancet Diabetes Endocrinol. 2017;8587(17):1‐14. 10.1016/S2213-8587(17)30014-1. [DOI] [PubMed] [Google Scholar]
- 6. Rabasa‐Lhoret R, Bourque J, Ducros F, Chiasson JL. Guidelines for premeal insulin dose reduction for postprandial exercise of different intensities and durations in type 1 diabetic subjects treated intensively with a basal‐bolus insulin regimen (ultralente‐lispro). Diabetes Care. 2001;24(4):625‐630. 10.2337/diacare.24.4.625. [DOI] [PubMed] [Google Scholar]
- 7. Moser O, Tschakert G, Mueller A, et al. Short‐acting insulin reduction strategies for continuous cycle ergometer exercises in patients with type 1 diabetes mellitus. Asian J Sports Med. 2017;8;e42160. [Google Scholar]
- 8. Moser O, Tschakert G, Mueller A, et al. Effects of high‐intensity interval exercise versus moderate continuous exercise on glucose homeostasis and hormone response in patients with type 1 diabetes mellitus using novel ultra‐long‐acting insulin. PLoS One. 2015;10(8):e0136489. 10.1371/journal.pone.0136489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(11):2065‐2079. 10.2337/dc16-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Campbell MD, Walker M, Bracken RM, et al. Insulin therapy and dietary adjustments to normalize glycemia and prevent nocturnal hypoglycemia after evening exercise in type 1 diabetes: a randomized controlled trial. BMJ Open Diabetes Res Care. 2015;3(1):e000085. 10.1136/bmjdrc-2015-000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arutchelvam V, Heise T, Dellweg S, Elbroend B, Minns I, Home PD. Plasma glucose and hypoglycaemia following exercise in people with type 1 diabetes: a comparison of three basal insulins. Diabet Med. 2009;26(10):1027‐1032. 10.1111/j.1464-5491.2009.02807.x. [DOI] [PubMed] [Google Scholar]
- 12. Baldi JC, Cassuto NA, Foxx‐Lupo WT, Wheatley CM, Snyder EM. Glycemic status affects cardiopulmonary exercise response in athletes with type I diabetes. Med Sci Sports Exerc. 2010;42(8):1454‐1459. 10.1249/MSS.0b013e3181d1fdb3. [DOI] [PubMed] [Google Scholar]
- 13. Yardley JE, Colberg SR. Update on management of type 1 diabetes and type 2 diabetes in athletes. Curr Sports Med Rep. 2017;16(1):38‐44. 10.1249/JSR.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 14. Breton MD, Cherñavvsky DR, Forlenza GP, et al. Closed‐loop control during intense prolonged outdoor exercise in adolescents with type 1 diabetes: the artificial pancreas ski study. Diabetes Care. 2017;40(12):1644‐1650. 10.2337/dc17-0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gomez AM, Gomez C, Aschner P, et al. Effects of performing morning versus afternoon exercise on glycemic control and hypoglycemia frequency in type 1 diabetes patients on sensor‐augmented insulin pump therapy. J Diabetes Sci Technol. 2015;9(3):619‐624. 10.1177/1932296814566233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tanenberg RJ, Newton CA, Drake AJ. Confirmation of hypoglycemia in the “dead‐in‐bed” syndrome, as captured by a retrospective continuous glucose monitoring system. Endocr Pract. 2010;16(2):244‐248. 10.4158/EP09260.CR. [DOI] [PubMed] [Google Scholar]
- 17. Tsalikian E, Mauras N, Beck RW, et al. Impact of exercise on overnight glycemic control in children with type 1 diabetes mellitus. J Pediatr. 2005;147(4):528‐534. 10.1016/j.jpeds.2005.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Campaigne BN, Gilliam TB, Spencer ML, Lampman RM, Schork MA. Effects of a physical activity program on metabolic control and cardiovascular fitness in children with insulin‐dependent diabetes mellitus. Diabetes Care. 1984;7(1):57‐62. 10.2337/DIACARE.7.1.57. [DOI] [PubMed] [Google Scholar]
- 19. Metcalf KM, Singhvi A, Tsalikian E, et al. Effects of moderate‐to‐vigorous intensity physical activity on overnight and next‐day hypoglycemia in active adolescents with type 1 diabetes. Diabetes Care. 2014;37(5):1272‐1278. 10.2337/dc13-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hofmann P, Pokan R, Preidler K, et al. Relationship between heart rate threshold, lactate turn point and myocardial function. Int J Sports Med. 1994;15(05):232‐237. 10.1055/s-2007-1021052. [DOI] [PubMed] [Google Scholar]
- 21. Hofmann P, Tschakert G. Special needs to prescribe exercise intensity for scientific studies. Cardiol Res Pract. 2011;2011:1‐10. 10.4061/2011/209302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tschakert G, Kroepfl J, Mueller A, Moser O, Groeschl W, Hofmann P. How to regulate the acute physiological response to “aerobic” high‐intensity interval exercise. J Sport Sci Med. 2015;14:29‐36. [PMC free article] [PubMed] [Google Scholar]
- 23. Gallen IW, Hume C, Lumb A. Fuelling the athlete with type 1 diabetes. Diabetes Obes Metab. 2011;13(2):130‐136. 10.1111/j.1463-1326.2010.01319.x. [DOI] [PubMed] [Google Scholar]
- 24. Workgroup on Hypoglycemia, American Diabetes Association . Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care. 2005;28(5):1245‐1249. http://www.ncbi.nlm.nih.gov/pubmed/15855602. Accessed June 9, 2017. [DOI] [PubMed] [Google Scholar]
- 25. Kennedy A, Nirantharakumar K, Chimen M, et al. Does exercise improve glycaemic control in type 1 diabetes? A systematic review and meta‐analysis. PLoS One. 2013;8(3):e58861 10.1371/journal.pone.0058861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chimen M, Kennedy A, Nirantharakumar K, Pang TT, Andrews R, Narendran P. What are the health benefits of physical activity in type 1 diabetes mellitus? A literature review. Diabetologia. 2012;55(3):542‐551. 10.1007/s00125-011-2403-2. [DOI] [PubMed] [Google Scholar]
- 27. Kirwan JP, Solomon TPJ, Wojta DM, Staten MA, Holloszy JO. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am J Physiol Metab. 2009;297(1):E151‐E156. 10.1152/ajpendo.00210.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rose AJ, Richter EA. Skeletal muscle glucose uptake during exercise: how is it regulated? Physiology (Bethesda). 2005;20:260‐270. 10.1152/physiol.00012.2005. [DOI] [PubMed] [Google Scholar]
- 29. Guelfi KJ, Ratnam N, Smythe GA, Jones TW, Fournier PA. Effect of intermittent high‐intensity compared with continuous moderate exercise on glucose production and utilization in individuals with type 1 diabetes. AJP Endocrinol Metab. 2006;292(3):E865‐E870. 10.1152/ajpendo.00533. [DOI] [PubMed] [Google Scholar]
- 30. Maran A, Pavan P, Bonsembiante B, et al. Continuous glucose monitoring reveals delayed nocturnal hypoglycemia after intermittent high‐intensity exercise in nontrained patients with type 1 diabetes. Diabetes Technol Ther. 2010;12(10):763‐768. 10.1089/dia.2010.0038. [DOI] [PubMed] [Google Scholar]
- 31. Walsh J, Roberts R, Heinemann L. Confusion regarding duration of insulin action: a potential source for major insulin dose errors by bolus calculators. J Diabetes Sci Technol. 2014;8(1):170‐178. 10.1177/1932296813514319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heise T, Hövelmann U, Brøndsted L, Adrian CL, Nosek L, Haahr H. Faster‐acting insulin aspart: earlier onset of appearance and greater early pharmacokinetic and pharmacodynamic effects than insulin aspart. Diabetes Obes Metab. 2015;17(7):682‐688. 10.1111/dom.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]


