Abstract
Objective
To investigate the impact of tumor necrosis factor inhibitor (TNFi) treatment and inflammation control on radiographic progression in early ankylosing spondylitis (AS) over 4 years.
Methods
We included a total of 215 patients with early AS (symptom duration <10 years) treated with TNFi (the TNFi group; n = 135) or with nonsteroidal antiinflammatory drugs (NSAIDs) (the control group; n = 80). Two blinded readers assessed radiographic progression using the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS). Inflammation control was inferred from C‐reactive protein (CRP) levels time‐averaged between 2 radiologic assessments. Linear mixed modeling was used to estimate mSASSS changes over radiographic intervals as well as the impact of clinical factors on outcomes.
Results
The TNFi group had longer disease duration, a higher baseline CRP level, and a higher Bath Ankylosing Spondylitis Disease Activity Index than did controls. The time‐averaged CRP level over radiographic intervals was lower with TNFi treatment than with NSAID treatment (mean ± SD 0.27 ± 0.30 mg/dl versus 0.61 ± 0.68 mg/dl; P < 0.001). Overall, mean ± SD mSASSS change over the 2‐year interval was 1.30 ± 2.97 units. In the multivariable model adjusted for age, smoking status, baseline CRP level, and the presence of syndesmophytes at baseline, the TNFi group showed less mSASSS change over the 2‐year interval (β = −0.90 [95% confidence interval {95% CI} −1.51, −0.29]). However, when a time‐averaged CRP level was additionally included, it significantly influenced the mSASSS change (β = 1.02 [95% CI 0.32, 1.71]), decreasing the estimated group difference (β = −0.52 [95% CI −1.17, 0.14]). NSAID indices of both groups were not associated with either time‐averaged CRP levels or mSASSS changes.
Conclusion
Effective suppression of inflammation by TNFi treatment decreases radiographic progression in early AS.
Introduction
It remains uncertain whether tumor necrosis factor inhibitors (TNFi) delay radiographic progression of ankylosing spondylitis (AS). Although TNFi effectively decrease spinal inflammation, previous studies from randomized controlled trials did not show any difference in radiographic progression between patients receiving TNFi and those using only nonsteroidal antiinflammatory drugs (NSAIDs) 1, 2, 3, 4. However, recent cohort studies suggested that early or long‐term TNFi treatment could slow down the process 5, 6, 7.
Some studies have supported the notion of a link between inflammation and pathologic new bone formation, especially during the early phase of the disease. Maksymowych et al showed that advanced inflammatory vertebral corner lesions on magnetic resonance imaging (MRI) are more likely to progress to syndesmophytes through a process of fat metaplasia in spite of TNFi treatment, while early lesions could be resolved without progression 8. In the Infliximab As First Line Therapy in Patients with Early Active Axial Spondyloarthritis Trial, which enrolled patients with axial spondyloarthritis of <3 years duration, ~70% of resolved vertebral inflammatory lesions did not progress to new fatty lesions 9. These results indicate that early, effective antiinflammatory treatment may reduce radiographic progression in AS. However, since these studies had relatively short observation periods, it was still uncertain whether TNFi treatment in the early phase of disease can lead to decreased syndesmophyte formation, the final end point of radiographic progression, compared with conventional NSAID treatment.
In the present study, we compared radiographic progression over 4 years of observation between TNFi and conventional NSAID treatment in patients with early AS. In addition, to disentangle the relationships among inflammation, treatment strategy, and radiographic progression, we also investigated the extent to which inflammation control contributes to the suppression of radiographic progression by TNFi versus NSAIDs.
Patients and methods
Patients. Patients’ clinical and radiographic data were extracted from 2 independent observational cohorts. AS patients receiving TNFi treatment (the TNFi group) were from a consecutive, single‐center cohort in Seoul National University Hospital, and patients receiving conventional NSAID treatment (the control group) were from another single‐center cohort in Seoul National University Bundang Hospital. All patients fulfilled the modified New York criteria for AS 10 at diagnosis. To precisely investigate radiographic progression in early AS, we included patients with initial onset of inflammatory back pain <10 years from the starting date of specific TNFi treatment (the TNFi group) or NSAID treatment (the control group) and with available sets of spine radiographs at baseline (defined as a starting date of group‐specific treatment) and after 2 and/or 4 years of their respective treatments 11.
The study was approved by the Institutional Review Boards (IRBs) of Seoul National University Hospital (approval no. 1611‐119‐810) and Seoul National University Bundang Hospital (approval no. B‐1604‐343‐112) and was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. The requirement for patient consent was waived by the IRBs due to the retrospective character of the study.
Clinical assessment during observation. All demographic and clinical data were extracted from the electronic medical database of each institution using common case report forms. Patients’ demographic factors, body mass index (BMI), smoking status, Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) 12, and serum C‐reactive protein (CRP) level were assessed at baseline. Disease activity was regularly monitored every 3 or 6 months in accordance with the preference of the treating physician. In the TNFi group, the BASDAI was regularly checked, and continuation of treatment was mainly determined based on whether a patient fulfilled the BASDAI criteria for 50% improvement (BASDAI 50) 13. In contrast, the BASDAI was not routinely scored in the NSAID group. Physicians in both hospitals routinely checked the name and dose of the prescribed NSAIDs as well as the average number of days per week that the NSAIDs were taken. Quantification of NSAID intake during the 2‐year interval was calculated as proposed in the Assessment of SpondyloArthritis international Society recommendations 14. Longitudinal control of inflammation during the 2‐year interval was estimated using time‐averaged CRP values, that is, the mean value of CRP levels determined every 6 months. The observation period in this study was 4 years from the baseline visit. However, for the precise estimation of treatment effect on radiographic progression and inflammation, observation was terminated if a patient stopped the TNFi, switched to another TNFi (the TNFi group), or started a new TNFi (the control group). Spine radiographs obtained during the observation period for each patient were used for analysis.
Measurement of serum CRP level. Serum high‐sensitivity CRP (hsCRP) level in patients in both centers was measured using a chemistry autoanalyzer latex‐enhanced turbidimetric immunoassay with CRP‐Latex reagent (Denkaseiken). This assay permitted the measurement of hsCRP levels as low as 0.01 mg/dl in both centers. Four times each year, both centers also perform external quality control for hsCRP for the College of American Pathologists.
Assessment of radiographic progression. Spine radiographs obtained in the 2 hospitals were given a unique code after all clinical information (including name of patient and hospital and date of examination) was deleted. After this processing, all radiographs were collected and delivered to each assessor in the form of a Digital Imaging and Communication in Medicine file. Radiographic progression was assessed using the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS) 15. Two trained assessors (JWP and MK) scored radiographs independently. If a radiograph had ≤3 missing vertebral corners, missing scores were replaced by the mean score of the corresponding corners of the visible segments. Radiographs with >3 missing vertebral corners were excluded from the scoring. The mean mSASSS of both readers was used for analysis. If a difference between scores measured by the 2 readers was >5 units (defined as a major disagreement), the same assessors rescored those radiographs. In case of persistent major disagreement after rescoring, an independent adjudicator (EYL) assigned a final score.
Statistical analysis. Interobserver reliability of the mSASSS was assessed using an intraclass correlation coefficient (ICC). The smallest detectable change (SDC) between the 2 readers was calculated to estimate reliably detectable radiographic progression given measurement error 16.
Progression of the mSASSS over time and impact of clinical factors on outcome were estimated using a linear mixed model. A “compound symmetry” correlation structure was selected based on Pearson correlation coefficients of the mSASSS at different time points. To estimate the impact of longitudinal inflammation control on radiographic progression, we constructed 2 different models. First, mSASSS changes over radiographic intervals were correlated with the baseline features of age, sex, BMI, smoking status (never versus ever), symptom duration, CRP level (mg/dl), BASDAI, HLA–B27 status, concomitant NSAID treatment, and the presence of syndesmophytes on baseline radiographs. Any clinical factors that showed relevant influence (P < 0.1) on the outcome were included in the multivariable model (model 1). Next, time‐averaged CRP level was added to this model, and changes in the effects of other covariates were analyzed (model 2). A Sobel test was also performed to estimate the indirect effect of TNFi on the outcome 17. Fitness of the model was estimated using Akaike's information criterion. To consider the bias due to measurement error between the assessors, mSASSS progression ≥2 units in 2 years (defined as definite radiographic progression) was also used as a dichotomous outcome and was modeled using a generalized linear mixed model.
Since patients mostly started TNFi treatment after the failure of first‐line NSAID treatment, it was expected that baseline features between the 2 groups were different. To minimize this confounding by indication, we performed 1:1 propensity score matching. This was carried out using age, disease duration, baseline CRP level, smoking status, and baseline mSASSS as predictors for choosing treatment, with a caliper of 0.2. Baseline BASDAI could not be included because its discrepancy between the 2 groups was so marked that propensity score matching including it as a covariate discarded most of the study population. Instead, the multivariable model performed after the matching was adjusted for the baseline BASDAI. After matching, 62 patients in each group were selected as the postmatched populations. In addition, the same analysis was performed in the subgroup of 88 patients (58 in the TNFi group and 30 in the NSAID group) who had a complete set of radiographs at all follow‐up time points during the observation period.
All statistical analyses were performed using IBM SPSS Statistics 20. P values less than 0.05 were considered significant.
Results
Patient characteristics. A total of 215 patients were included (135 in the TNFi group and 80 controls). The number of evaluated sets of radiographs was 328 in the TNFi group and 190 in the control group. The mean ± SD symptom duration was 4.2 ± 2.8 years. One hundred seventy‐one patients were male (79.5%), and 190 patients (88.4%) were HLA–B27 positive.
Clinical and radiographic features of included patients are presented in Table 1. Briefly, patients in the TNFi group had significantly longer disease duration (mean ± SD 2.7 ± 2.6 years versus 0.7 ± 1.8 years) and higher CRP levels (mean ± SD 2.2 ± 2.7 mg/dl versus 1.1 ± 1.3 mg/dl). However, other clinical factors such as age, sex, BMI, smoking status, and HLA–B27 positivity were comparable between the 2 groups. The mean ± SD baseline mSASSS was 6.2 ± 9.9 in the TNFi group and 7.3 ± 10.8 in the control group, which was not significantly different. In the postmatched population, baseline CRP level and disease duration were well balanced between the 2 groups,
Table 1.
Baseline characteristics of the patientsa
| TNFi group (n = 135) | Control group (n = 80) | P | |
|---|---|---|---|
| Age, years | 32.8 ± 11.5 | 34.4 ± 11.9 | 0.335 |
| Male sex, no. (%) | 110 (81.5) | 61 (76.2) | 0.358 |
| Body mass index | 23.3 ± 3.3 | 23.3 ± 3.5 | 0.980 |
| Symptom duration, yearsb | 4.3 ± 2.7 | 4.1 ± 2.9 | 0.679 |
| Disease duration, years | 2.7 ± 2.6 | 0.7 ± 1.8 | <0.001 |
| Ever smoker, no. (%) | 53 (39.3) | 32 (40.0) | 0.914 |
| HLA–B27 positive, no. (%) | 119 (88.1) | 71 (88.8) | 0.894 |
| BASDAI, 0–10 | 6.7 ± 1.6 | 3.2 ± 1.6 | <0.001 |
| Serum CRP level, mg/dl | 2.2 ± 2.7 | 1.1 ± 1.3 | <0.001 |
| Presence of syndesmophytes, no. (%) | 37 (27.4) | 19 (23.8) | 0.555 |
| Number of syndesmophytes | 1.6 ± 3.4 | 1.4 ± 3.1 | 0.591 |
| mSASSS, 0–72 | 6.2 ± 9.9 | 7.3 ± 10.8 | 0.445 |
Except where indicated otherwise, values are the mean ± SD. TNFi = tumor necrosis factor inhibitor; BASDAI = Bath Ankylosing Spondylitis Disease Activity Index; CRP = C‐reactive protein; mSASSS = modified Stoke Ankylosing Spondylitis Spine Score.
Time interval between initial onset of inflammatory back pain and baseline visit.
but imbalances in the BASDAI persisted (see Supplementary Table 1, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40661/abstract).
Radiographic progression over time. Among a total of 518 evaluations of sets of radiographs, mSASSS scores in 66 sets yielded major disagreement, and subsequently 12 of them were ultimately scored by the adjudicator. Interobserver ICCs for individual mSASSS scores, and for mSASSS change over time intervals, were 0.95 (95% confidence interval [95% CI] 0.92, 0.97) and 0.90 (95% CI 0.88, 0.92), respectively. The SDC for all mSASSS changes was 1.86. A Bland‐Altman plot is shown in Supplementary Figure 1, http://onlinelibrary.wiley.com/doi/10.1002/art.40661/abstract.
The mean ± SD mSASSS change over 2 years in the whole population was 1.30 ± 2.97 units. Estimated rates of progression in the univariable mixed model during the 0–2‐year interval and 2–4‐year interval were comparable (1.36 [95% CI 0.82, 1.89] and 1.25 [95% CI 0.82, 1.68], respectively; P = 0.757). Among a total of 321 radiographic intervals, definite radiographic progression occurred in 81 (25.2%). The proportion of intervals showing definite radiographic progression was higher in the TNFi group (37 of 119 [31.1%] versus 44 of 202 [21.8%]; P = 0.064).
In the univariable analysis, age (β = 0.07 [95% CI 0.04, 0.10]), baseline CRP level (β = 0.15 [95% CI 0.01, 0.29]), ever smoking (β = 0.81 [95% CI 0.15, 1.47]), and the presence of syndesmophytes at baseline (β = 2.49 [95% CI 1.83, 3.16]) were significantly associated with rapid radiographic progression. Patients’ sex, BMI, HLA–B27 positivity, and baseline BASDAI did not show any relevant effect (see Supplementary Table 2, http://onlinelibrary.wiley.com/doi/10.1002/art.40661/abstract). The TNFi group showed numerically less mSASSS change over radiographic intervals than did the control group (β = −0.68 [95% CI −1.36, 0.002]). In model 1, which included age, baseline CRP level, smoking status, and the presence of syndesmophytes at baseline, the TNFi group showed significantly slower radiographic progression over 2‐year intervals than did the control group (β = −0.90 [95% CI −1.51, −0.29]) (Table 2).
Table 2.
Impact of time‐averaged CRP level on difference of mSASSS progression between TNFi treatment and NSAID treatment during 2‐year radiographic intervala
| β (95% CI)b | P | |
|---|---|---|
| Model 1c | ||
| Age, years | 0.02 (−0.004, 0.05) | 0.092 |
| Ever smoking (vs. never) | 0.30 (−0.30, 0.89) | 0.326 |
| Baseline CRP level, mg/dl | 0.16 (0.03, 0.29) | 0.019 |
| Presence of syndesmophytes at baseline (vs. absence) | 2.09 (1.32, 2.86) | <0.001 |
| TNFi group (vs. control group) | −0.90 (−1.51, −0.29) | 0.004 |
| Model 2c | ||
| Age, years | 0.03 (−0.002, 0.05) | 0.066 |
| Ever smoking (vs. never) | 0.28 (−0.31, 0.86) | 0.356 |
| Baseline CRP level, mg/dl | 0.12 (−0.01, 0.25) | 0.079 |
| Presence of syndesmophytes at baseline (vs. absence) | 1.86 (1.09, 2.63) | <0.001 |
| TNFi group (vs. control group) | −0.52 (−1.17, 0.14) | 0.123 |
| Time‐averaged CRP level in 2‐year interval, mg/dl | 1.02 (0.32, 1.71) | 0.004 |
TNFi = tumor necrosis factor inhibitor; NSAID = nonsteroidal antiinflammatory drug; 95% CI = 95% confidence interval.
Indicates difference in modified Stoke Ankylosing Spondylitis Spine Score (mSASSS) change in 2‐year radiographic interval between the 2 groups (dichotomous variable) or when a covariate increases by 1 unit (continuous variable).
Any clinical factors that showed significant association (P < 0.1) in the univariable analysis were included in the multivariable model (model 1). Time‐averaged C‐reactive protein (CRP) level was then added to this model, and changes in the effects of other covariates were analyzed (model 2). Akaike's information criterion, an estimate of model fitness, was 1,473.740 for model 1 and 1,457.817 for model 2.
Influence of time‐averaged CRP level on radiographic progression. The time‐averaged CRP level over radiographic intervals was lower with TNFi treatment than with NSAID treatment (mean ± SD 0.27 ± 0.30 mg/dl versus 0.61 ± 0.68 mg/dl; P < 0.001). And also, it was significantly lower in the TNFi group in both the 0–2‐year interval and 2–4‐year interval (mean ± SD 0.24 ± 0.27 mg/dl versus 0.62 ± 0.61 mg/dl and 0.31 ± 0.36 mg/dl versus 0.59 ± 0.78 mg/dl, respectively). The proportion of radiographic intervals with time‐averaged CRP level <0.2 mg/dl was also significantly higher in the TNFi group (57.3% versus 27.5%; P < 0.001).
The time‐averaged CRP level was significantly associated with rapid radiographic progression over relevant radiographic intervals (β = 1.68 [95% CI 1.00, 2.36]). When all intervals were stratified by time‐averaged CRP level status, the mean ± SD mSASSS change in intervals with time‐averaged CRP level <0.2 mg/dl was 0.58 ± 2.42 units, while it was 1.98 ± 3.28 units in the remaining intervals (Figure 1). Furthermore, definite radiographic progression also occurred significantly less frequently in the intervals with lower time‐averaged CRP level (15.7% versus 33.7%; P < 0.001).
Figure 1.
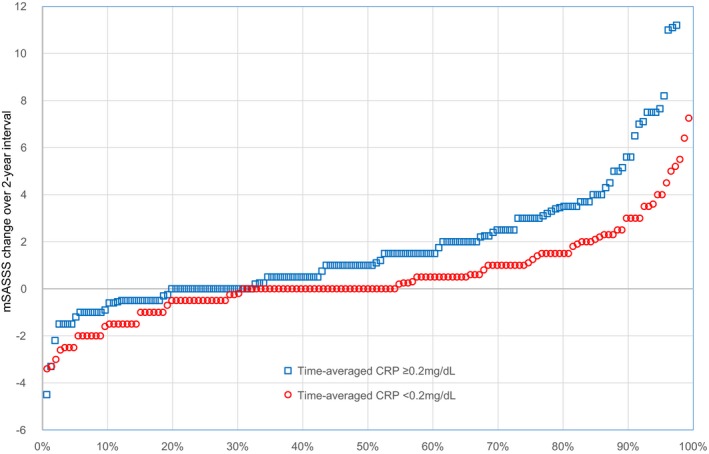
Cumulative probability plot showing radiographic progression during 2‐year time intervals according to time‐averaged C‐reactive protein (CRP) levels (<0.2 mg/dl versus ≥0.2 mg/dl) over individual intervals. mSASSS = modified Stoke Ankylosing Spondylitis Spine Score.
Interestingly, when mSASSS progression was analyzed after stratification by the presence of syndesmophytes at baseline and time‐averaged CRP level (based on 0.2 mg/dl), each subgroup showed a significantly different course of radiographic progression (Figure 2). In patients without syndesmophytes at baseline, the estimated mSASSS change in radiographic intervals with time‐averaged CRP level <0.2 mg/dl was 0.23 units (95% CI −0.22, 0.69) per 2‐year interval, and definite radiographic progression occurred in only 10% of patients. However, this progression rate was significantly increased in those with a higher time‐averaged CRP level (1.12 units [95% CI 0.61, 1.62]). The effect of time‐averaged CRP level in patients with syndesmophytes at baseline was also consistent.
Figure 2.
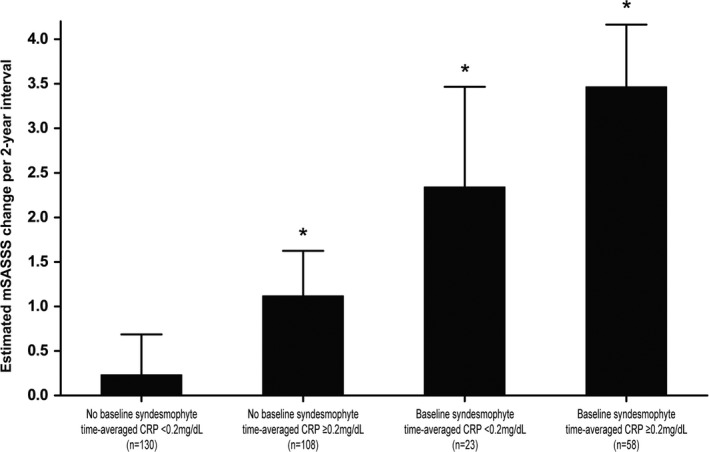
Different radiographic progression in 2‐year intervals according to the presence or absence of syndesmophytes at baseline and time‐averaged C‐reactive protein (CRP) level (<0.2 mg/dl or ≥0.2 mg/dl). Values are the mean and upper margin of the 95% confidence interval. * = P < 0.05 versus patients without syndesmophytes at baseline and with lower time‐averaged CRP levels. mSASSS = modified Stoke Ankylosing Spondylitis Spine Score.
In multivariable model 2, an increase of 1 mg/dl in time‐averaged CRP level resulted in an increase of 1.02 mSASSS units (95% CI 0.32, 1.72) per 2‐year interval. In contrast, the impact of TNFi treatment (versus NSAID treatment) was decreased and lost its statistical significance (β = −0.52 [95% CI −1.17, 0.14]) (Table 2). Mediation analysis also showed that TNFi treatment significantly reduced radiographic progression through an indirect effect mediated by time‐averaged CRP level (Z score for indirect effect = −2.08, P = 0.037). The effect of the interaction between treatment group and time‐averaged CRP level on mSASSS progression was not significant (P = 0.309), which suggests that the effect of time‐averaged CRP level did not differ by treatment regimen.
The impact of time‐averaged CRP level was consistent in the generalized linear mixed model, in which definite radiographic progression was used as the outcome variable. The presence of syndesmophytes at baseline and time‐averaged CRP level were associated with increased odds of progression (odds ratios [ORs] of 5.71 and 3.02, respectively). In contrast, TNFi treatment did not significantly reduce the probability of definite radiographic progression (OR 0.76 [95% CI 0.35, 1.64]) (Figure 3).
Figure 3.
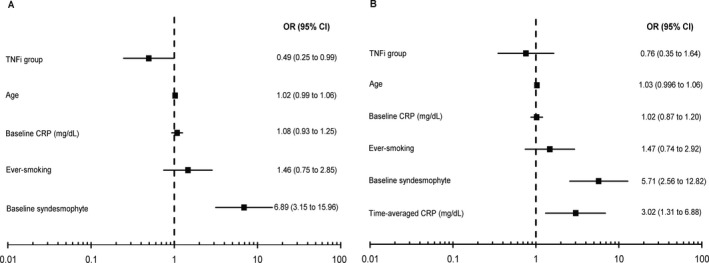
Forest plots indicating effect of clinical factors in 2 different multivariable models on odds of the occurrence of definite radiographic progression (defined as change of ≥2 units in modified Stoke Ankylosing Spondylitis Spine Score over 2 years). A, In model 1 including relevant baseline factors, treatment with tumor necrosis factor inhibitors (TNFi) was associated with significantly reduced odds of radiographic progression. B, This effect was decreased when the factor of time‐averaged C‐reactive protein (CRP) level was added (model 2). OR = odds ratio; 95% CI = 95% confidence interval.
Impact of NSAID index on radiographic progression. The mean ± SD NSAID index for the control group during 0–2‐year and 2–4‐year intervals was 46.3 ± 23.3 and 42.0 ± 24.2, respectively (difference not significant). The proportion of users of high amounts of NSAIDs (NSAID index ≥50) was lower in the 2–4‐year interval than in the 0–2‐year interval (31.5% versus 46.6%). In the control group, the NSAID index for radiographic intervals was not associated with mSASSS change irrespective of the time effect (β = −0.006 [95% CI −0.03, 0.02]) (see Supplementary Table 2, http://onlinelibrary.wiley.com/doi/10.1002/art.40661/abstract).
Among the 268 2‐year intervals in the TNFi group, concomitant NSAID therapy was administered in 190 (70.9%). The NSAID index (mean ± SD) of these 190 intervals was 23.3 ± 23.6, without any difference between 0–2‐year and 2–4‐year intervals. As in the control group, both concomitant NSAID use and NSAID index were not associated with mSASSS progression during radiographic intervals in the TNFi group (unadjusted β = −0.11 [95% CI −0.97, 0.74] and −0.007 [95% CI −0.03, 0.01], respectively) (see Supplementary Table 2, http://onlinelibrary.wiley.com/doi/10.1002/art.40661/abstract).
Interestingly, in the linear mixed model to investigate clinical factors affecting time‐averaged CRP level, the presence of syndesmophytes at baseline (adjusted β = 0.20 [95% CI 0.07, 0.33]), higher baseline CRP level (adjusted β = 0.05 [95% CI 0.03, 0.08]), and the control group (adjusted β = 0.36 [95% CI 0.16, 0.56]) were associated with increased time‐averaged CRP level. However, the NSAID index, irrespective of the group, did not significantly influence the outcome (adjusted β = 0.15 [95% CI −0.18, 0.49]).
Sensitivity analysis. In the postmatched population, the TNFi group showed numerically less radiographic progression than did the control group in model 1 (β = −0.64 [95% CI −1.84, 0.56]). The effect of TNFi treatment on the outcome was significantly decreased after adjustment for time‐averaged CRP level (β = −0.02 [95% CI −1.28, 1.24]). In contrast, the significant effect of time‐averaged CRP level on mSASSS progression was not changed (β = 1.15 [95% CI 0.27, 2.03]) (see Supplementary Table 3, http://onlinelibrary.wiley.com/doi/10.1002/art.40661/abstract). Other sensitivity analyses performed in the subgroup of 88 patients with complete sets of radiographs also showed consistent results (see Supplementary Table 4, http://onlinelibrary.wiley.com/doi/10.1002/art.40661/abstract).
Since the correlation between baseline and time‐averaged CRP level could influence the result of multivariable model 2, we further analyzed their relationship and its significance. The correlation between baseline and time‐averaged CRP level was significant, but the strength of correlation was very weak (r = 0.18, P = 0.001). In addition, when interaction between baseline and time‐averaged CRP level was added to multivariable model 2 as a covariate, it was not statistically significant (β = −0.01 [95% CI −0.29, 0.27]), and the effect of time‐averaged CRP level showed little change (β = 1.04 [95% CI 0.05, 2.04]). Correlations between age and symptom duration and between age and disease duration were also not significant (r = 0.11, P = 0.106 and r = 0.03, P = 0.717, respectively), and neither of these interactions influenced radiographic progression (data not shown).
Discussion
Finding the answer to the question of whether radiographic progression can be prevented through effective treatment of AS is a task still remaining for rheumatologists 18. To the best of our knowledge, this is the first study that analyzes the relative contributions to radiographic progression, in early AS, of time‐averaged CRP levels and direct TNFi treatment effects. Patients in the TNFi group showed less radiographic progression than those receiving conventional NSAID treatment. This was mainly linked to the differences in time‐averaged CRP levels between the 2 groups.
Overall, an increase of 1 mg/dl time‐averaged CRP level led to an increase of 1.02 mSASSS units per 2‐year interval. In contrast, a previous study that investigated a longitudinal relationship between disease activity and mSASSS progression in the Outcome in AS International Study (OASIS) cohort showed that an increase of 1 mg/dl time‐averaged CRP level led to additional mSASSS progression of 0.2 units over the same interval 19. Considering that patients in the OASIS cohort had significantly longer symptom durations (~20 years), it is probable that tight control of inflammation during the early phase of the disease is key to minimizing radiographic progression, supporting the notion of a “window of opportunity” 8. In fact, patients with low time‐averaged CRP levels between radiologic assessments and with no syndesmophytes at baseline showed a minimal mSASSS change over time, and definite radiographic progression occurred in only 10% of them.
In the present study, the control group showed significantly higher time‐averaged CRP levels than the TNFi group, which mediated rapid radiographic progression. However, the NSAID index was not significantly associated with time‐averaged CRP level or mSASSS change, irrespective of the treatment group. It would be premature to conclude that NSAIDs alone cannot reduce the time‐averaged CRP level based on this result, because this study did not compare the effect of NSAIDs with that of no treatment on time‐averaged CRP level. However, this result is consistent with that of a recent randomized clinical trial that compared radiographic progression in patients receiving continuous NSAID treatment with that in patients receiving NSAID treatment on demand 20. The proportion of patients with a high NSAID index was only 39% over all radiographic intervals in the control group, so inadequate dosages of NSAIDs could have led to less‐than‐optimal therapeutic effects. However, maintaining a high NSAID index is not well tolerated in daily clinical practice 21. Furthermore, a recent study showed that full‐dose NSAID treatment did not achieve a favorable response or a relevant decrease in sacroiliitis on MRI 22. Therefore, if a patient showed elevated CRP levels despite conventional NSAID treatment, timely switching to or adding TNFi treatment could be a proper strategy for inflammation control and inhibition of radiographic progression in early AS.
Some previous studies suggested that the Ankylosing Spondylitis Disease Activity Score (ASDAS) 23, which includes patient‐reported outcome and CRP level, could be better than CRP level alone for predicting radiographic progression 24, 25. Although a statistical model using the ASDAS showed a slightly better fit than one using CRP level in those studies, it would be premature to generalize from this result because patient‐reported outcome could be influenced by clinical factors unrelated to disease activity such as concomitant fibromyalgia 26. In contrast, a previous study by Machado et al showed that spinal inflammation detected by MRI correlated better with CRP level than with other measures of disease activity 27. Therefore, we think that time‐averaged CRP level could optimally represent the degree of inflammation during treatment and could help us to estimate the precise contribution of inflammation in radiographic progression. Unfortunately, the ASDAS was not routinely measured in our study, so we could not compare the power of the 2 markers to predict radiographic progression.
It is also interesting that only 57.3% of total radiographic intervals in the TNFi group had time‐averaged CRP values <0.2 mg/dl. This result suggests that significant numbers of patients receiving TNFi treatment did not achieve an optimal antiinflammatory effect in the real world. In contrast, all patients in the TNFi group fulfilled the BASDAI 50 response criteria over the entire observation period. This discrepancy could explain why patient‐reported outcomes could not precisely predict radiographic progression 19. Therefore, to minimize radiographic progression in AS, switching to other TNFi or interleukin‐17–blocking agents should be considered based on the objective degree of antiinflammatory effectiveness rather than on subjective outcome. This is consistent with the recently updated “treat‐to‐target” strategy, which consists of measuring disease activity, optimally using the ASDAS, and adjusting therapy accordingly 24, 28.
Our study has some limitations. First, the baseline features between the 2 groups were significantly different, and this could lead to confounding by indication. This was a major, but not unexpected, drawback of the study, because starting TNFi treatment in patients with AS is indicated after encountering intolerance or inadequate response to NSAID treatment in daily clinical practice. Although we performed propensity score matching to minimize this bias, the BASDAI could not be used in the matching process because there was little overlap in the ranges of these factors between the 2 groups. A randomized, head‐to‐head comparison of TNFi treatment with conventional NSAID treatment as initial treatment in patients with early AS would be optimal to demonstrate the different effect of time‐averaged CRP level on radiographic progression in the 2 groups. However, such a study is less than feasible under real‐world conditions.
Second, since this was not a randomized study, radiographic progression could be influenced by a number of unmeasured confounders such as patient compliance with treatment and physician preferences. For example, patients in the control group did not regularly complete the BASDAI during treatment, so it is possible that patient‐reported outcomes could differ between the 2 groups. However, in daily clinical practice, a physician would consider TNFi treatment if a patient does not fulfill the BASDAI 50 response criteria under NSAID treatment, so this imbalance should not be significant. In addition, it is possible that the NSAID index might have been underestimated because of use of over‐the‐counter NSAIDs. However, all AS patients in the Republic of Korea are covered by a national medical insurance system, and the patient pays only 10% of the price of all prescribed medication for the treatment of AS. Because of easy access to low‐cost medical care in the Republic of Korea, we think that underestimation of the NSAID index due to use of over‐the‐counter medicine is less likely 29.
Finally, because the present study included a relatively small number of patients in the control group, the effect of NSAID treatment could be insignificant due to Type II error. Although the 95% CI of the beta value regarding the effect of the NSAID index on the time‐averaged CRP level was relatively far from the criterion for statistical significance, this result should be confirmed in future studies with a larger sample size.
In conclusion, we show that TNFi treatment in early AS can reduce radiographic progression, mainly by effective inflammation control. Although these results should be replicated in future (preferably randomized) studies, they may support the notion that early effective suppression of inflammation using TNFi could inhibit pathologic new bone formation in AS.
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. E. Y. Lee had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design.
J. W. Park, E. Y. Lee.
Acquisition of data.
J. W. Park, Kim, Ha, J. K. Park, Kang, Y. J. Lee, Song, E. Y. Lee.
Analysis and interpretation of data.
J. W. Park, Kim, J. S. Lee, Y. J. Lee, E. Y. Lee.
Supporting information
Acknowledgments
We deeply appreciate the statistical assistance of the medical research collaboration center at Seoul National University Hospital. We also thank Soo Young Moon, MD for his comment regarding the quality control program of hsCRP measurement of 2 institutions.
Supported by the Korea Health Technology R & D Project, funded by the Ministry of Health & Welfare, Republic of Korea (grant HI14C1277).
References
- 1. Braun J, Landewe R, Hermann KG, Han J, Yan S, Williamson P, et al. Major reduction in spinal inflammation in patients with ankylosing spondylitis after treatment with infliximab: results of a multicenter, randomized, double‐blind, placebo‐controlled magnetic resonance imaging study. Arthritis Rheum 2006;54:1646–52. [DOI] [PubMed] [Google Scholar]
- 2. Van der Heijde D, Landewe R, Baraliakos X, Houben H, van Tubergen A, Williamson P, et al. Radiographic findings following two years of infliximab therapy in patients with ankylosing spondylitis. Arthritis Rheum 2008;58:3063–70. [DOI] [PubMed] [Google Scholar]
- 3. Van der Heijde D, Landewe R, Einstein S, Ory P, Vosse D, Ni L, et al. Radiographic progression of ankylosing spondylitis after up to two years of treatment with etanercept. Arthritis Rheum 2008;58:1324–31. [DOI] [PubMed] [Google Scholar]
- 4. Van der Heijde D, Salonen D, Weissman BN, Landewé R, Maksymowych WP, Kupper H, et al. Assessment of radiographic progression in the spines of patients with ankylosing spondylitis treated with adalimumab for up to 2 years. Arthritis Res Ther 2009;11:R127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haroon N, Inman RD, Learch TJ, Weisman MH, Lee M, Rahbar MH, et al. The impact of tumor necrosis factor α inhibitors on radiographic progression in ankylosing spondylitis. Arthritis Rheum 2013;65:2645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baraliakos X, Haibel H, Listing J, Sieper J, Braun J. Continuous long‐term anti‐TNF therapy does not lead to an increase in the rate of new bone formation over 8 years in patients with ankylosing spondylitis. Ann Rheum Dis 2014;73:710–5. [DOI] [PubMed] [Google Scholar]
- 7. Maas F, Arends S, Brouwer E, Essers I, van der Veer E, Efde M, et al. Reduction in spinal radiographic progression in ankylosing spondylitis patients receiving prolonged treatment with tumor necrosis factor inhibitors. Arthritis Care Res (Hoboken) 2017;69:1011–9. [DOI] [PubMed] [Google Scholar]
- 8. Maksymowych WP, Morency N, Conner‐Spady B, Lambert RG. Suppression of inflammation and effects on new bone formation in ankylosing spondylitis: evidence for a window of opportunity in disease modification. Ann Rheum Dis 2013;72:23–8. [DOI] [PubMed] [Google Scholar]
- 9. Poddubnyy D, Listing J, Sieper J. Brief report: course of active inflammatory and fatty lesions in patients with early axial spondyloarthritis treated with infliximab plus naproxen as compared to naproxen alone: results from the infliximab as first line therapy in patients with early active axial spondyloarthritis trial. Arthritis Rheumatol 2016;68:1899–903. [DOI] [PubMed] [Google Scholar]
- 10. Van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis: a proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8. [DOI] [PubMed] [Google Scholar]
- 11. Rudwaleit M, Haibel H, Baraliakos X, Listing J, Märker‐Hermann E, Zeidler H, et al. The early disease stage in axial spondylarthritis: results from the German Spondyloarthritis Inception Cohort. Arthritis Rheum 2009;60:717–27. [DOI] [PubMed] [Google Scholar]
- 12. Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994;21:2286–91. [PubMed] [Google Scholar]
- 13. Braun J, Davis J, Dougados M, Sieper J, van der Linden S, van der Heijde D. First update of the international ASAS consensus statement for the use of anti‐TNF agents in patients with ankylosing spondylitis. Ann Rheum Dis 2006;65:316–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dougados M, Simon P, Braun J, Burgos‐Vargas R, Maksymowych WP, Sieper J, et al. ASAS recommendations for collecting, analysing and reporting NSAID intake in clinical trials/epidemiological studies in axial spondyloarthritis. Ann Rheum Dis 2011;70:249–51. [DOI] [PubMed] [Google Scholar]
- 15. Creemers MC, Franssen MJ, van't Hof MA, Gribnau FW, van de Putte LB, van Riel PL. Assessment of outcome in ankylosing spondylitis: an extended radiographic scoring system. Ann Rheum Dis 2005;64:127–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bruynesteyn K, Boers M, Kostense P, van der Linden S, van der Heijde D. Deciding on progression of joint damage in paired films of individual patients: smallest detectable difference or change. Ann Rheum Dis 2005;64:179–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mackinnon DP, Warsi G, Dwyer JH. A simulation study of mediated effect measures. Multivariate Behav Res 1995;30:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Machado P. Anti–tumor necrosis factor and new bone formation in ankylosing spondylitis: the controversy continues. Arthritis Rheum 2013;65:2537–40. [DOI] [PubMed] [Google Scholar]
- 19. Ramiro S, van der Heijde D, van Tubergen A, Stolwijk C, Dougados M, van den Bosch F, et al. Higher disease activity leads to more structural damage in the spine in ankylosing spondylitis: 12‐year longitudinal data from the OASIS cohort. Ann Rheum Dis 2014;73:1455–61. [DOI] [PubMed] [Google Scholar]
- 20. Sieper J, Listing J, Poddubnyy D, Song IH, Hermann KG, Callhoff J, et al. Effect of continuous versus on‐demand treatment of ankylosing spondylitis with diclofenac over 2 years on radiographic progression of the spine: results from a randomised multicentre trial (ENRADAS). Ann Rheum Dis 2016;75:1438–43. [DOI] [PubMed] [Google Scholar]
- 21. Molto A, Granger B, Wendling D, Dougados M, Gossec L. Use of nonsteroidal anti‐inflammatory drugs in early axial spondyloarthritis in daily practice: data from the DESIR cohort. Joint Bone Spine 2017;84:79–82. [DOI] [PubMed] [Google Scholar]
- 22. Varkas G, Jans L, Cypers H, Van Praet L, Carron P, Elewaut D, et al. Six‐week treatment of axial spondyloarthritis patients with an optimal dose of nonsteroidal antiinflammatory drugs: early response to treatment in signal intensity on magnetic resonance imaging of the sacroiliac joints. Arthritis Rheumatol 2016;68:672–8. [DOI] [PubMed] [Google Scholar]
- 23. Lukas C, Landewé R, Sieper J, Dougados M, Davis J, Braun J, et al, for the Assessment of SpondyloArthritis international Society . Development of an ASAS‐endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis 2009;68:18–24. [DOI] [PubMed] [Google Scholar]
- 24. Molnar C, Scherer A, Baraliakos X, de Hooge M, Micheroli R, Exer P, et al. TNF blockers inhibit spinal radiographic progression in ankylosing spondylitis by reducing disease activity: results from the Swiss Clinical Quality Management cohort. Ann Rheum Dis 2018;77:63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Poddubnyy D, Protopopov M, Haibel H, Braun J, Rudwaleit M, Sieper J. High disease activity according to the Ankylosing Spondylitis Disease Activity Score is associated with accelerated radiographic spinal progression in patients with early axial spondyloarthritis: results from the GErman SPondyloarthritis Inception Cohort. Ann Rheum Dis 2016;75:2114–8. [DOI] [PubMed] [Google Scholar]
- 26. Molto A, Etcheto A, Gossec L, Boudersa N, Claudepierre P, Roux N, et al. Evaluation of the impact of concomitant fibromyalgia on TNF α blockers’ effectiveness in axial spondyloarthritis: results of a prospective, multicentre study. Ann Rheum Dis 2018;77:533–40. [DOI] [PubMed] [Google Scholar]
- 27. Machado P, Landewe RB, Braun J, Baraliakos X, Hermann KG, Hsu B, et al. MRI inflammation and its relation with measures of clinical disease activity and different treatment responses in patients with ankylosing spondylitis treated with a tumour necrosis factor inhibitor. Ann Rheum Dis 2012;71:2002–5. [DOI] [PubMed] [Google Scholar]
- 28. Smolen JS, Schols M, Braun J, Dougados M, FitzGerald O, Gladman DD, et al. Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force. Ann Rheum Dis 2018;77:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Park K, Park J, Kwon YD, Kang Y, Noh JW. Public satisfaction with the healthcare system performance in South Korea: universal healthcare system. Health Policy 2016;120:621–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


