Abstract
Objective
Treat‐to‐target strategies have improved outcomes in rheumatic diseases. In psoriatic arthritis (PsA), the proposed targets are the multidimensional target minimal disease activity (MDA) and the articular target Disease Activity index for PsA (DAPSA). The aim of this study was to compare the disease burden of PsA in patients with low disease activity according to the 2 definitions, MDA and DAPSA low disease activity (DAPSA‐LDA), 1 year after diagnosis.
Methods
We obtained data on MDA, DAPSA‐LDA and disease burden 1 year after diagnosis for patients included in the Dutch southwest early PsA cohort. Disease burden was assessed in 2 domains: “Body functions,” including the Short Form 36 bodily pain (SF‐36 BP) measure, and “Activity,” including the Health Assessment Questionnaire (HAQ).
Results
Among the 292 patients included, 48% achieved MDA and 74% achieved DAPSA‐LDA. Average scores for Body functions and Activity were better in patients who achieved MDA and those who achieved DAPSA‐LDA. The scores were significantly better in the 46% of patients who achieved both MDA and DAPSA‐LDA than in the 29% of patients who achieved only DAPSA‐LDA. The average SF‐36 BP score was higher in patients achieving both targets (73.8; 95% confidence interval [95% CI] 71.1‐76.5) than in patients achieving only DAPSA‐LDA (57.6; 95% CI 54.5‐60.8). Similarly, mean HAQ scores measuring Activity were 0.21 (95% CI 0.15‐0.26) and 0.63 (95% CI 0.53‐0.72), respectively.
Conclusion
Among patients with newly diagnosed PsA, 48% achieved MDA and 74% achieved DAPSA‐LDA after 1 year of receiving usual care. The average disease burden was better in patients who achieved MDA and those who achieved DAPSA‐LDA. Also, patients who achieved only DAPSA‐LDA reported worse outcomes than those who also achieved MDA.
Introduction
Psoriatic arthritis (PsA) belongs to the group of spondyloarthritides and is a heterogenic disease that involves both the skin (skin and nail psoriasis) and musculoskeletal features (arthritis, enthesitis spondylitis, and dactylitis) 1, 2. Without treatment, most patients with PsA experience progressive joint damage and increasing disability and have a reduced life expectancy 3, 4, 5. Outcomes in rheumatic diseases have improved substantially over the last decades, largely due to the introduction of new therapies 6 and their use in treat‐to‐target strategies 7. The objective of treat‐to‐target strategies is to achieve remission or a low or minimal disease activity state.
Such a state of disease signifies that the disease burden at that time is low and long‐term worsening of functioning, quality of life and joint erosion are prevented. The TICOPA study has shown that a treat‐to‐target strategy is also effective in PsA cases 8. In clinical practice, however, the treat‐to‐target approach has not been implemented in PsA as successfully as in rheumatoid arthritis. One of the main reasons is the lack of consensus on what the target should be in PsA. This is among other things related to the heterogenic nature of PsA. In the first trials in PsA, treatment effect was measured with targets used in rheumatoid arthritis and consequently mainly effects on arthritis were measured 9. However, other disease manifestations, such as psoriasis, enthesitis, and dactylitis, are considered important as well 10. It is unknown whether inclusion of manifestations other than arthritis in a treatment target is associated with better patient outcomes.
Significance & Innovations.
The average psoriatic arthritis burden was higher in patients who achieved Disease Activity Index for Psoriatic Arthritis Low Disease Activity (DAPSA‐LDA) and those who achieved minimal disease activity (MDA).
MDA was more difficult to achieve.
Patients who achieved both DAPSA‐LDA and MDA had a lower burden of disease compared with those who achieved only DAPSA‐LDA.
Different disease activity measures have been proposed as treatment targets, such as the Psoriatic Arthritis Disease Activity Score, the Composite Psoriatic arthritis Disease Activity Index, the Disease activity index for PsA (DAPSA), and minimal disease activity (MDA) 11, 12, 13, 14. Of these measures, both MDA and DAPSA were recently recommended by international experts to be the main targets 15. A patient is considered to have MDA if at least 5 of 7 remission criteria are met. Remission is assessed by the tender joint count in 68 joints (TJC68) and the swollen joint count in 66 joints (SJC66), psoriasis, enthesitis, patient's global and pain assessment as measured on a visual analog scale (VAS), and the Health Assessment Questionnaire (HAQ) 16. The DAPSA score is calculated using TJC68 and SJC66 scores, patient's global and pain scores on a VAS, and the C‐reactive protein (CRP) level 11. A DAPSA score of ≤14 represents a state of low disease activity (DAPSA‐LDA), and a score of ≤4 represents remission (DAPSA‐REM) 17. In contrast with MDA, DAPSA is a unidimensional target that mainly measures articular involvement 15. Before choosing either one of the targets, more information is needed regarding how they relate to outcomes relevant to patients. Therefore, the aim of our study was to compare the PsA disease burden in patients with LDA according to the 2 different definitions, MDA and DAPSA‐LDA, 1 year after diagnosis. In addition, we sought to determine which aspect of disease activity prevented patients from achieving MDA or DAPSA‐LDA. Disease burden was assessed using the International Classification of Functioning, Disability and Health (ICF) domains “Body functions” and “Activity” 18.
Patients and Methods
Patients and setting
We used data collected from the Dutch south west Early Psoriatic Arthritis Registry (DEPAR). Patients with newly diagnosed PsA were invited to participate. The diagnosis of PsA was made according to the expert opinion of the participating rheumatologists. All eligible patients with newly diagnosed PsA (ages ≥18 years and with no current treatment with disease‐modifying antirheumatic drugs [DMARDs] for joint symptoms) were asked to participate. Use of DMARDs for psoriasis and use of nonsteroidal antiinflammatory drugs were allowed. Patients were recruited at centers in the southwest region of the Netherlands (1 academic hospital, 10 general hospitals, and 1 treatment center specializing in rheumatic disease care). Written informed consent was obtained from all participants according to the Declaration of Helsinki. The study was approved by the local medical research ethics committee at Erasmus Medical Center Rotterdam (approval no. MEC‐2012‐549). Data for patients who were included between August 2013 and June 2017 were used. For this analysis, we used data 1 year after diagnosis and inclusion in DEPAR.
Patient and disease characteristics
Trained research nurses collected clinical data, including the SJC66 and the TJC68, enthesitis at the time of clinical examination (Leeds Enthesitis Index [LEI] 19), dactylitis (Leeds Dactylitis Index [LDI] 20), and psoriasis (Psoriasis Area and Severity Index [PASI] 21). Patients filled out questionnaires shortly before or after their visit to the research nurse. Multiple questionnaires are used to measure patient‐reported disease activity and different outcomes, such as health‐related quality of life. For this analysis, we used the Short Form 36 (SF‐36) 22, the HAQ 23, patient's global and pain scores on a VAS, and the Bristol Rheumatoid Arthritis Fatigue (BRAF) questionnaire 24.
Primary outcome
The impact of disease activity status as measured by MDA and DAPSA on the burden of disease was assessed in 2 domains of the World Health Organization (WHO) ICF 18. The primary outcomes in the Body function domain were bodily pain (BP) as measured with the SF‐36 and fatigue as measured using the BRAF questionnaire. In the Activity domain, the primary outcomes were physical functioning (PF) as measured by the SF‐36 and HAQ scores. The range of scores for both SF‐36 BP and SF‐36 PF is 0–100, with a higher score representing better health status. For BRAF scores (range 0–70) and HAQ scores (range 0–3), a lower score represents better health status.
Disease activity status
Disease activity status was assessed using MDA and DAPSA. MDA is defined as having met at least 5 of 7 remission criteria (SJC66 ≤1, TJC68 ≤1, LEI score ≤1, PASI score ≤1, patient's global score 2.0 cm [VAS], patient's pain score 1.5 cm [VAS], HAQ score ≤0.5). In addition, we determined which patients had achieved very Low disease activity (VLDA), which is a stricter form of MDA in which all remission criteria for MDA must be met 25. The DAPSA score was calculated as TJC68 + SJC66 + patient's global assessment score (VAS, cm) + patient's pain assessment score (VAS, cm) + CRP. Categories of DAPSA were DAPSA‐LDA (DAPSA score ≤14) and DAPSA‐REM (DAPSA score ≤4) 11, 17. Residual disease activity in the SJC66, TJC68, LEI, and PASI, patient's global assessment score, patient's pain assessment score, HAQ, dactylitis, and CRP level was determined.
Statistical analysis
Patient characteristics and fulfillment of targets were described using simple descriptive analysis techniques. First, the average disease burden in patients who had achieved MDA, DAPSA‐LDA, VLDA, or DAPSA‐MDA was compared. Second, we tested whether patients with MDA had also achieved DAPSA‐LDA and vice versa, or whether patients had a different disease status according to the 2 definitions. We compared disease burden according to these as well, using analysis of variance and t‐tests. Stata version 14.0 was used for the analyses.
Results
Achieving low disease activity
Two hundred ninety‐two (58%) of 504 patients had attended their 12‐month follow‐up visit at the time of the analysis. Their mean ± SD age was 51 ± 14 years, 150 (51%) were male, and the median symptom duration before diagnosis was 12 months (interquartile range 4–32) (Table 1). At the 12‐month follow‐up visit, 48% of patients had achieved MDA, and 64% had achieved DAPSA‐LDA. According to stricter criteria, 13% of patients had achieved VLDA, and 21% had achieved DAPSA‐REM (Figure 1). In 234 of the 292 patients, both DAPSA and MDA status were known. Reasons for missing data included incomplete questionnaires (40 patients) and missing CRP values (18 patients). Of these 234 patients, 78 (33%) had achieved neither MDA nor DAPSA, 47 (20%) had achieved DAPSA‐LDA but not MDA, 7 (3%) had achieved MDA but not DAPSA‐LDA, and 102 (44%) had achieved both MDA and DAPSA‐LDA.
Table 1.
Characteristics of the 292 patients at the time of diagnosisa
| Age, mean ± SD years | 51 ± 14 |
| Male sex, no. (%) | 150 (51) |
| Duration of symptoms, median (IQR) months | 12 (4–32) |
| TJC68, median (IQR) | 3 (1–8) |
| SJC66, median (IQR) | 2 (1–5) |
| HAQ score, median (IQR)b | 0.63 (0.25–1) |
| Elevated CRP level, no. (%)c | 71 (29) |
IQR = interquartile range; TJC68 = tender joint count in 68 joints; SJC66 = swollen joints count in 66 joints; HAQ = Health Assessment Questionnaire; CRP = C‐reactive protein.
Questionnaires for 41 patients were missing.
Values for 103 patients were missing.
Figure 1.
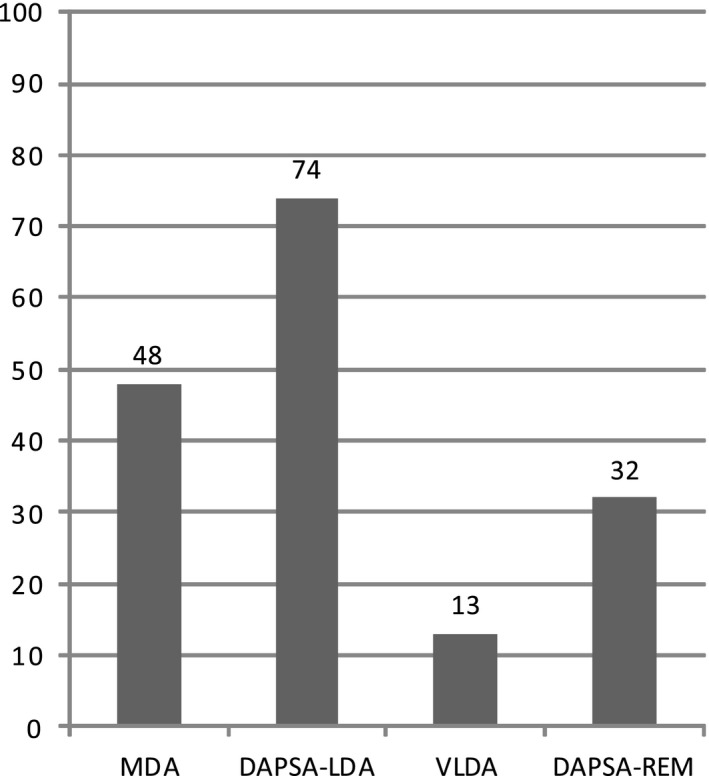
Percentages of patients fulfilling definitions of low disease activity. MDA = minimal disease activity; DAPSA‐LDA = Disease Activity Index for Psoriatic Arthritis Low Disease Activity (DAPSA ≤14), VLDA = very low disease activity; DAPSA‐REM = DAPSA remission (DAPSA ≤4).
Disease burden according to different definitions of low disease activity
Disease burden was assessed using the WHO ICF domains Body functions and Activity, and the average scores of patients with a low disease status according to different definitions were compared. Body functions were similar in patients with MDA and patients with DAPSA‐LDA, as measured using the SF‐36 BP and BRAF scores. These scores were slightly better in patients fulfilling the stricter VLDA and DAPSA‐REM criteria. The mean SF‐36 BP score was 73.9 (95% confidence interval [95% CI] 70.9–76.3) in patients who achieved MDA, 69.7 (95% CI 67.1–72.2) in those with DAPSA‐LDA, 83.0 (95% CI 78.5–87.4) in those with VLDA, and 77.4 (95% CI 73.1–81.8) in those who achieved DAPSA‐REM (Figure 2). Similarly, the impact on the Activity domain did not differ significantly among patients who achieved MDA and those who achieved DAPSA‐LDA and was better in those with VLDA and those with DAPSA‐REM (Figure 3). The mean SF‐36 PF scores were 85.8 (95% CI 83.1–88.4) in patients who achieved MDA, 81.6 (95% CI 78.9–84.3) in those with DAPSA‐LDA, 92.8 (95% CI 89.6–96.0) in those with VLDA, and 89.5 (95% CI 85.5–93.5) in those who achieved DAPSA‐REM. The mean HAQ scores were 0.22 (95% CI 0.17–0.27) in the MDA group, 0.32 (95% CI 0.26–0.38) in the DAPSA‐LDA group, 0.06 (95% CI 0.02–0.10) in the VLDA group, and 0.15 (95% CI 0.08–0.21) in the DAPSA‐REM group.
Figure 2.
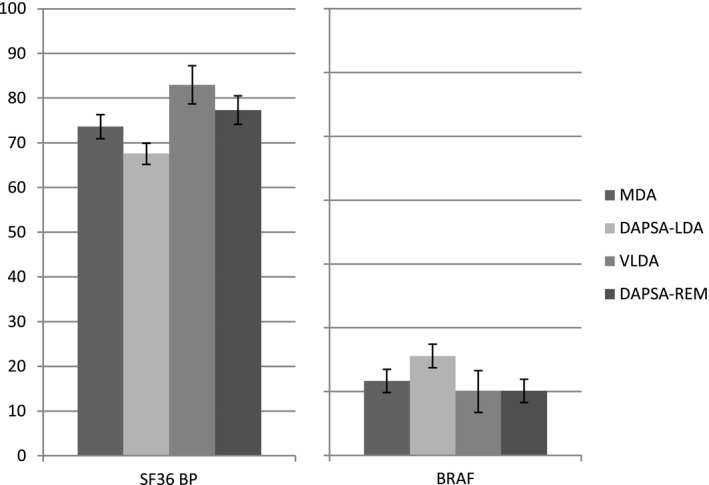
“Body functions” within different definitions of low disease activity. Values are the percentages and 95% confidence intervals. SF‐36 = Short Form 36; BP = bodily pain; BRAF = Bristol Rheumatoid Arthritis Fatigue (see Figure 1 for other definitions).
Figure 3.
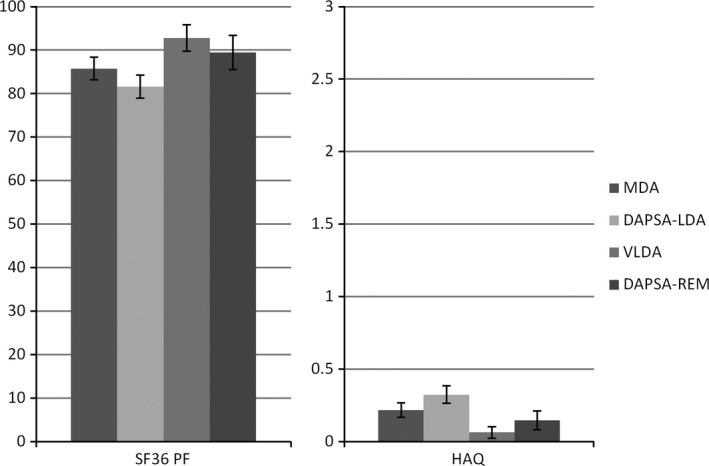
“Activity” within different definitions of low disease activity. Values are the percentages and 95% confidence intervals. SF‐36 = Short Form 36, PF = physical functioning; HAQ = Health Assessment Questionnaire (see Figure 1 for other definitions).
Measures of Body functions were significantly better in patients with both MDA and DAPSA‐LDA than in patients with only DAPSA‐LDA: mean SF‐36 BP scores 74.7 (95% CI 72.0–77.4) and 58.7 (95% CI 54.8–62.6), respectively, and mean BRAF scores 11.4 (95% CI 9.3–13.5) and 19.7 (95% CI 15.9–23.4), respectively. A similar outcome was observed for measures of Activity: mean SF‐36 PF scores 86.8 (95% CI 84.0–89.6) and 70.3 (95% CI 65.7–74.9), respectively, and mean HAQ scores 0.19 (95% CI 0.14–0.25) and 0.61 (95% CI 0.48–0.73), respectively. There was no statistically significant difference in Body functions and Activity between patients who achieved both MDA and DAPSA‐LDA and those who achieved only MDA (see Supplementary Figures 1 and 2, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.23571/abstract).
Residual disease activity
In patients with low disease activity according to criteria for MDA or DAPSA, the main indicators of residual disease activity were tender joints, psoriasis, patient's global assessment and pain assessment scores, and HAQ scores. The percentages of patients with residual disease activity were slightly higher in patients who achieved DAPSA‐LDA than in those who achieved only MDA. None of the patients with VLDA had any residual disease activity. Clinical enthesitis, dactylitis, and an elevated CRP level were rarely observed in patients in all categories of low disease activity (Table 2). In patients who had not achieved MDA, the domains of residual disease activity with the highest prevalence were patient's pain score (91%), patient's global score (86%), HAQ score (67%), TJC68 (61%), and psoriasis (59%) (Table 3). More than 1 joint was swollen in 27% of patients, and more than 1 clinical manifestation of enthesitis was present in 24% (LEI score >1). Residual disease activity in patients who did not fulfill the DAPSA‐LDA criteria was similar to that in patients who did not fulfill the MDA criteria, except more patients in the former group had an elevated CRP level (29%). Dactylitis was rarely observed. In patients who achieved only DAPSA, the domains of residual activity with the highest prevalence were patient's pain assessment score (89%) and patient's global assessment score (83%), which prevented them from achieving MDA (see Supplementary Table 1, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.23571/abstract).
Table 2.
Residual disease activity in patients with low disease activitya
| MDA (n = 127) | DAPSA ≤14 (n = 149) | VLDA (n = 34) | DAPSA ≤4 (n = 49) | |
|---|---|---|---|---|
| TJC68 >1 | 9 | 17 | 0 | 2 |
| SJC66 >1 | 6 | 10 | 0 | 4 |
| PASI score >1 | 34 | 42 | 0 | 39 |
| Pain score >1.5 (0–10‐cm VAS) | 26 | 43 | 0 | 10 |
| Global score >2.0 (0–10‐cm VAS) | 18 | 38 | 0 | 4 |
| HAQ score >0.5 | 7 | 20 | 0 | 8 |
| LEI score >1 | 1 | 5 | 0 | 2 |
| Dactylitis | 2 | 2 | 0 | 0 |
| Elevated CRP level | 6 | 2 | 0 | 0 |
A Disease Activity index for Psoriatic Arthritis (DAPSA) score of ≤14 represents low disease activity, and a score of ≤4 represents remission. Values are the percentage of patients with residual disease activity. MDA = minimal disease activity; VLDA = very low disease activity; TJC68 = tender joint count in 68 joints; SJC66 = swollen joint count in 66 joints; PASI = Psoriasis Area and Severity Index; VAS = visual analog scale; HAQ = Health Assessment Questionnaire; LEI = Leeds Enthesitis Index, CRP = C‐reactive protein.
Table 3.
Residual disease activity in patients with high disease activitya
| No MDA (n = 137) | DAPSA >14 (n = 85) | No VLDA (n = 218) | DAPSA >4 (n = 185) | |
|---|---|---|---|---|
| TJC68 >1 | 61 | 73 | 42 | 47 |
| SJC66 >1 | 27 | 32 | 20 | 22 |
| PASI score >1 | 59 | 55 | 54 | 49 |
| Pain score >1.5 (0–10‐cm VAS) | 91 | 93 | 70 | 75 |
| Global score >2.0 (0–10‐cm VAS) | 86 | 84 | 63 | 68 |
| HAQ score > 0.5 | 67 | 74 | 45 | 48 |
| LEI score >1 | 24 | 26 | 31 | 15 |
| Dactylitis severity score >1 | 3 | 4 | 3 | 3 |
| CRP level elevated | 16 | 29 | 14 | 15 |
A Disease Activity index for Psoriatic Arthritis (DAPSA) score of >14 represents …., and a score of ….. represents ….. Values are the percentage of patients with residual disease activity. MDA = minimal disease activity; DAPSA = Disease Activity Index for Psoriatic Arthritis; VLDA = very low disease activity; TJC68 = tender joint count in 68 joints; SJC66 = swollen joint count in 66 joints; PASI = Psoriasis Area and Severity Index; VAS = visual analog scale; HAQ = Health Assessment Questionnaire; LEI = Leeds Enthesitis Index, CRP = C‐reactive protein.
Discussion
In patients receiving usual care, in which treatment is not a target directed at achieving a specific PsA target, we did not observe large differences in the average disease burden between PsA patients who achieved either MDA or DAPSA‐LDA. In addition, we showed that patients with MDA usually also had DAPSA‐LDA. In contrast, not all patients with DAPSA‐LDA had achieved MDA. In a subsequent analysis of the burden of disease in these patients, we showed that patients achieving both targets reported better Body functions and Activity compared with those achieving only DAPSA‐LDA. It seems that this subgroup of patients warranted more attention but were regarded as having low disease activity as determined using the DAPSA score. No or very little residual disease activity was measured in patients achieving VLDA or DAPSA‐REM. However, only 13% of patients receiving usual care reached that target.
Our study is unique in the sense that we compared different targets and their relationship to the disease burden in patients with early PsA. Previous studies have shown high agreement between MDA and DAPSA; however, the investigators in those studies were not able to determine whether these groups differed in terms of disease burden 26, 27. Investigators in other studies did assess the burden of disease in patients fulfilling only 1 of the targets compared with those not fulfilling that target. It was shown in those studies that patients achieving the target had better scores for patient‐reported outcomes compared with patients in whom the target was not achieved 28, 29, 30, 31.
Besides the target being related to better outcomes, it should also be feasible to use in clinical practice. In patients with MDA, the presence of enthesitis, dactylitis, and psoriasis must be assessed additionally, although such an assessment probably was done during routine care, because the recommended treatment depends on the domain involved 32, 33. In contrast, a high DAPSA score indicates that treatment should be intensified, but it is less informative for determining how it should be intensified, because the choice of treatment depends on the domains involved. One of the reasons why patients who achieve both MDA and DAPSA report better outcomes than patients who achieve only DAPSA‐LDA is that the HAQ is also included in the criteria for MDA. A higher HAQ score in the patients who achieve DAPSA only could be the result of these patients not achieving MDA because of a high HAQ score. However, patients with only DAPSA‐LDA also had higher PASI scores, global and pain scores on a VAS, and higher SJC66 and TJC68 scores. The impact of psoriasis was recently demonstrated in a study by Mease et al 29, in which patients enrolled in the Consortium of Rheumatology Researchers of North America Registry who had greater skin involvement more often had not achieved MDA and also had poorer functional status compared with patients with less skin involvement. Furthermore, the overall burden of disease in patients with MDA did not change when we excluded the HAQ score as a remission criterion for MDA.
Studying the true value of different targets in the treatment of PsA ideally would be investigated in a large randomized clinical trial studying treat‐to‐target strategies and randomizing patients to treat‐to‐target care with different targets. Such data are currently not available. Therefore, we used the second‐best alternative, a large observational cohort of patients with early PsA. Use of this approach causes some challenges in interpreting the results. Rheumatologists were not instructed to treat patients according to a certain target. We do not know whether treatment would have differed between patients treated to target with either MDA or DAPSA if they would have been randomized.
In the current study, after 12 months of treatment, patients achieving only DAPSA‐LDA had a higher disease burden compared with patients who achieved both targets. Whether this gap would have been resolved with treatment remains to be determined. For example, comorbidities could influence the subjective elements of disease activity 34, 35. Irrespective of the disease activity measure that is used, in daily clinical care, disease activity always needs to be interpreted by the treating physician. A second challenge in the interpretation of our results is that in this real‐life cohort study, selection bias may have occurred. Some patients were lost to follow‐up and did not have a 12‐month assessment. This occurrence could be related to disease activity as well, because some patients were discharged from care by the rheumatologist and subsequently did not visit the research nurse when invited. The data we present are representative of the population visiting the rheumatologist 1 year after diagnosis, instead of all patients 1 year after diagnosis.
In conclusion, in our study of early PsA, no large differences were observed in the disease burden between patients achieving MDA or DAPSA‐LDA while receiving usual care, because most patients who achieve MDA also achieved DAPSA‐LDA. However, patients who reached both targets reported better Body function and Activity level compared with those who achieved only DAPSA‐LDA. It appears that MDA is more difficult to achieve, but aiming for MDA even after DAPSA‐LDA is achieved potentially results in better outcomes for patients.
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Vis had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Wervers, Vis, Tchetverikov, Gerards, Kok, Appels, van der Graaff, van Groenendael, Korswagen, Veris‐van Dieren, Luime.
Acquisition of data
Wervers, Vis, Tchetverikov, Gerards, Kok, Appels, van der Graaff, van Groenendael, Korswagen, Veris‐van Dieren, Hazes, Luime.
Analysis and interpretation of data
Wervers, Vis, Hazes, Luime.
Supporting information
Acknowledgements
We gratefully thank all participating patients and participating rheumatologists and research nurses. In addition, we would like to thank Esther Röder and the research team for their support.
Supported by The Netherlands Organisation for Health Research and Development.
References
- 1. Mease PJ. Psoriatic arthritis: update on pathophysiology, assessment and management. Ann Rheum Dis 2011;70 Suppl 1:i77–84. [DOI] [PubMed] [Google Scholar]
- 2. Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis 2005;64 Suppl 2:ii14–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Husted JA, Gladman DD, Farewell VT, Long JA, Cook RJ. Validating the SF‐36 health survey questionnaire in patients with psoriatic arthritis. J Rheumatol 1997;24:511–7. [PubMed] [Google Scholar]
- 4. Gladman DD, Shuckett R, Russell ML, Thorne JC, Schachter RK. Psoriatic arthritis (PSA): an analysis of 220 patients. Q J Med 1987;62:127–41. [PubMed] [Google Scholar]
- 5. Wong K, Gladman DD, Husted J, Long JA, Farewell VT. Mortality studies in psoriatic arthritis: results from a single outpatient clinic. I. Causes and risk of death. Arthritis Rheum 1997;40:1868–72. [DOI] [PubMed] [Google Scholar]
- 6. Mease PJ, Armstrong AW. Managing patients with psoriatic disease: the diagnosis and pharmacologic treatment of psoriatic arthritis in patients with psoriasis. Drugs 2014;74:423–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stoffer MA, Schoels MM, Smolen JS, Aletaha D, Breedveld FC, Burmester G, et al. Evidence for treating rheumatoid arthritis to target: results of a systematic literature search update. Ann Rheum Dis 2016;75:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coates LC, Moverley AR, McParland L, Brown S, Navarro‐Coy N, O'Dwyer JL, et al. Effect of tight control of inflammation in early psoriatic arthritis (TICOPA): a UK multicentre, open‐label, randomised controlled trial. Lancet 2015;386:2489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Palominos PE, Gaujoux‐Viala C, Fautrel B, Dougados M, Gossec L. Clinical outcomes in psoriatic arthritis: a systematic literature review. Arthritis Care Res (Hoboken) 2012;64:397–406. [DOI] [PubMed] [Google Scholar]
- 10. Orbai AM, de Wit M, Mease P, Shea JA, Gossec L, Leung YY, et al. International patient and physician consensus on a psoriatic arthritis core outcome set for clinical trials. Ann Rheum Dis 2017;76:673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schoels M, Aletaha D, Funovits J, Kavanaugh A, Baker D, Smolen JS. Application of the DAREA/DAPSA score for assessment of disease activity in psoriatic arthritis. Ann Rheum Dis 2010;69:1441–7. [DOI] [PubMed] [Google Scholar]
- 12. Coates LC, Helliwell PS. Validation of minimal disease activity criteria for psoriatic arthritis using interventional trial data. Arthritis Care Res (Hoboken) 2010;62:965–9. [DOI] [PubMed] [Google Scholar]
- 13. Helliwell PS, FitzGerald O, Fransen J, Gladman DD, Kreuger GG, Callis‐Duffin K, et al. The development of candidate composite disease activity and responder indices for psoriatic arthritis (GRACE project). Ann Rheum Dis 2013;72:986–91. [DOI] [PubMed] [Google Scholar]
- 14. Mumtaz A, Gallagher P, Kirby B, Waxman R, Coates LC, Veale JD, et al. Development of a preliminary composite disease activity index in psoriatic arthritis. Ann Rheum Dis 2011;70:272–7. [DOI] [PubMed] [Google Scholar]
- 15. Smolen JS, Schols M, Braun J, Dougados M, FitzGerald O, Gladman DD, et al. Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force. Ann Rheum Dis 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coates LC, Fransen J, Helliwell PS. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis 2010;69:48–53. [DOI] [PubMed] [Google Scholar]
- 17. Schoels MM, Aletaha D, Alasti F, Smolen JS. Disease activity in psoriatic arthritis (PsA): defining remission and treatment success using the DAPSA score. Ann Rheum Dis 2016;75:811–8. [DOI] [PubMed] [Google Scholar]
- 18. International classification of functioning, disability, and health: ICF. Geneva: World Health Organization; 2001. [Google Scholar]
- 19. Healy PJ, Helliwell PS. Measuring clinical enthesitis in psoriatic arthritis: assessment of existing measures and development of an instrument specific to psoriatic arthritis. Arthritis Rheum 2008;59:686–91. [DOI] [PubMed] [Google Scholar]
- 20. Healy PJ, Helliwell PS. Measuring dactylitis in clinical trials: which is the best instrument to use? J Rheumatol 2007;34:1302–6. [PubMed] [Google Scholar]
- 21. Fredriksson T, Pettersson U. Severe psoriasis: oral therapy with a new retinoid. Dermatologica 1978;157:238–44. [DOI] [PubMed] [Google Scholar]
- 22. Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 23. Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum 1980;23:137–45. [DOI] [PubMed] [Google Scholar]
- 24. Nicklin J, Cramp F, Kirwan J, Greenwood R, Urban M, Hewlett S. Measuring fatigue in rheumatoid arthritis: a cross‐sectional study to evaluate the Bristol Rheumatoid Arthritis Fatigue Multi‐Dimensional questionnaire, visual analog scales, and numerical rating scales. Arthritis Care Res (Hoboken) 2010;62:1559–68. [DOI] [PubMed] [Google Scholar]
- 25. Coates LC, Helliwell PS. Defining low disease activity states in psoriatic arthritis using novel composite disease instruments. J Rheumatol 2016;43:371–5. [DOI] [PubMed] [Google Scholar]
- 26. Chimenti MS, Triggianese P, Conigliaro P, Tonelli M, Gigliucci G, Novelli L, et al. A 2‐year observational study on treatment targets in psoriatic arthritis patients treated with TNF inhibitors. Clin Rheumatol 2017;36:2253–60. [DOI] [PubMed] [Google Scholar]
- 27. Michelsen B, Diamantopoulos AP, Hoiberg HK, Soldal DM, Kavanaugh A, Haugeberg G. Need for improvement in current treatment of psoriatic arthritis: study of an outpatient clinic population. J Rheumatol 2017;44:431–6. [DOI] [PubMed] [Google Scholar]
- 28. Van Mens LJ, Turina MC, van de Sande MG, Nurmohamed MT, van Kuijk AW, Baeten DL. Residual disease activity in psoriatic arthritis: discordance between the rheumatologist's opinion and minimal disease activity measurement. Rheumatology (Oxford) 2018;57:283–90. [DOI] [PubMed] [Google Scholar]
- 29. Mease PJ, Karki C, Palmer JB, Etzel CJ, Kavanaugh A, Ritchlin CT, et al. Clinical and patient‐reported outcomes in patients with psoriatic arthritis (PsA) by body surface area affected by psoriasis: results from the Corrona PsA/Spondyloarthritis Registry. J Rheumatol 2017;44:1151–8. [DOI] [PubMed] [Google Scholar]
- 30. Queiro R, Canete JD, Montilla C, Abad M, Montoro M, Gomez S, et al. Minimal disease activity and impact of disease in psoriatic arthritis: a Spanish cross‐sectional multicenter study. Arthritis Res Ther 2017;19:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aletaha D, Alasti F, Smolen JS. Disease activity states of the DAPSA, a psoriatic arthritis specific instrument, are valid against functional status and structural progression. Ann Rheum Di. 2017;76:418–21. [DOI] [PubMed] [Google Scholar]
- 32. Orbai AM, Mease PJ, de Wit M, Kalyoncu U, Campbell W, Tillett W, et al. Report of the GRAPPA‐OMERACT Psoriatic Arthritis Working Group from the GRAPPA 2015 Annual Meeting. J Rheumatol 2016;43:965–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gossec L, Smolen JS. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. 2016;75:499–510. [DOI] [PubMed] [Google Scholar]
- 34. Brikman S, Furer V, Wollman J, Borok S, Matz H, Polachek A, et al. The effect of the presence of fibromyalgia on common clinical disease activity indices in patients with psoriatic arthritis: a cross‐sectional study. J Rheumatol 2016;43:1749–54. [DOI] [PubMed] [Google Scholar]
- 35. Ballegaard C, Hojgaard P, Dreyer L, Cordtz R, Jorgensen TS, Skougaard M, et al. The impact of comorbidities on tumor necrosis factor inhibitor therapy in psoriatic arthritis: a population‐based cohort study. Arthritis Care Res (Hoboken) 2018;70:592–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


