Abstract
Aims
To assess the benefits of intensive statin therapy on reducing cardiovascular (CV) events in patients with type 2 diabetes complicated with hyperlipidaemia and retinopathy in a primary prevention setting in Japan. In the intension‐to‐treat population, intensive therapy [targeting LDL cholesterol <1.81 mmol/L (<70 mg/dL)] was no more effective than standard therapy [LDL cholesterol ≥2.59 to <3.10 mmol/L (≥100 to <120 mg/dL)]; however, after 3 years, the intergroup difference in LDL cholesterol was only 0.72 mmol/L (27.7 mg/dL), and targeted levels were achieved in <50% of patients. We hypothesized that the intergroup difference in CV events would have been statistically significant if more patients had been successfully treated to target.
Materials and Methods
This exploratory post hoc analysis focused on intergroup data from patients who achieved their target LDL cholesterol levels. The primary endpoint was the composite incidence of CV events. A Cox proportional hazards model was used to estimate hazard ratios (HRs) for incidence of the primary endpoint in patients who achieved target LDL cholesterol levels in each group.
Results
Data were analysed from 1909 patients (intensive: 703; standard: 1206) who achieved target LDL cholesterol levels. LDL cholesterol at 36 months was 1.54 ± 0.30 mmol/L (59.7 ± 11.6 mg/dL) in the intensive group and 2.77 ± 0.46 mmol/L (107.1 ± 17.8 mg/dL) in the standard group (P < 0.05). After adjusting for baseline prognostic factors, the composite incidence of CV events or deaths associated with CV events was significantly lower in the intensive than the standard group (HR 0.48; 95% confidence interval 0.28‐0.82; P = 0.007).
Conclusions
This post hoc analysis suggests that achieving LDL cholesterol target levels <1.81 mmol/L may more effectively reduce CV events than achieving target levels ≥2.59 to <3.10 mmol/L in patients with hypercholesterolaemia and diabetic retinopathy.
Keywords: cardiovascular disease, clinical trial, diabetic retinopathy, dyslipidaemia, lipid‐lowering therapy
1. INTRODUCTION
Aging populations and modern lifestyles have been increasingly associated with higher levels of dyslipidaemia and impairment of glucose metabolism in diseases such as type 2 diabetes around the world. Each of these conditions is a known risk factor for cardiovascular disease (CVD), and the risk of a cardiovascular (CV) event is even higher in patients with both conditions.1, 2, 3 Among patients with diabetes, the CV risk is known to be further increased in patients whose diabetes is complicated by retinopathy; such patients are recognized to be at very high risk for CVD.4, 5
The EMPATHY study is the first to assess the benefits of intensive statin therapy in patients with type 2 diabetes with hyperlipidaemia and diabetic retinopathy in a primary prevention setting, and also the first large‐scale clinical study to evaluate the effectiveness of the treat‐to‐target approach. The study compared the benefits of intensive and standard statin therapy for reducing a composite of CV events or deaths from CV events (the primary endpoint). Analysis of the intention‐to‐treat (ITT) population showed that lipid‐lowering therapy targeting LDL cholesterol <1.81 mmol/L (<70 mg/dL) did not have a more beneficial effect on the primary endpoint than therapy targeting LDL cholesterol ≥2.59 to <3.10 mmol/L (≥100 to <120 mg/dL) (hazard ratio [HR] 0.84, 95% confidence interval [CI] 0.67‐1.07; P = 0.15).6 These findings appeared to contradict earlier findings that showed benefits of lower LDL cholesterol in patients with diabetes.7, 8, 9 Notably, however, the LDL cholesterol target in the EMPATHY study was achieved by fewer than half of the patients in either group. In addition, a large percentage of patients on standard therapy in the original study (targeting ≥2.59 to <3.10 mmol/L) actually achieved LDL cholesterol levels below the target range (Figure 1). These factors may have contributed to masking the efficacy of the intensive therapy.
Figure 1.
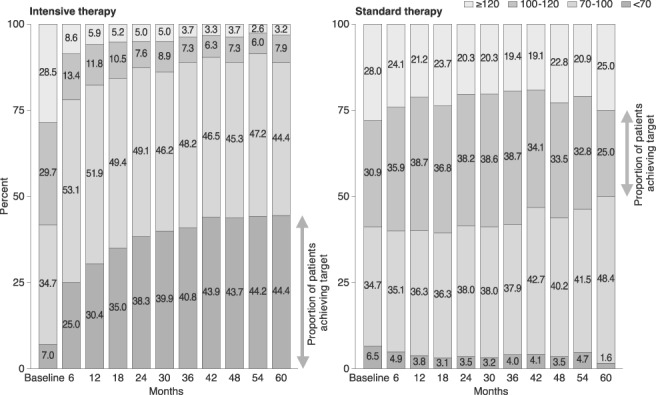
Distribution of LDL cholesterol in the intensive and standard therapy groups in the original study
To further investigate the efficacy of intensive therapy, we conducted additional exploratory analyses of between‐group comparisons. Although previous large‐scale clinical studies of statins have included exploratory (post hoc) analyses stratified by lipid levels achieved, in all cases, these sub‐analyses were for dose comparison studies. More importantly, none of the studies assessed whether the patients achieved prespecified goals for LDL cholesterol levels.10, 11
We limited our sub‐analyses to those patients whose LDL cholesterol levels were within the targeted range, in order to better assess the effects of the treat‐to‐target approach in these patient populations. Our hypothesis was that intensive therapy in patients who achieved their target (LDL cholesterol <1.81 mmol/L) would be superior to standard therapy (LDL cholesterol target ≥2.59 to <3.10 mmol/L) in reducing the incidence of composite CV events.
2. MATERIALS AND METHODS
2.1. Study design
The EMPATHY study was conducted to determine whether intensive lipid‐lowering therapy is superior to standard therapy in reducing the incidence of CV events or death from CV events in patients with type 2 diabetes complicated by hyperlipidaemia and diabetic retinopathy and without a history of CVD.6, 12 The study used a multicentre, prospective, randomized, open‐label, blinded endpoint (PROBE) design. It was conducted in Japan in accordance with the Declaration of Helsinki and Japanese ethical guidelines for clinical studies. The protocol was reviewed and approved by the institutional review board of each participating centre. The study was registered with the University Hospital Medical Information clinical trials registry (UMIN000003486).
The sub‐analysis design was based on the results of the primary analysis of the EMPATHY study, in which patients were initially treated to ≥2.59 to <3.10 mmol/L (run‐in period) and were then randomized (1:1) to intensive therapy targeting LDL cholesterol <1.81 mmol/L or standard therapy targeting ≥2.59 to <3.10 mmol/L (treatment period). The primary endpoint was a composite of the incidence of CV events (cardiac, cerebral, renal and vascular events) and death from CV events, compared between the two groups.
2.2. Patients
This sub‐analysis was performed on data collected from patients in the EMPATHY study who achieved mean LDL cholesterol levels of <1.81 mmol/L in the intensive therapy group in the original study (the intensive group) and ≥2.59 to <3.10 mmol/L in the standard therapy group in the original study (the standard group). The mean LDL cholesterol for each patient was defined as the mean value of measurements obtained at scheduled visits, starting 6 months after randomization to the intensive therapy group or the standard therapy group in the original study, and continuing to the final visit for those who developed no events or to the nearest day before onset for those who developed any events.
2.3. Procedures
Analysis included all patients who had at least one scheduled visit during the period starting 6 months after randomization. For reference, in comparison to these mean values, additional analysis was performed on data collected from patients who showed the target LDL cholesterol level at their last visit. The last visit was defined to be the nearest day before onset of an event for patients who developed any events, or the date of the final visit for patients who did not develop any events during the scheduled visits, starting 6 months after randomization to a treatment group.
2.4. Outcomes
In the EMPATHY study, the primary outcome was the composite incidence of CV events, including cardiac, cerebral, renal and vascular events, or death associated with CV events. The secondary outcomes included death from any cause; individual incidence of the events defined as CV events for the primary endpoint; incidence of stroke; change in laboratory variables related to chronic kidney disease; and safety. Primary and secondary endpoints were adjudicated by an event evaluation committee whose members were unaware of the treatment allocation. In this sub‐analysis, we analysed only the primary outcome and safety because of the small number of CV events.
2.5. Statistical analysis
A Cox proportional hazards model was used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for the incidence of the primary endpoint in patients who achieved target LDL cholesterol levels in the intensive and standard groups. Because this additional analysis was performed in a sub‐group of patients, a Cox proportional hazards model was applied, with study group and baseline influencing factors as explanatory variables, to adjust for these factors.
A stepwise method was used with the Cox proportional hazards model in the full analysis set (ITT population) to select influencing factors; the primary endpoint was the objective variable, and prognostic factors were the explanatory variables. In this analysis, 15 potential prognostic factors were evaluated: gender, age, body mass index, compliance with lipid‐lowering agents (including statins) from enrolment, smoking status (current smoker, past smoker, non‐smoker), family history of coronary artery disease, family history of cerebrovascular disease, duration of diabetes, presence or absence of diabetic nephropathy, presence or absence of diabetic neuropathy, presence or absence of hypertension, funduscopic findings at enrolment (simple retinopathy, pre‐proliferative retinopathy, proliferative retinopathy), glycated haemoglobin (HbA1c) at informed consent, LDL cholesterol at randomization, and estimated glomerular filtration rate (eGFR) at enrolment (<60, ≥60 mL/min/1.73 m2).
3. RESULTS
3.1. Study participants
Of the 5144 patients randomized to the intensive and standard therapy groups in the EMPATHY study, a total of 1909 were included in this sub‐analysis (703 in the intensive group and 1206 in the standard group). A total of 70 patients (25 in the intensive group and 45 in the standard group) had only one scheduled visit at least 6 months after randomization.
3.2. Baseline characteristics
Some of the demographic characteristics of the patients in both groups at baseline were similar (age, family history of coronary artery disease and/or cerebrovascular disease, presence of neuropathy and/or nephropathy, severity of retinopathy, HbA1c levels and eGFR), while other characteristics differed between the groups (Table 1). In comparison to the standard therapy group, a higher proportion of patients in the intensive therapy group was male (51.9% vs. 43.5%), received no lipid‐lowering treatment before study enrolment (54.9% vs. 39.1%), were current smokers (19.3% vs. 16.9%), and had hypertension at enrolment (75.7% vs. 70.6%). Other differences between the two groups included higher mean BMI in the intensive group, and longer duration of diabetes and higher mean LDL cholesterol level at enrolment in the standard group.
Table 1.
Baseline demographic characteristics (potential prognostic factors): for patients achieving LDL cholesterol target in mean value
| Intensive group | Standard group | ||
|---|---|---|---|
| (n = 703) | (n = 1206) | P | |
| Male | 365 (51.9) | 525 (43.5) | <0.001* |
| Age, yearsa | 62.7 (10.8) | 63.6 (10.1) | 0.23 † |
| Body mass index, kg/m2 | 26.2 (4.2) | 25.5 (4.2) | <0.001 † |
| Lipid‐lowering agentsb | |||
| None | 386 (54.9) | 472 (39.1) | <0.001* |
| 1 drug | 316 (45.0) | 733 (60.8) | |
| ≥2 drugs | 1 (0.1) | 1 (0.1) | |
| Statinsb | <0.001* | ||
| No | 428 (60.9) | 511 (42.4) | |
| Yes | 275 (39.1) | 695 (57.6) | |
| Smokingc | 136 (19.3) | 204 (16.9) | 0.01* |
| Family history | |||
| Coronary artery disease | 86 (12.2) | 165 (13.7) | 0.37* |
| Cerebrovascular disease | 146 (20.8) | 261 (21.6) | 0.65* |
| Duration of diabetes, years | 12.3 (8.3) | 13.4 (9.1) | 0.02 † |
| Diabetic complications | |||
| Neuropathy | 217 (30.9) | 382 (31.7) | 0.71* |
| Nephropathy | 385 (54.8) | 614 (50.9) | 0.10* |
| Hypertension | 532 (75.7) | 852 (70.6) | 0.02* |
| Funduscopyd | |||
| Simple retinopathy | 454 (64.6) | 785 (65.1) | 0.99* |
| Preproliferative retinopathy | 141 (20.1) | 243 (20.1) | |
| Proliferative retinopathy | 103 (14.7) | 170 (14.1) | |
| Othere | 3 (0.4) | 5 (0.4) | |
| HbA1c, %a | 7.71 (1.20) | 7.71 (1.19) | 0.91 † |
| LDL cholesterol, mmol/Lf | 2.42 (0.62) | 2.79 (0.61) | <0.001 † |
| eGFR, mL/min/1.73 m2 | 75.1 (21.7) | 74.6 (19.6) | 0.81 † |
Abbreviations: HbA1c, glycated haemoglobin; eGFR, estimated glomerular filtration rate.
Data are mean (SD) or n (%).
*The χ2 test without Yates' correction.
†Wilcoxon rank sum test.
Values obtained at the time of consent.
Values obtained at provisional enrolment.
Not including past smokers.
Diagnosed by ophthalmologists based on the modified Davis classification.
Includes five patients who had a history of laser therapy but no funduscopic findings at enrolment. The remaining three patients were found to be retinopathy‐negative after enrolment.
Values were calculated using the Friedewald equation; LDL cholesterol = total cholesterol − [HDL cholesterol + triglycerides/5].
The demographic characteristics of the patients who were at their target LDL cholesterol level at the last visit were similar to those who were at their mean target LDL cholesterol level, with the exception of nephropathy (Table S1, Appendix S1).
The proportion of patients using atorvastatin, rosuvastatin or pitavastatin was about the same in the two groups at baseline (48.2% in the intensive group and 53.1% in the standard group), and the proportion using pravastatin, fluvastatin or simvastatin was 51.2% and 46.7%, respectively. At the end of the study, the proportion of atorvastatin, rosuvastatin or pitavastatin users remained nearly unchanged in the standard group (50.9%), but had risen to 98.2% in the intensive group. Dose levels at baseline were similar in the intensive and standard groups for all statins. In the intensive group, the dose increased for all statin types over the course of the study. The doses did not change for the standard group (Table S2, Appendix S1). It should be noted that the statin dose for “intensive” therapy in Japan is lower than in the United States and Europe.
3.3. Laboratory values
The changes in levels of LDL cholesterol, total cholesterol, HDL cholesterol and triglycerides in the sub‐analysis are shown in Figure 2. In the intensive group, the mean level of LDL cholesterol decreased significantly from baseline (2.42 ± 0.62 mmol/L) to the first measurement at 6 months (1.72 ± 0.36 mmol/L) and then remained at this level or lower (1.54 ± 0.30 mmol/L at 36 months) to 60 months after the start of treatment (1.46 ± 0.42 mmol/L). In the standard group, the LDL cholesterol level after 6 months of treatment was slightly higher (2.83 ± 0.45 mmol/L) than the baseline level (2.79 ± 0.61 mmol/L) and remained at or near that level (2.77 ± 0.46 mmol/L at 36 months) throughout the course of the study to 60 months after the start of treatment, when it dropped slightly to near‐baseline level (2.78 ± 0.58 mmol/L). Total cholesterol showed a similar pattern to LDL cholesterol in both groups. Triglyceride levels were slightly higher in the intensive group at baseline, but that gap diminished somewhat after the start of the study. HDL cholesterol remained substantially unchanged throughout the study in both groups.
Figure 2.
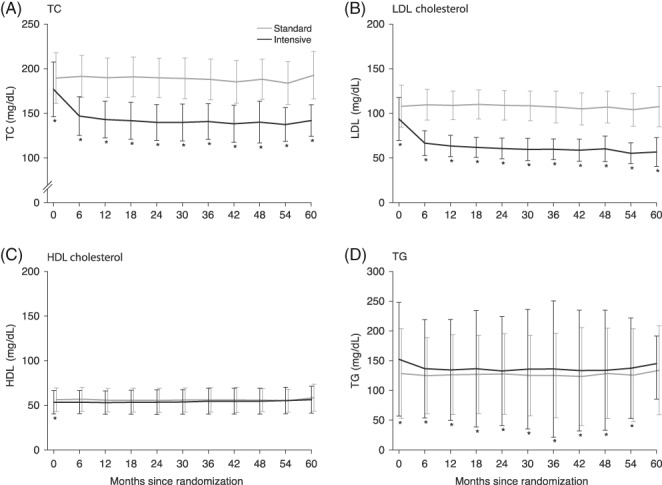
Changes in lipid variables over time. Data are mean and SD values. The black line shows findings for the intensive group and the gray line shows findings for the standard group. *P < 0.05, calculated using a mixed model repeated measures approach. The model included group, observation time point, and interaction between group and observation time point as fixed effects. TC, total cholesterol; TG, triglycerides
No changes were noted for either group during the study in blood pressure, HbA1c, creatinine or creatine kinase; however, in the intensive group, high‐sensitivity C‐reactive protein (hsCRP) levels were significantly reduced at all time points except 60 months, and there was a significant difference between the groups in hsCRP (Table S3, Appendix S1).
3.4. Efficacy endpoints
Since stepwise variable selection showed that eight factors were statistically related to the primary outcome among the 15 potential prognostic factors, the analysis was adjusted for these variables: gender; smoking status (current smoker, past smoker, non‐smoker); presence or absence of diabetic nephropathy, neuropathy, or hypertension; funduscopic findings at enrolment; HbA1c at informed consent; and eGFR at enrolment (<60, ≥60 mL/min/1.73 m2). Baseline LDL cholesterol was not found to be a prognostic factor. We adjusted for these eight prognostic factors to estimate HRs and 95% CIs for the incidence of CV events (the primary endpoint of the EMPATHY study).
In this sub‐analysis, a significantly smaller proportion of patients in the intensive group (18/703 patients) experienced CV events or death associated with CV events than in the standard group (56/1206 patients; HR 0.48, 95% CI 0.28‐0.82; P = 0.007 [Figure 3 and Table S4, Appendix S1]). This difference between the groups started at ~12 months after randomization. These findings remained unchanged even if baseline LDL cholesterol was added as a ninth prognostic factor (data not shown).
Figure 3.
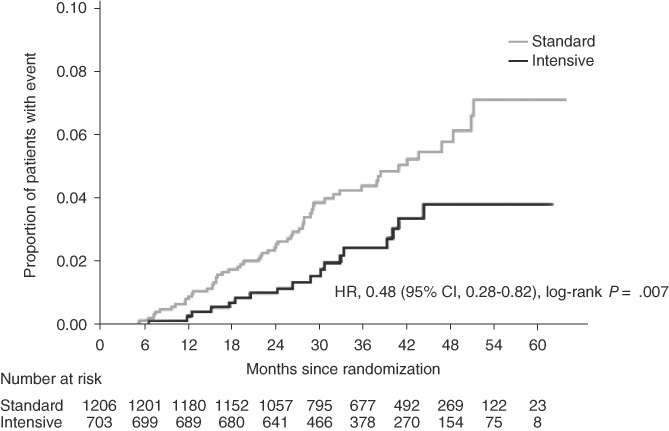
Cumulative event curve for the primary endpoint in the intensive and standard groups (patients achieving LDL cholesterol target, mean value). Hazard ratio (HR), 95% confidence interval (CI) and P value were estimated using a stratified Cox proportional hazards model, with gender (male, female), smoking status, presence or absence of diabetic nephropathy, presence or absence of diabetic neuropathy, presence or absence of hypertension, funduscopic findings, and baseline glycated haemoglobin (<8.4, ≥8.4%) and estimated glomerular filtration rate (<60, ≥60 [mL/min/1.73 m2]) as covariates
In the above sub‐analysis, we used mean LDL cholesterol values to determine whether each patient achieved the target range. We then repeated our analysis using LDL cholesterol values at the last visit. In this analysis we also noted a significant difference in the primary endpoint between the intensive group and the standard group (HR 0.43, 95% CI 0.27‐0.68; P < 0.001 [Figure 4]).
Figure 4.
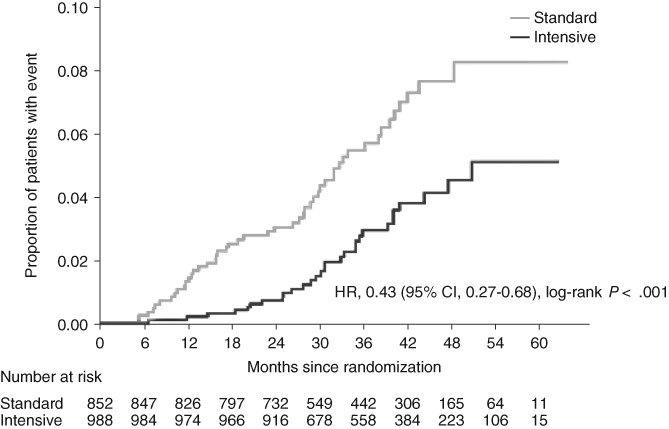
Cumulative event curve for the primary endpoint in the intensive and standard groups (patients achieving LDL cholesterol target at the last visit). Hazard ratio (HR), 95% confidence interval (CI) and P value are estimated using a Cox proportional hazards model with gender (male, female), smoking status, presence or absence of diabetic nephropathy, presence or absence of diabetic neuropathy, presence or absence of hypertension, funduscopic findings, and baseline glycated haemoglobin (<8.4, ≥8.4%) and estimated glomerular filtration rate (< 60, ≥60 mL/min/1.73 m2) as covariates
3.5. Safety
The safety endpoints examined in this analysis were adverse events (AEs), serious AEs, adverse drug reactions (ADRs) and serious ADRs. There was no significant difference in the incidence rates for each of these endpoints between the two groups. The major AEs were hepatobiliary disorders, renal and urinary disorders, rhabdomyolysis, myopathy and cancer (Table S5, Appendix S1). Overall, the occurrence of these events in the two groups was similar except for renal and urinary disorders, which were more common in the standard group (9.2%) than in the intensive group (5.7%).
4. DISCUSSION
The EMPATHY study assessed the benefits of intensive statin monotherapy for lipid management in patients with type 2 diabetes complicated by hypercholesterolaemia and diabetic retinopathy in a primary prevention setting. The study also evaluated the appropriateness of the treat‐to‐target approach in this patient population. Results from the EMPATHY study showed that intensive lipid‐lowering therapy targeting <1.81 mmol/L of LDL cholesterol was no more effective in reducing a composite of incidence of CV events or death from CV events than standard therapy targeting ≥2.59 to <3.10 mmol/L (HR 0.84, 95% CI 0.67‐1.07)6; however, the ITT method may lead to underestimation of intergroup differences in efficacy in situations where the treatment goals have not been properly achieved. In the present study, in particular, fewer than half of the patients in each group had LDL cholesterol levels within their target range, and nearly half in the standard group had LDL cholesterol levels below the target.
Our planned between‐group difference in LDL cholesterol was ~1.03 mmol/L (~40 mg/dL) (<1.81 mmol/L for the intensive therapy group compared with ~2.84 mmol/L (~110 mg/dL) for the standard therapy group) in the original study, with a predicted HR of 0.65; however, after 3 years of treatment, the actual LDL cholesterol difference was 0.72 mmol/L (1.98 vs. 2.69 mmol/L). We hypothesized that the smaller‐than‐expected difference may have been attributable, at least in part, to the unexpectedly low number of patients who achieved their LDL target. Our exploratory post hoc analyses were designed to investigate this hypothesis by comparing findings between patients whose LDL cholesterol was within the target range for their group.
The sub‐analysis involved differences in some prognostic factors between the patient group meeting their target LDL cholesterol levels of <1.81mmol/L under intensive therapy and the patient group meeting their LDL cholesterol levels of 2.59 to 3.10 mmol/L under standard therapy. We adjusted for eight factors that had been identified as potentially affecting the primary endpoint: gender (male, female); smoking status; presence or absence of diabetic nephropathy; presence or absence of diabetic neuropathy; presence or absence of hypertension; funduscopic findings; baseline HbA1c; and eGFR. We also found some significant intergroup differences for body mass index, use of lipid‐lowering agents, use of statins, duration of diabetes, and baseline LDL cholesterol level; however, because they did not affect the primary endpoints in this study, we did not adjust for those factors. After adjusting for the eight selected prognostic factors, the results of the analysis showed that the intensive lipid‐lowering therapy targeting <1.81 mmol/L LDL cholesterol significantly reduced the primary endpoint (the composite of incidence of CV events or death from CV events). Because of the low number of events (74), in this analysis we limited the number of factors, using a stepwise method for adjustment in the analytical model. We did this to avoid potentially non‐reproducible and unstable results. For further confirmation, we also performed an analysis with all variables included; similar results were obtained (HR 0.51; 95% CI 0.29‐0.89; P < 0.05 [Table S6, Appendix S1]). Safety events occurred at approximately the same rate in the two groups.
We used mean values for LDL cholesterol in patients who achieved their target levels because we thought it was important to ensure that patients were exposed to a specific concentration of LDL cholesterol for a certain period of time. Our results, although exploratory, suggest that achieving a target of <1.81 mmol/L LDL cholesterol lowers the risk of CV events significantly more than achieving a target of 2.59 to 3.10 mmol/L. For reference, we have also provided a summary of our findings for the proportion of patients who achieved their target LDL cholesterol level at the last visit. Results were similar to those based on mean values.
In the main results paper, we performed post hoc analysis, which involved classifying patient data into four subcategories (mean LDL cholesterol <1.81, 1.81 to <2.59, 2.59 to <3.10, and ≥3.10 mmol/L during the study). That analysis tended to show event prevention at lower LDL cholesterol values in both the intensive and standard therapy groups in the original study6; the results of the present sub‐analysis are consistent with those findings. This fact supports the reliability of our sub‐analysis. Although exploratory, we believe that these findings could meaningfully impact lipid management in clinical practice for the primary prevention of CV events in patients with type 2 diabetes with hyperlipidaemia and diabetic retinopathy.
Previous large‐scale clinical studies of statins have also used LDL cholesterol levels as a basis for post hoc sub‐analyses,8, 9, 10, 11 and usefulness was demonstrated in groups achieving lower target levels; however, all of these sub‐analyses were in dose comparison studies, and none assessed whether patients had achieved their target LDL cholesterol levels. To the best of our knowledge, no other analyses have been reported that show the effect of specified target LDL cholesterol levels using statin monotherapy on the occurrence of CV events or CV‐related deaths. Although this was an exploratory analysis, our data are valuable when assessing the importance of the treat‐to‐target approach in lipid management.
In the ITT analysis for the EMPATHY study, the difference in LDL cholesterol between the two groups was 0.72 mmol/L, and the HR for the primary endpoint was 0.84 (95% CI 0.67‐1.07; P = 0.15).6 In this sub‐analysis, LDL cholesterol at 36 months was 1.54 mmol/L in the intensive group and 2.77 mmol/L in the standard group, a difference of 1.23 mmol/L (47.4 mg/dL) between the two groups, and the HR was 0.48 (95% CI 0.28‐0.82; P = 0.007). In this sub‐analysis, aggressive treatment with the goal of lowering LDL cholesterol to 1.81 mmol/L was clearly effective in reducing the number of occurrences of the primary endpoint. The actual difference in LDL cholesterol exceeded the planned difference of ~1.03 mmol/L, which meant that the actual HR was also higher than the planned HR of 0.65. The main analysis did not detect a significant difference in primary endpoint occurrence between the two groups. These sub‐analysis findings indicate that we were unable to obtain significant results from the main analysis because of failure to achieve target LDL cholesterol levels.
No major differences were noted between groups in the incidence of AEs or ADRs. It thus appears unlikely that specific safety concerns will occur when intensive statin monotherapy is used to reduce LDL cholesterol below 1.81 mmol/L. We found no marked increase in cerebral haemorrhage in the intensive group (two patients in the intensive group, one patient in the standard group), nor any increase in HbA1c associated with statin use in this study.
These study findings are limited because they are derived from an exploratory analysis which included only those patients whose LDL cholesterol was within the target range for their assigned group: LDL cholesterol <1.81 mmol/L in the intensive therapy group in the original study and ≥2.59 to <3.10 mmol/L in the standard therapy group in the original study. In the EMPATHY study, <50% of patients reached their target LDL cholesterol. This can be attributed in part to the fact that over half of the investigators were general practitioners, rather than lipid specialists. Many Japanese physicians who treat hyperlipidaemia as part of their routine clinical practice are not lipid management experts and are concerned about adverse effects such as intracranial haemorrhage from intensive LDL cholesterol‐lowering. Such concerns may have affected some of the investigators in the present study, making them reluctant to prescribe high‐dose statin therapy even when the protocol stipulated the aggressive target of 1.81 mmol/L. Because of the small number of events, secondary endpoints were not assessed (Table S4, Appendix S1). In addition, although we detected and adjusted for eight prognostic factors, there may be additional unmeasured factors or confounding factors that should be considered.
In conclusion, the results from this exploratory post hoc analysis suggest that achievement of LDL cholesterol levels below 1.81 mmol/L is associated with more effective reduction of CV events than levels of 2.59 to 3.10 mmol/L in patients with type 2 diabetes with retinopathy and hyperlipidaemia who are at high coronary risk.4, 5 There were no major increases in AEs or ADRs when statin monotherapy was used to reduce LDL cholesterol below 1.81 mmol/L. Our results indicate the importance of targeting LDL cholesterol below 1.81 mmol/L, and then meeting that target consistently, for the reduction of CV events in this high‐risk patient population; however, this analysis was exploratory and must be substantiated in randomized clinical trials. A feasible approach is also needed for achieving these target levels in a clinical setting.
CONFLICTS OF INTEREST
H.I. reports grants and personal fees from Shionogi & Co., Ltd during the course of the study, and grants and personal fees from Takeda Pharmaceutical Co. Ltd, Nippon Boehringer Ingelheim Co., Ltd, Daiichi Sankyo Co., Ltd, MSD K.K., Mitsubishi Tanabe Pharma Corporation, Shionogi & Co., Ltd and Taisho Toyama Pharmaceutical Co., Ltd, as well as grants from Sumitomo Dainippon Pharma Co., Ltd, Astellas Pharma Inc., Kyowa Hakko Kirin Co., Ltd, Teijin Pharma Ltd, Mochida Pharmaceutical Co., Ltd, Ono Pharmaceutical Co., Ltd, Chugai Pharmaceutical Co., Ltd, Eli Lilly Japan K.K. and personal fees from Nipro Corporation and SBI Pharmaceuticals Co., Ltd outside the submitted work. I.K. reports personal fees from Shionogi & Co., Ltd during the course of the study, grants and personal fees from Takeda Pharmaceutical Co. Ltd, Nippon Boehringer Ingelheim Co., Ltd, Astellas Pharma Inc., Daiichi Sankyo Co., Ltd, and Otsuka Pharmaceutical Co., Ltd and grants from MSD K.K., Shionogi & Co., Ltd, GlaxoSmithKline K.K., Sanofi K.K., Genzyme Japan K.K., Sumitomo Dainippon Pharma Co., Ltd, Mitsubishi Tanabe Pharma Corporation and Bristol‐Myers Squibb Co. outside the submitted work. M.T. reports personal fees from Shionogi & Co., Ltd during the course of the study. T.A. reports personal fees from Shionogi & Co., Ltd during the course of the study, grants and personal fees from St. Jude Medical Japan Co., Ltd, Terumo Corporation, Daiichi Sankyo Co., Ltd and Abbott Vascular Japan Co., Ltd, grants from Goodman Co., Ltd, Otsuka Pharmaceutical Co., Ltd, Pfizer Japan Inc., Bayer Yakuhin, Ltd and Boston Scientific Corporation, and personal fees from Nippon Boehringer Ingelheim Co., Ltd outside the submitted work. H.D. reports grants and personal fees from Shionogi & Co., Ltd during the course of the study, grants and personal fees from AstraZeneca K.K., Astellas Pharma Inc., Abbott Vascular Japan Co., Ltd, Otsuka Pharmaceutical Co., Ltd, Kaken Pharmaceutical Co., Ltd, Kissei Pharmaceutical Co., Ltd, Kyowa Hakko Kirin Co., Ltd, Kowa Pharmaceutical Co. Ltd, Sanofi K.K., Daiichi Sankyo Co., Ltd, Sumitomo Dainippon Pharma Co., Ltd, Takeda Pharmaceutical Co. Ltd, Terumo Corporation, Nippon Boehringer Ingelheim Co., Ltd, Bayer Yakuhin, Ltd, Pfizer Japan Inc., Philips Respironics GK, Bristol‐Myers Squibb Co., Sanwa Kagaku Kenkyusho Co., Ltd, Mitsubishi Tanabe Pharma Corporation, MSD K.K. and GlaxoSmithKline K.K., grants from Eisai Co., Ltd, Teijin Pharma Ltd, Nippon Shinyaku Co., Ltd, VitalAire Japan K.K., Fujifilm RI Pharma Co., Ltd, Boston Scientific Corporation, Fuji Chemical Industries Co., Ltd, Fukuda Denshi Co., Ltd and Actelion Pharmaceuticals Japan Ltd, and personal fees from Aska Pharmaceutical Co., Ltd, Chugai Pharmaceutical Co., Ltd, Taisho Toyama Pharmaceutical Co., Ltd, Toa Eiyo Ltd, Ono Pharmaceutical Co., Ltd, Medtronic Japan Co., Ltd and Mochida Pharmaceutical Co., Ltd outside the submitted work. Y.E. reports non‐financial support from Shionogi & Co., Ltd during the course of the study. H.F. reports other fees (consultant) from Mehergen Group Holdings, Inc. outside the submitted work. J.H. reports grants and personal fees from Shionogi & Co., Ltd during the course of the study, and grants and personal fees from Astellas Pharma Inc., Nippon Boehringer‐Ingelheim Co., Ltd, Mochida Pharmaceutical Co., Ltd, Daiichi Sankyo Co., Ltd, Takeda Pharmaceutical Co., Ltd, Sumitomo Dainippon Pharma Co., Ltd, MSD K.K., Teijin Pharma Ltd, Actelion Pharmaceuticals Japan Ltd, Otsuka Pharmaceutical Co., Ltd, Novartis Pharma K.K. and Sanwa Kagaku Kenkyusho Co., Ltd outside the submitted work. K.H. reports personal fees and non‐financial support from Shionogi & Co., Ltd during the course of the study, grants and personal fees from Daiichi Sankyo Co., Ltd, Mochida Pharmaceutical Co., Ltd, grants from Actelion Pharmaceuticals Japan Ltd, Eisai Co., Ltd, Otsuka Pharmaceutical Co., Ltd, Sumitomo Dainippon Pharma Co., Ltd, Takeda Pharmaceutical Co. Ltd, Nippon Boehringer Ingelheim Co., Ltd, Bayer Yakuhin, Ltd, Sysmex Corporation, Medtronic Japan Co., Ltd and St. Jude Medical Japan Co., Ltd, and personal fees from Kowa Pharmaceutical Co. Ltd outside the submitted work. S.Is. reports grants and personal fees from Shionogi & Co., Ltd, during the course of the study, grants and personal fees from Amgen Astellas BioPharma K.K., Astellas Pharma Inc., Daiichi Sankyo Co., Ltd, Eli Lilly Japan K.K., Kowa Pharmaceutical Co. Ltd, Nippon Boehringer Ingelheim Co., Ltd, Kissei Pharmaceutical Co., Ltd, MSD K.K., Novartis Pharma K.K., Mitsubishi Tanabe Pharma Corporation, Ono Pharmaceutical Co. Ltd, Sanofi K.K., Takeda Pharmaceutical Co. Ltd, Taisho Toyama Pharmaceutical Co., Ltd, and Teijin Pharma Ltd, grants from Fujifilm Pharma Co., Ltd, Sumitomo Dainippon Pharma Co., Ltd, and Kyowa Hakko Kirin Co., Ltd, and personal fees from AstraZeneca K.K., Bayer Yakuhin, Ltd, Novo Nordisk Pharma Ltd, Pfizer Japan Inc., and Sanwa Kagaku Kenkyusho Co. Ltd outside the submitted work. T.I. reports personal fees and non‐financial support from Shionogi & Co., Ltd during the course of the study, grants and personal fees from Sanofi K.K., Sumitomo Dainippon Pharma Co., Ltd and Daiichi Sankyo Co., Ltd, grants from Takeda Pharmaceutical Co. Ltd and Mitsubishi Tanabe Pharma Corporation, and personal fees from Astellas Pharma Inc., AstraZeneca K.K. and MSD K.K. outside the submitted work. S.It. reports grants, personal fees and non‐financial support from Shionogi & Co., Ltd during the course of the study. A.K. reports personal fees and non‐financial support from Shionogi & Co., Ltd during the course of the study and personal fees from Astellas Pharma Inc., Sunstar Group Ltd, Eli Lilly Japan K.K., Sanofi K.K., AstraZeneca K.K., Takeda Pharmaceutical Co. Ltd, Taisho Toyama Pharmaceutical Co., Ltd, Nippon Boehringer Ingelheim Co., Ltd, Kowa Pharmaceutical Co. Ltd, and Sanwa Kagaku Kenkyusho Co. Ltd outside the submitted work. S.K. reports grants from Shionogi & Co., Ltd during the course of the study. K.K. reports grants and personal fees from Shionogi & Co., Ltd during the course of the study. M.Ki. reports grants and personal fees from Shionogi & Co., Ltd during the course of the study, grants and personal fees from Astellas Pharma Inc., Sanofi K.K., Pfizer Japan Inc., Ono pharmaceutical Co. Ltd, Novartis Pharma K.K., Mitsubishi Tanabe Pharma Corporation, Kyowa Hakko Kirin Co., Ltd, Abbott Japan Co., Ltd, and Otsuka Pharmaceutical Co., Ltd, grants from the Japanese government, Japan Heart Foundation, Japan Cardiovascular Research Foundation, Calpis Co., Ltd and Nihon Kohden Corporation, and personal fees from Daiichi Sankyo Co., Ltd, Bayer Yakuhin Ltd, Nippon Boehringer Ingelheim Co., Ltd, Kowa Pharmaceutical Co. Ltd, Sumitomo Dainippon Pharma Co., Ltd, Sawai Pharmaceutical Co., Ltd, MSD K.K., Shionogi & Co., Ltd, AstraZeneca K.K., Asahi Kasei Medical Co., Ltd, Novo Nordisk Pharma Ltd, Fujifilm RI Pharma Co., Ltd, and Japan Medical Data, outside the submitted work. T.K. reports grants and personal fees from Shionogi & Co., Ltd, during the course of the study, grants and personal fees from Daiichi Sankyo Co., Ltd and Bayer Yakuhin Ltd, and grants from Merck & Co., Inc., Novartis Pharma K.K., Astellas Pharma Inc., and Pfizer Japan Inc. outside the submitted work. M.Ku. reports personal fees from Shionogi & Co., Ltd during the course of the study and grants and personal fees from Shionogi & Co., Ltd outside the submitted work. K.M. reports other (meeting attendance fee) from Shionogi & Co., Ltd during the course of the study. T.Mura. reports personal fees from Shionogi & Co., Ltd during the course of the study. T.Muro. reports personal fees from Shionogi & Co., Ltd during the course of the study, grants and personal fees from Daiichi Sankyo Co., Ltd, Pfizer Japan Inc., Kowa Pharmaceutical Co. Ltd, MSD K.K., and Mitsubishi Tanabe Pharma Corporation, and personal fees from AstraZeneca K.K. outside the submitted work. K.N. reports non‐financial support from Shionogi & Co., Ltd during the course of the study. S.O. reports personal fees and non‐financial support from Shionogi & Co., Ltd during the course of the study. Y.Sa. reports grants, personal fees, and non‐financial support from Shionogi & Co., Ltd during the course of the study, grants, personal fees and other (advisory boards) from MSD K.K., Ono Pharmaceutical Co., Ltd, Mitsubishi Tanabe Pharma Corporation, Pfizer Japan Inc., Novartis Pharma K.K., grants and personal fees from Daiichi Sankyo Co., Ltd, Bayer Yakuhin, Ltd, Otsuka Pharmaceutical Co., Ltd, Sumitomo Dainippon Pharma Co., Ltd, Astellas Pharma Inc., and Takeda Pharmaceutical Co. Ltd, and grants from Baxter Ltd, Kyowa Hakko Kirin Co., Ltd, Teijin Pharma Ltd, Eisai Co., Ltd, Zeria Pharmaceutical Co., Ltd, Nihon Medi‐Physics Co., Ltd, Chugai Pharmaceutical Co., Ltd, Genzyme Japan K.K., and Medtronic Japan Co., Ltd, outside the submitted work. Y.Se. reports personal fees from Shionogi & Co., Ltd during the course of the study, grants and personal fees from Otsuka Pharmaceutical Co. and Ltd, Nippon Boehringer Ingelheim Co., Ltd, and grants from Mitsubishi Tanabe Pharma Co., Ltd, Fujifilm RI Pharma Co., Ltd, Roche Diagnostics K.K., MSD K.K., Pfizer Japan Inc., Bayer Yakuhin, Ltd, and Shionogi & Co., Ltd outside the submitted work. T.S. reports personal fees and non‐financial support from Shionogi & Co., Ltd during the course of the study. S.Sh. reports personal fees and non‐financial support from Shionogi & Co., Ltd during the course of the study. M.S. reports personal fees and non‐financial support from Shionogi & Co., Ltd during the course of the study. S.Su. reports personal fees from Shionogi & Co., Ltd during the course of the study, and grants from The Ministry of Education, Culture, Sports, Science, and Technology in Japan outside the submitted work. Y.T. reports personal fees from Shionogi & Co., Ltd during the course of the study, grants and personal fees from Astellas Pharma Inc., AstraZeneca K.K., Bayer Yakuhin, Ltd, Daiichi Sankyo Co., Ltd, Sumitomo Dainippon Pharma Co., Ltd, Eli Lilly Japan K.K., Kissei Pharmaceutical Co., Ltd, Kowa Pharmaceutical Co. Ltd, Kyowa Hakko Kirin Co., Ltd, MSD K.K., Mitsubishi Tanabe Pharma Corporation, Nippon Boehringer Ingelheim Co., Ltd, Novo Nordisk Pharma Ltd, Ono Pharmaceutical Co., Ltd, Sanwa Kagaku Kenkyusho Co., Ltd, Sanofi K.K., Shionogi & Co., Ltd, Taisho Toyama Pharmaceutical Co., Ltd, and Takeda Pharmaceutical Co. Ltd, and personal fees from Novartis Pharma K.K. outside the submitted work. H.T. reports personal fees and non‐financial support from Shionogi & Co., Ltd during the course of the study, grants and personal fees from Daiichi Sankyo Co., Ltd, and Takeda Pharmaceutical Co. Ltd, grants from Novartis Pharma K.K. and Astellas Pharma Inc., and personal fees from MSD K.K., Otsuka Pharmaceutical Co., Ltd, Pfizer Japan Inc., Mitsubishi Tanabe Pharma Corporation, Teijin Pharma Ltd, Nippon Boehringer Ingelheim Co., Ltd, and Bayer Yakuhin, Ltd, BMS outside the submitted work. K.Ue. reports other (contracted work) from Shionogi & Co., Ltd during the course of the study, and personal fees from Shionogi & Co., Ltd outside the submitted work. K.Ut. reports personal fees and non‐financial support from Shionogi & Co., Ltd during the course of the study, and grants from Sanofi K.K., MSD K.K., Taisho Toyama Pharmaceutical Co., Ltd, Nippon Boehringer lngelheim Co., Ltd, Takeda Pharmaceutical Co. Ltd, Eli Lilly Japan K.K. and Novo Nordisk Pharma Ltd outside the submitted work. M.Y. reports personal fees from Shionogi & Co., Ltd during the course of the study, and other (donation) from Shionogi & Co., Ltd outside the submitted work. T.Y. reports other (lecture fee) from Shionogi & Co., Ltd during the course of the study. S.Y. reports other (contracted work) from Shionogi & Co., Ltd during the course of the study. K.Yok. reports personal fees from Shionogi & Co., Ltd during the course of the study, grants, personal fees and non‐financial support from MSD K.K., grants and personal fees from Astellas Pharma Inc., Daiichi Sankyo Co., Ltd, Sumitomo Dainippon Pharma Co., Ltd, Kyowa Hakko Kirin Co., Ltd, Mochida Pharmaceutical Co., Ltd, Nippon Boehringer lngelheim Co., Ltd, Ono Pharmaceutical Co. Ltd, Pfizer Japan Inc., Shionogi & Co., Ltd, Taisho Toyama Pharmaceutical Co., Ltd, Takeda Pharmaceutical Co. Ltd, and Mitsubishi Tanabe Pharma Corporation, grants from Bristol‐Myers Squibb Co., Eli Lilly Japan K.K., Teijin Pharma Ltd, and Toyama Chemical Co., Ltd, and personal fees from AstraZeneca K.K., Eisai Co., Ltd, Kowa Co., Ltd, Kowa Pharmaceutical Co., Ltd, Sanofi K.K., and Sanwa Kagaku Kenkyusho Co., Ltd outside the submitted work. K.Yos. reports personal fees and non‐financial support from Shionogi & Co., Ltd during the course of the study. M.Yo. has nothing to disclose during the course of the study, and reports grants and personal fees from Shionogi & Co., Ltd outside the submitted work. N.Y. reports personal fees from Shionogi & Co., Ltd during the course of the study, and personal fees from Shionogi & Co., Ltd outside the submitted work. K.N. reports other (contracted) work from Shionogi & Co., Ltd during the course of the study, and grants from Takeda Pharmaceutical Co., Ltd and Fujifilm Pharma Co., Ltd outside the submitted work. R.N. reports personal fees from Shionogi & Co., Ltd during the course of the study, and personal fees from Astellas Pharma Inc., Sumitomo Dainippon Pharma Co., Ltd, MSD K.K., Ono Pharmaceutical Co., Ltd, Kowa Pharmaceutical Co. Ltd, Mitsubishi Tanabe Pharma Corporation, Nippon Boehringer Ingelheim Co., Ltd, Toa Eiyo Ltd, Eisai Co., Ltd, Nippon Chemiphar Co., Ltd outside the submitted work.
Author contributions
H.I., I.K., H.D., H.F., S.K., T.Muro., K.Ut., and T.Y. contributed to design, conduct/data collection and writing the manuscript. M.T. contributed to conduct/data collection, analysis and writing the manuscript. T.A., J.H., T.I., A.K., M.Ki., T.K., M.Ku., K.No., S.O., Y.Sa., Y.Se., T.S., S.Sh., H.T., S.Y. and N.Y. contributed to writing the manuscript. Y.E. contributed to conduct/data collection. K.H., S.It., S.Su., K.Ue., K.Yok., K.Na. and R.N. contributed to design and writing the manuscript. S.Is., K.K., M.S., Y.T., M.Ya., K.Yos. and M.Yo. contributed to conduct/data collection and writing the manuscript. K.M. contributed to design and conduct/data collection. T.Mura. contributed to design.
Supporting information
Table S1. Baseline demographic characteristics (potential prognostic factors): for patients achieving LDL‐C target at the last visit.
Table S2. Mean dose by statin type at baseline and last visit.
Table S3. Non‐lipid parameters over time.
Table S4. Events for primary and secondary endpoints
Table S5. Adverse events and key safety data.
Table S6. Intergroup analysis of primary endpoint in patients who achieved target LDL‐C (including all covariates).
ACKNOWLEDGMENTS
This study was funded by Shionogi & Co., Ltd EDIT, Inc. (Tokyo, Japan) who provided medical writing and editing.
Itoh H, Komuro I, Takeuchi M, et al. Achieving LDL cholesterol target levels <1.81 mmol/L may provide extra cardiovascular protection in patients at high risk: Exploratory analysis of the Standard Versus Intensive Statin Therapy for Patients with Hypercholesterolaemia and Diabetic Retinopathy study. Diabetes Obes Metab. 2019;21:791–800. 10.1111/dom.13575
Funding information Shionogi & Co., Ltd provided support for this research but was not involved in analysis, data interpretation, or manuscript preparation.
REFERENCES
- 1. Turner RC, Millns H, Neil HA, et al. Risk factors for coronary artery disease in non‐insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ. 1998;316:823‐828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sone H, Tanaka S, Tanaka S, et al. Serum level of triglycerides is a potent risk factor comparable to LDL cholesterol for coronary heart disease in Japanese patients with type 2 diabetes: subanalysis of the Japan Diabetes Complications Study (JDCS). J Clin Endocrinol Metab. 2011;96:3448‐3456. [DOI] [PubMed] [Google Scholar]
- 3. Gæde P, Pedersen O. Intensive integrated therapy of type 2 diabetes: implications for long‐term prognosis. Diabetes. 2004;53(suppl 3):S39‐S47. [DOI] [PubMed] [Google Scholar]
- 4. Kramer CK, Rodrigues TC, Canani LH, Gross JL, Azevedo MJ. Diabetic retinopathy predicts all‐cause mortality and cardiovascular events in both type1and 2 diabetes: meta‐analysis of observational studies. Diabetes Care. 2011;34:1238‐1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ohno T, Kinoshita O, Fujita H, et al. Detecting occult coronary artery disease followed by early coronary artery bypass surgery in patients with diabetic retinopathy: report from a diabetic retinocoronary clinic. J Thorac Cardiovasc Surg. 2010;139:92‐97. [DOI] [PubMed] [Google Scholar]
- 6. Itoh H, Komuro I, Takeuchi M, et al. EMPATHY Investigators. Intensive treat‐to‐target statin therapy in high‐risk Japanese patients with hypercholesterolemia and diabetic retinopathy: report of a randomized study. Diabetes Care. 2018;41:1275‐1284. [DOI] [PubMed] [Google Scholar]
- 7. Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol‐lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta‐analysis. Lancet. 2008;371:117‐125. [DOI] [PubMed] [Google Scholar]
- 8. LaRosa JC, Grundy SM, Kastelein JJ, Kostis JB, Greten H, Treating to New Targets (TNT) Steering Committee and Investigators . Safety and efficacy of atorvastatin‐induced very low‐density lipoprotein cholesterol levels in patients with coronary heart disease (a post hoc analysis of the treating to new targets [TNT] study). Am J Cardiol. 2007;100:747‐752. [DOI] [PubMed] [Google Scholar]
- 9. Shepherd J, Barter P, Carmena R, et al. Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: the Treating to New Targets (TNT) study. Diabetes Care. 2006;29:1220‐1226. [DOI] [PubMed] [Google Scholar]
- 10. Hsia J, MacFadyen JG, Monyak J, Ridker PM. Cardiovascular event reduction and adverse events among subjects attaining low‐density lipoprotein cholesterol <50 mg/dl with rosuvastatin. The JUPITER trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin). J Am Coll Cardiol. 2011;57:1666‐1675. [DOI] [PubMed] [Google Scholar]
- 11. Olsson AG, Lindahl C, Holme I, et al. LDL cholesterol goals and cardiovascular risk during statin treatment: the IDEAL study. Eur J Cardiovasc Prev Rehabil. 2011;18:262‐269. [DOI] [PubMed] [Google Scholar]
- 12. Ueshima K, Itoh H, Kanazawa N, et al. Rationale and design of the Standard Versus Intensive Statin Therapy for Hypercholesterolemic Patients with Diabetic Retinopathy (EMPATHY) Study: a randomized controlled trial. J Atheroscler Thromb. 2016;23:976‐990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline demographic characteristics (potential prognostic factors): for patients achieving LDL‐C target at the last visit.
Table S2. Mean dose by statin type at baseline and last visit.
Table S3. Non‐lipid parameters over time.
Table S4. Events for primary and secondary endpoints
Table S5. Adverse events and key safety data.
Table S6. Intergroup analysis of primary endpoint in patients who achieved target LDL‐C (including all covariates).


