Abstract
Background
Long‐term management of moderate‐to‐severe psoriasis is usually discussed in terms of continuous administration; however, there are many situations in clinical practice where treatment may be withdrawn with subsequent retreatment.
Objective
To assess the clinical course after ixekizumab treatment withdrawal and retreatment, as well as the effectiveness of ixekizumab retreatment, in Japanese patients with plaque psoriasis.
Methods
This single‐arm, open‐label study (UNCOVER‐J; NCT01624233) comprised 78 patients with plaque psoriasis. After ixekizumab treatment (160‐mg loading dose, 80 mg every 2 weeks for the first 12 weeks, and then 80 mg every 4 weeks (IXE Q4W) until Week 52), 70 patients achieved a Psoriasis Area Severity Index (PASI)75 response at Week 52. These 70 patients withdrew from ixekizumab treatment from Weeks 52 to 100. Patients who relapsed (PASI ≤50) during the Treatment Withdrawal Period were retreated with IXE Q4W for 192 weeks.
Results
At Weeks 52, 76 and 100, PASI75 response rates were 100%, 26% and 7%; PASI90 response rates were 87%, 11% and 3%; and PASI100 response rates were 53%, 0% and 0%. After treatment withdrawal, 87% of patients relapsed; median time to relapse was 143 days. After 12 weeks of retreatment with IXE Q4W, 83% of relapsed patients achieved PASI75, 68% achieved PASI90 and 25% achieved PASI100; improvements were maintained up to 120 weeks of retreatment. Treatment‐emergent adverse events and serious adverse events were reported in 56% and 4% of patients during the Treatment Withdrawal Period, and in 88% and 14% of patients during the Retreatment Period.
Conclusion
In patients withdrawn from ixekizumab after achieving PASI75, approximately half relapsed within 5 months of withdrawal; however, most patients recaptured response within 12 weeks, and response was maintained for up to 120 weeks of retreatment.
Introduction
Long‐term management of moderate‐to‐severe psoriasis, a chronic inflammatory skin disease, is usually discussed in terms of continuous administration.1, 2 However, treatment interruptions may occur in clinical practice owing to infection, pregnancy, compliance issues, scheduled drug‐free intervals or insurance coverage, etc.1, 2, 3, 4 Psoriasis plaques frequently relapse when oral and biologic systemic treatments are interrupted, and retreatment may be insufficient to recapture initial response.5, 6, 7, 8 Therefore, thorough assessment of the efficacy and safety of biologic therapies during treatment withdrawal and retreatment is important to provide useful data for physicians in case they need to interrupt treatment in routine clinical practice.
Ixekizumab is a high‐affinity monoclonal antibody that selectively targets interleukin (IL)‐17A.9 It has been widely approved for the treatment of plaque psoriasis and psoriatic arthritis, and it is also approved for generalized pustular psoriasis and erythrodermic psoriasis in Japan. The effectiveness of continuous ixekizumab treatment for moderate‐to‐severe plaque psoriasis has been demonstrated in several phase III studies (UNCOVER‐1, UNCOVER‐2 and UNCOVER‐3).10, 11 Treatment withdrawal after 12 weeks of ixekizumab was assessed in a pooled analysis of responding patients [Static Physician Global Assessment (sPGA) of 0 or 1 at Week 12] in UNCOVER‐1 and UNCOVER‐2.12 Most patients who were withdrawn from ixekizumab treatment relapsed (sPGA ≥3), and the majority of these patients recaptured response after 24 weeks of retreatment. These results suggest that ixekizumab can restore clinical response upon retreatment; although longer‐term data (>12 weeks of continuous therapy before treatment withdrawal and >24 weeks of retreatment) addressing the efficacy and safety of interrupted ixekizumab treatment are lacking. Here we report the results of an analysis of a phase III trial (UNCOVER‐J) that evaluated efficacy and safety outcomes among Japanese patients who were withdrawn after 52 weeks of treatment, and who were retreated with ixekizumab for up to 120 weeks after experiencing a relapse. Data up to 52 weeks in UNCOVER‐J have been reported previously in Saeki et al.13
Materials and methods
Study design
This was a phase III, multicentre, single‐arm, open‐label and long‐term study (ClinicalTrials.gov number NCT01624233; first patient enrolled 21 June 2012). The protocol was approved by the local institutions’ ethics committees and conforms to the provisions of the Declaration of Helsinki. All patients provided written informed consent. Data up to 24 weeks in UNCOVER‐J were first reported in Saeki et al.14
Study population
Eligibility criteria have been described in detail previously.14 Key inclusion criteria for patients with plaque psoriasis were as follows: male and female Japanese patients with moderate‐to‐severe psoriasis, aged ≥20 years, confirmed diagnosis of the disease, a Psoriasis Area Severity Index (PASI) score ≥12, sPGA score ≥3 and ≥10% body surface area involvement at screening and baseline.
Treatment protocol
Ixekizumab (Eli Lilly and Company, Indianapolis, IN, USA) was administered as subcutaneous (SC) injections as follows: at baseline (Week 0), 160 mg; from Weeks 2 to 12, 80 mg every 2 weeks (Induction Dosing Period); from Weeks 16 to 52, 80 mg every 4 weeks (Maintenance Dosing Period); from Weeks 52 to 100, all responders [patients achieving ≥75% improvement from baseline in PASI (PASI75) at Week 52] discontinued ixekizumab at Week 52 (last dose Week 48) and were followed until Week 100 (Treatment Withdrawal Period); and from Week 100 up to an additional 192 weeks, 80 mg ixekizumab every 4 weeks (Retreatment Period). If a patient experienced a relapse (defined as PASI decrease from PASI75 at Week 52 to PASI ≤50) at any visit before Week 100, the patient immediately entered the Retreatment Period (Fig. 1).
Figure 1.
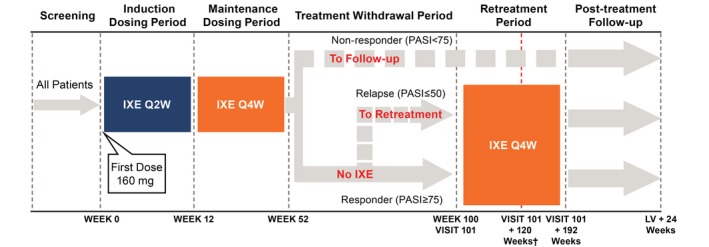
Study design (patients with plaque psoriasis). All patients received 160‐mg doses of ixekizumab at Week 0 (Visit 2; all doses during the study were delivered by SC injection) and 80 mg IXE Q2W from Week 2 (Visit 4) to Week 12 (Induction Dosing Period). During the Maintenance Dosing Period and Retreatment Period, patients received 80 mg IXE Q4W. At Week 52, patients with plaque psoriasis were classified as a responder (PASI75) or non‐responder (PASI <75). Non‐responders entered the Post‐treatment Follow‐up Period. Responders moved to the Treatment Withdrawal Period. Patients who completed the Treatment Withdrawal Period (Week 100) entered the Retreatment Period. If a patient experienced a relapse (loss of response; PASI ≤50) at any visit prior to Week 100, the patient moved to the Retreatment Period with a duration of 192 weeks. †Nine months after marketing authorization was received in Japan, all patients stopped administration of ixekizumab and moved to post‐treatment follow‐up. Patients who had completed the Retreatment Period or were ongoing were counted as having completed the Retreatment Period. At the Retreatment Period cut‐off date (3 April 2017), all completed patients had at least 120 weeks of retreatment. IXE, ixekizumab; IXE Q2W, 80 mg ixekizumab every 2 weeks; IXE Q4W, 80 mg ixekizumab every 4 weeks; LV, last regularly scheduled visit or early termination visit; PASI50/75, ≥50%/≥75% improvement in the Psoriasis Area Severity Index; SC, subcutaneous.
Outcome measures
Efficacy measures included the following: proportion of patients achieving PASI75, PASI90, PASI100; proportion of patients achieving sPGA (0,1) and sPGA (0); time to relapse (loss of response; PASI ≤50) following ixekizumab withdrawal in patients with a PASI75 response at Week 52; change from baseline in PASI scores; Dermatology Life Quality Index (DLQI);15 and Psoriasis Scalp Severity Index (PSSI; for patients with scalp psoriasis at baseline). Safety measures included drug‐free‐emergent adverse events [DEAEs; for adverse events (AEs) occurring during the Treatment Withdrawal Period, coded and summarized using the Medical Dictionary for Regulatory Activities, version 20.0]; treatment‐emergent adverse events (TEAEs; AEs occurring during the Retreatment Period, coded and summarized as per DEAEs); AEs of special interest (AESIs; infections including Pneumocystis jirovecii pneumonia, allergic reactions/hypersensitivities, injection‐site reactions, hepatic disorders, cytopenias, depression, malignancies, cerebrocardiovascular events, interstitial lung disease); serious AEs (SAEs); AEs that led to study discontinuation; and immunogenicity [antibody production against ixekizumab, defined as drug‐free‐emergent anti‐drug antibody (DE‐ADA; Treatment Withdrawal Period) or treatment‐emergent anti‐drug antibody (TE‐ADA; Retreatment Period)].
Statistical analysis
This analysis focuses on the Treatment Withdrawal Period and the Retreatment Period (Fig. 1). Efficacy data for the Induction Dosing Period and the Maintenance Dosing Period for patients who achieved PASI75 at Week 52 are presented for reference. The responder population, which included all patients with plaque psoriasis who achieved PASI75 at Week 52, was used for the analyses of the Induction Dosing Period, Maintenance Dosing Period and Treatment Withdrawal Period. The relapse population, which included all patients with plaque psoriasis who experienced a relapse (PASI ≤50 from PASI75 at Week 52) during the Treatment Withdrawal Period, was used for the analyses of the Retreatment Period (except TE‐ADA, which are reported for all patients entering the Retreatment Period). Sample size determination has been described previously.14 Continuous data are summarized by descriptive statistics. Categorical data are summarized by frequency counts and percentages, with missing data imputed using non‐responder imputation (NRI). Statistical analyses were carried out using SAS version 9.2 (Cary, NC, USA).
Results
Disposition
A total of 70/78 (89.7%) enrolled patients with plaque psoriasis completed the Maintenance Dosing Period and entered the Treatment Withdrawal Period at Week 52 (responder population; Table 1). Of these, 61 patients relapsed (PASI ≤50) during the Treatment Withdrawal Period. Of the 70 patients, three patients (two relapsed, one did not relapse) did not enter the following Retreatment Period owing to an AE or physician's decision, and 67 patients (59 relapsed, eight did not relapse) entered the Retreatment Period. The 59 patients who relapsed during the Treatment Withdrawal Period and entered the following Retreatment Period were included in the relapse population. The eight patients who did not relapse (i.e. maintaining PASI >50) completed the entire Treatment Withdrawal Period, to Week 100. Of the 67 patients who entered the Retreatment Period, seven patients (10.4%) discontinued before Week 192, and 60 (89.6%) patients completed the Retreatment Period. (Note: 9 months after marketing authorization was received in Japan, all patients discontinued ixekizumab and entered post‐treatment follow‐up.) Patients who had completed the Retreatment Period or who were ongoing were counted as having completed the Retreatment Period. At the cut‐off date for the Retreatment Period (3 April 2017), all completers had at least 120 weeks of retreatment.
Table 1.
Patient disposition of the responder population during the Treatment Withdrawal Period and Retreatment Period in patients with plaque psoriasis (UNCOVER‐J)
| n (%) | Treatment withdrawal period (N = 70) | Retreatment period (N = 67) |
|---|---|---|
| Completed period | 67 (95.7) | 60 (89.6) |
| Relapsed | 61 (87.1) | NA |
| Discontinued | 3 (4.3) | 7 (10.4) |
| Adverse event | 1 (1.4) | 3 (4.5) |
| Physician decision | 2 (2.9) | 1 (1.5) |
| Patient decision | 0 | 2 (3.0) |
| Lack of efficacy | 0 | 1 (1.5) |
Of the 70 patients who entered the Treatment Withdrawal Period, three patients did not enter the following Retreatment Period (one patient who discontinued owing to an adverse event before Week 100 but did not relapse; one patient who discontinued owing to a physician decision before Week 100 and relapsed; one patient who relapsed before Week 100 but did not enter the Retreatment Period owing to a physician decision).
n, number in group; N, population size; NA, not applicable.
Demographic and baseline clinical characteristics
The overall demographic and baseline clinical characteristics for patients with plaque psoriasis (N = 78) have been described previously.13, 14 Demographic and baseline characteristics for the responder population (N = 70; Table 2) were consistent with those for the overall population with plaque psoriasis.
Table 2.
Baseline demographics for the responder population (relapse and non‐relapse) in patients with plaque psoriasis (UNCOVER‐J)
| Characteristic | Relapse population (N = 61) | Non‐relapse population (N = 9) | Responder population total (N = 70) |
|---|---|---|---|
| Age, years | 43.8 (11.18) | 53.3 (9.27) | 45.1 (11.35) |
| Male, n (%) | 49 (80.3) | 7 (77.8) | 56 (80.0) |
| Weight, kg | 74.6 (16.66) | 65.6 (12.91) | 73.4 (16.43) |
| Duration of psoriasis symptoms, years | 15.5 (9.81) | 12.0 (9.11) | 15.0 (9.73) |
| Prior systemic therapy, n (%) | |||
| Non‐biologic only | 34 (55.7) | 6 (66.7) | 40 (57.1) |
| Biologic only | 1 (1.6) | 0 | 1 (1.4) |
| Non‐biologic and biologic | 12 (19.7) | 0 | 12 (17.1) |
| PASI score | 26.9 (9.02) | 24.2 (6.52) | 26.5 (8.75) |
| sPGA score | 3.8 (0.57) | 3.8 (0.44) | 3.8 (0.55) |
| Scalp psoriasis, n (%) | 60 (98.4) | 9 (100.0) | 69 (98.6) |
| PSSI score in patients with scalp psoriasis | 26.0 (15.66)† | 24.3 (14.50) | 25.8 (15.42)‡ |
| Nail psoriasis, n (%) | 32 (52.5) | 6 (66.7) | 38 (54.3) |
| NAPSI score in patients with nail psoriasis | 32.2 (22.27) | 36.2 (25.40) | 32.8 (22.47) |
| PsA, n (%) | 9 (14.8) | 1 (11.1) | 10 (14.3) |
| Pain VAS in patients with PsA | 63.2 (22.35) | 30.0 (NA) | 59.9 (23.55) |
| DLQI score | 11.3 (6.39) | 4.3 (2.35) | 10.4 (6.46) |
| Itch NRS score | 6.3 (2.58) | 4.6 (2.01) | 6.1 (2.57) |
†Baseline scores only available for 58 patients.
‡Baseline scores only available for 67 patients.
Data are mean (SD) unless otherwise stated. A total of 61/70 patients who entered the Treatment Withdrawal Period relapsed by Week 100.
DLQI, Dermatology Life Quality Index; NA, not applicable; NAPSI, Nail Psoriasis Severity Index; NRS, numeric rating scale; PASI, Psoriasis Area Severity Index; PsA, psoriatic arthritis; PSSI, Psoriasis Scalp Severity Index; SD, standard deviation; sPGA, Static Physician Global Assessment; VAS, visual analogue scale.
Efficacy outcomes
Induction dosing period
For the responder population, all outcome measures showed improved efficacy from baseline to Week 12. At Week 12, PASI75, 90 and 100 response rates were 100%, 87% and 34% (NRI), respectively. At Week 12, sPGA (0,1) and sPGA (0) response rates were 90% and 39% (NRI), respectively. At baseline, mean PASI, DLQI and PSSI scores were 26.5, 10.4 and 25.8, respectively, and improved at Week 12 to 1.0, 1.7 and 1.6, respectively (mean PASI, DLQI and PSSI are presented for all treatment periods in Fig. 2a–c, respectively).
Figure 2.

Mean PASI (a), DLQI (b) and PSSI (c) data (up to at least 120 weeks from the start of retreatment). Data, including the Induction Dosing Period, are from PASI75 responders at Week 52. The number of patients at selected weeks is shown below each graph. DLQI, Dermatology Life Quality Index; IXE, ixekizumab; IXE Q2W, 80 mg ixekizumab every 2 weeks; IXE Q4W, 80 mg ixekizumab every 4 weeks; PASI, Psoriasis Area Severity Index; PASI75, ≥75% improvement in the Psoriasis Area Severity Index; PSSI, Psoriasis Scalp Severity Index.
Maintenance dosing period
For the responder population, most outcome measures were stable or further improved from Week 12 at Week 52. At Week 52, PASI75, 90 and 100 response rates were 100%, 87% and 53% (NRI), respectively. At Week 52, sPGA (0,1) and sPGA (0) response rates were 90% and 57% (NRI), respectively. At Week 52, mean PASI, DLQI and PSSI scores were 0.9, 1.3 and 2.9, respectively.
Treatment withdrawal period
For the responder population, all outcome measures showed reduced efficacy after ixekizumab treatment was withdrawn at Week 52. At Week 76 (24 weeks after ixekizumab withdrawal) and Week 100 (48 weeks after ixekizumab withdrawal), PASI75 response rates, which did not include retreatment data after relapse, were 26% and 7%, PASI90 response rates were 11% and 3%, and PASI100 response rates were 0% and 0% (NRI), respectively (Fig. 3a). At Week 100, sPGA (0,1) and sPGA (0) response rates were 4.3% and 0% (NRI), respectively (Fig. 3b). For the 61 (87.1%) patients with PASI75 at Week 52 who relapsed (PASI ≤50) following the withdrawal of ixekizumab, the median time to relapse was 143 days. Of the 61 patients, for the patients with ≥PASI75 ~<90 (n = 8), ≥PASI90 ~<100 (n = 20) and PASI100 (n = 33) at Week 52, the median time to relapse was 85, 135 and 165 days, respectively. At Week 100, mean PASI, DLQI and PSSI scores increased to 5.7, 3.9 and 9.2, respectively.
Figure 3.
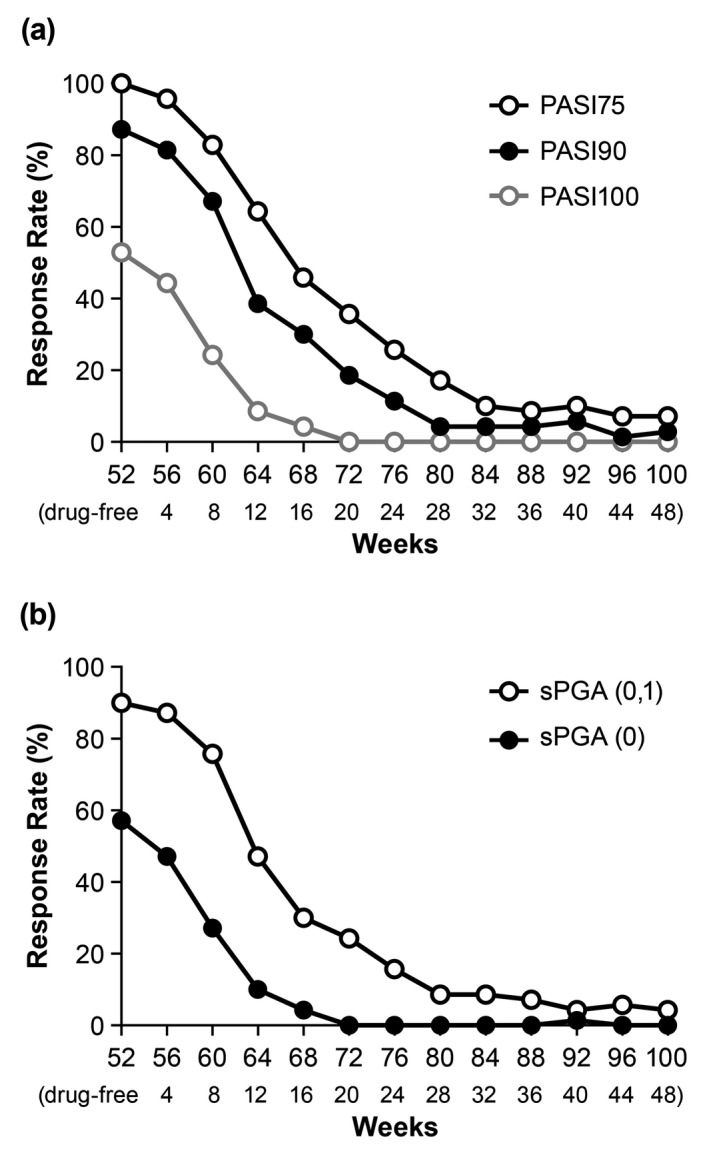
PASI (a) and sPGA (b) response rates (up to Week 100) using NRI during the Treatment Withdrawal Period. Patients were treated with 80 mg ixekizumab every 2 weeks up to Week 12, and then 80 mg ixekizumab every 4 weeks up to Week 52. At Week 52, responders (PASI75) were withdrawn from treatment and followed until Week 100. Data are from PASI75 responders at Week 52. The number of weeks drug‐free are shown at the bottom of each graph. NRI, non‐responder imputation; PASI75/90/100, ≥75%/≥90%/100% improvement in Psoriasis Area Severity Index; sPGA, Static Physician Global Assessment.
Retreatment period
For the relapse population, all outcome measures showed improved efficacy with ixekizumab retreatment and were maintained up to 120 weeks. At Week 12 and Week 120 of retreatment, PASI75 response rates were 83% and 83%, PASI90 response rates were 68% and 68%, and PASI100 response rates were 25% and 39% (NRI), respectively (Fig. 4a). At Week 12 and Week 120 of retreatment, sPGA (0,1) response rates were 69% and 68%, and sPGA (0) response rates were 27% and 41% (NRI), respectively (Fig. 4b). At Week 0 of retreatment, mean PASI, DLQI and PSSI scores were 17.9, 9.5 and 24.0, respectively, which improved to 2.9, 2.7 and 2.6 at Week 12 of retreatment, respectively, and were maintained at Week 120 of retreatment (1.9, 1.6 and 2.5, respectively).
Figure 4.
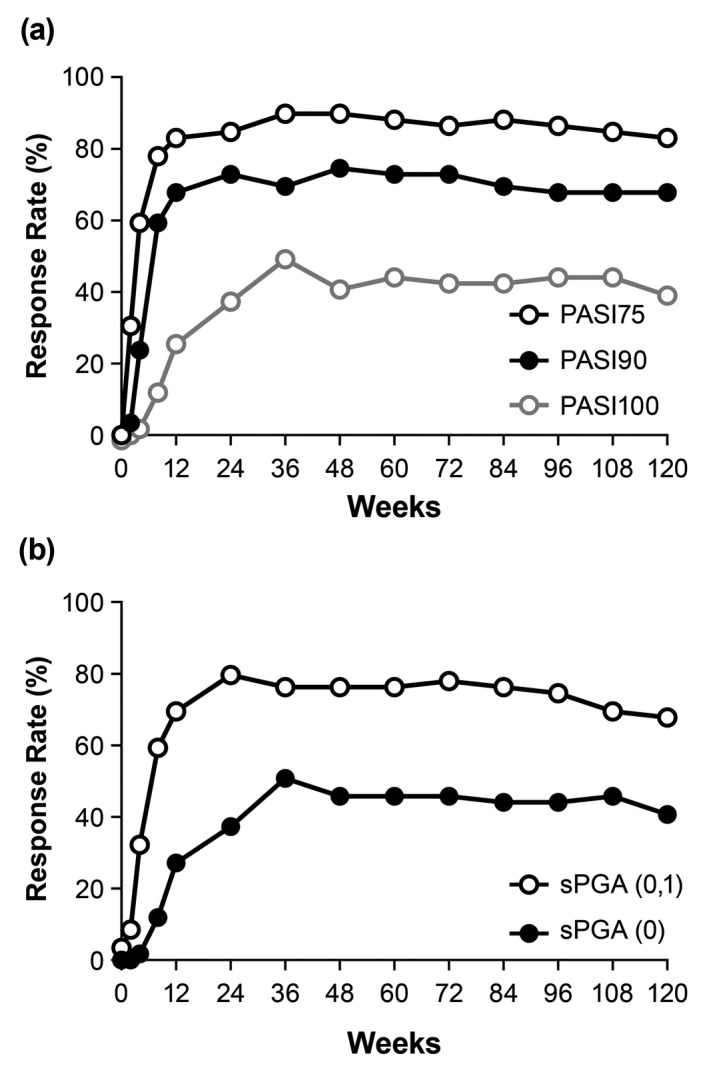
PASI (a) and sPGA (b) response rates using NRI in relapsed and retreated patients. Patients were treated with 80 mg ixekizumab every 2 weeks up to Week 12, and then 80 mg ixekizumab every 4 weeks up to Week 52. At Week 52, responders (PASI75) were withdrawn from treatment. At relapse (PASI ≤50), patients were retreated with 80 mg ixekizumab every 4 weeks. Relapse Week 0 includes all relapse patients, regardless of the week in which the relapse occurred. NRI, non‐responder imputation; PASI75/90/100, ≥75%/≥90%/100% improvement in Psoriasis Area Severity Index; sPGA, Static Physician Global Assessment.
Safety
Treatment withdrawal period
For the responder population, approximately half of patients who withdrew from ixekizumab treatment reported DEAEs during the Treatment Withdrawal Period, during which ixekizumab was not administered to patients (Table 3). The most common DEAEs were viral upper respiratory tract infections [6/70 (8.6%)], followed by influenza, aspartate aminotransferase increased, gamma‐glutamyltransferase increased and urticaria [3/70 (4.3%) each]. There were no deaths, few severe DEAEs [2/70 (2.9%)], and only one patient (1.4%) discontinued the study owing to an AE (SAE of gastric cancer). Three patients reported SAEs (gastric cancer, hand fracture and cytomegalovirus infection). AESIs were reported by 2/70 (28.6%) patients; the most common AESIs were infections.
Table 3.
Safety overview: treatment withdrawal period (responder population) and Retreatment Period (relapse population) in patients with plaque psoriasis (UNCOVER‐J)
| AE, n (%) | Treatment withdrawal period (no ixekizumab; n = 70) | Retreatment period (IXE Q4W; n = 59) |
|---|---|---|
| DEAEs/TEAEsa | 39 (55.7) | 52 (88.1) |
| AE leading to discontinuation | 1 (1.4) | 3 (5.1) |
| Deaths | 0 | 0 |
| SAEs | 3 (4.3) | 8 (13.6) |
| AESI | 20 (28.6) | 40 (67.8) |
| Infections | 15 (21.4) | 34 (57.6) |
| Hepatic | 5 (7.1) | 8 (13.6) |
| Allergic reactions/hypersensitivity | 4 (5.7) | 11 (18.6) |
| Malignancies | 1 (1.4) | 0 |
| Injection‐site reaction | 0 | 2 (3.4) |
| Cytopenias | 0 | 0 |
| Depression | 0 | 1 (1.7) |
| Cerebrocardiovascular disease | 0 | 1 (1.7) |
| Pneumocystis jirovecii pneumonia | 0 | 0 |
| Interstitial lung disease | 0 | 0 |
DEAEs are reported for the Treatment Withdrawal Period and TEAEs are reported for the Retreatment Period.
Patients with multiple occurrences of the same event were counted under the highest severity; in the case where the severity has an additional category ‘more severe than baseline’ collected, the ‘more severe than baseline’ and ‘severe’ categories were combined for analysis and presentation.
Of the 70 patients who entered the Treatment Withdrawal Period, three patients did not enter the following Retreatment Period (one patient who discontinued owing to an AE before Week 100 but did not relapse; one patient who discontinued owing to a physician decision before Week 100 and relapsed; one patient who relapsed before Week 100 but did not enter the Retreatment Period owing to a physician decision). Of these 70 patients, 61 patients relapsed during the Treatment Withdrawal Period. A total of 59 patients who relapsed during the Treatment Withdrawal Period and who entered the following Retreatment Period were included in the relapse population.
AE, adverse event; AESI, adverse event of special interest; DEAE, drug‐free‐emergent adverse event; IXE Q4W, 80 mg ixekizumab every 4 weeks; SAE, serious adverse event; TEAE, treatment‐emergent adverse event.
Retreatment period
For the relapse population, most patients reported TEAEs during the Retreatment Period (Table 3). The most common TEAEs were viral upper respiratory tract infections [10/59 (16.9%)], followed by influenza and asteatotic eczema [8/59 (13.6%) each], hypertension [6/59 (10.2)], and folliculitis and eczema [5/59 (8.5%) each]. There were no deaths, few severe TEAEs [6/59 (10.2%)] and only three patients (5.1%) who discontinued the study owing to an AE. Eight patients reported 11 SAEs during the Retreatment Period (ankle fracture, cholelithiasis, diverticulum intestinal haemorrhagic, haemangioma, hand fracture, hyperplastic cholecystopathy, intervertebral disc protrusion, large intestine polyp, pharyngotonsillitis, pneumonia and spondylolisthesis); none of these SAEs led to study discontinuation. AESIs were reported by 40 (28.6%) patients; the most common AESIs were infections.
Immunogenicity
During the Treatment Withdrawal Period, 14/69 (20.3%) patients were DE‐ADA positive, but all were neutralizing antibody negative. For those who entered the Retreatment Period, 13/67 (19.4%) patients were TE‐ADA positive. One patient was neutralizing antibody positive at the point just before the retreatment start. The anti‐drug antibody (ADA) titre when detected the neutralizing antibody was moderate. This patient achieved PASI75, 90 and 100 at Retreatment Week 36–60 and maintained PASI 75 up to Retreatment Week 120.
Discussion
This is the first clinical study to report long‐term data on ixekizumab treatment withdrawal and retreatment in patients with moderate‐to‐severe psoriasis. We found that Japanese patients who previously responded to continuous ixekizumab treatment (PASI75 at Week 52) showed reduced efficacy in all parameters after ixekizumab withdrawal, with approximately half of patients relapsing within 5 months of treatment interruption. However, there were eight patients who completed the entire Treatment Withdrawal Period to Week 100 without relapse. Further, by Week 12 of ixekizumab retreatment (80 mg every 4 weeks), we observed an improvement in all efficacy parameters including PASI and DLQI scores and maintenance of response up to Week 120 of retreatment. Notably, improvements during retreatment occurred without use of the approved loading (160 mg) or induction doses for ixekizumab (80 mg every 2 weeks). Furthermore, we found that PSSI scores generally showed the same pattern of improvement as PASI scores with treatment withdrawal and retreatment. Ixekizumab was also well tolerated, with no new safety concerns identified during treatment withdrawal or retreatment. These results highlight the potential of ixekizumab as a treatment for Japanese patients with moderate‐to‐severe psoriasis under conditions that reflect the treatment interruptions that might occur in clinical practice.
In this analysis of UNCOVER‐J, response and relapse criteria were based on PASI scores in accordance with both the primary endpoint for the overall study (PASI75 at Week 12)14 and common practices in clinical trials in Japan. Although different response criteria were used in our earlier pooled analysis of ixekizumab treatment interruption,12 the findings for Japanese patients in the current study were generally similar to the global population. In the earlier pooled analysis, responders were defined as those who achieved sPGA (0,1) at Week 12. Similar to the current study, we observed that among patients interrupting treatment at Week 12, most (>80%) had relapsed (sPGA ≥3) with treatment withdrawal by Week 60, with a median time to relapse of approximately 20 weeks. Further, 80%, 56% and 35% (observed) of patients recaptured a PASI75, 90 and 100 at 12 weeks of retreatment, respectively, and response rates were maintained to Week 24 of retreatment in our pooled analysis. The results of the current study confirm our earlier findings and may be considered to more closely replicate clinical practice (longer‐term period of treatment before treatment discontinuation), but importantly they show that response rates are maintained with long‐term retreatment (up to 120 weeks). In addition, irrespective of the selected response/relapse criteria and differences in timing of treatment withdrawal (i.e. Week 12 for the UNCOVER studies and Week 52 in the current study), results from both studies show that most patients relapse with ixekizumab withdrawal and recapture the response with retreatment. However, not all patients recaptured the same degree of efficacy when ixekizumab was re‐introduced. The approved loading (160 mg) and induction doses (80 mg every 2 weeks) of ixekizumab were not used for retreatment in both studies, and the impact of these doses on clinical outcomes could not be evaluated. Further evaluation of interrupted ixekizumab therapy with different treatment strategies (e.g. withdrawal times and doses) is warranted.
Studies of other IL‐17 inhibitors using different treatment strategies demonstrate varying relapse and recapture of response profiles with interrupted therapy. A pooled analysis of the ERASURE and FIXTURE trials of secukinumab, including patients who achieved PASI75 at Week 52, demonstrated that most patients relapsed (loss of >50% of the maximum PASI gain compared with baseline) and recaptured response with retreatment.16 Only 16% (secukinumab 300 mg–placebo treatment group) of patients did not relapse after treatment withdrawal, and the median time to relapse was approximately 28 weeks. Upon retreatment using weekly dosing to Week 3 and every 4 weeks thereafter, 95%, 70% and 38% of patients recaptured PASI75, 90 and 100, respectively, at Week 12. The SCULPTURE trial of secukinumab, using retreatment‐as‐needed vs. a fixed‐interval maintenance regimen, demonstrated that most responding patients (PASI75 at Week 12) relapsed with treatment withdrawal.7 Overall, 85% (secukinumab 300 mg retreatment‐as‐needed group) relapsed (loss of >20% of the maximum PASI gain compared with baseline), and the median time to relapse was approximately 24 weeks. Most patients recaptured response with retreatment (69% recaptured PASI75 at first retreatment and 91% of patients recaptured PASI75 at Week 12). A pooled analysis of the AMAGINE‐1, AMAGINE‐2 and AMAGINE‐3 trials of brodalumab, an anti‐IL‐17 receptor antibody, demonstrated more rapid relapse upon treatment discontinuation compared with the anti‐IL‐17 antibodies, ixekizumab and secukinumab.17 At brodalumab treatment discontinuation, 96% of patients had achieved PASI75. Median time to relapse (defined as the request of a patient to initiate a new treatment after brodalumab withdrawal) was 6–7 weeks, and only 22% of patients had not relapsed at 3 months (all had relapsed by 9 months). Brodalumab retreatment was not conducted. Differences in response/relapse criteria make strict comparison between these studies difficult. However, similar to ixekizumab, the results from these studies7, 16, 17 show a loss of response with treatment withdrawal and recapture of response with retreatment, with lower response rates than initial treatment.
Some studies have shown an increased risk for the development of ADAs with intermittent therapy with biologics.4, 18, 19, 20 In the current study, the incidence of TE‐ADAs during the initial 52‐week Maintenance Treatment Period was 12.8% (10/78), while the incidence of TE‐ADAs during the Retreatment Period (maximum 192 weeks) was 19.4% (13/67). Taking into account the longer duration of the Retreatment Period, the incidence of TE‐ADAs during the Retreatment Period has not increased from that of the initial 52‐week Maintenance Treatment Period. In addition, similar to a previous study of interrupted ixekizumab therapy,12 few patients in the current study developed neutralizing antibodies. In the previous study of ixekizumab, there were no meaningful differences in the per cent PASI improvement upon retreatment in patients with or without TE‐ADAs.12 Considering the small number of evaluated patients, the absence of a reference arm and the open‐label nature of the current study, no additional conclusions can be drawn.
Important strengths of this analysis include the prospective and multicentre design of the UNCOVER‐J study, the low discontinuation rate and the length of follow‐up with retreatment. However, there are several limitations to this analysis that are inherent in the study design, including the lack of a control group, open‐label treatment, and the fact that the study was conducted in Japanese patients only, with a relatively small sample size.
In conclusion, we found that in Japanese patients with an initial response to ixekizumab, approximately half relapsed within 5 months of treatment withdrawal; however, most of them subsequently recaptured responses within 12 weeks, and that response was maintained up to 120 weeks of retreatment. Furthermore, interrupted ixekizumab therapy was well tolerated.
Authors’ contributions
YM was involved in the study conception, design and data analysis. YU was an investigator in the study. All authors participated in the interpretation of the study results, and in the drafting, critical revision and approval of the final version of the manuscript.
Acknowledgements
Medical writing assistance was provided by Cassandra Haley, PhD, CMPP and Luke Carey, PhD of ProScribe – Envision Pharma Group, and was funded by Eli Lilly Japan K.K. ProScribe's services complied with international guidelines for Good Publication Practice (GPP3).
Other contributors/acknowledgements: The authors would like to thank all study participants and the Japanese Ixekizumab Study Group: Toshihide Akasaka of Iwate Medical University Hospital, Yoshihide Asano of The University of Tokyo Hospital, Takafumi Etoh of Tokyo Teishin Hospital, Yasuyuki Fujita of Hokkaido University Hospital, Takashi Hashimoto of Kurume University Hospital, Mari Higashiyama of Nissay Hospital, Atsuyuki Igarashi of NTT Medical Center Tokyo, Hironobu Ihn of Kumamoto University, Keiji Iwatsuki of Okayama University Hospital, Kenji Kabashima of Kyoto University Hospital, Akira Kawada of Kinki University Faculty of Medicine, Makoto Kawashima of Tokyo Women's Medical University, Koichiro Nakamura of Saitama Medical University Hospital, Yukari Okubo of Tokyo Medical University Hospital, Ryuhei Okuyama of Shinshu University School of Medicine, Akira Ozawa of Tokai University School of Medicine, Koji Sayama of Ehime University Graduate School of Medicine, Mariko Seishima of Gifu University Graduate School of Medicine, Tetsuo Shiohara of Kyorin University Hospital, Masakazu Takahara of Kyusyu University Hospital, Hidetoshi Takahashi of Asahikawa Medical University Hospital, Kazuhiko Takehara of Kanazawa University Hospital, Keiji Tanese of Keio University Hospital, Mamori Tani of Osaka University Hospital, Yoshinori Umezawa of The Jikei University Hospital, Hideaki Watanabe of Showa University School of Medicine, and Keiichi Yamanaka of Mie University Graduate School of Medicine.
Role of the sponsor: Eli Lilly Japan K.K. was involved in the study design, data collection, data analysis and preparation of the manuscript.
Conflict of interest
Authors’ conflict of interest disclosures are attached in the Journal requirement form.
Funding source
This study was sponsored by Eli Lilly Japan K.K., the manufacturer/licensee of Taltz®.
Contributor Information
N. Akashi, Email: akashi_naotsugu@lilly.com
the Japanese Ixekizumab Study Group:
Toshihide Akasaka, Yoshihide Asano, Takafumi Etoh, Yasuyuki Fujita, Takashi Hashimoto, Mari Higashiyama, Atsuyuki Igarashi, Hironobu Ihn, Keiji Iwatsuki, Kenji Kabashima, Akira Kawada, Makoto Kawashima, Koichiro Nakamura, Yukari Okubo, Ryuhei Okuyama, Akira Ozawa, Koji Sayama, Mariko Seishima, Tetsuo Shiohara, Masakazu Takahara, Hidetoshi Takahashi, Kazuhiko Takehara, Keiji Tanese, Mamori Tani, Hideaki Watanabe, and Keiichi Yamanaka
References
- 1. Brezinski EA, Armstrong AW. Off‐label biologic regimens in psoriasis: a systematic review of efficacy and safety of dose escalation, reduction, and interrupted biologic therapy. PLoS ONE 2012; 7: e33486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ramirez‐Fort MK, Levin AA, Au SC, Gottlieb AB. Continuous versus intermittent therapy for moderate‐to‐severe psoriasis. Clin Exp Rheumatol 2013; 31: S63–S70. [PubMed] [Google Scholar]
- 3. Mrowietz U, de Jong EM, Kragballe K et al A consensus report on appropriate treatment optimization and transitioning in the management of moderate‐to‐severe plaque psoriasis. J Eur Acad Dermatol Venereol 2014; 28: 438–453. [DOI] [PubMed] [Google Scholar]
- 4. Papp K, Crowley J, Ortonne JP et al Adalimumab for moderate to severe chronic plaque psoriasis: efficacy and safety of retreatment and disease recurrence following withdrawal from therapy. Br J Dermatol 2011; 164: 434–441. [DOI] [PubMed] [Google Scholar]
- 5. Bissonnette R, Iversen L, Sofen H et al Tofacitinib withdrawal and retreatment in moderate‐to‐severe chronic plaque psoriasis: a randomized controlled trial. Br J Dermatol 2015; 172: 1395–1406. [DOI] [PubMed] [Google Scholar]
- 6. Griffiths CE, Luger TA, Brault Y, Germain JM, Mallbris L. Retreatment in patients with psoriasis achieving response with etanercept after relapse due to treatment interruption: results from the CRYSTEL study. J Eur Acad Dermatol Venereol 2015; 29: 468–473. [DOI] [PubMed] [Google Scholar]
- 7. Mrowietz U, Leonardi CL, Girolomoni G et al Secukinumab retreatment‐as‐needed versus fixed‐interval maintenance regimen for moderate to severe plaque psoriasis: a randomized, double‐blind, noninferiority trial (SCULPTURE). J Am Acad Dermatol 2015; 73: 27–36.e21. [DOI] [PubMed] [Google Scholar]
- 8. Papp K, Menter A, Poulin Y, Gu Y, Sasso EH. Long‐term outcomes of interruption and retreatment vs. continuous therapy with adalimumab for psoriasis: subanalysis of REVEAL and the open‐label extension study. J Eur Acad Dermatol Venereol 2013; 27: 634–642. [DOI] [PubMed] [Google Scholar]
- 9. Liu L, Lu J, Allan BW et al Generation and characterization of ixekizumab, a humanized monoclonal antibody that neutralizes interleukin‐17A. J Inflamm Res 2016; 9: 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gordon KB, Blauvelt A, Papp KA et al Phase 3 trials of ixekizumab in moderate‐to‐severe plaque psoriasis. N Engl J Med 2016; 375: 345–356. [DOI] [PubMed] [Google Scholar]
- 11. Griffiths CE, Reich K, Lebwohl M et al Comparison of ixekizumab with etanercept or placebo in moderate‐to‐severe psoriasis (UNCOVER‐2 and UNCOVER‐3): results from two phase 3 randomised trials. Lancet 2015; 386: 541–551. [DOI] [PubMed] [Google Scholar]
- 12. Blauvelt A, Papp KA, Sofen H et al Continuous dosing versus interrupted therapy with ixekizumab: an integrated analysis of two phase 3 trials in psoriasis. J Eur Acad Dermatol Venereol 2017; 31: 1004–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saeki H, Nakagawa H, Nakajo K et al Efficacy and safety of ixekizumab treatment for Japanese patients with moderate to severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis: results from a 52‐week, open‐label, phase 3 study (UNCOVER‐J). J Dermatol 2017; 44: 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saeki H, Nakagawa H, Ishii T et al Efficacy and safety of open‐label ixekizumab treatment in Japanese patients with moderate‐to‐severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis. J Eur Acad Dermatol Venereol 2015; 29: 1148–1155. [DOI] [PubMed] [Google Scholar]
- 15. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19: 210–216. [DOI] [PubMed] [Google Scholar]
- 16. Blauvelt A, Langley R, Szepietowski J et al Secukinumab withdrawal leads to loss of treatment responses in a majority of subjects with plaque psoriasis with retreatment resulting in rapid regain of responses: a pooled analysis of two phase 3 trials. J Am Acad Dermatol 2016; 74: AB273. [Google Scholar]
- 17. Masson Regnault M, Konstantinou MP, Khemis A et al Early relapse of psoriasis after stopping brodalumab: a retrospective cohort study in 77 patients. J Eur Acad Dermatol Venereol 2017; 31: 1491–1496. [DOI] [PubMed] [Google Scholar]
- 18. Garcês S, Demengeot J, Benito‐Garcia E. The immunogenicity of anti‐TNF therapy in immune‐mediated inflammatory diseases: a systematic review of the literature with a meta‐analysis. Ann Rheum Dis 2013; 72: 1947–1955. [DOI] [PubMed] [Google Scholar]
- 19. Menting SP, van Lümig PP, de Vries AC et al Extent and consequences of antibody formation against adalimumab in patients with psoriasis: one‐year follow‐up. JAMA Dermatol 2014; 150: 130–136. [DOI] [PubMed] [Google Scholar]
- 20. Reich K, Wozel G, Zheng H, van Hoogstraten HJ, Flint L, Barker J. Efficacy and safety of infliximab as continuous or intermittent therapy in patients with moderate‐to‐severe plaque psoriasis: results of a randomized, long‐term extension trial (RESTORE2). Br J Dermatol 2013; 168: 1325–1334. [DOI] [PubMed] [Google Scholar]


