Abstract
Background
Erythrodermic and generalized pustular psoriasis are rare, difficult to treat forms of psoriasis. In previous reports, we documented 24‐ and 52‐week findings of an open‐label, phase 3 trial (UNCOVER‐J) of ixekizumab in Japanese patients with erythrodermic or generalized pustular psoriasis; most patients responded to treatment and maintained response through 52 weeks.
Objective
To assess the long‐term (>3 years) efficacy and safety of ixekizumab in Japanese patients with erythrodermic or generalized pustular psoriasis.
Methods
These subgroup analyses were of a partial population of patients from UNCOVER‐J (NCT01624233; Sponsored by Eli Lilly and Company), specifically those with erythrodermic psoriasis (N = 8) or generalized pustular psoriasis (N = 5). These patients received 160 mg ixekizumab at Week 0, ixekizumab 80 mg every 2 weeks through Week 12, and ixekizumab 80 mg every 4 weeks thereafter up to Week 244. This regimen is consistent with the regimen approved in Japan for plaque, erythrodermic, and generalized pustular psoriasis and psoriatic arthritis. Efficacy assessments included Global Improvement Score (GIS), Psoriasis Area and Severity Index (PASI), dermal symptoms (for patients with generalized pustular psoriasis), Dermatology Life Quality Index (DLQI) and Itch Numeric Rating Scale (NRS). Safety assessments included treatment‐emergent adverse events and adverse events of special interest.
Results
Most patients had a GIS of resolved or improved from Week 12 onwards, and all patients had early and sustained improvement in PASI and dermal symptom (generalized pustular psoriasis only) scores. Mean improvements in DLQI and Itch NRS at Week 12 were sustained through Week 244. Ixekizumab was well tolerated over 3 years of treatment in patients with erythrodermic psoriasis or generalized pustular psoriasis, and no new safety concerns were identified.
Conclusion
These findings suggest that ixekizumab can be an effective long‐term treatment option for erythrodermic or generalized pustular psoriasis.
Short abstract
Linked article: This article is commented on G. Egawa et al., p. 259 in this issue. To view this article visit https://doi.org/10.1111/jdv.15416
Introduction
Plaque psoriasis is a relatively common, immune‐mediated skin condition that affects 0.3% of the Japanese population.1 Erythrodermic and generalized pustular psoriasis are relatively rare forms of psoriasis (1.5% and 1.8% prevalence, respectively, of all psoriasis in Japan2) that can be more severe and challenging to treat. Further, relapses are common with these forms of psoriasis and systemic treatment is often necessary. Unfortunately, treatment options for erythrodermic and generalized pustular psoriasis are limited and there is little information available in the literature on the effectiveness of treatments, in particular from clinical trials. In addition, data from long‐term (>52 weeks) studies have not been reported.
Ixekizumab is a high‐affinity monoclonal antibody that selectively targets interleukin (IL)‐17A.3 Based on the efficacy and safety findings from three multinational, phase 3 trials (UNCOVER‐1, UNCOVER‐2 and UNCOVER‐3),4, 5 ixekizumab has been widely approved for the treatment of moderate‐to‐severe plaque psoriasis. More recently, following the completion of two additional, multinational, phase 3 trials (SPIRIT‐P1 and SPIRIT‐P2),6, 7 ixekizumab has been approved for the treatment of active psoriatic arthritis. We previously reported 24‐ and 52‐week findings from a multicentre, open‐label, phase 3 trial (UNCOVER‐J) of ixekizumab in Japanese patients with moderate‐to‐severe plaque psoriasis, erythrodermic psoriasis, or generalized pustular psoriasis8, 9 Most patients with erythrodermic psoriasis or generalized pustular psoriasis responded to treatment with ixekizumab and maintained response through 52 weeks. At Week 52, all patients had Global Improvement Scores (GIS) of improved or resolved.9 Ixekizumab has since been approved in Japan for the treatment of pustular and erythrodermic psoriasis (note: ixekizumab is also approved in Japan for the treatment of plaque psoriasis and psoriatic arthritis in patients who have an inadequate response to current systemic therapy).
Patients in UNCOVER‐J continued the trial after Week 52 for the evaluation of long‐term (>3 years) efficacy and safety of ixekizumab in patients with erythrodermic psoriasis or generalized pustular psoriasis, and in patients with plaque psoriasis who underwent withdrawal and retreatment with ixekizumab. The withdrawal/retreatment findings were recently published10; here, we report long‐term (>3 years) follow‐up efficacy and safety findings for patients with erythrodermic psoriasis or generalized pustular psoriasis in UNCOVER‐J.
Materials and methods
Study design
UNCOVER‐J was a phase 3, multicentre, single‐arm, open‐label study carried out in Japan (ClinicalTrials.gov: NCT01624233).
The study protocol was approved by relevant local institution ethics committees and was implemented in accordance with the Helsinki Declaration of 1975, as revised in 1983. All study patients provided written informed consent.
Study population
The eligibility criteria for UNCOVER‐J have been described in detail previously.8 Briefly, key inclusion criteria were male and female Japanese patients with psoriasis, age ≥20 years, candidates for phototherapy or systemic therapy, and a confirmed diagnosis of plaque psoriasis, erythrodermic psoriasis or generalized pustular psoriasis. Erythrodermic psoriasis was defined as ≥80% body surface area involvement with inflammatory erythema at screening and baseline. Generalized pustular psoriasis was defined by the criteria set by the Japanese Ministry of Health, Labour and Welfare.11 Patients were excluded if they were concurrently using or had recently used any biologic agent within the following periods: etanercept <28 days; infliximab, adalimumab, or alefacept <60 days; golimumab <90 days; ustekinumab <8 months; rituximab <12 months; or any other biologic agent <5 half‐lives prior to baseline. Topical treatments and oral corticosteroids (≤10 mg/day of prednisone or its equivalent) were permitted. Concomitant use of cyclosporine was permitted until Week 2 only in patients with erythrodermic psoriasis.
Note: the methods and results described in this report apply to patients with erythrodermic or generalized pustular psoriasis only.
Treatment protocol
In the Induction Dosing Period (Week 0 to Week 12), patients received ixekizumab 160 mg [given as two 80 mg subcutaneous (sc) injections] at Week 0 and then ixekizumab 80 mg sc every 2 weeks (Fig. 1). In the Maintenance Dosing (Week 12 to Week 52) and Retreatment Periods (Week 52 onwards for up to 192 weeks), patients received ixekizumab 80 mg sc every 4 weeks. Note: patients with erythrodermic psoriasis or generalized pustular psoriasis received the same treatment regimen throughout the Maintenance Dosing and Retreatment Periods. The wording ‘Retreatment’ was used because patients with plaque psoriasis in UNCOVER‐J (not included in this subanalysis) underwent retreatment during this period if certain criteria were met.8 The treatment regimen used in this study is consistent with the treatment regimen approved in Japan for plaque, erythrodermic, and generalized pustular psoriasis and psoriatic arthritis.
Figure 1.
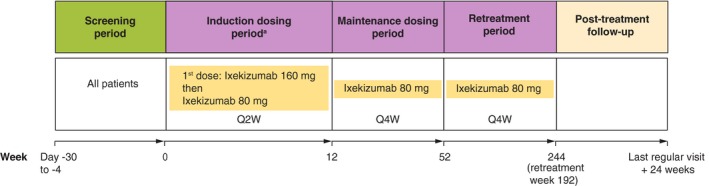
Study design. aPatients received ixekizumab 160 mg (given as two 80 mg subcutaneous injections) at Week 0 and then ixekizumab 80 mg Q2W until Week 12. Note: patients with erythrodermic psoriasis or generalized pustular psoriasis received the same treatment regimen throughout the Maintenance Dosing and Retreatment Periods. The wording ‘Retreatment’ was used because patients with plaque psoriasis in UNCOVER‐J (not included in this subanalysis) underwent retreatment during this period if certain criteria were met. Q2W, every 2 weeks; Q4W, every 4 weeks.
Note: All patients stopped administration of ixekizumab (and moved to the post‐treatment follow‐up period) 9 months after marketing authorization in Japan. Patients who had completed the Retreatment Period or who were ongoing at this time were counted as having completed the Retreatment Period.
Outcome measures
Efficacy
Efficacy outcomes included GIS, Psoriasis Area and Severity Index (PASI), dermal symptoms (for patients with generalized pustular psoriasis), Dermatology Life Quality Index (DLQI),12 and Itch Numeric Rating Scale (NRS).13 For GIS, psoriatic lesions were rated from 1 (resolved) to 4 (worsened). Dermal symptoms were assessed based on Japanese Dermatological Association Practice Guidelines for the treatment of generalized pustular psoriasis,11, 14 where skin symptoms were evaluated by areas of erythema, confluent pustules and skin oedema. Scores for each ranged from 0 to 3 (total: 0 to 9), with higher scores indicating more severe disease.
Safety
Safety outcomes included treatment‐emergent adverse events (TEAEs) and adverse events of special interest (AESIs), specifically cytopenias, liver function test abnormalities, infection, injection‐site reactions, allergic reactions/hypersensitivities, cerebrocardiovascular events, malignancies, depression, Pneumocystis jirovecii pneumonia and interstitial lung disease. TEAEs were coded and summarized using the Medical Dictionary for Regulatory Activities (Version 20.0).
Statistical analysis
Analyses were carried out on the full analysis set, which comprised all patients who received at least one dose of ixekizumab and who had at least one postbaseline PASI measurement.
Sample size determination for UNCOVER‐J has been described previously.8
Continuous data are summarized by descriptive statistics, whereas categorical data are summarized by frequency counts and percentages. Missing continuous data were imputed using the last observation carried forward (LOCF) method. As this was a single‐arm study, no statistical tests for treatment comparisons were performed. Analyses were carried out using SAS software, version 9.2 or later (SAS Institute Inc., Cary, NC, USA).
Results
Disposition
Of 91 patients enrolled in UNCOVER‐J, eight patients had erythrodermic psoriasis and five patients had generalized pustular psoriasis; all completed the study through to Week 52. Of the eight patients with erythrodermic psoriasis who entered the Retreatment Period, six completed the period and two patients discontinued because of TEAEs (mycobacterium tuberculosis and abnormal hepatic function, both n = 1). Of the five patients with generalized pustular psoriasis patients who entered the Retreatment Period, four completed the period and one withdrew as he/she was not able attend the follow‐up visits.
Demographic and baseline clinical characteristics
The overall demographic and baseline clinical characteristics of patients with erythrodermic psoriasis or generalized pustular psoriasis have been described previously.8 All patients had used or were using nonbiologic systemic therapy to treat their conditions prior to enrolling in the study [per the study protocol, all were discontinued before the study started (exceptions were oral corticosteroids, which could be continued, and cyclosporine, which was permitted until Week 2 in patients with erythrodermic psoriasis)]. Individual patient data are summarized in Table 1.
Table 1.
Demographic and clinical characteristics
| Patient | Age, years | Sex | Weight, kg | Duration of psoriasis, years | PASI | Assessment of skin symptomsa | DLQI | Itch NRS | Prior biologic systemic therapy | Prior nonbiologic systemic therapy | CRP, mg/L |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Erythrodermic psoriasis | |||||||||||
| E1 | 69 | M | 68 | 37.7 | 28.3 | NA | 3 | 1 | None | Etretinate, Other | 3.81 |
| E2 | 61 | M | 53 | 35.5 | 29.4 | NA | 6 | 6 | None | Other | 0.56 |
| E3 | 35 | M | 105 | 10.5 | 49.8 | NA | 8 | 3 | None | Cyclosporine, Etretinate | 2.07 |
| E4 | 57 | M | 74 | 2.9 | 43.6 | NA | 12 | 7 | None | Cyclosporine, Etretinate, Other | 7.86 |
| E5 | 53 | M | 82 | 26.7 | 31.7 | NA | 11 | 3 | Infliximab | Cyclosporine, Etretinate, Methotrexate, Other | 0.50 |
| E6 | 36 | M | 100 | 20.8 | 52.2 | NA | 9 | 2 | Adalimumab, Ustekinumab | Cyclosporine, Etretinate, Other | 6.77 |
| E7 | 53 | F | 55 | 2.2 | 48.6 | NA | 14 | 7 | None | Other | 7.03 |
| E8 | 36 | M | 92 | 10.6 | 58.8 | NA | 28 | 7 | Ustekinumab | Other | 10.20 |
| Generalized pustular psoriasis | |||||||||||
| P1 | 37 | F | 58 | 30.5 | 10.4 | 1 | 1 | 6 | Infliximab | Cyclosporine, Etretinate | 1.89 |
| P2 | 29 | F | 53 | 12.9 | 8.2 | 2 | 9 | 10 | None | Cyclosporine, Other | 0.28 |
| P3 | 45 | M | 55 | 0.8 | 22.2 | 6 | 19 | 9 | None | Cyclosporine, Other | 14.30 |
| P4 | 61 | F | 43 | 41.6 | 10.4 | 3 | 11 | 7 | Adalimumab, Infliximab | Cyclosporine, Etretinate, Other | 23.00 |
| P5 | 67 | M | 71 | 20.6 | 12.8 | 2 | 8 | 4 | None | Cyclosporine, Other | 0.20 |
Score range: 0–9 (least to most severe disease) in patients with generalized pustular psoriasis only.
CRP, C‐reactive protein; DLQI, Dermatology Life Quality Index; F, female; M, male; NA, not applicable; NRS, Numeric Rating Scale; PASI, Psoriasis Area and Severity Index.
Efficacy
Erythrodermic psoriasis
Most patients had a GIS of resolved or improved (from baseline) from Week 12 onwards (Fig. 2a). By Week 64 (Retreatment Week 12), 2/7 (observed) patients had a GIS of resolved and 5/7 patients had a GIS of improved. One patient had a GIS of worsened at Weeks 112 and 124 (Retreatment Weeks 60 and 72).
Figure 2.
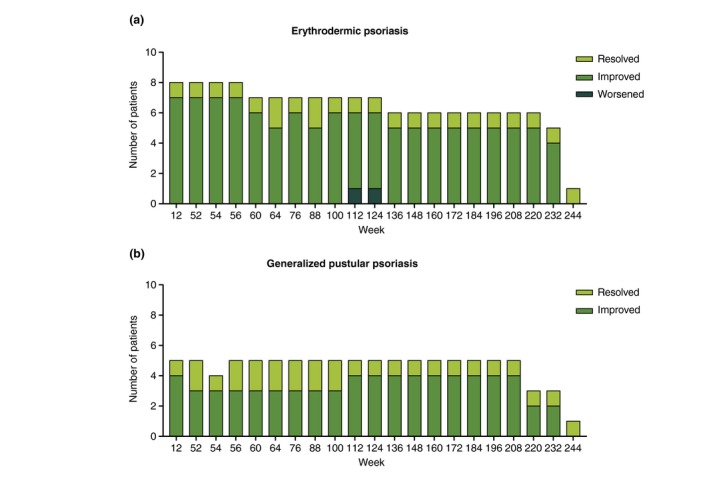
Number of patients with erythrodermic psoriasis (a) or generalized pustular psoriasis (b) who had Global Improvement Scores categorized as resolved, improved or worsened throughout the study.
All patients had early and sustained improvement in PASI scores (Fig. 3a). The mean PASI score was 42.8 at baseline, 3.0 at Week 52 (LOCF), and 5.0 at Week 244 (LOCF) (Retreatment Week 192). Two patients, E3 and E6, had relatively (vs. other patients) high PASI scores during the study; both of these patients also had high PASI scores at baseline and weighed ≥100 kg.
Figure 3.
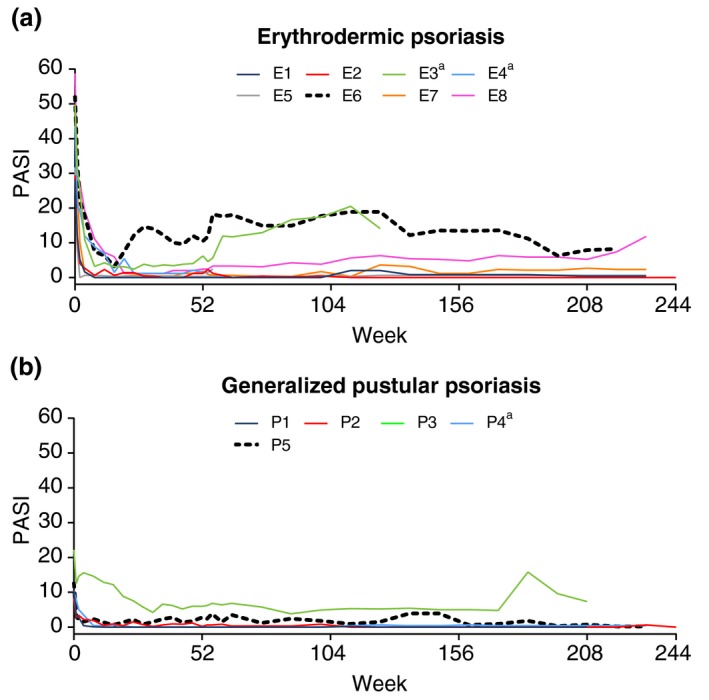
Psoriasis Area and Severity Index (PASI) scores in patients with erythrodermic psoriasis (a) or generalized pustular psoriasis (b). Each coloured line represents scores for an individual patient. aPatient discontinued during Retreatment Period. E, erythrodermic psoriasis patient; P, generalized pustular psoriasis patient.
Mean improvements in DLQI and Itch NRS at Week 12 were sustained through Week 244 (LOCF, Table 2) (Retreatment Week 192).
Table 2.
DLQI and Itch NRS scores in patients with erythrodermic psoriasis or generalized pustular psoriasis
| Mean (SD) | Erythrodermic psoriasis (N = 8) | Generalized pustular psoriasis (N = 5) | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Week 12 | Week 52 | Week 244a | Baseline | Week 12 | Week 52 | Week 244a | |
| DLQI | 11.4 (7.6) | 1.8 (3.0) | 1.9 (1.7) | 2.1 (2.5) | 9.6 (6.5) | 4.2 (6.6) | 3.8 (4.4) | 3.6 (4.8) |
| Itch NRS | 4.5 (2.5) | 1.0 (0.5) | 1.1 (0.8) | 1.3 (0.7) | 7.2 (2.4) | 2.0 (1.7) | 1.8 (3.0) | 1.6 (2.1) |
Retreatment Week 192.
Missing data were imputed using the LOCF method.
DLQI, Dermatology Life Quality Index; NRS, Numeric Rating Scale; SD, standard deviation.
Generalized pustular psoriasis
All patients had a GIS of resolved or improved from Week 12 onwards (Fig. 2b). By Week 64 (Retreatment Week 12), 2/5 patients had a GIS of resolved and 3/5 patients had a GIS of improved. No patients had a GIS of unchanged or worsened during the Retreatment Period.
All patients had early and sustained improvement in PASI scores (Fig. 3b). The mean PASI score was 12.8 at baseline, 1.8 at Week 52 (LOCF) and 1.6 at Week 244 (LOCF) (Retreatment Week 192). One patient, P3, had relatively (vs. other patients) high PASI scores during the study; this patient also had a high PASI score at baseline.
All patients had early and sustained improvement in dermal symptoms (Fig. 4). The mean total dermal symptoms score was 2.8 at baseline, 0.8 at Week 52 (LOCF) and 0.6 at Week 244 (LOCF) (Retreatment Week 192). One patient, P3, had relatively (vs. other patients) high dermal symptom scores during the study; this patient also had a high dermal symptom score at baseline.
Figure 4.
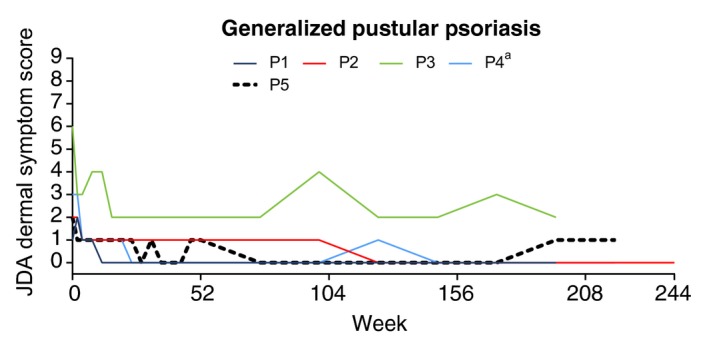
Japanese Dermatological Association (JDA) dermal symptom scores in patients with generalized pustular psoriasis. Each coloured line represents scores for an individual patient. Dermal symptoms were evaluated by areas of erythema, confluent pustules and skin oedema. Scores for each ranged from 0 to 3 (total: 0 to 9) with higher scores indicating more severe disease. aPatient discontinued during Retreatment Period. P, generalized pustular psoriasis patient.
Mean improvements in DLQI and Itch NRS at Week 12 were sustained through Week 244 (LOCF, Table 2) (Retreatment Week 192).
Safety
All patients in both subgroups reported TEAEs after 52 weeks (Table 3; TEAEs reported before Week 52 have been previously described in more detail8, 9). No severe TEAEs, serious adverse events or deaths were reported after Week 52. A total of 2/8 patients with erythrodermic psoriasis discontinued the study due to TEAEs. Patient E3 discontinued due to abnormal hepatic function 701 days after first receiving study treatment. This patient had baseline aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels of 27 and 38 U/L, respectively, and Day 700 AST and ALT levels of 58 and 139 U/L, respectively. The event was moderate in severity, but did not resolve; causality was unknown. Patient E4 discontinued due to a positive mycobacterium tuberculosis test 358 days after first receiving study treatment (no confirmed diagnosis of active or latent tuberculosis was reported). The event was mild in severity, did not resolve, despite treatment with rifampicin, and was considered possibly related to study treatment. The overall safety profile after 52 weeks was similar to the overall safety profile before Week 52.
Table 3.
Safety overview in patients with erythrodermic psoriasis or generalized pustular psoriasis
| Adverse event,a n (%) | Erythrodermic psoriasis (N = 8) | Generalized pustular psoriasis (N = 5) | ||
|---|---|---|---|---|
| 0–52 weeksb | After 52 weeksc | 0–52 weeksb | After 52 weeksc | |
| Patients with ≥ 1 TEAE | 7 (87.5) | 8 (100) | 5 (100) | 5 (100) |
| Mild | 3 (37.5) | 4 (50.0) | 2 (40.0) | 3 (60.0) |
| Moderate | 3 (37.5) | 4 (50.0) | 3 (60.0) | 2 (40.0) |
| Severe | 1 (12.5) | 0 | 0 | 0 |
| AE leading to discontinuation | 0 | 2 (25.0) | 0 | 0 |
| Deaths | 0 | 0 | 0 | 0 |
| SAEs | 0 | 0 | 0 | 0 |
Adverse events were included regardless of their relationship with the study drug.
Note: these data, except for the severity data, have been published previously.9
After 52‐week data do not include AEs that occurred between 0 and 52 weeks.
AE, adverse event; SAEs, serious adverse events; TEAE, treatment‐emergent dverse event.
The most common TEAEs by system organ class (SOC) after 52 weeks in patients with erythrodermic psoriasis were infections and infestations and skin and subcutaneous tissue disorders (both 4/8 patients). The most common TEAEs by SOC after 52 weeks in patients with generalized pustular psoriasis were general disorders and administration‐site conditions, infections and infestations and musculoskeletal disorders (all 4/5 patients).
AESIs were reported by 4/8 patients with erythrodermic psoriasis and by 4/5 patients with generalized pustular psoriasis. Specific AESIs reported by patients with erythrodermic psoriasis included infections (4/8 patients), abnormal hepatic function (2/8 patients) and allergic reaction/hypersensitivity (1/8 patients). The AESIs categorized as infection included viral upper respiratory tract infection (two events) and folliculitis, gastroenteritis, periodontitis, gingivitis, otitis externa, tonsillitis and helicobacter infection (all one event). The AESI categorized as allergic reaction/hypersensitivity was eczema (one event, mild in severity). Specific AESIs reported by patients with generalized pustular psoriasis included infections (4/5 patients), allergic reaction/hypersensitivity (2/5 patients), and injection‐site reaction and depression (both 1/5 patients). The AESIs categorized as infection included viral upper respiratory tract infection (two events) and periodontitis, angular cheilitis, conjunctivitis, oral herpes and paronychia (all one event). The AESIs categorized as allergic reaction/hypersensitivity were nonanaphylactic eczema (two events, both mild in severity), contact dermatitis (one event, mild in severity) and allergic rhinitis (one event, moderate in severity). The AESI of depression was mild in severity and was not considered related to study treatment. Of note, there were no AESIs of inflammatory bowel disease or malignancy reported.
Discussion
Ours is the first clinical study to report on the long‐term (244‐week) efficacy and safety of an IL‐17 inhibitor for the treatment of erythrodermic or generalized pustular psoriasis. We found that ixekizumab demonstrated evidence of clinical efficacy over 3 years of treatment in Japanese patients with these forms of psoriasis, while safety findings were consistent with the known safety profile of ixekizumab in patients with psoriasis. These results support the use of ixekizumab for the treatment of erythrodermic or generalized pustular psoriasis.
We found that ixekizumab had a rapid onset of efficacy, and that this efficacy was sustained for more than 3 years in patients with erythrodermic or generalized pustular psoriasis. Efficacy was demonstrated by multiple measures, including GIS, PASI, assessment of dermal symptoms (in patients with generalized pustular psoriasis only), DLQI, and Itch NRS. Of note, patients who discontinued nonbiologic systemic therapies before starting ixekizumab also experienced subsequent improvement in their symptoms without resuming nonbiologic systemic therapies. To date, such long‐term (beyond 52 weeks) efficacy data have not been reported from studies of other IL‐17 inhibitors, including secukinumab and brodalumab. Specifically, previous studies of secukinumab and brodalumab have examined 52‐week, open‐label efficacy in patients with generalized pustular psoriasis (N = 12)15 and in patients with erythrodermic (N = 18) or generalized pustular psoriasis (N = 12),16 respectively.
Ixekizumab was well tolerated over 3 years of treatment in patients with erythrodermic psoriasis or generalized pustular psoriasis and no new safety concerns were identified. Notably, no patients experienced severe TEAEs after 52 weeks of treatment, and there were no serious adverse events reported. Further, the incidence of TEAEs, overall and by severity, was generally similar between the periods comprising the first 52 weeks of treatment and treatment thereafter. Finally, although there were only a small number of patients, the overall safety profile in patients with erythrodermic psoriasis or generalized pustular psoriasis appeared to be consistent with that for ixekizumab in general among patients with psoriasis.17
Our study has a number of noteworthy strengths including the multicentre design, length of follow‐up, and low rate of discontinuation. However, several limitations must be acknowledged, including the open‐label design, lack of a control group and small sample size. The small sample size reflects the prevalence of these forms of psoriasis in the general population, but, nevertheless, means that the results should be interpreted with some degree of caution. Further, the inclusion of Japanese patients only means that the results may not be generalizable to other populations. In addition, our inclusion criteria for generalized pustular psoriasis did not require assessment of systemic manifestations and laboratory results (i.e. fever, white blood cell count, serum C‐reactive protein, serum albumin), although, overall, our exclusion criteria were set to enrol medically stable patients. A final limitation is that all patients stopped administration of ixekizumab 9 months after marketing authorization in Japan (patients who had completed the Retreatment Period or who were ongoing at this time were counted as having completed the study).
In conclusion, we found that the previously reported efficacy and acceptable safety profile of ixekizumab in patients with erythrodermic or generalized pustular psoriasis after 52 weeks of treatment9 was sustained through 3 years of treatment. These findings suggest that ixekizumab can be an effective long‐term treatment option for erythrodermic or generalized pustular psoriasis.
Author Contributions
YO, TM and KI were study investigators and were involved in data collection. All authors participated in the interpretation of study results, and in the drafting, critical revision and approval of the final version of the manuscript.
Acknowledgments
Role of the sponsor: Eli Lilly Japan K.K. was involved in the study design, data collection, data analysis and preparation of the manuscript.
Other contributors/acknowledgments: The authors would like to thank all study participants and all members of the Japanese Ixekizumab Study Group who enrolled patients with erythrodermic psoriasis or generalized pustular psoriasis: Dr Takashi Hashimoto of Kurume University Hospital, Dr Atsuyuki Igarashi of NTT Medical Center Tokyo, Dr Kazuhiko Takehara of Kanazawa University Hospital, Dr Yoshinori Umezawa of The Jikei University Hospital and Dr Keiichi Yamanaka of Mie University Graduate School of Medicine.
Conflict of Interest
YO has been a consultant, scientific advisor, and/or investigator for Eli Lilly Japan K. K., Kyowa Hakko Kirin Co., Ltd, Mitsubishi Tanabe Pharma Corporation, Maruho Co., Ltd, Celgene K.K., Janssen Pharmaceutical K.K., AbbVie GK, Eisai Co., Ltd, Torii Pharmaceutical Co., Ltd, Leo Pharma, MSD K.K. and Boehringer Ingelheim Japan, Inc. TM has received honoraria from Maruho Co. Ltd. KI has nothing to disclose. HTI, KN, YM and HE are employed by Eli Lilly. KN and HE own shares in Eli Lilly and Company.
Funding Sources
This study was sponsored by Eli Lilly Japan K.K., the manufacturer/licensee of Taltz®. Medical writing assistance was provided by Luke Carey, PhD and Serina Stretton, PhD, CMPP of ProScribe – Envision Pharma Group, and was funded by Eli Lilly Japan K.K. ProScribe's services complied with international guidelines for Good Publication Practice (GPP3).
References
- 1. Kubota K, Kamijima Y, Sato T et al Epidemiology of psoriasis and palmoplantar pustulosis: a nationwide study using the Japanese national claims database. BMJ Open 2015; 5: e006450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ito T, Takahashi H, Kawada A, Iizuka H, Nakagawa H, Japanese Society for Psoriasis Research . Epidemiological survey from 2009 to 2012 of psoriatic patients in Japanese Society for Psoriasis Research. J Dermatol 2018; 45: 293–301. [DOI] [PubMed] [Google Scholar]
- 3. Liu L, Lu J, Allan BW et al Generation and characterization of ixekizumab, a humanized monoclonal antibody that neutralizes interleukin‐17A. J Inflamm Res 2016; 9: 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Griffiths CE, Reich K, Lebwohl M et al Comparison of ixekizumab with etanercept or placebo in moderate‐to‐severe psoriasis (UNCOVER‐2 and UNCOVER‐3): results from two phase 3 randomised trials. Lancet 2015; 386: 541–551. [DOI] [PubMed] [Google Scholar]
- 5. Gordon KB, Blauvelt A, Papp KA et al Phase 3 trials of ixekizumab in moderate‐to‐severe plaque psoriasis. N Engl J Med 2016; 375: 345–356. [DOI] [PubMed] [Google Scholar]
- 6. Mease PJ, van der Heijde D, Ritchlin CT et al Ixekizumab, an interleukin‐17A specific monoclonal antibody, for the treatment of biologic‐naive patients with active psoriatic arthritis: results from the 24‐week randomised, double‐blind, placebo‐controlled and active (adalimumab)‐controlled period of the phase III trial SPIRIT‐P1. Ann Rheum Dis 2017; 76: 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nash P, Kirkham B, Okada M et al Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24‐week randomised, double‐blind, placebo‐controlled period of the SPIRIT‐P2 phase 3 trial. Lancet 2017; 389: 2317–2327. [DOI] [PubMed] [Google Scholar]
- 8. Saeki H, Nakagawa H, Ishii T et al Efficacy and safety of open‐label ixekizumab treatment in Japanese patients with moderate‐to‐severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis. J Eur Acad Dermatol Venereol 2015; 29: 1148–1155. [DOI] [PubMed] [Google Scholar]
- 9. Saeki H, Nakagawa H, Nakajo K et al Efficacy and safety of ixekizumab treatment for Japanese patients with moderate to severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis: results from a 52‐week, open‐label, phase 3 study (UNCOVER‐J). J Dermatol 2017; 44: 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Umezawa Y, Torisu‐Itakura H, Morisaki Y et al Long‐term efficacy and safety results from an open‐label phase III study (UNCOVER‐J) in Japanese plaque psoriasis patients: impact of treatment withdrawal and retreatment of ixekizumab. J Eur Acad Dermatol Venereol 2018. 10.1111/jdv.15292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iwatsuki K, Terui T, Ozawa A. Practice guidelines for generalized pustular psoriasis (GPP): treatment guidelines incorporating TNF‐α inhibitor. Jpn J Dermatol 2010; 120: 815–839. [Google Scholar]
- 12. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19: 210–216. [DOI] [PubMed] [Google Scholar]
- 13. Phan NQ, Blome C, Fritz F et al Assessment of pruritus intensity: prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta Derm Venereol 2012; 92: 502–507. [DOI] [PubMed] [Google Scholar]
- 14. Terui T, Akiyama M, Ikeda S et al Guidelines for treatment of generalized pustular psoriasis (2014 edition). Jpn J Dermatol 2015; 125: 2211–2257. [Google Scholar]
- 15. Imafuku S, Honma M, Okubo Y et al Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: A 52‐week analysis from phase III open‐label multicenter Japanese study. J Dermatol 2016; 43: 1011–1017. [DOI] [PubMed] [Google Scholar]
- 16. Yamasaki K, Nakagawa H, Kubo Y, Ootaki K, Japanese Brodalumab Study Group . Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52‐week, open‐label study. Br J Dermatol 2017; 176: 741–751. [DOI] [PubMed] [Google Scholar]
- 17. Strober B, Leonardi C, Papp KA et al Short‐ and long‐term safety outcomes with ixekizumab from 7 clinical trials in psoriasis: etanercept comparisons and integrated data. J Am Acad Dermatol 2017; 76: 432–440 e417. [DOI] [PubMed] [Google Scholar]


