Abstract
Aim
In anatomic studies of the embryo, it has been established that during the development of the lower limb, several changes in foot position can be observed defined as a temporary ‘physiological clubfoot’. The aim of this study was to develop and test a measurement tool for objective documentation of the first trimester foot position in vivo and made an attempt to create a chart for first trimester foot position.
Methods
We developed a virtual orthopedic protractor for measuring foot positioning using three‐dimensional virtual reality visualization. Three‐dimensional ultrasound volumes of 112 pregnancies of women examined during the first trimester were studied in a BARCO I‐Space. The frontal angle (plantar flexion) and the lateral angle (adduction) between the leg and foot were measured from 8 until 13 weeks gestational age.
Results
We observed that the frontal angle steadily decreases, whereas the lateral angle first increases, resulting in transient physiological clubfeet position at 10‐ to 11‐week gestation, followed by a decrease to a normal foot position.
Conclusion
A transient clubfoot position is present during the normal development of the lower limbs, and it has been measured in vivo for the first time. This study emphasizes that a diagnosis of congenital clubfoot should not be made in the first trimester of pregnancy.
Keywords: clubfoot, pregnancy, prenatal diagnosis, ultrasonography, virtual reality
Introduction
There is an increasing interest in the detection of structural abnormalities in the first trimester of pregnancy.1, 2 First trimester screening and technical improvement of ultrasound equipment have contributed to this shift of interest from the second trimester toward the first trimester of pregnancy. Detailed knowledge on sonographic appearance of normal first trimester development is essential, not only for early diagnosis of congenital anomalies but for the establishment of embryonic health.3
An idiopathic congenital clubfoot is one of the most common observed congenital anomalies with a reported incidence of 1 in 1000 live births.4 Congenital clubfoot, or talipes equinovarus, is described as a fixation of the foot in adduction, in supination and in varus, with a medial rotation in relation to the talus of the calcaneus, navicular and cuboid bones, which are held in adduction and inversion by the ligaments and tendons.5 Congenital clubfeet may be isolated but in approximately half of the cases they are associated with other anomalies in which case the prognosis is usually poor, e.g., neurological disorders (spina bifida), and chromosomal abnormalities and genetic factors.6, 7, 8 In addition, congenital clubfoot is also associated with a relative lack of space in utero (e.g., twin pregnancy, oligohydramnios and early amniocentesis),6 which was already presumed by Hippocrates in Ancient Greece.9
In anatomical studies of the embryo, it was established that during the development of the lower limb, several changes in foot position can be recognized, temporarily leading to a ‘physiological clubfoot’.10 Although different developmental pathways have been proposed, in all embryos a decrease in the angle of the foot with the frontal side of the leg (plantar flexion), and at first an increase followed by a decrease in the angle of the foot with the lateral side of the leg (adduction) is observed.
As the diagnosis of a congenital clubfoot is based on the subjective assessment of the ultrasound images, there is, especially at the end of the first trimester, a need for a measurement tool for objective documentation of the foot position. Recent developments in three‐dimensional (3D) sonographic imaging techniques have resulted in remarkable progress in the visualization of the developing fetus. Moreover, by using virtual reality (VR), e.g., the BARCO I‐Space (Barco N.V.), it is possible to immerse the viewer in a computer‐generated 3D environment, allowing him to perceive depth and to interact with volume‐rendered (ultrasound) data in a more natural and intuitive manner than is possible with 3D views displayed on a two‐dimensional (2D) screen.11 This technique has provided new insights into normal as well as abnormal fetal growth and development.11, 12, 13
We developed a method for measuring the position of the foot by using a virtual orthopedic protractor in VR, as the third dimension is necessary and essential for defining parallelism of two straight lines. This innovative foot positioning tool was tested for practical use in the first trimester, including reproducibility of measurements and developing reference curves for scientific and diagnostic purposes.
The aim of this study was to develop and evaluate a three‐dimensional ultrasound in VR (3D‐VR) measurement tool for objective documentation of first trimester foot position in vivo using VR and to construct reference data for foot position in ongoing pregnancies.
Methods
Study population and sample
This study has been conducted in a periconception cohort study at a university hospital for which women were enrolled for first trimester longitudinal 3D ultrasound measurements to evaluate fetal growth and development using 3D ultrasound and VR. Pregnant women who participated were enrolled via the outpatient clinic of the Department of Obstetrics and Gynecology and local midwifery practices. All women received weekly 3D ultrasound scans between 6 + 0 and 12 + 6 weeks gestational age (GA). A 3D ultrasound image of the total embryo was taken and stored when the embryo was at rest and did not move. Only women less than 8‐week pregnant with a singleton pregnancy entered the study for further analysis. All participants signed a written informed consent form and the local medical and ethical review committee approved the study protocol (MEC 2004–227 – December 10, 2009, combining: METC 232.394/2003/177, METC 323.395/2003/178, MEC 2004–227).
We selected from the cohort 141 women who were enrolled in 2009 and from whom at least one ultrasound volume was obtained and digitally stored in the so called ‘virtual embryo collection’. Two pregnancies complicated with trisomy 21 and three with congenital anomalies were excluded. Three multiple pregnancies, three drop outs, 16 miscarriages and two cases with an intrauterine fetal demise had to be excluded as well, leaving 112 inclusions for analysis (Fig. 1).
Figure 1.
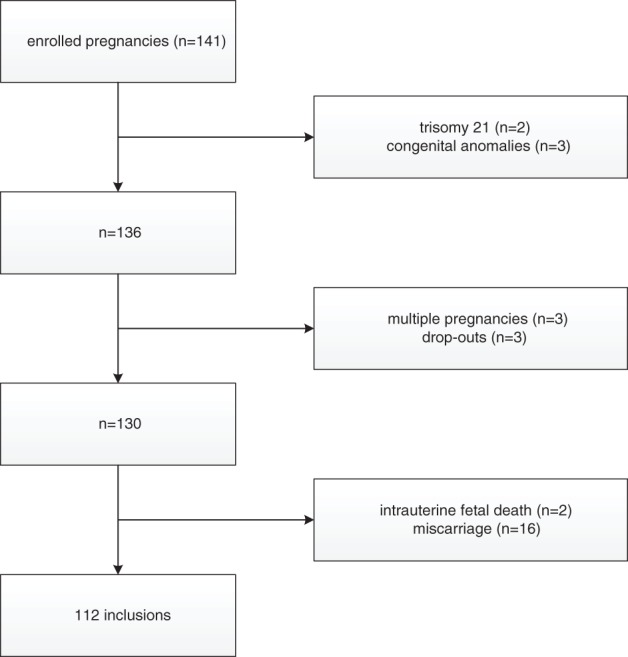
Flowchart illustrating inclusions and exclusions of the study population.
Pregnancy dating
The GA was calculated according to the first day of the last menstrual period (LMP) in case of a regular menstrual cycle of 28 days and adjusted for a longer or shorter cycle.14 In case of a discrepancy in GA of more than 7 days between crown rump length (CRL) and the LMP, or an unknown LMP, the GA was calculated by using CRL at 12‐week GA. In case of assisted reproductive technology, GA was determined by the date of oocyte retrieval plus 14 days in pregnancies conceived via in vitro fertilization with or without intracytoplasmic sperm injection procedures, from the LMP or insemination date plus 14 days in pregnancies conceived through intrauterine insemination, and from the day of embryo transfer plus 17 or 18 days in pregnancies originating from the transfer of cryopreserved embryos, depending on the number of days between oocyte retrieval and cryopreservation of the embryo.
Material
The sonographic volumes were acquired using a Voluson E8 ultrasound machine (GE Medical Systems) and obtained with a transvaginal scan (GE‐probe RIC‐6‐12‐D [4.5–11.9 MHz]). With regard to the safety aspects of first trimester ultrasound, the thermal index and mechanical index were kept below 1.0, the examiners were qualified and experienced, and the as‐low‐as‐reasonable‐practicable principle was respected: the duration of the examination did not exceed 30 min, and 3D images were stored for offline evaluation in order to reduce the exposure to ultrasound as much as possible.15 The 3D datasets were collected when the fetus was at rest. The 3D volumes were transferred to the BARCO I‐Space (Barco N.V.) and visualized in 3D using our V‐Scope software.16 The hologram, visualized through polarizing glasses, can be manipulated by a wireless joystick tracked by an optical tracking system. This joystick also controls a measuring tool to trace lines and measure angles and volumes. For our study, the 3D volumes were resized (enlarged), rotated and cropped when necessary and gray‐scale and opacity values were adjusted for optimal image quality.
Measurements
We measured the frontal angle, being a measure for plantar flexion of the foot, between the ventral side of the lower leg and the ventral side of the foot by drawing a line from the ventral side of the knee joint to the instep and a line from the instep to the end of the middle toe using the joystick. The computer calculated the angle between both lines. Thereafter we measured the lateral angle, being a measure for adduction of the foot, between the lateral side of the leg and the lateral side of the foot by drawing a line from the lateral side of the fifth to the medial side of the first toe and a line from the medial side of the first toe upwards and parallel in all directions to the lower leg, using the line of the first measurement as a reference (Fig. 2). The possibility to draw this line is a unique feature of VR, which offers the opportunity to check for parallelism in all directions (Video). The measurement procedure was, when possible, performed separately for both feet and in that case the difference between both feet was calculated as well.
Figure 2.
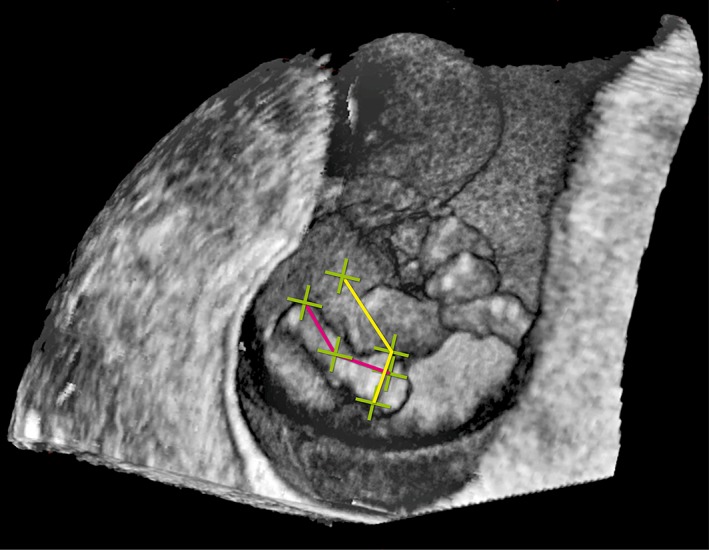
Measurements on the fetal foot performed in the I‐Space; frontal angle: a line from the ventral side of the knee joint to the instep and a line from the instep to the end of the middle toe (pink line). Lateral angle: a line from the lateral side of the fifth to the medial side of the first toe and a line from the medial side of the first toe upwards and parallel in all directions to the lower leg (yellow line).
Statistical analysis
Interclass and intraclass correlations were calculated from a two‐way model using R 2.51.1.17 Reference curves were estimated using the GAMLSS package.18 For each outcome curves were fitted using a normal, scaled t and the Box‐Cox transformed normal and several numbers of degrees of freedom in the splines for the location and dispersion were tried19 and the best fitting model was selected using the Akaike information criterion (AIC). The AIC is a measure for the goodness‐of‐fit of a model that corrects for model complexity. By using the model with the best AIC we made sure that we selected a well‐fitting model that is not more complex than necessary. Here, we treated the data as if all observations were independent (since no standard methods for reference curves correlated observations exist).
Reproducibility
All measurements were performed offline and for each angle measurement 30 randomly chosen measurements, by using a numeric computer‐generated sequence, were repeated by the same observer (Maria S. Rifouna) and the same 30 measurements were measured by another observer (H. B.) to determine reproducibility of the new measurement technique. The measurements used for intra‐ and interobserver analysis were repeated after an interval of at least 2 weeks in the same stored ultrasound volume as used for the original measurements.
Results
From the 112 pregnancies, 530 volumes were obtained for evaluation. Patient characteristics and success rates are presented in Tables 1 and 2, respectively.
Table 1.
General characteristics
| Characteristic | Median (range) or percentage |
| Mothers (n = 112) | |
| Maternal age (years) | 32.9 (18.9–42.7) |
| Gravidity | 2 (1–10) |
| Parity | |
| 0 | 62.5% |
| 1 | 27.7% |
| ≥2 | 9.8% |
| Miscarriages ≥2 | 25.9% |
| Conception mode | |
| Natural | 70.5% |
| IVF or IVF/ICSI | 27.7% |
| Intrauterine insemination | 1.8% |
| Gestational diabetes | 5.4% |
| Hypertensive disorders | 8.9% |
| Fetal growth restriction | 3.6% |
| Newborns (n = 112) | |
| Female | 52.7% |
| Birth weight (grams) | 3390 (450–4700) |
| GA at delivery (weeks) | 39 + 4 (26 + 4 to 42 + 0) |
GA, gestational age; ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilization.
Table 2.
Success percentages of angle measurements (measurements/number of images; %) by gestational age (expressed in complete weeks)
| Week | Right leg | % | Left leg | % |
|---|---|---|---|---|
| 8 | 14/104 | 13.5 | 16/104 | 15.4 |
| 9 | 28/109 | 25.7 | 25/109 | 22.9 |
| 10 | 56/108 | 51.9 | 53/108 | 49.1 |
| 11 | 69/108 | 63.9 | 58/108 | 53.7 |
| 12 | 40/101 | 39.6 | 37/101 | 36.6 |
The measurements of the angles are plotted against the GA in Figure 3. Both curves of the right and left foot look similar, showing the same pattern for both frontal and lateral angles. Regarding the frontal angles of the left and right foot, a decrease of the angle with advancing GA is observed from approximately 155° to 110°. However, the lateral angles show a different curve: the angles first increase from approximately 105° to 125° and reach a peak at about 11‐week GA, resulting in a physiological clubfoot position, followed by a decrease to 110° thereafter. The lateral curves flatten from 12‐week GA onwards and a continuing decrease is observed thereafter. Left and right differences of frontal and lateral angles in the same cases are depicted in Figure 4. No preponderance of left or right feet is observed. Differences in frontal angles appeared to be smaller than in lateral angles.
Figure 3.
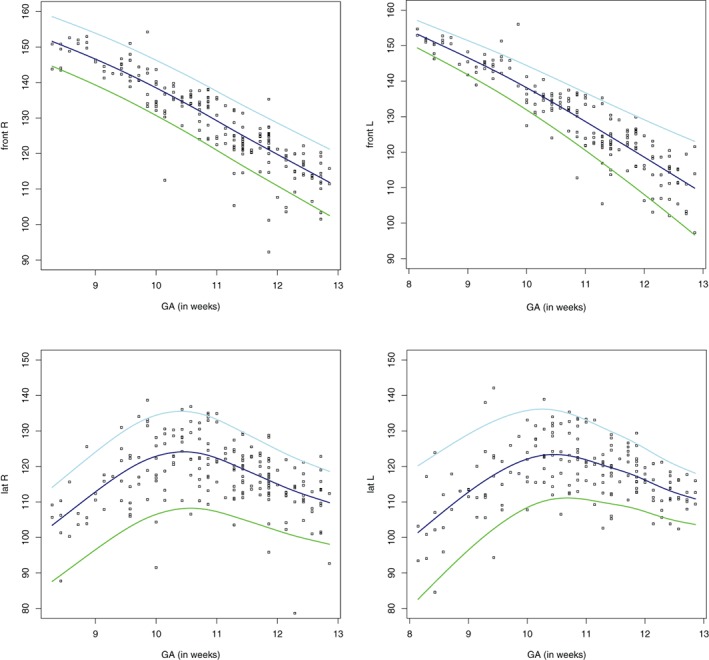
Individual measurements of upper left: the frontal angle of the right foot (using TF, t‐family); upper right: the frontal angle of the left foot (using TF, t‐family); lower left: the lateral angle of the right foot (using BCCG, Box‐Cox Cole and Green); lower right: the lateral angle of the left foot (using NO, Normal).  , P5,
, P5,  , P50,
, P50,  , P95. GA, gestational age.
, P95. GA, gestational age.
Figure 4.
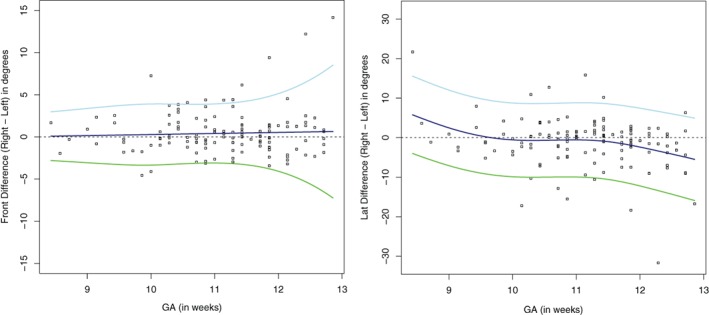
Left and right differences of frontal and lateral angles in the same cases; outliers belong to different fetuses.  , P5,
, P5,  , P50,
, P50,  , P95. GA, gestational age.
, P95. GA, gestational age.
Reproducibility was good. The mean differences, the 95% limits of agreement and the intraclass correlation coefficients of intra‐ and interobserver variability are shown in Table S1. Bland and Altman plots are shown in Figures S1 and S2.
Discussion
We investigated the fetal development of the lower limb by means of VR and succeeded in measuring the first trimester physiological clubfoot in vivo objectively. Our findings are consistent with the in vitro study on the development of the legs and feet of Victoria‐Diaz and Victoria‐Diaz, who described the changes in the foot position of embryos from legal abortions already three decades ago (Fig. S3).10 In the first stage of development, the foot is in line with the leg. Because of the relatively large growth of the fibula compared to the tibia, in the second stage the foot is displaced in inversion and dorsiflexion, resulting in a physiological clubfoot (‘fibular phase’). The fibular phase is observed when the length of the embryo is 21 to 30 mm, which roughly corresponds to 8.5‐ to 10‐week GA. In the third‐stage growth, acceleration of the tibia causes the foot to attain its normal position (‘tibial phase’). The embryonic length is 31–50 mm during the tibial phase, which roughly corresponds to 10‐ to 11.5‐week GA. At this early stage of pregnancy the length of the fibula and tibia cannot be reliable measured with ultrasound as the bones mainly consist of less echogenic cartilage. However, since it is possible to measure the angles of the foot position, using VR the observation of a transient clubfoot position in our study is confirmed. The lateral angles of the feet first increase, which corresponds to the fibular phase (Fig. S4). Next the lateral angles decrease again to attain a normal foot position, corresponding to the tibial phase. It is assumed that failure to move on from the fibular to the tibial phase results in a congenital clubfoot.
Another anatomical study was performed by Kawashima and Uhthoff: they investigated in vitro 189 feet in fetuses ranging from 9‐ to 22‐week GA.20 They observed a steady decline in the frontal angle until the end of 12‐week GA as well. From 13‐week GA, no changes in foot position were observed. The lateral angle first increased until 11‐week GA followed by a decrease thereafter. Changes in foot position during the physiological clubfoot occurred mainly at the talar level and they concluded that clubfeet might be caused by a developmental arrest of the talus. Regardless of etiology, the changes in foot position are the same and correspond with our results.
The hypothesis that clubfeet are caused by a growth disturbance is sustained by Dietz.21 He explained this by the differences in muscle fibers and cellular content in the lower leg in patients with clubfeet, possibly caused by a regional abnormality of the tibial nerve.
Duce et al.22 investigated clubfeet in mice by means of micro magnetic resonance imaging. The peroneal muscle atrophy (pma) mouse mutant has, due to atrophy of the anterior and lateral muscle compartments in the hind limbs, a clubfoot position. Although several differences in anatomy between this mutant mouse and humans affected by congenital clubfoot are seen, the authors observed an arrest in the development of the hind foot. This development was not completed in pma mice compared to the wild type, suggesting a comparable etiology of this anomaly.
Over the years, congenital clubfeet are increasingly detected using prenatal ultrasound,6, 23 although there remains a false positive diagnosis in about 10% of the cases.24 Glotzbecker et al.25 tried to decrease this by using a 2D angle measurement classification system. In a sagittal view, the angle between the long axis of the leg, respectively, the foot (resembling our ‘lateral angle’), was measured. False positive rate dropped dramatically using a cut off of 80°. Grande et al.26 performed a large (n = 13 723) retrospective study to assess sensitivity of detecting anomalies in the first trimester. They found an overall detection rate for minor skeletal anomalies like clubfeet of only 9%. Our data show that an isolated clubfoot position suspected at the time of the nuchal translucency scan might be a transient finding.
Our results gain more insight in the normal development of the foot position and its sonographic appearance, being a prerequisite for diagnosing abnormalities like a pathological clubfoot. Further research is required to determine the foot position in fetuses in the first trimester that later on appear to have a congenital clubfoot. Continuation of collection of first trimester volumes might include fetuses, which later appear to be affected by clubfeet. This will allow comparison of normal to abnormal foot position and increase our understanding of the development and etiology of this common anomaly.
To our best knowledge, we are the first to measure the foot position in vivo using ultrasound.
The characteristics of this subgroup are comparable to those of women enrolled between 2010 and 2014.27 The approach we used in this study appeared to be a reproducible measurement technique to evaluate the fetal foot position in early pregnancy. Yet, this might be an overestimation since we evaluated the same dataset and we evaluated only the datasets with the best volume quality.
The observed differences between right and left feet (Fig. 4) could be caused by a difference in growth or development between the right and left foot in individual cases. Limbs develop in a craniocaudal fashion but we are not aware of a left–right or right–left gradient. We are yet not able to explain this difference, albeit small.
The proposed angle measurement technique cannot be carried out using ultrasound equipment with a 2D display, limiting its applicability. However, specialized 3D software is available to perform angle measurements on ultrasound machines or desktop computers. Also, a user friendly and cheap 3D‐VR desktop system has been developed for routine use of diagnostic 3D‐VR ultrasound on a separate nonexpensive desktop computer in an outpatient clinic, allowing precise length, volume and angle measurements.28
A limitation is that only data were available until 13‐week GA. The still decreasing lateral angles at the end of 12‐week GA suggest a continuing developing foot position, although Kawashima and Uhthoff did not observe this in their anatomical studies. Although a fetus with a congenital clubfoot has not been observed in our study, it seems reasonable to defer the prenatal diagnosis of this anomaly to at least a GA of more than 13 weeks.
Another limitation is the low success rate of the angle measurements, especially at 8‐ and 9‐week GA, possibly due to the relatively small fetal extremities at that moment. (Table 2). Although ultrasound generally is regarded as a safe instrument to use in early pregnancy, in order to keep the exposure to ultrasound as short as possible, in our study only already stored nontargeted 3D sweeps of the entire fetus were used. This may have hampered the quality of the volumes we acquired. Dedicated volume acquisition of the fetal feet might result in higher feasibility figures. Also artifacts like acoustic shadowing furthermore reduced the success rate. Also movement artifacts of the fetus could have impeded accurate visualization. All these aspects contributed to the suboptimal success rate limiting both generalization of our findings as well as clinical implementation. The extent to which these influences were decisive for the final result will appear in subsequent studies.
By using 3D VR, we developed a measurement tool for foot position assessment and made a first attempt to create a chart for the first trimester foot position. Future studies should aim at improving the success rate of measurements. The angle measurements would not have been possible without the use of this innovative technique. A transient clubfoot position is present during the normal development of the lower limbs, and it has been measured in vivo for the first time. Our data show that in early pregnancy a clubfoot may be physiological and diagnosis of a pathological clubfoot should not be made in the first trimester of pregnancy.
Disclosure
This research was solely funded by Erasmus MC, University Medical Centre, Rotterdam, the Netherlands. No external funding was obtained. All authors report no conflicts of interest.
Supporting information
Appendix S1. Supporting information
Figure S1 Bland and Altman plots showing the intraobserver variability of the four different angles. Upper limit (UL) and lower limit (LL), both of the 95% limits of agreement; mean and difference both in degrees
Figure S2 Bland and Altman plots showing the interobserver variability of the four different angles.
Upper limit (UL) and lower limit (LL), both of the 95% limits of agreement; mean and difference both in degrees
Figure S3 Embryonic development of the lower limb, derived from earlier publication (Victoria‐Diaz and Victoria‐Diaz, 1984), reprinted with permission from the publisher
Figure S4 Physiological clubfoot position at 10 weeks and 2‐day gestational age.
Video S1 Measurements on the fetal foot carried out in the I‐Space (provided as a separate file)
Acknowledgments
We would like to thank Mr Tom de Vries Lentsch for his help with processing Figures 2 and S4. This research was solely funded by Erasmus MC, University Medical Centre, Rotterdam, the Netherlands. No external funding was obtained.
References
- 1. Syngelaki A, Chelemen T, Dagklis T, Allan L, Nicolaides KH. Challenges in the diagnosis of fetal non‐chromosomal abnormalities at 11‐13 weeks. Prenat Diagn 2011; 31: 90–102. [DOI] [PubMed] [Google Scholar]
- 2. Chaoui R, Nicolaides KH. Detecting open spina bifida at the 11‐13‐week scan by assessing intracranial translucency and the posterior brain region: Mid‐sagittal or axial plane? Ultrasound Obstet Gynecol 2011; 38: 609–612. [DOI] [PubMed] [Google Scholar]
- 3. Steegers‐Theunissen RP, Steegers EA. Embryonic health: New insights, mHealth and personalised patient care. Reprod Fertil Dev 2015; 27: 712–715. [DOI] [PubMed] [Google Scholar]
- 4. Werler MM, Yazdy MM, Mitchell AA et al Descriptive epidemiology of idiopathic clubfoot. Am J Med Genet A 2013; 161A: 1569–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miedzybrodzka Z. Congenital talipes equinovarus (clubfoot): A disorder of the foot but not the hand. J Anat 2003; 202: 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Offerdal K, Jebens N, Blaas HG, Eik‐Nes SH. Prenatal ultrasound detection of talipes equinovarus in a non‐selected population of 49 314 deliveries in Norway. Ultrasound Obstet Gynecol 2007; 30: 838–844. [DOI] [PubMed] [Google Scholar]
- 7. Bakalis S, Sairam S, Homfray T, Harrington K, Nicolaides K, Thilaganathan B. Outcome of antenatally diagnosed talipes equinovarus in an unselected obstetric population. Ultrasound Obstet Gynecol 2002; 20: 226–229. [DOI] [PubMed] [Google Scholar]
- 8. Cohen‐Overbeek TE, Grijseels EW, Lammerink EA, Hop WC, Wladimiroff JW, Diepstraten AF. Congenital talipes equinovarus: Comparison of outcome between a prenatal diagnosis and a diagnosis after delivery. Prenat Diagn 2006; 26: 1248–1253. [DOI] [PubMed] [Google Scholar]
- 9. Sanzarello I, Nanni M, Faldini C. The clubfoot over the centuries. J Pediatr Orthop B 2017; 26: 143–151. [DOI] [PubMed] [Google Scholar]
- 10. Victoria‐Diaz A, Victoria‐Diaz J. Pathogenesis of idiopathic clubfoot. Clin Orthop Relat Res 1984; 185: 14–24. [PubMed] [Google Scholar]
- 11. Baken L, van Heesch PN, Wildschut HI et al First‐trimester crown‐rump length and embryonic volume of aneuploid fetuses measured in virtual reality. Ultrasound Obstet Gynecol 2013; 41: 521–525. [DOI] [PubMed] [Google Scholar]
- 12. Gijtenbeek M, Bogers H, Groenenberg IA et al First trimester size charts of embryonic brain structures. Hum Reprod 2014; 29: 201–207. [DOI] [PubMed] [Google Scholar]
- 13. Baken L, Benoit B, Koning AHJ, van der Spek PJ, Steegers EAP, Exalto N. First‐trimester crown‐rump length and embryonic volume of fetuses with structural congenital abnormalities measured in virtual reality: An observational study. Biomed Res Int 2017; 2017: 1953076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Steegers‐Theunissen RP, Verheijden‐Paulissen JJ, van Uitert EM et al Cohort profile: The Rotterdam periconceptional cohort (predict study). Int J Epidemiol 2016; 45: 374–381. [DOI] [PubMed] [Google Scholar]
- 15. Knez J, Day A, Jurkovic D. Ultrasound imaging in the management of bleeding and pain in early pregnancy. Best Pract Res Clin Obstet Gynaecol 2014; 28: 621–636. [DOI] [PubMed] [Google Scholar]
- 16. Rousian M, Exalto N, Koning A, Steegers EAP. Three‐dimensional virtual reality in early pregnancy In: Farquharson RG, Stephenson MD. (eds). Early Pregnancy, 2nd edn. Cambridge: Cambridge University Press, 2017; 208–219. [Google Scholar]
- 17. RCoreTeam . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2015. [Google Scholar]
- 18. Rigby RA, Stasinopoulos DM. Generalized additive models for location, scale and shape. J Roy Stat Soc C 2005; 54: 507–544. [Google Scholar]
- 19. Hastie TJ. Generalized additive models In: Chambers JM, Hastie TJ (eds). Statistical Models in S. Boca Raton, FL: Wadsworth & Brooks/Cole, 1992; 249–308. [Google Scholar]
- 20. Kawashima T, Uhthoff HK. Development of the foot in prenatal life in relation to idiopathic club foot. J Pediatr Orthop 1990; 10: 232–237. [PubMed] [Google Scholar]
- 21. Dietz FR. On the pathogenesis of clubfoot. Lancet 1985; 1: 388–390. [DOI] [PubMed] [Google Scholar]
- 22. Duce S, Madrigal L, Schmidt K et al Micro‐magnetic resonance imaging and embryological analysis of wild‐type and pma mutant mice with clubfoot. J Anat 2010; 216: 108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liao YM, Li SL, Luo GY et al Routine screening for fetal limb abnormalities in the first trimester. Prenat Diagn 2016; 36: 117–126. [DOI] [PubMed] [Google Scholar]
- 24. Lauson S, Alvarez C, Patel MS, Langlois S. Outcome of prenatally diagnosed isolated clubfoot. Ultrasound Obstet Gynecol 2010; 35: 708–714. [DOI] [PubMed] [Google Scholar]
- 25. Glotzbecker MP, Estroff JA, Spencer SA et al Prenatally diagnosed clubfeet: Comparing ultrasonographic severity with objective clinical outcomes. J Pediatr Orthop 2010; 30: 606–611. [DOI] [PubMed] [Google Scholar]
- 26. Grande M, Arigita M, Borobio V, Jimenez JM, Fernandez S, Borrell A. First‐trimester detection of structural abnormalities and the role of aneuploidy markers. Ultrasound Obstet Gynecol 2012; 39: 157–163. [DOI] [PubMed] [Google Scholar]
- 27. Parisi F, Rousian M, Koning AH et al Periconceptional maternal biomarkers of one‐carbon metabolism and embryonic growth trajectories: The Rotterdam Periconceptional cohort (predict study). Fertil Steril 2017; 107: 691–698. [DOI] [PubMed] [Google Scholar]
- 28. Baken L, van Gruting IM, Steegers EA, van der Spek PJ, Exalto N, Koning AH. Design and validation of a 3D virtual reality desktop system for sonographic length and volume measurements in early pregnancy evaluation. J Clin Ultrasound 2015; 43: 164–170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information
Figure S1 Bland and Altman plots showing the intraobserver variability of the four different angles. Upper limit (UL) and lower limit (LL), both of the 95% limits of agreement; mean and difference both in degrees
Figure S2 Bland and Altman plots showing the interobserver variability of the four different angles.
Upper limit (UL) and lower limit (LL), both of the 95% limits of agreement; mean and difference both in degrees
Figure S3 Embryonic development of the lower limb, derived from earlier publication (Victoria‐Diaz and Victoria‐Diaz, 1984), reprinted with permission from the publisher
Figure S4 Physiological clubfoot position at 10 weeks and 2‐day gestational age.
Video S1 Measurements on the fetal foot carried out in the I‐Space (provided as a separate file)


