Abstract
Aims
To assess the cardiovascular (CV) safety of oral semaglutide, the first tablet formulation of a glucagon‐like peptide‐1 receptor agonist.
Materials and methods
PIONEER 6 is a multinational, randomized, placebo‐controlled, double‐blind trial in patients with type 2 diabetes at high risk of CV events (defined as being aged ≥50 years and having established CV disease [CVD] or moderate [stage 3] chronic kidney disease [CKD], or being aged ≥60 years with ≥1 other CV risk factor). Patients were randomized to once‐daily oral semaglutide (up to 14 mg) or placebo added to standard of care. The primary composite endpoint is time to first occurrence of CV death or non‐fatal myocardial infarction or non‐fatal stroke. The primary hypothesis was to exclude an excess in CV risk with oral semaglutide by assessing non‐inferiority versus placebo for the primary endpoint (non‐inferiority margin of 1.8 for the upper boundary of the 95% confidence interval of the hazard ratio). PIONEER 6 is event‐driven, with follow‐up continuing until accrual of at least 122 primary outcome events. There is no pre‐defined minimal duration.
Results
Overall, 3183 patients have been enrolled (mean age 66.1 years, 31.6% females) in 214 sites across 21 countries. At baseline, the mean duration of diabetes was 14.9 years, mean glycated haemoglobin concentration was 66 mmol/mol (8.2%), and 84.6% of patients had established CVD/moderate CKD.
Conclusions
PIONEER 6 will provide evidence regarding the CV safety of oral semaglutide in patients with type 2 diabetes and high CV risk.
Keywords: cardiovascular disease, cardiovascular outcomes trial, GLP‐1 receptor agonist, oral semaglutide, type 2 diabetes
1. INTRODUCTION
Glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs) are glucose‐lowering agents recommended for the treatment of type 2 diabetes that have been shown to lower glycated haemoglobin (HbA1c) levels and body weight effectively, and are associated with a low risk of hypoglycaemia and improved systolic blood pressure.1, 2, 3, 4 Despite these benefits, GLP‐1RAs appear to be used less frequently than other new glucose‐lowering agents, such as dipeptidyl peptidase‐4 inhibitors.5
An oral GLP‐1RA formulation may improve acceptance and adherence6 for some patients compared with injectable GLP‐1RAs and may lead to earlier initiation of GLP‐1RA treatment in type 2 diabetes. However, successful delivery of peptides, such as GLP‐1RAs, through the oral route is challenging, because of numerous barriers to absorption, including degradation by gastrointestinal enzymes, pH‐induced conformational changes and limited protein permeability of the intestinal membrane.7, 8
Semaglutide, a glucagon‐like peptide‐1 (GLP‐1) analogue shown to improve glycaemic control and reduce body weight when administered subcutaneously (s.c.) in patients with type 2 diabetes9, 10, 11 and recently approved for use in Europe,12 Canada,13 Japan14 and the United States,15 is being developed as a once‐daily oral tablet formulation. Oral semaglutide is co‐formulated with sodium N‐(8‐[2‐hydroxybenzoyl] amino) caprylate (SNAC), an absorption enhancer that promotes the absorption of semaglutide across the gastric mucosa via effects on transcellular pathways.16
An oral formulation of semaglutide with similar efficacy and safety to the s.c. formulation has the potential to increase the access of GLP‐1 therapies to patients with a preference for oral administration. In a 26‐week, phase II trial conducted in 632 patients with type 2 diabetes and sub‐optimal glycaemic control, oral semaglutide (doses ranging from 2.5 to 40 mg once daily) reduced HbA1c levels compared with placebo in a dose‐dependent manner (−0.7% to −1.9% compared with −0.3% with placebo).17 In the same trial, oral semaglutide also demonstrated dose‐dependent weight reduction (−2.1 to −6.9 kg vs. −1.2 kg with placebo).17 The phase II study also compared oral semaglutide with s.c. semaglutide 1 mg, with similar reductions in HbA1c and comparable tolerability observed with higher doses of oral semaglutide versus s.c. semaglutide.17 Based on a combined evaluation of the efficacy and tolerability data in the phase II trial, three doses of oral semaglutide (3, 7 and 14 mg once daily) were selected for evaluation in the PIONEER (Peptide InnOvatioN for Early DiabEtes TReatment) phase IIIa programme, which is ongoing to further investigate the efficacy and safety of oral semaglutide in a large and broad population of patients with type 2 diabetes.
Cardiovascular disease (CVD) is the leading cause of death in patients with type 2 diabetes.18 Guidelines have been issued by regulatory authorities to the pharmaceutical industry regarding the evaluation of cardiovascular (CV) safety to exclude an excess in CV risk with new therapies to treat type 2 diabetes.19, 20, 21 In previous CV outcomes trials, treatment with both s.c. liraglutide and s.c. semaglutide has shown a CVD risk reduction compared with placebo in patients with type 2 diabetes and high CV risk,22, 23 with some guidelines recommending liraglutide for second‐line use after metformin in these patients.1 As part of the PIONEER programme, and in accordance with regulatory guidance, PIONEER 6 is a pre‐approval CV outcomes trial that is being conducted to specifically explore CV safety of oral semaglutide compared with placebo. In the present paper, we describe the design of the PIONEER 6 trial and report the baseline characteristics of the trial population.
2. METHODS
2.1. Trial design and oversight
PIONEER 6 is an ongoing, randomized, double‐blind, placebo‐controlled, parallel‐group, international, multicentre CV outcomes trial to compare the CV safety of oral semaglutide with placebo. PIONEER 6 is being conducted in 214 sites across 21 countries in Africa, Asia, Europe, Latin America, North America and the Middle East. The trial is registered with ClinicalTrials.gov (NCT02692716) and is being conducted in compliance with International Conference on Harmonisation Good Clinical Practice guidelines,24 applicable regulatory requirements and in accordance with the Declaration of Helsinki.25
Approval by relevant local independent ethics committees and institutional review boards, and national regulatory authorities was mandated prior to the commencement of the trial at each site. All patients provided written informed consent prior to any trial‐related activity.
A panel of lead principal investigators and selected trial coordinators was nominated to provide advice on the conduct of the trial to the sponsor. An independent data monitoring committee provides an ongoing review of accumulating data and subsequent guidance on trial continuation.
2.2. Participants
Male and female patients diagnosed with type 2 diabetes were eligible for inclusion in the trial if they were considered at high risk of CV events, as defined by one the following two categories. (a) Presence of established CVD or moderate (stage 3) chronic kidney disease (CKD): age ≥50 years and established CVD (prior myocardial infarction [MI]; prior stroke or transient ischaemic attack; prior coronary, carotid or peripheral arterial revascularization; >50% stenosis on angiography or imaging of coronary, carotid or lower extremity arteries; documented history of symptomatic coronary heart disease; documented asymptomatic cardiac ischaemia or chronic heart failure (New York Heart Association [NYHA] class II–III); or moderate renal impairment (estimated glomerular filtration rate [eGFR] 30–59 mL/min/1.73 m2 using the Chronic Kidney Disease Epidemiology Collaboration equation26). (b) CV risk factor(s) only (ie,. no prior CVD or moderate CKD): age ≥60 years and the presence of at least one of the following CV risk factors: microalbuminuria or proteinuria (diagnosed according to local or commonly accepted guidelines); hypertension and left ventricular hypertrophy (documented by ECG or imaging); left ventricular systolic or diastolic dysfunction (by imaging); or ankle/brachial index <0.9. Eligibility criteria were evaluated by the investigator and clinical criteria were accordingly defined in line with local practice and/or guidelines.
Following receipt of informed consent, a screening visit was completed during which patient demographics, medical history, concomitant medications and concomitant illnesses were recorded, and a physical examination was performed. Key criteria for exclusion from the trial were: treatment with any GLP‐1RA, dipeptidyl peptidase‐4 inhibitor or pramlintide within 90 days prior to screening; NYHA class IV heart failure; planned coronary, carotid or peripheral artery revascularization; MI, stroke or hospitalization for unstable angina or transient ischaemic attack within 60 days prior to screening; history of major surgical procedures involving the stomach that may affect drug absorption; chronic/intermittent haemodialysis or peritoneal dialysis, or severe renal impairment (eGFR <30 mL/min/1.73 m2); history of diabetic ketoacidosis; and proliferative retinopathy or maculopathy requiring acute treatment. Proliferative retinopathy or maculopathy requiring acute treatment was verified by an eye examination (fundus photography or dilated fundoscopy) performed within 90 days before screening or during the screening period. Other exclusion criteria were typical for a clinical trial in this patient population and are detailed in the Supplementary Appendix.
2.3. Trial treatment and procedures
Eligible patients were randomized in a 1:1 ratio to once‐daily treatment with either oral semaglutide or placebo (Figure 1), with randomization stratified based on evidence of established CVD/moderate CKD at screening or CV risk factors only. Randomization could occur on the same day as the screening visit or up to 3 weeks afterwards. Randomization was performed using an interactive voice/web response system (IV/WRS) and blinding of trial staff is maintained by using the same IV/WRS for dispensing of trial drug and through the use of visually identical oral semaglutide and placebo tablets in identical packaging.
Figure 1.
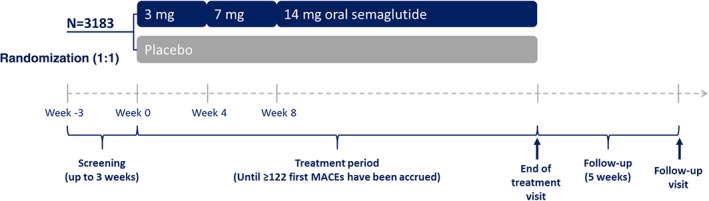
Trial design. MACE, major adverse cardiovascular event
The dose for the evaluation of the CV safety of oral semaglutide was 14 mg, which is the maximum dose chosen for study in the PIONEER programme. To mitigate the potential for adverse gastrointestinal events, patients followed a dose‐escalation regimen receiving 3 mg oral semaglutide/placebo once daily for 4 weeks, followed by 7 mg oral semaglutide/placebo once daily for 4 weeks, and then 14 mg oral semaglutide/placebo once daily for the remainder of the trial (Figure 1). Extensions of the dose‐escalation period, dose reductions (and subsequent re‐escalation) and treatment pauses (and subsequent re‐initiation) were permitted during the trial if, based on the investigator's opinion, unacceptable adverse events (AEs) occurred. Patients were instructed to take the tablet in the morning in a fasting state with up to half a glass of water (approximately 120 mL) and to wait at least 30 minutes before eating, drinking or taking other oral concomitant medication.
The study flow and visit schedule is presented in Figure 1 and Supplementary Table 1. Trial visits occurred at weeks 2, 4 and 8 following randomization, and approximately every 6 or 7 weeks thereafter (Figure 1). A variety of assessments and procedures are performed at different intervals (Supplementary Table 1), including vital signs, body weight, ECG, blood sampling (for evaluation of HbA1c, fasting plasma glucose, lipids, biochemistry, haematology and calcitonin levels), assessment of trial treatment compliance and collection of reports for serious AEs, AEs leading to discontinuation of trial treatment and other events of special interest. In addition to the eye examination performed immediately before or as part of the screening procedures, eye examinations are also being performed at around year 1 and at the end‐of‐treatment visit.
Investigators are responsible for the management of glycaemic control in each patient. Background therapy with glucose‐lowering agents to maintain/optimize glycaemic control according to standard of care in local or international guidelines is encouraged during the trial at the investigator's discretion; however, the use of other GLP‐1RAs, dipeptidyl peptidase‐4 inhibitors and pramlintide is not permitted. Glycaemic management is guided by review of fasting plasma glucose and HbA1c results from the trial site, and self‐measured blood glucose levels by the patient. Ongoing management of CVD, risk factors and potential microvascular complications is conducted by the investigators in accordance with local standards of care. Investigators received written guidance to encourage them in maintaining standard of care for the management of glycaemic control and CV risk factors, including achievement of HbA1c ≤53 mmol/mol (7%) (or individualized target, depending on the patient characteristics), blood pressure targets of 140/90 mmHg (angiotensin‐receptor blockers or angiotensin‐converting enzyme inhibitors are suggested as first line, but other antihypertensive agents may be considered based on individual patient needs; lower blood pressure targets may also be considered as appropriate for some individuals), and treatment with statins and aspirin as recommended.
2.4. Endpoints and assessments
The primary endpoint is time from randomization to first occurrence of a major adverse CV event (MACE) composite endpoint consisting of CV death, non‐fatal MI or non‐fatal stroke (the classic three‐point MACE). Secondary endpoints include: time from randomization to first occurrence of an expanded composite CV endpoint (encompassing the same events as the primary endpoint, plus unstable angina requiring hospitalization or hospitalization for heart failure); time from randomization to first occurrence of the individual components of the expanded composite CV endpoint; time to first occurrence of a composite of all‐cause death, non‐fatal MI or non‐fatal stroke; time to all‐cause death; time to permanent trial drug discontinuation due to AEs; number of serious AEs; and changes from baseline in a variety of laboratory and clinical assessments. CV events and other selected types of events are adjudicated by an independent blinded external event adjudication committee.
2.5. Trial duration and follow‐up
PIONEER 6 is an event‐driven trial and will continue until at least 122 adjudication‐confirmed first MACEs have occurred. There is no pre‐specified duration of exposure to trial medication and so it is likely that there will be fewer primary endpoint events than seen in the similar CV outcome trials for liraglutide and s.c. semaglutide.22, 23 Follow‐up visits will be held 5 weeks after the end‐of‐treatment visit to allow safety assessments after wash‐out of the trial drug. Patients who discontinue trial drug prematurely will continue to be followed in accordance with the planned visit schedule. No interim analyses are planned, other than those conducted by an independent data monitoring committee.
2.6. Statistical methods
The trial aims to confirm that treatment with oral semaglutide does not result in an unacceptable increase in CV risk compared with placebo (upper limit of the two‐sided 95% confidence interval [CI] does not exceed 1.8) in patients with type 2 diabetes and at high CV risk. This is in line with the US Food and Drug Administration (FDA) guidance for approval of new glucose‐lowering agents in type 2 diabetes.21 Analysis of the primary endpoint (time from randomization to first MACE) will include all of the first MACEs occurring during the trial from randomization to the patient's last visit (at follow‐up approximately 5 weeks after the last planned dose of the trial product), regardless of adherence to trial treatment and procedures or adjustment of background medication. A stratified Cox proportional hazards model will be used for the primary analysis, with treatment group as a fixed factor, and stratification based on evidence of CVD or advanced CKD at screening. Estimated hazard ratio and the two‐sided 95% CI will be determined, with non‐inferiority confirmed in accordance with FDA guidance, as outlined earlier.21 If non‐inferiority is confirmed, a test for superiority versus placebo will be performed, thereby preserving the type 1 error in the strong sense at 5% (two‐sided) when using the hierarchical testing strategy. Sensitivity analyses will explore the robustness of the primary analysis. All primary and secondary analyses will be performed on the full analysis set, which will include all randomized patients. According to the study protocol, no interim analyses are planned before the database is locked.
2.7. Sample size
A log‐rank test determined that to confirm non‐inferiority for the primary endpoint with 90% power, a total of 122 first MACEs are required. Based on the anticipated event rate and chosen sample size, it is estimated that the treatment period for each patient will be between 12 and 19 months, depending on recruitment time and accrual rate of first MACEs. For a total of 122 first MACEs to occur within a trial duration of approximately 19 months (including an expected 7‐month recruitment period), we calculated that 3176 patients would require randomization. The sample size calculation was guided by assumptions derived from data from the LEADER and SUSTAIN 6 CV outcomes trials,22, 23 including an anticipated first MACE rate of 3 per 100 patient years across both treatment groups and a loss‐to‐follow‐up rate of 1% per year. A maximum of 650 patients (approximately 20%) was permitted within the CV risk factors only stratum to ensure sufficient CV risk within the trial population to accrue the sufficient number of events.
3. RESULTS
The first patient was randomized on 17th January 2017 and recruitment was completed on 29th August 2017, representing a recruitment period of just over 7 months. In total, 3418 patients underwent screening, with 3183 randomized to treatment. The majority of patients (84.6%) were aged ≥50 years and had established CVD or moderate CKD, while the remainder (15.4%) were aged ≥60 years with the presence of CV risk factors only.
The baseline characteristics of the enrolled patients were compiled in a blinded manner after completion of enrolment and before data cleaning. One third of the randomized patients were female (31.6%) and the mean age at baseline was 66.1 years (Table 1). The mean body mass index was 32.3 kg/m2. The majority of participants were white (72.3%). The average duration of diabetes at baseline was 14.9 years, and the most frequently used glucose‐lowering agents were biguanides (metformin; 76.6% of patients), insulin (61.0%), sulphonylureas (31.6%) and sodium‐glucose co‐transporter‐2 (SGLT2) inhibitors (9.5%; Table 1). The mean HbA1c level was 66 mmol/mol (8.2%). The mean eGFR was 74.2 mL/min/1.73 m2 and just over one quarter of patients (26.2%) had an eGFR <60 mL/min/1.73 m2. Approximately one third of patients were reported by the investigators as having diabetic nephropathy (33.5%), with diabetic retinopathy and neuropathy also reported to be present in 27.2% and 36.0% of patients, respectively.
Table 1.
Demographics and baseline characteristics
| Total randomized population (N = 3,183) | |
|---|---|
| Female, n (%) | 1007 (31.6) |
| Age, ys | 66.1 (7.1) |
| ≥65 ys, n (%) | 1848 (58.1) |
| ≥75 ys, n (%) | 410 (12.9) |
| Race, n (%) | |
| American Indian or Alaska Native | 29 (0.9) |
| Asian | 630 (19.8) |
| Black or African American | 192 (6.0) |
| Native Hawaiian or other Pacific Islander | 6 (0.2) |
| Other | 26 (0.8) |
| White | 2300 (72.3) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 514 (16.1) |
| Body weight, kg | 90.9 (21.2) |
| Body mass index, kg/m2 | 32.3 (6.5) |
| HbA1c, mmol/mol | 66 (18) |
| HbA1c, % | 8.2 (1.6) |
| Fasting plasma glucose, mmol/L | 8.7 (3.3) |
| Diabetes duration, ys | 14.9 (8.5) |
| Glucose‐lowering medication, n (%) | |
| Insulin | 1943 (61.0) |
| Biguanides | 2437 (76.6) |
| SGLT2 inhibitors | 301 (9.5) |
| Sulphonylureas | 1007 (31.6) |
| Other | 264 (8.3) |
| CVD status, n (%) | |
| Established CVD | 2692 (84.6) |
| CV risk factors only | 491 (15.4) |
| Antihypertensive medication, n (%) | 3034 (95.3) |
| Lipid‐lowering medication, n (%) | 2750 (86.4) |
| Antithrombotic medication, n (%) | 2516 (79.0) |
| Smoking history, n (%) | |
| Current | 348 (10.9) |
| Previous | 1399 (44.0) |
| Never | 1436 (45.1) |
| Investigator‐reported microvascular complications, n (%) | |
| Diabetic retinopathy | 866 (27.2) |
| Diabetic nephropathy | 1066 (33.5) |
| Diabetic neuropathy | 1145 (36.0) |
| eGFR,a mL/min/1.73 m2, Mean (SD) | 74.2 (21.0) |
| <30 mL/min/1.73 m2, n (%) | 29 (0.9) |
| ≥30 to <60 mL/min/1.73 m2, n (%) | 827 (26.1) |
| ≥60 to <90 mL/min/1.73 m2, n (%) | 1389 (43.9) |
| ≥90 mL/min/1.73 m2, n (%) | 918 (29.0) |
| Vital signs | |
| Systolic blood pressure, mmHg | 135.6 (17.6) |
| Diastolic blood pressure, mmHg | 76.0 (10.1) |
| Cholesterol | |
| LDL cholesterol, mmol/L | 2.2 (0.9) |
| HDL cholesterol, mmol/L | 1.1 (0.3) |
| Triglycerides, mmol/L | 2.0 (1.5) |
Abbreviations: CV, cardiovascular; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; SGLT2, sodium‐glucose co‐transporter‐2.
Data are mean (SD) unless otherwise indicated.
eGFR assessed using Chronic Kidney Disease Epidemiology Collaboration formula in serum at baseline.
The mean baseline blood pressure was 136/76 mm Hg, and the mean fasting lipid levels were as follows: low‐density lipoprotein (LDL) cholesterol, 2.2 mmol/L; high‐density lipoprotein (HDL) cholesterol, 1.1 mmol/L; and triglycerides, 2.0 mmol/L. The majority of patients were treated with antihypertensive drugs (95.3%), lipid‐lowering therapies (86.4%), and antithrombotic (antiplatelet and/or anticoagulant) medication (79.0%).
4. DISCUSSION
Four CV outcomes trials of GLP‐1RAs have been published as of June 2018: ELIXA (once‐daily s.c. lixisenatide),27 LEADER (once‐daily s.c. liraglutide),22 SUSTAIN 6 (once‐weekly s.c. semaglutide)23 and EXSCEL (once‐weekly extended‐release s.c. exenatide28; Table 2). In all four trials, CV safety was demonstrated for the GLP‐1RA versus placebo.22, 23, 27, 28 Moreover, s.c. liraglutide and s.c. semaglutide showed significant reductions in the classic three‐point MACE endpoint versus placebo (hazard ratios 0.87 [95% CI 0.78, 0.97] and 0.74 [95% CI 0.58, 0.95], respectively)22, 23 and a recent meta‐analysis has reported a significant 10% relative risk reduction in the classic three‐point MACE endpoint with GLP‐1RAs versus placebo.36
Table 2.
Summary of completed and ongoing cardiovascular outcomes trials of glucagon‐like peptide‐1 receptor agonists in type 2 diabetes
| Completed CV outcomes trials with GLP‐1RAs | Unpublished/ongoing CV outcomes trials with GLP‐1RAs | |||||||
|---|---|---|---|---|---|---|---|---|
| SUSTAIN 623 | LEADER22, 29 | ELIXA27, 30 | EXSCEL28, 31 | PIONEER 6 | FREEDOM CVO32 | REWIND33, 34 | HARMONY OUTCOMES35 | |
| Number of randomized patients | 3297 | 9340 | 6068 | 14 752 | 3183 | 4156 | 9901 | 9575 |
| Clinicaltrials.gov identifier | NCT01720446 | NCT01179048 | NCT01147250 | NCT01144338 | NCT02692716 | NCT01455896 | NCT01394952 | NCT02465515 |
| Interventions | Semaglutide s.c. once weekly vs. placebo | Liraglutide s.c. once daily vs. placebo | Lixisenatide s.c. once daily vs. placebo | Exenatide s.c. once weekly vs. placebo | Oral semaglutide once daily vs. placebo | Exenatide continuous delivery (ITCA 650) vs. placebo | Dulaglutide s.c. once weekly vs. placebo | Albiglutide s.c. once weekly vs. placebo |
| HbA1c eligibility criterion | ≥7.0% | ≥7.0% | 5.5–11.0% | 6.5–10.0% | – | >6.5% | ≤9.5% | >7.0% |
| Main CV risk‐related inclusion criteria | ≥50 ys with established CVD or CKD Stage ≥3 or ≥60 ys with ≥1 CV risk factor | ≥50 ys with established CVD or CKD Stage ≥3 or ≥60 ys with ≥1 CV risk factor | Acute coronary event ≤180 d | With or without previous CV events | ≥50 ys with established CVD or CKD Stage 3 or ≥60 ys with ≥1 CV risk factor | ≥40 ys with established CVD | 50–54 ys with previous CVD or 55–59 ys with previous CVD or evidence of other vascular or renal disease or ≥60 ys plus as above or ≥2 CV risk factors | ≥40 ys with established CVD |
| Trial phase | III (Pre‐approval) | III/IV(Post‐approval) | III (Pre‐approval) | III/IV (Post‐approval) | III (Pre‐approval) | III (Pre‐approval) | III/IV (Post‐approval) | IV (Post‐approval; albiglutide now in discontinuation process) |
| Median follow‐up/planned | 2.1 ys | 3.8 ys | 2.1 ys | 3.2 ys | Treatment period of 12–19 mo | 2 ys | 7–8 ys | Max. 3–5 ys |
| Events planned/observed, n | ≥122/254 | ≥611/1302 | 844/805 | 1360/1744 | ≥122/NA | NA | ≥1200/NA | NA |
| Key baseline characteristics | ||||||||
| Agea, ys | 64.6 | 64.2–64.4 | 60.3 | 63b | 66.1 | NA | 66.2 | NA |
| Female, % | 39.3 | 35.5–36.0 | 30.7 | 38 | 31.6 | NA | 46.3 | NA |
| HbA1ca, % | 8.7 | 8.7 | 7.7 | 8.0b | 8.2 | NA | 7.3 | NA |
| Diabetes durationa, ys | 13.9 | 12.8 | 9.3 | 12.0b | 14.9 | NA | 10.0 | NA |
| Established CVDc, % | 83.0 | 81.3 | 100 | 73.1 | 84.6 | 100 | 31.4 | NA |
| Primary outcome, HR (95%CI), P value | 3‐point MACE 0.74 (0.58, 0.95) P < 0.001 for non‐inferiority P = 0.02 for superiority but not pre‐specified | 3‐point MACE 0.87 (0.78, 0.97) P < 0.001 for non‐inferiority P = 0.01 for superiority | 4‐point MACE 1.02 (0.89, 1.17) P < 0.001 for non‐inferiority P = 0.81 for superiority | 3‐point MACE 0.91 (0.83, 1.00) P < 0.001 for non‐inferiority P = 0.06 for superiority | 3‐point MACE Assessing for non‐inferiority | 4‐point MACE | 3‐point MACE | 3‐point MACE |
| Completion/estimated date of completion | March 2016 | December 2015 | February 2015 | May 2017 | October 2018 | March 2016 (currently unpublished) | July 2018 | March 2018 |
Abbreviations: CI, confidence interval; CKD, chronic kidney disease; CV, cardiovascular; CVD, cardiovascular disease; GLP‐1RA, glucagon‐like peptide 1‐receptor agonist; HR, hazard ratio; MACE, major adverse cardiovascular event; NA, not available; s.c., subcutaneous.
Mean unless indicated otherwise.
Median.
Established CVD includes history of CKD in SUSTAIN 6, LEADER and PIONEER 6 studies.
The mechanism whereby GLP‐1RAs may reduce CV risk is not fully elucidated. Preclinical studies suggest various potential mechanisms for the CV benefit of these agents.37 in vitro studies suggest these agents may have effects on adhesion molecules and endothelial function that may inhibit atherosclerosis and thrombosis.38 A randomized trial in patients with newly diagnosed and treatment‐naïve type 2 diabetes found that six months of liraglutide treatment improved arterial stiffness and left ventricular myocardial strain, and reduced N‐terminal pro b‐type natriuretic peptide independently of weight loss by reducing oxidative stress.39 A meta‐analysis in patients with type 2 diabetes has also indicated that GLP‐1‐based therapy is associated with an increase in flow‐mediated dilation, reductions in atherosclerotic markers such as C‐reactive protein, plasminogen activator inhibitor‐1 and B‐type natriuretic peptide, as well as reductions in LDL cholesterol and triglycerides.40
The PIONEER 6 study will add to this evidence base for the CV safety of GLP‐1RAs and uses the same primary endpoint as SUSTAIN‐6. To rule out an unacceptable increase in CV risk caused by oral semaglutide, and in line with FDA guidance,21 PIONEER 6 enrolled a population of patients with type 2 diabetes at high risk of CV events. The eligibility criteria are similar to those of the CV outcomes trial with once‐weekly s.c. semaglutide (SUSTAIN 6),23 the same molecular entity in a different formulation, including patients with established CVD/moderate CKD, and also some with well‐established CV risk factors and advanced age. Such a population at elevated risk of CV events was selected to ensure accruing of events so that the primary objective of the trial could be reached within a reasonable timeframe. Similarly to SUSTAIN 6, the majority (>80%) of patients recruited in PIONEER 6 had prior CVD/moderate CKD, with less than one sixth of participants having only CV risk factors (Table 2). Moreover, the presence of CV risk factors and use of CV medications was similar in PIONEER 6 compared with SUSTAIN 6 (Table S2). Compared with SUSTAIN 6, PIONEER 6 included a greater proportion of patients receiving SGLT‐2 inhibitors (0.2% vs. 9.5%),23 reflecting the increasing use of this drug class. In contrast to SUSTAIN 6,23 there was no lower threshold for HbA1c in PIONEER 6, which could explain why baseline HbA1c was lower (66 mmol/mol [8.2%]) than in the LEADER and SUSTAIN 6 studies (72 mmol/mol [8.7%] for both).22, 23
The populations of PIONEER 6 and SUSTAIN 623 are very similar to that used in LEADER,22 but differ from that studied in the ELIXA CV outcomes trial with once‐daily s.c. lixisenatide, which included only patients with type 2 diabetes and acute coronary syndrome within the previous 180 days (Table 2).27 The EXSCEL CV outcomes trial with once‐weekly exenatide included a patient population with a slightly wider range of CV risk, with ~73% of the population having prior CVD.28, 31 As opposed to the other GLP‐1RA trials, the ongoing REWIND CV outcomes trial with once‐weekly dulaglutide includes a markedly lower proportion of patients with established CVD (31% in REWIND vs. 85% in PIONEER 6), a higher proportion of females (46.3% in REWIND vs. 31.6% in PIONEER 6), a lower mean HbA1c (56 mmol/mol [7.3%] in REWIND vs. 66 mmol/mol [8.2%] in PIONEER 6) and a longer trial duration (7–8 years).33 The differences in patient populations and trial design from various GLP‐1RA CV outcomes trials indicate that care should be taken when comparing outcomes between trials or generalizing data to a broader diabetes population.
Time from randomization to the three‐point MACE composite endpoint (CV death, non‐fatal MI or non‐fatal stroke) was selected as the primary endpoint of PIONEER 6, an approach that captures the three CV events mandated by the FDA for such trials21 and is consistent with most recent CV outcomes trials with GLP‐1RAs (Table 2).19 Although PIONEER 6 has recruited a similar high CV risk patient population to those included in other recent CV outcomes trials in diabetes and includes a similar primary endpoint, differences in trial design will influence interpretation of the final data. PIONEER 6 is powered as a non‐inferiority trial to exclude an unacceptable increase in CV risk compared with placebo, as set by the FDA (upper limit of the 95% CI below 1.8). PIONEER 6 is solely event‐driven (close down of the trial will be initiated once the estimated number of first MACEs [n = 122] needed to rule out an excess risk have been accrued) and does not include a minimum trial duration. PIONEER 6 will therefore accrue substantially fewer CV events and have a shorter treatment duration than other diabetes treatment CV outcomes trials, such as SUSTAIN 6 and LEADER, which had a minimum trial duration after the last patient was randomized (the median observation time in SUSTAIN 6 and LEADER was 2.1 and 3.8 years respectively [Table 2]).19, 22, 23 In both the LEADER and SUSTAIN 6 trials, more endpoint events were accrued (approximately double) than the number needed to achieve statistical power,22, 23 as a result of the minimum duration and higher than expected event rates. The accrual of more events in SUSTAIN 6 allowed that trial to identify a significantly lower risk of the primary outcome (albeit not pre‐specified before trial initiation) of three‐point MACE among patients treated with s.c. semaglutide compared with placebo (P = 0.02 for superiority of s.c. semaglutide).23 It has been suggested that trial duration is a factor in being able to demonstrate CV risk reductions in diabetes CV outcomes trials.39 Nevertheless, a relatively short trial duration was used for PIONEER 6 to allow the CV safety of oral semaglutide to be determined early in the development programme and because the CV safety of semaglutide as a s.c. formulation had already been demonstrated in SUSTAIN 6; however, due to the short duration and expected accrual of fewer events than SUSTAIN 6, a statistically significant CV risk reduction with oral semaglutide versus placebo may be less likely.
5. CONCLUSION
In summary, PIONEER 6 is a pre‐approval non‐inferiority trial that will investigate the CV safety of oral semaglutide compared with placebo and will, therefore, provide an important insight for regulatory approval. The trial has completed enrolment of 3183 patients with type 2 diabetes who are at high CV risk and will conclude once a minimum of 122 adjudication‐confirmed first MACEs needed to determine CV safety are accrued.
CONFLICT OF INTEREST
S.C.B. has received honoraria, teaching and research sponsorship/grants from Abbott, AstraZeneca, Boehringer Ingelheim, BMS, Cellnovo, Diartis, Eli Lilly, GlaxoSmithKline, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, Roche, Sanofi‐Aventis, Schering‐Plough, Servier and Takeda, has received funding for educational programmes from Cardiff University, Doctors.net, Elsevier, Onmedica, Omnia‐Med and Medscape, and has an ownership interest in Gycosmedia (a diabetes on‐line news service). O.M. has received honoraria for advisory boards for Novo Nordisk, Eli Lilly, Sanofi, Merck Sharp & Dohme, Boehringer Ingelheim, Jansen and Jansen, Novartis and AstraZeneca, is on Speaker's Bureau for AstraZeneca, Bristol‐Myers Squibb, Novo Nordisk, Eli Lilly, Sanofi, Novartis, Merck Sharp & Dohme, Boehringer Ingelheim, has grants paid to her institution as study physician by AstraZeneca and Bristol‐Myers Squibb, and has received research grant support through Hadassah Hebrew University Hospital from Novo Nordisk. R.A. has received honoraria for advisory boards for Novo Nordisk, Eli Lilly, Merck Sharp & Dohme, Sanofi, speaking fees from AstraZeneca, Novo Nordisk, Eli Lilly, Merck Sharpe & Dohme, Sanofi, and research support from AstraZeneca, Novo Nordisk, Eli Lilly, Merck Sharpe & Dohme, Sanofi. Ab.C. has served as an advisory panel/board member for Novo Nordisk, Sanofi, Eli Lilly, Merck Sharp & Dohme and AstraZeneca, has received research support from Novo Nordisk, AstraZeneca, Novartis, MSD and Boehringer Ingelheim, and has served on the Speaker's Bureau for Novo Nordisk, Sanofi, Eli Lilly, AstraZeneca and Merck Sharp & Dohme. Ag.C. has received lecture fees and fees for serving on advisory boards from Novo Nordisk, Eli Lilly, AstraZeneca, Sanofi Aventis, Merck Sharp & Dohme and Takeda, and grant support to his institution from AstraZeneca, Eli Lilly and Novo Nordisk. K.D. has received consulting fees from GlaxoSmithKline and Sanofi, research support from AstraZeneca, GlaxoSmithKline, Novo Nordisk, and Sanofi, and participated in CME‐sponsored events funded by Novo Nordisk, Sanofi, Merck, Eli Lilly and AstraZeneca. M.C.F. has received consultancy fees from advisory boards for Novo Nordisk, Sanofi, AstraZeneca, Boehringer Ingelheim, Servier and Pfizer, and honoraria for speaking from Novo Nordisk, Sanofi, AstraZeneca, Boehringer Ingelheim and Servier. M.E.F. has received research support from Amgen and Novo Nordisk. D.R.F. has received honoraria from advisory boards for Sanofi, Eli Lilly, Abbott and Pfizer, and speaking engagements for Sanofi, Novo Nordisk, Eli Lilly, Merk Sharp & Dohme and Medtronic, and has received research grants from Novo Nordisk, AstraZeneca, Bristol‐Myers Squibb, Eli Lilly, Sanofi, Novartis, Merck Sharp & Dohme, Boehringer Ingelheim, Amgen and OPKO. C.G. has participated in scientific advisory boards and received consulting/speaking fees from AstraZeneca, Bayer AG, Boehringer Ingelheim, Eli Lilly, Merck KGaA, Merck Sharp & Dohme, Novo Nordisk, and Sanofi. J.G. has received research funding from Merck Sharp & Dohme, Sanofi Aventis, Eli Lilly, and Novo Nordisk. P.J. has received consultancy fees from advisory boards for Novo Nordisk, Sanofi, AstraZeneca, Boehringer Ingelheim, Servier, Pfizer, Bayer, Eli Lilly, GlaxoSmithKline and Novartis, and honoraria for speaking from Novo Nordisk, Sanofi, Pfizer, Roche, Boehringer Ingelheim and Servier. R.M. has received speaker fees and advisory board honoraria from Novo Nordisk. J.F.M.‐T. has participated in advisory panels for Merck, AstraZeneca and Novo Nordisk, has received research support from Merck, Novo Nordisk, Abbott, Nutricia and Vegenat, and is on the speakers' bureau for Merck, Novo Nordisk, Sanofi, AstraZeneca and Menarini. M.A.N. is a member of advisory boards or has consulted with AstraZeneca, Boehringer Ingelheim, Eli Lilly & Co., Fractyl, GlaxoSmithKline, Intarcia, Menarini/Berlin Chemie, Merck, Sharp & Dohme and Novo Nordisk, has served on the speakers' bureau of AstraZeneca, Boehringer Ingelheim, Eli Lilly & Co., GlaxoSmithKline, Menarini/Berlin Chemie, Merck, Sharp & Dohme, and Novo Nordisk A/S, and his institution has received grant support from AstraZeneca, Boehringer Ingelheim, Eli Lilly & Co., GlaxoSmithKline, Intarcia, Menarini/Berlin‐Chemie, Merck, Sharp & Dohme, Novartis and Novo Nordisk A/S. S.D.P has received consulting fees and honoraria for speaking from Novo Nordisk, AstraZeneca, Boehringer Ingelheim, Merck, Sanofi, Abbott, Eli Lilly, Valeant and Janssen. R.J.S. has received consultancy fees from Novo Nordisk and is on the Speaker's Bureau for AstraZeneca, Eli Lilly and Novo Nordisk. W.H.‐H.S. has received advisor and/or speaker fees from AstraZeneca, Bayer HealthCare, Boehringer Ingelheim Pharmaceuticals, Daiichi‐Sankyo, Eli Lilly and Company, Merck Sharp & Dohme, Mitsubishi Tanabe Pharma Corporation, Novartis, Novo Nordisk, Pfizer, Sanofi‐Aventis and Takeda Pharmaceutical Company. C.J.T. (or the institute with which he is associated) has received research grants, served as a consultant for, or gave lectures organised by Merck, AstraZeneca and Novo Nordisk. M.H. has received investigator‐initiated research grants on projects related to GLP‐1 biology from AstraZeneca, Merck, Novo Nordisk, has received consulting fees or honoraria from AstraZeneca, Boehringer‐Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk and Roche, and is co‐inventor of two provisional patents related to GLP‐1. M.T., M.S. and O.K.J. are employees of Novo Nordisk A/S. P.B., C.D. and N.T. have no competing interests.
Author contributions
S.C.B., O.M., R.A., P.B., Ab.C., Ag.C., C.D., K.D., M.C.F., M.E.F., D.R.F., J.G., C.G., P.J., J.F.M.‐T., M.A.N., S.D.P., W.H.‐H.S., R.S., C.J.T., N.T. and M.H. were principal study investigators (S.C.B. and M.H. were signatory investigators) involved in the conduct of the trial, interpreting the data and in writing, critically reviewing and approving the final manuscript. O.K.J. was the trial statistician and was involved in analysing the data and in writing, critically reviewing and approving the final manuscript. M.T. was involved in the design and conduct of the trial, interpreting the data, and in writing, critically reviewing and approving the final manuscript. M.S. was involved in the coordination and conduct of the trial, interpreting the data and in writing, critically reviewing and approving the final manuscript.
Supporting information
Appendix S1. Supplementary material
Table S1. Flow chart of assessments by patient visit.
Table S2. Demographics and baseline characteristics for the PIONEER 6 and SUSTAIN 6 trials.
ACKNOWLEDGMENTS
This trial was funded by Novo Nordisk A/S. Medical writing and editorial support provided by Graham Allcock and Emma Marshman, Spirit Medical Communications Ltd, was funded by Novo Nordisk A/S. The authors would like to thank the patients participating in this trial, and the investigators, coordinators and trial site staff. The authors would also like to thank Morten Donsmark of Novo Nordisk for critically reviewing the manuscript.
Bain SC, Mosenzon O, Arechavaleta R, et al. Cardiovascular safety of oral semaglutide in patients with type 2 diabetes: Rationale, design and patient baseline characteristics for the PIONEER 6 trial. Diabetes Obes Metab. 2019;21:499–508. 10.1111/dom.13553
Funding information This trial was funded by Novo Nordisk A/S. Medical writing and editorial support provided by Graham Allcock and Emma Marshman, Spirit Medical Communications Ltd, was funded by Novo Nordisk A/S.
REFERENCES
- 1. American Diabetes Association . 8. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes‐2018. Diabetes Care. 2018;41(suppl 1):S73‐S85. [DOI] [PubMed] [Google Scholar]
- 2. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient‐centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58(3):429‐442. [DOI] [PubMed] [Google Scholar]
- 3. Goud A, Zhong J, Peters M, Brook RD, Rajagopalan S. GLP‐1 agonists and blood pressure: a review of the evidence. Current Hypertens Rep. 2016;18(2):16. [DOI] [PubMed] [Google Scholar]
- 4. Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon‐like peptide‐1 receptor agonists and dipeptidyl peptidase‐4 inhibitors. Diabetes Obes Metab. 2016;18(3):203‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fu H, Curtis BH, Schuster DP, Festa A, Kendall DM. Treatment patterns among older patients with type 2 diabetes in the United States: a retrospective cohort study. Diabetes Technol Ther. 2014;16(12):833‐839. [DOI] [PubMed] [Google Scholar]
- 6. Cooke CE, Lee HY, Tong YP, Haines ST. Persistence with injectable antidiabetic agents in members with type 2 diabetes in a commercial managed care organization. Curr Med Res Opin. 2010;26(1):231‐238. [DOI] [PubMed] [Google Scholar]
- 7. Araujo F, Fonte P, Santos HA, Sarmento B. Oral delivery of glucagon‐like peptide‐1 and analogs: alternatives for diabetes control? J Diabetes Sci Technol. 2012;6(6):1486‐1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ma S, We L, Yang H, Deng S, Jevnikar AM. Emerging technologies to achieve oral delivery of GLP‐1 and GLP‐1 analogs for treatment of type 2 diabetes mellitus (T2DM). Can J Biotechnol. 2017;1(1):1‐10. [Google Scholar]
- 9. Ahren B, Masmiquel L, Kumar H, et al. Efficacy and safety of once‐weekly semaglutide versus once‐daily sitagliptin as an add‐on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56‐week, double‐blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5(5):341‐354. [DOI] [PubMed] [Google Scholar]
- 10. Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once‐weekly semaglutide versus once‐daily insulin glargine as add‐on to metformin (with or without sulfonylureas) in insulin‐naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open‐label, parallel‐group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(5):355‐366. [DOI] [PubMed] [Google Scholar]
- 11. Sorli C, Harashima SI, Tsoukas GM, et al. Efficacy and safety of once‐weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double‐blind, randomised, placebo‐controlled, parallel‐group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(4):251‐260. [DOI] [PubMed] [Google Scholar]
- 12. European Medicines Agency . Ozempic: EPAR – public assessment report. 2018. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_report/human/004174/WC500244165.pdf. Accessed March 15, 2018.
- 13. Health Canada . Regulatory decision summary – Ozempic – Health Canada. 2018. https://hpr‐rps.hres.ca/reg‐content/regulatory‐decision‐summary‐detail.php?linkID=RDS00317. Accessed March 19, 2018.
- 14. Pharmaceuticals and Medical Devices Agency . Iryō‐yō iyakuhin shōsai hyōji Ozenpikku hika chū 2 mg [Detailed display of medical drugs, Ozenic table subcutaneous injection 2 mg]. 2018. http://www.pmda.go.jp/PmdaSearch/iyakuDetail/620023_24994A6G1029_1_02#CONTRAINDICATIONS. Accessed April 11, 2018.
- 15. Food and Drug Administration . Ozempic® – Prescribing information. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209637lbl.pdf. Accessed January 5, 2018.
- 16. Buckley ST, Schéele SG, Kirk RK, Knudsen LB. Mechanism of absorption mediated by SNAC in an oral formulation of semaglutide. Diabetes. 2017;66(suppl 1):1206 ‐P. [Google Scholar]
- 17. Davies M, Pieber TR, Hartoft‐Nielsen ML, Hansen OKH, Jabbour S, Rosenstock J. Effect of oral semaglutide compared with placebo and subcutaneous semaglutide on glycemic control in patients with type 2 diabetes: a randomized clinical trial. JAMA. 2017;318(15):1460‐1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. American Diabetes Association . 9. Cardiovascular disease and risk management: standards of medical care in diabetes‐2018. Diabetes Care. 2018;41(suppl 1):S86‐S104. [DOI] [PubMed] [Google Scholar]
- 19. Cefalu WT, Kaul S, Gerstein HC, et al. Cardiovascular outcomes trials in Type 2 diabetes: where do we go from here? reflections from a diabetes care editors' expert forum. Diabetes Care. 2018;41(1):14‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. European Medicines Agency . Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus. 2012. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500129256.pdf. Accessed January 5, 2018.
- 21. Food and Drug Administration . Guidance for industry diabetes mellitus — Evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. 2008. https://www.fda.gov/downloads/Drugs/Guidances/ucm071627.pdf. Accessed January 5, 2018.
- 22. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in Type 2 diabetes. N Engl J Med. 2016;375(4):311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with Type 2 diabetes. N Engl J Med. 2016;375(19):1834‐1844. [DOI] [PubMed] [Google Scholar]
- 24. International Conference on Harmonisation . ICH harmonised tripartite guideline. Guideline for good clinical practice E6(R1), step 4. 1996.
- 25. World Medical Association . Declaration of Helsinki. Ethical principles for medical research involving human subjects. Last amended by the 64th WMA General Assembly; Fortaleza, Brazil. 2013.
- 26. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with Type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247‐2257. [DOI] [PubMed] [Google Scholar]
- 28. Holman RR, Bethel MA, Mentz RJ, et al. Effects of once‐weekly exenatide on cardiovascular outcomes in Type 2 diabetes. N Engl J Med. 2017;377(13):1228‐1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marso SP, Poulter NR, Nissen SE, et al. Design of the liraglutide effect and action in diabetes: evaluation of cardiovascular outcome results (LEADER) trial. Am Heart J. 2013;166(5):823‐830. [DOI] [PubMed] [Google Scholar]
- 30. Bentley‐Lewis R, Aguilar D, Riddle MC, et al. Rationale, design, and baseline characteristics in evaluation of LIXisenatide in Acute Coronary Syndrome, a long‐term cardiovascular end point trial of lixisenatide versus placebo. Am Heart J. 2015;169(5):631‐638. [DOI] [PubMed] [Google Scholar]
- 31. Mentz RJ, Bethel MA, Gustavson S, et al. Baseline characteristics of patients enrolled in the Exenatide Study of Cardiovascular Event Lowering (EXSCEL). Am Heart J. 2017;187:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Clinicaltrials.gov. NCT01455896. A study to evaluate cardiovascular outcomes in patients with type 2 diabetes treated with ITCA 650. https://clinicaltrials.gov/ct2/show/NCT01455896. Accessed February 20, 2018.
- 33. Gerstein HC, Colhoun HM, Dagenais GR, et al. Design and baseline characteristics of participants in the Researching cardiovascular Events with a Weekly INcretin in Diabetes (REWIND) trial on the cardiovascular effects of dulaglutide. Diabetes Obes Metab. 2018;20(1):42‐49. [DOI] [PubMed] [Google Scholar]
- 34. Clinicaltrials.gov. NCT01394952. Researching cardiovascular events with a weekly incretin in diabetes (REWIND). https://clinicaltrials.gov/ct2/show/NCT01394952. Accessed February 20, 2018. [DOI] [PubMed]
- 35. Clinicaltrials.gov. NCT02465515. Effect of albiglutide, when added to standard blood glucose lowering therapies, on major cardiovascular events in subjects with Type 2 diabetes mellitus. https://clinicaltrials.gov/ct2/show/NCT02465515. Accessed February 20, 2018.
- 36. Bethel MA, Patel RA, Merrill P, et al. Cardiovascular outcomes with glucagon‐like peptide‐1 receptor agonists in patients with type 2 diabetes: a meta‐analysis. Lancet Diabetes Endocrinol. 2018;6(2):105‐113. 10.1016/S2213-8587(17)30412-6. [DOI] [PubMed] [Google Scholar]
- 37. Nauck MA, Meier JJ, Cavender MA, Abd El Aziz M, Drucker DJ. Cardiovascular actions and clinical outcomes with glucagon‐like peptide‐1 receptor agonists and dipeptidyl peptidase‐4 inhibitors. Circulation. 2017;136(9):849‐870. [DOI] [PubMed] [Google Scholar]
- 38. Gaspari T, Liu H, Welungoda I, Hu Y, et al. A GLP‐1 receptor agonist liraglutide inhibits endothelial cell dysfunction and vascular adhesion molecule expression in an ApoE−/− mouse model. Diab Vasc Dis Res. 2011;8(2):117‐124. [DOI] [PubMed] [Google Scholar]
- 39. Lambadiari V, Pavlidis G, Kousathana F, et al. Effects of 6‐month treatment with the glucagon like peptide‐1 analogue liraglutide on arterial stiffness, left ventricular myocardial deformation and oxidative stress in subjects with newly diagnosed type 2 diabetes. Cardiovasc Diabetol. 2018;17(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Song X, Jia H, Jiang Y, et al. Anti‐atherosclerotic effects of the glucagon‐like peptide‐1 (GLP‐1) based therapies in patients with type 2 diabetes mellitus: a meta‐analysis. Sci Rep. 2015;5:10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supplementary material
Table S1. Flow chart of assessments by patient visit.
Table S2. Demographics and baseline characteristics for the PIONEER 6 and SUSTAIN 6 trials.


