Abstract
This methodological review suggests what to do and what not to do in short‐chain fatty acid (SCFA) research for researchers, supervisors, scientific reviewers, and regulatory officers. High viscosity of gut contents, existence of bacterial biofilm and of mucus layer at the mucosal surface, and rapid absorption of SCFAs make it difficult to know their concentrations at the very surface of the mucosa. As lumen or fecal concentration of SCFAs does not reflect their rate of production, these parameters should not be used as measures of SCFA production or absorption. Effects of SCFAs can vary and even become opposite at different dose, time of/after exposure or time of the day. Thus, results without dose–response, time‐course, and diurnal variance experiments can be seriously misleading. It is also to note that too much emphasis on n‐butyrate should be avoided.
Keywords: acetate, butyrate, large intestine, physiology, propionate
1. INTRODUCTION
Studies lacking in careful methodological consideration can result in inappropriate feeding/food policies and to feeding/eating behavior of general public.
Short‐chain fatty acids such as acetic, propionic, and n‐butyric acids are produced from carbohydrates by microbes in the forestomach and/or in the cecum or colon (Macfarlane & Macfarlane, 1997; Wallace, 1995). Short‐chain fatty acids (SCFAs) are important energy nutrient for many species such as ruminants, camelids, or marsupials (Ichikawa & Sakata, 1997a; Sakata, 2000; Stevens & Hume, 1995). At the same time, SCFAs are important modulators of physiological functions such as absorption, motility, mucus release, or gut epithelial cell proliferation of host animals (Cummings, Rombeau, & Sakata, 1995; Ichikawa & Sakata, 1997a; Inagaki & Sakata, 2001; Sakata, 1987a, 1994a,1994b). A considerable proportion of the effects of indigestible carbohydrates and gut microbes should be mediated via the production of SCFAs (Ichikawa & Sakata, 1997a; Inagaki & Sakata, 2001; Sakata, 1987a, 1994a,1994b). As a matter of fact SCFAs might be the first example of a signal nutrient, that is, nutrient that can modulate the function of animals/humans, as shown by Tamate, McGilliard, Jacobson, and Getty (1962, 1964). Thus, the interest on indigestible carbohydrates, gut bacteria, and probiotics attracted attentions on SCFAs.
Methodological validity is the prerequisite for an acceptable experimental study and the basis for legislative decision. Interests on the large intestine, gut fermentation, dietary fibers, and probiotics has lead to a large number of studies concerning SCFAs such as acetic, propionic, and n‐butyric acids produced in the large intestine by gut bacteria. However, inappropriate design of studies often resulted in a misleading conclusion. Therefore, I picked up potential pitfalls in studies of SCFAs and suggest what to do and what not to do in this field of research, partly based on my previous publications (Inagaki & Sakata, 2001; Sakata, 2010). I sincerely ask students, researchers, supervisors, reviewers, and regulatory officers to observe these pitfalls seriously.
2. DEFINITION
Sound science requires appropriate definition of terms. First of all, an excellent writing on chemical nature of SCFAs and other carboxylic acids (Fukushima, 1995) should be quite helpful in this regard.
I dare to adopt the most strict definition by Wrong (Wrong, 1995), that is, “short‐chain fatty acids (SCFAs)” would describe “saturated unbranched alkyl monocarboxylic acids of 2 to 4 carbon atoms” in the present paper. This definition would exclude branched chain fatty acids such as iso‐butyric acid, oxy‐acids such as lactic acid, or di‐carboxylic acids such as succinic acids, which can be produced by gut microbes. Branched‐chain fatty acids can be produced from branched‐chain amino acids (Macfarlane & Gibson, 1995). Lactic acid and succinic acid are often produced at low pH (Ushida & Sakata, 1998), for example, when the entry rate of readily fermentable carbohydrate into the forestomach or large intestine is faster than the absorption rate (Wallace, 1995). Influence of these non‐SCFA acids can sometimes be the same as SCFA, but sometimes differ from those of SCFA (Ichikawa & Sakata, 1997b; Inagaki & Sakata, 2003). Accordingly, it is important that authors define what they mean by “SCFA”.
3. ESTIMATION OF SCFA PRODUCTION
There are three major difficulties when we wish to know the SCFAs concentration and their production rates in the large bowel lumen; slow intraluminal diffusion rate (Takahashi, 2011; Takahashi & Sakata, 2002, 2018), rapid mucosal absorption (von Engelhardt, 1995; von Engelhardt, Rönau, Rechkemmer, & Sakata, 1989) and metabolism of SCFA by epithelial cells (Jørgensen & Brøbech, 2009; Livesey & Elia, 1995; Livesey et al., 1995; Nakatani, Inoue, Tomonaga, Fukuta, & Tsukahara, 2018; Roediger, 1982, 1995; Tsukahara, Matsukawa, Tomonaga, Inoue, & Ushida, 2014).
It is important to measure the rate of production of each SCFA and non‐SCFA acids in the gut lumen to clarify the mechanism that regulates bacterial fermentation in the large intestine (Inagaki & Sakata, 2003). SCFA being the major energy source, the production rate of SCFA in the reticulo‐rumen has been meticulously studied (Annison & Armstrong, 1970; Ørskov, 1995). However, the methodology for large bowel fermentation is yet primitive. Thus, I am discussing the methodology for the estimation of SCFA production in the large bowel below.
3.1. Lumen or fecal concentration
As most SCFAs produced in the large bowel are absorbed (von Engelhardt et al., 1989), it is not reasonable to estimate their production rates from their lumen or fecal concentrations. Lumen concentrations of SCFA are the result of dynamic balance between their production rates and the rates of their disappearance by absorption and by translocation due to the flow of contents toward the anus. Thus, the lumen concentrations of SCFA can be affected by both rates of production and disappearance. Since more than 95% of SCFA produced in the large bowel lumen is absorbed (von Engelhardt et al., 1989), measurement of fecal SCFA concentration should reflect just 5% of produced SCFA left unabsorbed.
Our earlier study demonstrated that lumen concentration of SCFA does not reflect entrance rate, but reflects disappearance rate of SCFA from the lumen (Ichikawa & Sakata, 1997b). We infused either a physiologic mixture of acetic, propionic, and n‐butyric acids (total 150 mmol/L) or lactic acid (150 mmol/L) continuously into the isolated cecum for 3 days and measured concentrations of these acids. We found nearly no SCFA, but approximately 80 mmol/L lactic acid in the lumen. Thus, the lumen concentration of SCFA did not reflect their production rate in the lumen, but reflected the rate of absorption. This was confirmed by the finding that fecal acetate was inversely related to acetate absorption from the human rectum and distal colon (Vogt & Wolever, 2003).
Under usual conditions gut contents contain a large proportion of solid materials such as food components, sloughed epithelial cells, and microbial cell bodies (Sakata & von Engelhardt, 1981a). Such solid particles make the gut contents very viscous non‐Newtonean fluid, inevitably of low diffusion coefficient (Takahashi, 2011; Takahashi & Sakata, 2002, 2018). Reynold's numbers of gut contents calculated from the measured viscosity of gut contents and measured rate of gut motility indicated the absence of macroscopic mixture in the gut lumen (Takahashi, 2011; Takahashi & Sakata, 2018), except for the pylorus (Takahashi & Sakata, 2018), and perhaps in the reticulo‐lumen. This together with the low diffusion coefficient leads to heterogeneous SCFA concentration in the lumen contents even at the same longitudinal segment, that is, lower in the periphery than in the core (Yajima & Sakata, 1992) (Figure 1). Thus, the SCFA concentration of “mixed” lumen contents can be by far higher than their actual concentration at the mucosal surface.
Figure 1.
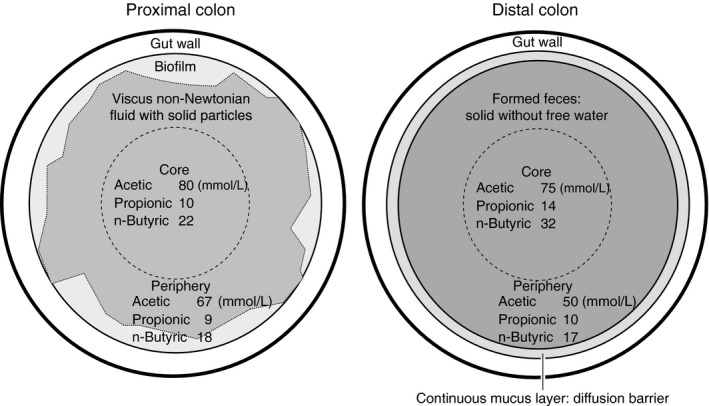
Schematic drawings of the cross section of the proximal (left) and distal (right) colon of the rats with concentrations of SCFAs in the core and periphery of contents (Sakata, 1987a; Sakata & von Engelhardt, 1981b; Takahashi & Sakata, 2002, 2018; Tsukahara et al., 2014; Yajima & Sakata, 1992)
It is also important to consider the time‐course of the lumen concentrations of SCFA. This is especially important in investigations using nocturnal rodents such as rats or mice, which often show a high luminal lactic acid just after the feeding of readily fermentable indigestible carbohydrates such as fructooligosaccharides (Inagaki, 1999) (Figure 2).
Figure 2.
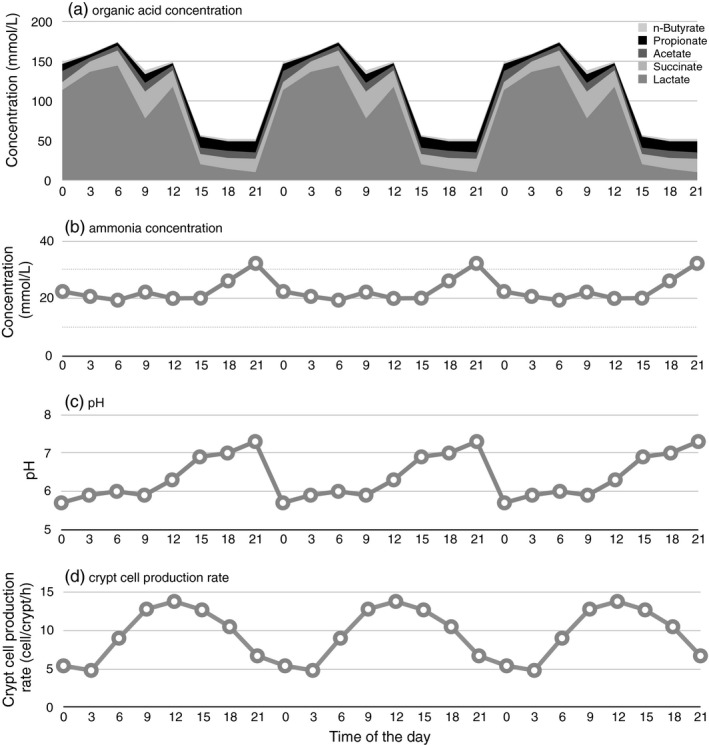
Means of (a) organic acid concentration, (b) ammonia concentration, and (c) pH in the cecal supernatant, and (d) crypt cell production rate of cecal epithelium of rats fed AIN‐93 diet added with 7.5 g/100 g fructooligosaccharides between 20:15 and 23:15. All parameters except for n‐butyrate concentration showed statistically significant diurnal variance (p < 0.05). The same data were plotted three times to show the periodicity (Inagaki, 1999)
3.2. Lumen pool size
Some researchers have adopted lumen “pool size” (lumen concentration × contents volume) to indicate SCFA production rate (Campbell, Fahey, & Wolf, 1997; Hara, Suzuki, Kobayashi, & Kasai, 1996; Topping et al., 1997). However, in addition to the danger of using lumen concentration as a part of this calculation, the pool size may be strongly influenced by the volume of the large intestine. Accordingly, “pool size” is a poor parameter for production rate.
3.3. Portal arterio‐venous (A‐V) difference
Watanabe and Murai found a large arterio‐veneous difference of volatile fatty acids (i.e., SCFAs) in the cecal and colonic vein and thereupon concluded that a significant production in the pig cecum and colon as early as in 1968 (Watanabe & Murai, 1968). Bloemen et al. (2009) also used this technique in humans under surgical operation to demonstrate the release of SCFAs from the gut into the portal vein.
It is possible to estimate the net gain of bacterial metabolites from portal‐drained organs by multiplying the concentration difference between arterial and portal blood by the portal flow rate (Hajivassiliou, Greer, Fisher, & Finlay, 1998). However, as a considerable part of SCFAs is consumed by epithelial cells (Jørgensen & Brøbech, 2009; Roediger, 1982), this method may result in an underestimation (Nakatani et al., 2018).
Care must be taken to maintain the normal blood supply to the mucosa when the portal concentration of organic acids is measured, because some SCFA absorption depends on active sodium transport, which in turn requires oxidative energy production in epithelial cells (Engelhardt, Bartels, Kirschberger, Meyer zu Düttingdorf, & Busche, 1998). Oxygen supply is also necessary for the oxidation of SCFAs in epithelial cells. In this regard, chronically catheterized animals/subjects without anesthesia should be used (Uhing & Kimura, 1995).
3.4. Isotope dilution
In theory, isotope dilution methods (Pouteau et al., 1998) can be the golden standard to measure the rates of consumption and production of organic acids by bacteria, their metabolism in epithelial cells, and their penetration into the organs, even in humans under normal life. However, this method is expensive and tedious, not yet sufficiently sensitive, and requires extensive mixing of the isotope in the lumen, a process that is not always easy to guarantee. Moreover, it is not always easy to establish steady state in the lumen of the large bowel of humans or of livestocks, who eat intermittently.
3.5. Fecal bacterial cell body
Bacteria in the large intestine obtain energy to synthesize protein, nucleic acid, membranes, and cell wall from anaerobic breakdown of carbohydrates and thereby excrete SCFAs. Thus, there should be a linear positive correlation among carbohydrate degradation, SCFA production and bacterial cell body production in the large intestine (Livesey & Elia, 1995). Accordingly, it is possible to estimate SCFA production in the large intestine from fecal excretion of bacterial cell mass or cell protein mass (Livesey & Elia, 1995).
Diaminopimelic acid produced specifically by bacteria has been a useful marker substance to estimate bacterial cell mass in ruminant science (Phillipson, 1964). This applies to the research on the large intestine as well. Thus, one may measure the daily fecal excretion of diaminopimelic acid and divide this by the average diaminopimelic acid content per bacterial cell mass or per bacterial cell protein to estimate net daily production of bacterial cell mass or bacterial cell protein, respectively. Then, we can estimate SCFA production from stoichiometric equation (Livesey & Elia, 1995; Macfarlane & Gibson, 1995; Wallace, 1995). This method can also be applied to estimate the amount of carbohydrates degraded by bacteria in the large intestine.
Another marker for bacteria‐specific component should be deoxyribonucleic acid, which was used to estimate digestibility of dietary fiber in pig upper digestive tract and in the hindgut (Rowan, Moughan, & Wilson, 1992).
Since the conversion efficiency of carbohydrate energy to bacterial energy is about 30 kJ/100 kJ of carbohydrate fermented (Livesey, 1993), and since bacteria consists largely of protein, so accounting for most of the nitrogen present in feces, an upper estimate to total SCFA production can be made from the following equation:
where Nf denotes fecal nitrogen (g) (Livesey, 1993).
This method based on fecal excretion of bacteria cannot specify the proportion of produced acids. It is especially serious when there is a significant production of lactic or succinic acid, which cannot be used by the host animal as energy source and has effects different from SCFAs (Inagaki & Sakata, 2003). Nevertheless, this method is relatively easy and rather accurate, and should attract more attention.
3.6. Ex vivo culture of large intestinal bacteria
Ex vivo cultures of large intestinal bacteria, as the slurry of feces (Barry et al., 1995; Monsma & Marlett, 1995), as diluted gut contents (Kikuchi & Sakata, 1992; Kiriyama, Hariu, & Sakata, 1992; Ushida & Sakata, 1998) or as washed bacteria (Sakata et al., 1999), have been used to measure the conversion of carbohydrates to SCFA and other acids.
It is evident that the mixing in such culture system does not precisely reproduce the mixing of gut contents in vivo (Takahashi & Sakata, 2018), and it is difficult to correspond culture time to in vivo retention time (Inagaki & Sakata, 2001). Most culture system does not simulate the absorption of SCFA, except for a few studies simulating absorption by removal using dialysis tubing or by continuous dilution (Czerkawski, 1986).
In spite of these drawbacks, ex vivo culture system has benefits. The system is relatively simple and easy to handle. It is easy to control substrate entry rate, dilution rate and mode of mixing. Therefore, ex vivo culture system can be considered as an acceptable alternative to estimate the conversion of carbohydrates to SCFAs and its regulatory mechanism (Inagaki & Sakata, 2001).
It is of critical importance to use bacterial composition not far from the in vivo condition both qualitatively and quantitatively (Inagaki & Sakata, 2001). In this regard, we should avoid too much dilution. Dilution should alter bacterial composition to favor rapidly proliferating species. We should also avoid too much free water in the system. Content of free water in large‐intestinal contents is rather limited due to the water holding capacity of feed residue (Sakata, 1987a). It is also important to think about the existence of solid surface, which is usually the case unless parenteral feeding or feeding of elemental diet. Because there are bacteria that require solid surface to adhere for their normal proliferation or metabolism, at least in the rumen, and therefore the lack of solid surface often leads to very unstable and unpredictable culture (Czerkawski, 1986).
3.6.1. Batch culture
Batch culture using either large intestinal contents (Kihara & Sakata, 1997; Kikuchi & Sakata, 1992; Monsma & Marlett, 1995; Sakata et al., 1999; Ushida & Sakata, 1998), feces (Barry et al., 1995; Monsma & Marlett, 1995) or bacteria isolated from such materials (Kiriyama et al., 1992; Sakata, Kojima, Fujieda, Takahashi, & Michibata, 2003; Sakata et al., 1999) is easy to perform and inexpensive. It is possible to conduct many concurrent cultures, even using the same source of inoculum (Sakata et al., 1999, 2003; Ushida & Sakata, 1998). This enables a mathematical description of the time‐course and kinetic analysis of metabolite production (Kikuchi & Sakata, 1992; Kiriyama et al., 1992). A batch culture method has been developed which uses as little as 0.1 ml (Kihara & Sakata, 1997, 2001), and this in turn permits the amounts of both innoculum and test substrate(s) to be reduced.
However, in batch cultures, the initial culture conditions cannot be maintained as the substrate is consumed and metabolic products accumulate during incubation (Kiriyama et al., 1992). Therefore, it is essential to make the culture short, say within 24 hr maximum.
It is important to use original mixed bacterial species to evaluate the fermentation in the hindgut (Sakata et al., 1999, 2003; Ushida & Sakata, 1998). Otherwise, it is impossible to simulate the complex and interactive metabolism of the bacterial ecosystem in the large intestine.
Earlier, we developed a batch culture method employing gas release from the culture as the measure for acid production, and hence carbohydrate breakdown (Kikuchi & Sakata, 1992), an adoption of Menke method in ruminant science (Menke, Raab, Salewski, & Steingass, 1979). This enables continuous monitor of the bacterial metabolism with a single culture, even second by second, if we use video monitoring (Kiriyama et al., 1992). Thus, it is quite easy to compare the time‐courses of bacterial metabolism between different conditions as many as 30.
3.6.2. Continuous culture
This method can employ various patterns of substrate supply. It is possible to alter the dilution rate, to regulate the pH, or to adapt the culture to certain substrate(s) or culture condition (Sakata et al., 2003). It is also possible to remove the fermentation products by dilution with buffer or through a dialysis membrane (Czerkawski, 1986). It is possible to use autoclaved cecal contents or ileal effluents as the basal vehicle to which the fermentation substrate(s) of interest will be added (Sakata et al., 2003).
Some researchers used serially connected plural fermenters sometimes with different dimensions, dilution rates or pH (Gibson, Cummings, & Macfarlane, 2018; Molly, Van de Woestyne, & Verstraete, 1993). The use of such complex systems needs special cares, because it is more tedious and error‐prone.
Anyway, continuous culture is more tedious, expensive and space consuming than batch cultures. Continuous culture methods require greater quantities of test substance(s) than batch cultures. The choice of different culture methods depends on the objective of the study, though over‐simulation is not always productive (Czerkawski, 1986).
4. STUDIES TO KNOW EFFECTS OF SCFAs
Most of the in vivo effect of SCFA is the sum of direct, local indirect and systemic effects (Sakata, 1994b). Systemic effect can supersede local effect (Galfi, Neogrady, & Sakata, 1990). Accordingly, in vitro results may not be directly extrapolated to in vivo conditions. As most effects of SCFA are dose dependent (Inagaki & Sakata, 2005; LeBlay et al., 2000) and vary between different acids (Inagaki & Sakata, 2003; Sakata, 1994b; Yajima, 1995), it is important to know both the nature of the acid and the extent of its production by bacterial population.
I am afraid that too much emphasis is placed on n‐butyrate, partly because of the reportedly preferred use of n‐butyrate by colonocytes (Roediger, 1995) and of the strongest effect of this acid to inhibit cell proliferation in vitro (Ginsburg, Salamon, Sreevalsan, & Freese, 1973; Kruh, Defer, & Tichonicky, 1995). However, studies using mucosal strip showed that acetate was the most preferred fuel for colonocytes (Jørgensen & Brøbech, 2009) and an A‐V difference study shows more‐or‐less indifferent use of three SCFAs by colonic tissue (Tsukahara et al., 2014). Furthermore, acetate and propionate are the strongest stimulants for intestinal blood flow (Mortensen & Nielsen, 1995) and phasic colonic motility (Yajima, 1985), respectively. Therefore, more studies on acetate and propionate are needed.
4.1. Correlation between lumen or fecal concentration and function of SCFAs
It is too dangerous to speculate effect(s) of SCFAs based on the correlation between lumen or fecal SCFA concentration and physiologic effects or symptoms.
First of all, such a correlation does not support causal relationship. Lumen or fecal SCFA concentration does not reflect the rate of SCFA production (see above) and often has little correlation with effects of SCFAs. For example, a continuous infusion of either physiologic mixture of SCFAs into the rat large intestine stimulated epithelial cell proliferation of the large intestine without increasing lumen concentration of SCFAs (Ichikawa & Sakata, 1997b). Thus, lumen concentration is a poor measure of the exposure to SCFA.
4.2. Need for direct exposure to SCFAs
We should keep it in mind that the production of SCFA in the large intestine accompanies the consumption of ammonia, hydrogen sulfide, and branched‐chain fatty acids for the synthesis of protein of bacterial cell body (Livesey & Elia, 1995; Macfarlane & Gibson, 1995). Thus, the apparent correlation between an effect and the increased SCFA production should not be directly interpreted that SCFAs are the causative agent for the effect. The decrease in ammonia, hydrogen sulfide, or branched‐chain fatty acids could be responsible for the effect.
It is also possible for some indigestible carbohydrate to stimulate calcium absorption by directly increasing epithelial permeability independent of the action of SCFAs, however, accompanying the increase in luminal or fecal SCFA concentration (Mineo, Hara, Kikuchi, Sakurai, & Tomita, 2001; Mineo, Hara, Shigematsu, Okuhara, & Tomita, 2002).
The direct administration of a SCFA or a mixture of SCFAs into the physiologic production site should be the first choice to test the effect of SCFA(s).
4.3. Dose and route
Effects of SCFAs can vary or even become opposite at different doses. For example, SCFAs stimulate phasic contraction of the colon (Yajima, 1985), and cell proliferation of isolated smooth muscle cells (LeBlay et al., 2000) or that of colonic epithelial cells in organ culture (Inagaki & Sakata, 2005) at physiologic low dose, however, become inhibitory at unphysiologically high dose (Cherbut et al., 1996; Ginsburg et al., 1973; Inagaki & Sakata, 2005; Kruh et al., 1995; LeBlay et al., 2000) (Figure 3).
Figure 3.
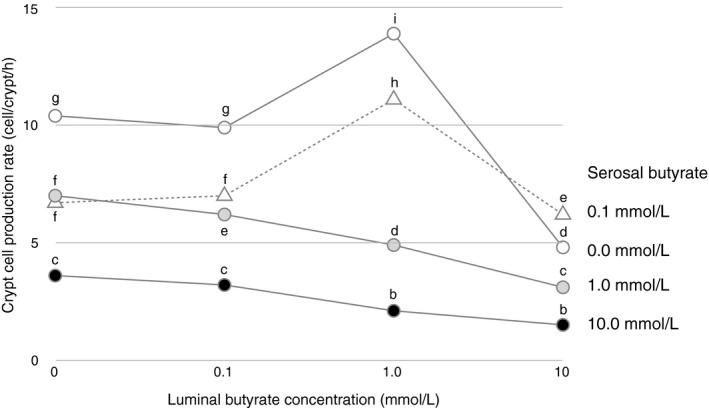
Mean crypt cell production rate of pig distal colonic mucosa exposed to various luminal and serosal n‐butyric acid concentrations in organ culture (Inagaki & Sakata, 2005) (n = 40 after jackknife resampling, pooled standard error of the mean = 1.45). Means not sharing a same letter differ significantly by Tukey's post‐hoc test (p < 0.05)
In vivo production rate of SCFA(s) should be a good measure to decide the dose of SCFA(s) for an in vivo or in situ experiment. Thus, it is recommended to administer SCFA(s) to animals with deficient SCFA(s) production, for example, by feeding diet with deficient fermentable indigestible carbohydrate (Sakata, 1987a), by hindgut bypass (Ichikawa & Sakata, 1998), by parenteral feeding, or using germ‐free animals (Sakata, 1987a). If we wish to know the effect of SCFAs produced in the hindgut, then we should administer SCFAs into the hindgut preferably with its ordinary contents (Sakata, 1987a).
It is essential to define the site of administration according to an anatomic marking such as the entrance of blood vessels (Sakata, 2007; Sakata & Setoyama, 1997).
Lack of solid matter or infusion of too much amount of water should abnormally reduce the viscosity of contents and thereby abnormally increase the rate of absorption (Takahashi, 2011; Takahashi & Sakata, 2002, 2018). Such approaches may also damage the barrier function of bacterial biofilm and mucus layer (Figure 1). Actually, too much amount of free water abnormally stimulated colonic mucus release in an in situ perfusion study using rats (Sakata & von Engelhardt, 1981b).
Portal or systemic venous administration (Velazquez et al., 1996) is desirable if we are interested in hepatic or systemic effects of absorbed SCFA, respectively. Naturally, the dose should observe the physiologic entry rate of SCFAs in these vessels (Nakatani et al., 2018; Tsukahara et al., 2014; Watanabe & Murai, 1968).
It is difficult to set an appropriate concentration of SCFAs for in vitro studies (Inagaki & Sakata, 2005). We do not know the concentrations of SCFAs at the very surface of both the luminal and baso‐lateral cell membranes of cecal or colonic epithelial cells (Figure 4).
Figure 4.
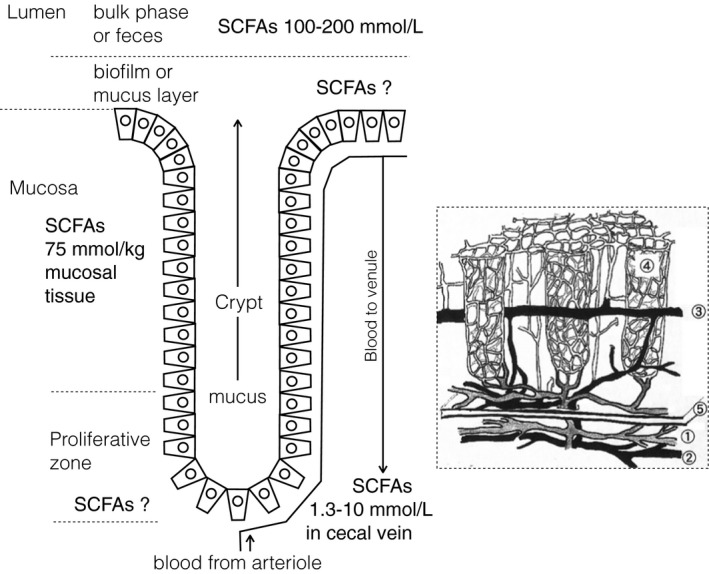
Compiled information about the luminal (Nakatani et al., 2018; Sakata, 1987a; Tsukahara et al., 2014; Yajima & Sakata, 1992), mucosal (Tsukahara et al., 2014), pericryptal, and cecal venus (Nakatani et al., 2018; Tsukahara et al., 2014; Watanabe & Murai, 1968) concentrations of SCFAs (left) with a schematic drawing of mucosal vascular system in the pig distal colon (Saito, 2003) (right inserted). Note the directions of mucus flow in the crypt lumen, which is against the entry of SCFAs via this pathway and of blood flow from the crypt bottom to the flat surface. The latter does not favor the delivery of SCFAs under the flat surface epithelium to the crypt bottom via local vascular network. Numbers 1–5 in the right panel show arteriole, venule, mid‐cryptal venule, pericryptal capillary network and muscularis mucosae, respectively
The high viscosity of lumen contents (Takahashi, 2011; Takahashi & Sakata, 2002, 2018), existence of bacterial biofilm (Macfarlane, McBain, & Macfarlane, 1997) or mucus layer (Sakata & von Engelhardt, 1981a) on the epithelial surface, and rapid absorption rate for SCFA of cecal or colonic epithelium (von Engelhardt, 1995) should make their concentration at the surface of luminal membrane far lower than those in the lumen bulk phase (Figure 4), even at the flat surface of the ceco‐colonic segment (Inagaki & Sakata, 2005). SCFA concentration at the surface of crypt lumen is more difficult to know. It is hard to take samples directly from the crypt lumen and there is a bottom‐to‐surface flow of mucus in the crypt lumen (Figure 4). Bottom‐to‐top pericryptal blood flow (Figure 4) does not favor the transport of SCFAs in the lamina propria under the flat surface toward the crypt bottom. Therefore, it is difficult to estimate the SCFA concentration at the baso‐lateral surface of cells in crypt bottom, where cell proliferation takes place. Even for the epithelial cells at the flat surface, we do not have reliable data of SCFA concentration at the baso‐lateral surface. However, that concentration may not be too much different from “mucosal concentration” measured by Tsukahara et al. (2014), which was approximately one half as the lumen bulk phase concentration. The concentration of SCFAs at the baso‐lateral surface of epithelial cells should lie somewhere between the concentrations at the luminal surface and venous blood (Nakatani et al., 2018; Tsukahara et al., 2014; Watanabe & Murai, 1968).
Therefore, it is essential to conduct a dose–response study covering both luminal and venous SCFA concentrations to know the effect of SCFAs on or via epithelial cells. In this regard, it may be safer to include very wide dose range of several orders (Inagaki & Sakata, 2005; LeBlay et al., 2000), when it is difficult to know the in situ concentration of SCFAs to which the target cell is directly exposed.
If we are interested in effects of SCFAs on peripheral cells, then we may soundly employ arterial concentration of SCFAs. In this regard, studies on arterial concentrations of SCFAs are badly needed.
4.4. Time‐course
It is a difficult question how long we should expose objective cells, tissue or organ to SCFAs. Some effect of SCFAs can reverse according to the length of exposure. For example, exposure of the luminal side of the distal colon to some SCFA leads to peristaltic contraction (Yajima, 1985), then to relaxation (Cherbut, 1995). It is also well known that the sensory mechanism for SCFAs in the colon adapts to multiple stimuli; the reaction to the second stimulus not so strong as the one to the first stimulus (Yajima, 1995).
It is also important if the duration of the effect is sufficiently long for physiological or nutritional significance. For instance, it may not be important if a SCFA stimulate gut epithelial cell proliferation just for 1 hr, however, it can be of some nutritional significance if SCFA can stimulate for several days (Sakata, 1987a) (Figure 5) or longer (Sakata, 1986).
Figure 5.
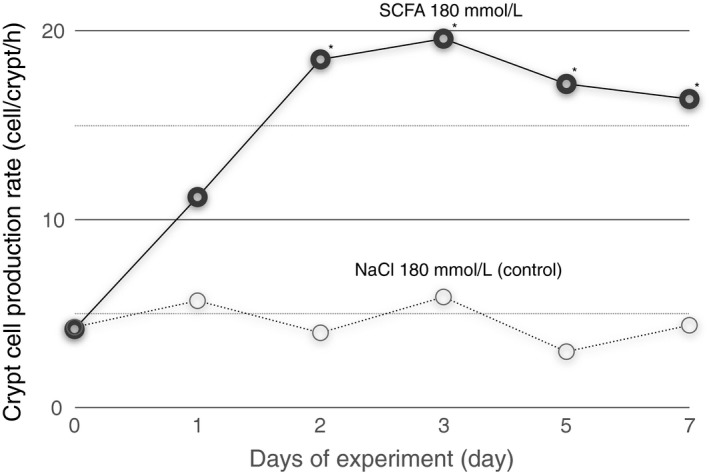
Mean crypt cell production rate of the distal colon in rats fed an elemental diet plus kaolin (9:1, w/w) and given acetic 100, propionic 20 and n‐butyric acid (mmol/L), or 180 mmol/L sodium chloride (control) both at pH 6.1, 3 ml twice daily into the cecum for 1 week (Sakata, 1987b). *significantly different from the control by Scheffe's test at p < 0.5
Thus, it is essential to conduct a series of time‐course study (Figure 5) to say something about nutritional or physiological effects of SCFAs in vivo.
4.5. Circadian fluctuation
Many functions of multicellular organisms have a circadian fluctuation. For instance, gut epithelial cell proliferation is low during actively moving period (light period in humans or ruminants, dark period in rats or mice) (Sakata, 1987a; Sakata & Tamate, 1978). Thus, we should think about the timing of the administration and that of observation. It is also important to observe if SCFA alters the peak value or bottom value.
For example, SCFAs increased the height of the peak in gut epithelial cell proliferation without changing their pattern of circadian fluctuation (Sakata, 1987a) (Figure 6). If I concluded based on the results at just one time point of the day, then it were possible to conclude both SCFAs had a stimulatory effect or they had no effect at all, depending on the time of observation (Sakata, 1987a).
Figure 6.

Mean crypt cell production rate of the distal colon in rats fed an elemental diet plus kaolin (9:1, w/w) and given acetic 100, propionic 20 and n‐butyric acid (mmol/L), or 180 mmol/L sodium chloride (control) both at pH 6.1, 3 ml twice daily into the cecum for 1 week (Sakata, 1987b). Data were plotted twice to show the diurnal pattern. *, **, ***significantly different from the control by Scheffe's test at p < 0.05, p < 0.01 or p < 0.001, respectively
We should also notice that researchers during their usual working time observe experimental rodents during their resting time, which is often disturbed by maintenance activities in the animal house.
4.6. Interaction with other metabolites
There are metabolites such as carbon dioxide, ammonia, hydrogen sulfide, or organic acids beside SCFAs in the hindgut of mammals or birds. Therefore, we should not neglect the influence of these substances on the effect of SCFAs.
For example, both SCFA and ammonia stimulate epithelial cell proliferation in the rat colon, however, with negative interaction effect (Ichikawa & Sakata, 1998). Effect of SCFA can also vary at different pH (Ichikawa & Sakata, 1997b). In other words, there is a reasonable possibility that an effect of SCFA can be modified by co‐existing acid or base.
Thus, we should take special care when we wish to extrapolate the effect of SCFA found in an experiment without co‐existing metabolites to in vivo condition. This is one of the reasons why I stick to the idea to administer SCFA into the hindgut with their contents containing other metabolites.
5. CONCLUDING REMARKS
The above discussion showed that research on SCFAs needs many cares. I do hope researchers, supervisors, and reviewers observe these points and remind that they are responsible for their output and outcomes of reviewing to tax‐paying citizens and to general consumers, who are sponsoring your research.
I dedicate the present article to two giant scientists in SCFA science, who lead me to the field of SCFA research, late Prof. Hideo Tamate and Prof. Wolfgang von Engelhardt with my deepest appreciation. I also wish to express my gratitude to Dr. Akiko Inagaki and Ms. Shihoko Saito for their kind permission to use their data or schematic drawing in their MSc. thesis, respectively.
Sakata T. Pitfalls in short‐chain fatty acid research: A methodological review. Anim Sci J. 2019;90:3–13. 10.1111/asj.13118
REFERENCES
- Annison, E. F. , & Armstrong, D. G. (1970). Volatile fatty acid metabolism and energy supply In Phillipson A. T., Annison E. F., Armstrong D. G., Balch C. C., Comline R. S., Hardy R. N., Hobson P. N., & Keynes R. D. (Eds.), Physiology of digestion and metabolism in the ruminant. New Castle upon Tyne, UK: Oriel Press. [Google Scholar]
- Barry, J. L. , Hoebler, C. , Macfarlane, G. T. , Macfarlane, S. , Mathers, J. C. , Reed, K. , … Romney, C. J. (1995). Estimation of the fermentability of dietary fibre in vitro: A European interlaboratory study. British Journal of Nutrition, 74, 303–322. 10.1079/BJN19950137 [DOI] [PubMed] [Google Scholar]
- Bloemen, J. G. , Venema, K. , van de Poll, M. C. , Olde Damink, S. W. , Buurman, W. A. , & Dejong, C. H. (2009). Short chain fatty acids exchange across the gut and liver in humans measured at surgery. Clinical Nutrition, 28, 657–661. 10.1016/j.clnu.2009.05.011 [DOI] [PubMed] [Google Scholar]
- Campbell, J. M. , Fahey, G. C. , & Wolf, B. W. (1997). Selected indigestible oligosaccharides affect large bowel mass, cecal and fecal short‐chain fatty acids, pH and microflora in rats. The Journal of Nutrition, 127, 130–136. 10.1093/jn/127.1.130 [DOI] [PubMed] [Google Scholar]
- Cherbut, C. (1995). Effects of short‐chain fatty acids on gastrointestinal motility In Cummings J., Rombeau J., & Sakata T. (Eds.), Physiological and clinical aspects of short‐chain fatty acids (pp. 191–207). Cambridge: Cambridge University Press. [Google Scholar]
- Cherbut, C. , Aube, A. , Blottiere, H. , Pacaud, P. , Sccargignato, C. , & Galmiche, J. (1996). In vitro contractile effects of short chain fatty acids in the rat terminal ileum. Gut, 8, 53–58. 10.1136/gut.38.1.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J. H., Rombeau J. L., & Sakata T. (Eds.) (1995). Physiological and clinical aspects of short chain fatty acids (pp. 191–207). Cambridge: Cambridge University Press. [Google Scholar]
- Czerkawski, J. W. (1986). An introduction to rumen studies. Oxford, UK: Pergamon Press Ltd. [Google Scholar]
- Fukushima, M. (1995). Chemistry of short‐chain fatty acids In Cummings J. H., Rombeau J. L., & Sakata T. (Eds.), Physiological and clinical aspects of short chain fatty acids (pp. 15–34). Cambridge: Cambridge University Press. [Google Scholar]
- Galfi, P. , Neogrady, S. , & Sakata, T. (1990). Effects of volatile fatty acids on the epithelial cell proliferation of the digestive tract and its hormonal mediation In Tsuda T., Sasaki Y., & Kawashima R. (Eds.), Physiological aspects of digestion and metabolism in ruminants (pp. 49–59). London: Academic Press. [Google Scholar]
- Gibson, G. R. , Cummings, J. H. , & Macfarlane, G. T. (2018). Use of a three‐stage continuouis culture system to study the effect of mucin on dissimilatory sulfate reduction and methanogenesis by mixed populations of human gut bacgteria. Appllied and Environmental Microbiology, 54, 2750–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg, E. , Salamon, D. , Sreevalsan, T. , & Freese, E. (1973). Growth inhibition and morphological changes caused by lipophilic acids in mammalian cells. Proceedings of the National Academy of Sciences of the United States of America, 70, 2457–2461. 10.1073/pnas.70.8.2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajivassiliou, C. A. , Greer, K. , Fisher, A. , & Finlay, I. G. (1998). Non‐invasive measurement of colonic blood flow distribution using laser Doppler imaging. The British Journal of Surgery, 85, 52–55. 10.1046/j.1365-2168.1998.00555.x [DOI] [PubMed] [Google Scholar]
- Hara, H. , Suzuki, K. , Kobayashi, S. , & Kasai, T. (1996). Fermentable property of dietary fiber may not determine cecal and colonic mucosal growth in fiber‐fed rats. The Journal of Nutritional Biochemistry, 7, 549–554. 10.1016/S0955-2863(96)00105-2 [DOI] [Google Scholar]
- Ichikawa, H. , & Sakata, T. (1997a). Physiological actions of short‐chain fatty acids (in Japanese). Journal of Oleo Science, 46, 143–150. [Google Scholar]
- Ichikawa, H. , & Sakata, T. (1997b). Effect of L‐lactic acid, short‐chain fatty acids, and pH in cecal infusate on morphometric and cell kinetic parameters of rat cecum. Digestive Diseases and Sciences, 42, 1598–1610. 10.1023/A:1018884625737 [DOI] [PubMed] [Google Scholar]
- Ichikawa, H. , & Sakata, T. (1998). Stimulation of epithelial cell proliferation of isolated distal colon of rats by continuous colonic infusion of ammonia or short‐chain fatty acids is nonadditive. The Journal of Nutrition, 128, 843–847. 10.1093/jn/128.5.843 [DOI] [PubMed] [Google Scholar]
- Inagaki, A. (1999). Time‐dependent changes in cecal organic acid concentration and cecal crypt cell production rate in rats meal‐fed a diet containing indigestible saccharides (in Japanese). Ishinomaki, Japan: Department of Life Sciences, Ishinomaki Senshu University. [Google Scholar]
- Inagaki, A. , & Sakata, T. (2001). Fermentation of oligosaccharides and influences of fermentation products In McCleary B. V., & Prosky L. (Eds.), Advanced dietary fibre technology (pp. 197–205). Oxford: Blackwell Science Ltd. [Google Scholar]
- Inagaki, A. , & Sakata, T. (2003). Influences of lactic and succinic acid on functions of the large intestine In Kritchevsky D., Bonfield C. T., & Edwards C. A. (Eds.), Dietary fiber in health and disease: 6th vahouny symposium (pp. 183–186). Vahouny Symposia, Delray Beach. [Google Scholar]
- Inagaki, A. , & Sakata, T. (2005). Dose‐dependent stimulatory and inhibitory effects of luminal and serosal n‐butyric acid on epithelial cell proliferation of pig distal colonic mucosa. Journal of Nutritional Sciences and Vitaminology, 51, 156–160. 10.3177/jnsv.51.156 [DOI] [PubMed] [Google Scholar]
- Jørgensen, J. , & Brøbech, P. (2009). Utilization of short‐chain fatty acids by colonic mucosal tissue strips: A new method of assessing colonic mucosal metabolism. Scandinavian Journal of Gastroenterology, 35, 659–666. [DOI] [PubMed] [Google Scholar]
- Kihara, M. , & Sakata, T. (1997). Fermentation of dietary carbohydrates to short‐chain fatty acids by gut microbes and its influence on intestinal morphology of a detritivorous teleost Tilapia (Oreochromis niloticus). Comparative Biochemistry & Physiology [A], 118, 1201–1207. 10.1016/S0300-9629(97)00052-2 [DOI] [Google Scholar]
- Kihara, M. , & Sakata, T. (2001). Influences of incubation temperature and various saccharides on the production of organic acids and gases by gut microbes of rainbow trout Oncorhynchus mykiss in a micro‐scale batch culture. Comparative Biochemistry & Physiology [B] 171, 441–447. [DOI] [PubMed] [Google Scholar]
- Kikuchi, H. , & Sakata, T. (1992). Qualitative and quantitative estimation of soluble indigestible polysaccharides as substrates for hindgut fermentation by mini‐scale batch culture. Journal of Nutritional Sciences and Vitaminology, 38, 287–296. 10.3177/jnsv.38.287 [DOI] [PubMed] [Google Scholar]
- Kiriyama, H. , Hariu, Y. , & Sakata, T. (1992). Comparison of in vitro productivities of short‐chain fatty acids and gases from aldoses and the corresponding alcohols by pig cecal bacteria. The Journal of Nutritional Biochemistry, 3, 447–451. 10.1016/0955-2863(92)90002-Z [DOI] [Google Scholar]
- Kruh, J. , Defer, N. , & Tichonicky, L. (1995). Effects of butyrate on cell proliferation and gene expression In Cummings J., Rombeau J., & Sakata T. (Eds.), Physiological and clinical aspects of short‐chain fatty acids (pp. 275–288). Cambridge: Cambridge University Press. [Google Scholar]
- LeBlay, G. , Blottiere, H. M. , Ferrier, L. , LeFoll, E. , Bonnet, C. , Galmiche, J. P. , & Cherbut, C. (2000). Short‐chain fatty acids induce cytoskeletal and extracellular protein modifications associated with modulation of proliferation on primary culture of rat intestinal smooth muscle cells; lecture; inagaki; project. Digestive Diseases and Sciences, 45, 1623–1630. 10.1023/A:1005529414765 [DOI] [PubMed] [Google Scholar]
- Livesey, G. (1993). Comments on the methods used to determine the energy values of carbohydrates: Dietary fibre, sugar alcohols and other bulking agents. International Journal of Food Sciences and Nutrition, 44, 221–241. 10.3109/09637489309017443 [DOI] [Google Scholar]
- Livesey, G. , & Elia, M. (1995). Short‐chain fatty acids as an energy source in the colon: Metabolism and clinical implications In Cummings J., Rombeau J., & Sakata T. (Eds.), Physiological and clinical aspects of short‐chain fatty acids (pp. 427–481). Cambridge: Cambridge University Press. [Google Scholar]
- Livesey, G. , Smith, T. , Eggum, B. , Tetens, I. , Nyman, M. , Roberfroid, M. , … Decombaz, J. (1995). Determination of digestible energy values and fermentabilities of dietary fibre supplements: A European interlaboratory study in vivo. British Journal of Nutrition, 74, 289–302. 10.1079/BJN19950136 [DOI] [PubMed] [Google Scholar]
- Macfarlane, G. T. , & Gibson, G. R. (1995). Microbiological aspects of the production of short‐chain fatty acids in the large bowel In Cummings J., Rombeau J., & Sakata T. (Eds.), Physiological and clinical aspects of short‐chain fatty acids (pp. 87‐105). Cambridge: Cambridge University Press. [Google Scholar]
- Macfarlane, G. T. , & Macfarlane, S. (1997). Human colonic microbiota: Ecology, physiology and metabolic potential of intestinal bacteria. Scandinavian Journal of Gastroenterology, 32, 3–9. 10.1080/00365521.1997.11720708 [DOI] [PubMed] [Google Scholar]
- Macfarlane, S. , McBain, A. J. , & Macfarlane, G. T. (1997). Consequences of biofilm and sessile growth in the large intestine. Advances in Dental Research, 11, 59–68. 10.1177/08959374970110011801 [DOI] [PubMed] [Google Scholar]
- Menke, K. H. , Raab, L. , Salewski, A. , & Steingass, H. (1979). The estimation of the digestibility and metabolizable energy content of ruminant feedingstuffs from the gas production when they are incubated with rumen liquor in vitro. The Journal of Agricultural Science, 93, 217–222. 10.1017/S0021859600086305 [DOI] [Google Scholar]
- Mineo, H. , Hara, H. , Kikuchi, H. , Sakurai, H. , & Tomita, F. (2001). Various indigestible saccharides enhance net calcium transport from the epithelium of the small and large intestine of rats in vitro. The Journal of Nutrition, 131, 3243–3246. 10.1093/jn/131.12.3243 [DOI] [PubMed] [Google Scholar]
- Mineo, H. , Hara, H. , Shigematsu, N. , Okuhara, Y. , & Tomita, F. (2002). Melibiose, difructose anhydride III and difructose anhydride IV enhance net calcium absorption in rat small and large intestinal epithelium by increasing the passage of tight junctions in vitro. The Journal of Nutrition, 132, 3394–3399. 10.1093/jn/132.11.3394 [DOI] [PubMed] [Google Scholar]
- Molly, K. , Van de Woestyne, M. , & Verstraete, W. (1993). Development of a 5‐step multi‐chamber reactor as a simulation of the human intestinal microbial ecosystem. Applied Microbiology and Biotechnology, 39, 254–258. 10.1007/BF00228615 [DOI] [PubMed] [Google Scholar]
- Monsma, D. , & Marlett, J. (1995). Rat cecal inocula produce different patterns of short‐chain fatty acids than fecal inocula in in vitro fermentations. The Journal of Nutrition, 125, 2463–2470. 10.1093/jn/125.10.2463 [DOI] [PubMed] [Google Scholar]
- Mortensen, F. V. , & Nielsen, H. (1995). In vivo and in vitro effects of short‐chain fatty acids on intestinal blood circulation In Cummings J., Rombeau J., & Sakata T. (Eds.), Physiological and clinical aspects of short‐chain fatty acids (pp. 391–400). Cambridge: Cambridge University Press. [Google Scholar]
- Nakatani, M. , Inoue, R. , Tomonaga, S. , Fukuta, K. , & Tsukahara, T. (2018). Production, absorption and blood flow dynamics of short‐chain fatty acids produced by fermentation in piglet hindgut during the suckling‐weaning period. Nutrients, 10(9), 1220 10.3390/nu10091220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ørskov, E. R. (1995). Utilization of short‐chain fatty acids in ruminants In Cummings J., Rombeau J., & Sakata T. (Eds.), Physiological and clinical aspects of short‐chain fatty acids (pp. 243–256). Cambridge: Cambridge University Press. [Google Scholar]
- Phillipson, A. T. (1964). CHAPTER 3 ‐ The Digestion and Absorption of Nitrogenous Compounds in the Ruminant In Munro H. N., & Allison J. B. (Eds.), Mammalian protein metabolism. Academic Press; 10.1016/B978-1-4832-3209-6.50010-4 [DOI] [Google Scholar]
- Pouteau, E. , Vahedi, K. , Messing, B. , Flourie, B. , Nguyen, P. , Darmaun, D. , & Krempf, M. (1998). Production rate of acetate during colonic fermentation of lactulose: A stable‐isotope study in humans. The American Journal of Clinical Nutrition, 68, 1276–1283. 10.1093/ajcn/68.6.1276 [DOI] [PubMed] [Google Scholar]
- Roediger, W. E. (1982). Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology, 83, 424–429. [PubMed] [Google Scholar]
- Roediger, W. E. (1995). The place of short‐chain fatty acids in colonocyte metabolism in health and ulcerative colitis: The impaired colonocyte barrier In Cummings J., Rombeau J., & Sakata T. (Eds.), Physiological and clinical aspects of short‐chain fatty acids (pp. 337–351). Cambridge: Cambridge University Press. [Google Scholar]
- Rowan, A. M. , Moughan, P. J. , & Wilson, M. N. (1992). The flows of deoxyribonucleic acid and diaminopimelic acid and the digestibility of dietary fibre components at the terminal ileum, as indicators of microbial activity in the upper digestive tract of ileostomised pigs. Animal Feed Science and Technology, 36, 129–141. 10.1016/0377-8401(92)90092-K [DOI] [Google Scholar]
- Saito, S. (2003). Structure of mucosal vascular network in pig distal colon (in Japanese). Ishinomaki, Japan: Department of Life Sciences, School of Science and Engineering, Ishinomaki Senshu University. [Google Scholar]
- Sakata, T. (1986). Effects of indigestible dietary bulk and short chain fatty acids on the tissue weight and epithelial cell proliferation rate of the digestive tract in rats. Journal of Nutritional Science and Vitaminology, 32, 355–362. 10.3177/jnsv.32.355 [DOI] [PubMed] [Google Scholar]
- Sakata, T. (1987a). Stimulatory effect of short‐chain fatty acids on epithelial cell proliferation in the rat intestine: A possible explanation for trophic effects of fermentable fibre, gut microbes and luminal tropic factors. British Journal of Nutrition, 58, 96–103. [DOI] [PubMed] [Google Scholar]
- Sakata, T. (1987b). Short‐chain fatty acids and water in the hindgut contents and feces of rats after hindgut bypass surgery. Scandinavian Journal of Gastroenterology, 22, 961–968. 10.3109/00365528708991943 [DOI] [PubMed] [Google Scholar]
- Sakata, T. (1994a). Message from gut microbes (in Japanese). Kagaku to Seibutsu, 32, 23–31. 10.1271/kagakutoseibutsu1962.32.23 [DOI] [Google Scholar]
- Sakata, T. (1994b). Short‐chain fatty acids as a physiological signal from gut microbes In Chivers D. J., & Langer P. (Eds.), The digestive system in mammals: Food, form and function (pp. 392–408). Cambridge: Cambridge University Press. [Google Scholar]
- Sakata, T. (2000). Microbial digestion: A strategy that allowed mammalian hervicory (in Japanese). Bulletin of Ishinomaki Senshu University, 11, 59–69. [Google Scholar]
- Sakata, T. (2007). Comparative anatomy of rat large intestine (in Japanese). Foods Food Ingredients Journal of Japan, 212, 53–56. [Google Scholar]
- Sakata, T. (2010). Tansashobousan kenkyu no otoshiana (in Japanese) In Hindgut‐Club‐Japan (Eds.), Shoukakan no eiyou seiri to tyounaisaikinn (pp. 27–32). Kyoto: Hindgut Club Japan. [Google Scholar]
- Sakata, T. , Kojima, T. , Fujieda, M. , Miyakozawa, M. , Takahashi, M. , & Ushida, K. (1999). Probiotic preparations dose‐dependently increase net production rates of organic acids and decrease that of ammonia by pig cecal bacteria in batch culture. Digestive Diseases and Sciences, 44, 1485–1493. 10.1023/A:1026624423767 [DOI] [PubMed] [Google Scholar]
- Sakata, T. , Kojima, T. , Fujieda, M. , Takahashi, M. , & Michibata, T. (2003). Influences of probiotic bacteria on organic acid production by pig caecal bacteria in vitro. Proceedings of the Nutrition Society, 62, 73–80. 10.1079/PNS2002211 [DOI] [PubMed] [Google Scholar]
- Sakata, T. , & Setoyama, H. (1997). Bi‐phasic allometric growth of the small intestine, cecum and the proximal, middle, and distal colon of rats (Rattus norvegicus Berkenhout, 1764) before and after weaning. Comparative Biochemistry & Physiology [A], 118, 897–902. 10.1016/S0300-9629(97)00222-3 [DOI] [PubMed] [Google Scholar]
- Sakata, T. , & Tamate, H. (1978). Presence of circadian rhythm in the mitotic index of the ruminal epithelium in sheep. Research in Veterinary Science, 24, 1–3. [PubMed] [Google Scholar]
- Sakata, T. , & von Engelhardt, W. (1981a). Luminal mucin in the large intestine of mice, rats and guinea pigs. Cell and Tissue Research, 219, 629–635. [DOI] [PubMed] [Google Scholar]
- Sakata, T. , & von Engelhardt, W. (1981b). Influence of short‐chain fatty acids and osmolality on mucin release in the rat colon. Cell and Tissue Research, 219, 371–377. [DOI] [PubMed] [Google Scholar]
- Stevens, C. E. , & Hume, I. D. (1995). Comparative physiology of the vertebrate digestive system, 2nd ed. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Takahashi, T. (2011). Flow behavior of digesta and the absorption of nutrients in the gastrointestine. Journal of Nutritional Sciences and Vitaminology, 57, 265–273. 10.3177/jnsv.57.265 [DOI] [PubMed] [Google Scholar]
- Takahashi, T. , & Sakata, T. (2002). Large particles increase viscosity and yield stress of pig cecal contents without changing basic viscoelastic properties. The Journal of Nutrition, 132, 1026–1030. 10.1093/jn/132.5.1026 [DOI] [PubMed] [Google Scholar]
- Takahashi, T. , & Sakata, T. (2018). Flow behavior of intestinal contents and manner of absorption of nutrients in the gastrointestine (in Japanese). Journal of Japanese Society for Parenteral & Enteral Nutrition, 57, 265–273. [Google Scholar]
- Tamate, H. , McGilliard, A. , Jacobson, N. , & Getty, R. (1962). Effect of various diets on the anatomical development of the stomach in the calf. Journal of Dairy Science, 45, 408–420. 10.3168/jds.S0022-0302(62)89406-5 [DOI] [Google Scholar]
- Tamate, H. , McGilliard, A. , Jacobson, N. , & Getty, R. (1964). Effect of various diets on the histological development of the stomach in the calf. Tohoku Journal of Agricultural Research, 14, 171–193. [Google Scholar]
- Topping, D. L. , Gooden, J. M. , Brown, I. L. , Biebrick, D. A. , McGrath, L. , Trimble, R. P. , … Illman, R. J. (1997). A high amylose (amylomaize) starch raises proximal large bowel starch and increases colon length in pigs. The Jouranal of Nutrition, 127, 615–622. 10.1093/jn/127.4.615 [DOI] [PubMed] [Google Scholar]
- Tsukahara, T. , Matsukawa, N. , Tomonaga, S. , Inoue, R. , & Ushida, K. (2014). High‐sensitivity detection of short‐chain fatty acids in procine ileal, cecal, portal and abdominal blood by gas chromatography‐mass spectrometry. Animal Science Journal, 85, 494–498. 10.1111/asj.12188 [DOI] [PubMed] [Google Scholar]
- Uhing, M. , & Kimura, R. (1995). The effect of surgical bowel manipulation and anesthesia on intestinal glucose absorption in rats. Journal of Clinical Investigation, 95, 2790–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushida, K. , & Sakata, T. (1998). Effect of pH on oligosaccharide fermentation by porcine cecal digesta. Journal of Animal Science and Technology, 69, 100–107. [Google Scholar]
- Velazquez, O. C. , Zhou, D. , Seto, R. W. , Jabbar, A. , Choi, J. , Lederer, H. M. , & Rombeau, J. L. (1996). In vivo crypt surface hyperproliferation is decreased by butyrate and increased by deoxycholate in normal rat colon: Associated in vivo effects on c‐Fos and c‐Jun expression [published erratum appears in JPEN J Parenter Enteral Nutr 1996 Nov‐Dec;20(6):428] [see comments]. Journal of Parenteral and Enteral Nutrition, 20, 243–250. 10.1177/0148607196020004243 [DOI] [PubMed] [Google Scholar]
- Vogt, J. A. , & Wolever, T. M. (2003). Fecal acetate is inversely related to acetate absorption from the human rectum and distal colon. The Journal of Nutrition, 133, 3145–3148. 10.1093/jn/133.10.3145 [DOI] [PubMed] [Google Scholar]
- von Engelhardt, W. (1995). Absorption of short‐chain fatty acids from the large intestine In Cummings J., Rombeau J., & Sakata T. (Eds.), Physiological and clinical aspects of short‐chain fatty acids (pp. 149–170). Cambridge: Cambridge University Press. [Google Scholar]
- von Engelhardt, W. , Bartels, J. , Kirschberger, S. , Meyer zu Düttingdorf, H. D. , & Busche, R. (1998). Role of short‐chain fatty acids in the hind gut. Veterinary Quarterly, 20(Suppl 3), S52–S59. 10.1080/01652176.1998.9694970 [DOI] [PubMed] [Google Scholar]
- von Engelhardt, W. , Rönau, K. , Rechkemmer, G. , & Sakata, T. (1989). Absorption of short‐chain fatty acids and their role in the hindgut of monogastric animals. Animal Feed Science and Technology, 23, 43–53. 10.1016/0377-8401(89)90088-6 [DOI] [Google Scholar]
- Wallace, R. J. (1995). Biochemistry and microbiology In Cummings J. H., Rombeau J. L., & Sakata T. (Eds.), Physiological and clinical aspects of short chain fatty acids (pp. 57–71). Cambridge: Cambridge University Press. [Google Scholar]
- Watanabe, Y. , & Murai, H. (1968). The production of volatile fatty acid, lactic acid and glucose and arterio‐veneous differences of volatile fatty acid in sections of the alimentary tract of pig (in Japanese with English abstract). Shinshu Daigaku Nougakubu Kiyou, 5, 29–36. [Google Scholar]
- Wrong, O. M. (1995). Definitions and history In Cummings J., Rombeau J., & Sakata T. (Eds.), Physiological and clinical aspects of short‐chain fatty acids (pp. 1–14). Cambridge: Cambridge University Press. [Google Scholar]
- Yajima, T. (1985). Contractile effect of short‐chain fatty acids on the isolated colon of the rat. Journal of Physiology, 368, 667–678. 10.1113/jphysiol.1985.sp015882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima, T. (1995). Sensory mechanism for short‐chain fatty acids in the colon In Cummings J., Rombeau J., & Sakata T. (Eds.), Physiological and clinical aspects of short‐chain fatty acids (pp. 209–221). Cambridge: Cambridge University Press. [Google Scholar]
- Yajima, T. , & Sakata, T. (1992). Core‐ and periphery‐concentrations of short‐chain fatty acids in luminal contents of the rat colon. Comparative Biochemistry & Physiology, 103A, 353–355. 10.1016/0300-9629(92)90593-F [DOI] [PubMed] [Google Scholar]


