Abstract
Interest has increased in comorbidities associated with psoriasis and their effects on health‐related quality of life (HRQoL). This study aimed to evaluate the prevalence of metabolic syndrome (MetS) and psoriatic arthritis (PsA) and to investigate HRQoL and the prevalence of hypertension, type 2 diabetes mellitus (T2DM), obesity and dyslipidemia. In a cross‐sectional design, patients diagnosed with plaque psoriasis answered an interview and standardized questionnaires (Dermatology Life Quality Index questionnaire [DLQI], 36‐Item Short Form Health Survey [SF‐36] and EuroQol Five‐Dimension Questionnaire Three‐Level version [EQ‐5D‐3L]). Physical examination and several tests to assess desired outcomes were performed by a dermatologist and a rheumatologist during three visits. The prevalence of MetS and PsA was 50.0% and 41.8%, respectively. Dyslipidemia was the most prevalent (74.5%) secondary comorbidity, followed by hypertension (61.8%), obesity (52.5%) and T2DM (30.9%). The mean (standard deviation) DLQI score was 6.5 (6.9), and mean physical and mental SF‐36 measures were 45.2 (10.4) and 45.5 (12.3), respectively, and for EQ‐5D‐3L, mean utility index and EQ‐VAS scores were 0.68 (0.27) and 72.7 (19.7), respectively. PsA and MetS are important comorbidities; a reduced HRQoL is noted among plaque psoriasis patients with these comorbidities, emphasizing the relevance of diagnosis and treatment beyond the care of skin lesions.
Keywords: metabolic syndrome, psoriasis, psoriatic arthritis, quality of life, systemic disease
Introduction
Psoriasis (PsO) can be defined as a chronic, systemic inflammatory disease that can affect the skin, semi‐mucosa, joints, and additional organs and tissues. Clinical manifestations can be presented as plaques on elbows, knees or scalp and/or superficial pustules on palms, soles or diffused over the body.1 There are several clinical variations of PsO; psoriasis vulgaris (also known as plaque psoriasis) is the most common (75–90%).2 Disease prevalence is as high as 11.8% in several reports from different countries.1, 3, 4, 5, 6 A recent telephone survey estimates a prevalence of 1.3% in Brazil.1, 3, 4, 5, 6
The pathophysiology of PsO involves an interaction between inflammatory components, elements of the innate and adaptive immune response, and abnormal proliferation and differentiation of keratinocytes.3 The immune‐mediated feature characterizes PsO as a systemic illness that contributes to a greater frequency of comorbidities among affected subjects.7, 8
Common comorbidities associated with PsO include metabolic syndrome (MetS), psoriatic arthritis (PsA), diabetes, obesity, hypertension and dyslipidemia. The estimated prevalence of MetS described in the general population varies from 0.2% to 43.9%; however, its association with PsO may be approximately fivefold higher, depending on the characteristics of the population studied.2, 9, 10, 11, 12, 13, 14, 15, 16 The prevalence of PsA among patients with plaque psoriasis ranges 5.9–48%, depending on the patient characteristics and criteria used.17, 18 The presence of MetS and/or PsA differs according to disease severity.19, 20, 21 Additionally, according to the published work, 8.3%, 18.4%, 19.6% and 20.0% of patients with plaque psoriasis had comorbid diabetes, obesity, hypertension and dyslipidemia, respectively.22
There is an increasing interest within the Brazilian research community to identify the comorbidities associated with PsO and understand the effect of the disease on quality of life. However, this subject is still scarcely researched. Thus, the primary objectives of this study were to evaluate the prevalence of MetS and PsA; secondary objectives were to evaluate patient health‐related quality of life (HRQoL) and the prevalence of hypertension, type 2 diabetes mellitus (T2DM), obesity and dyslipidemia among patients with plaque psoriasis.
Methods
Study design and eligibility criteria
This was a cross‐sectional observational study conducted in nine tertiary centers, located in southeastern, southern and northern Brazilian regions, specializing in PsO treatment, in the following Brazilian cities (with respective states): São Paulo, Santo André and Ribeirão Preto (state of São Paulo); Rio de Janeiro and Niterói (state of Rio de Janeiro); Curitiba (state of Paraná); Porto Alegre (state of Rio Grande do Sul); and Belém (state of Pará). Eligible patients were those diagnosed with plaque psoriasis reported by medical records and aged 18 years and older. Patients unable to provide informed consent and/or answer the interview, with confirmed/suspected pregnancy and/or enrolled in a drug‐interventional study in the past 12 months were excluded.
Data collection
Data collection was performed between April 2014 and May 2015 over three study visits within a 30‐day interval between each visit (±10 days).
During visit 1, patients answered a structured interview, which included questions about sociodemographic and disease characteristics, comorbidities and PsO treatment. Additionally, three standardized questionnaires were used to assess HRQoL: Dermatological Life Quality Index (DLQI), 36‐Item Short Form Health Survey (SF‐36) and EuroQol Five‐Dimension Questionnaire Three‐Level version (EQ‐5D‐3L). Dermatologists performed a physical examination of patients, including blood pressure measurements, radiograph of hands, wrists and feet, and laboratory tests. A pregnancy test was required for all women with child‐bearing potential. Disease severity was classified according to Finlay's Rule of Tens; patients with a Psoriasis Area and Severity Index (PASI) score of more than 10 and/or DLQI score of more than 10 or body surface area involvement of more than 10% were considered to have severe PsO.23 Patients not fulfilling this criterion were considered to have mild to moderate PsO.
At visit 2, a rheumatologist performed a physical examination, including blood pressure measurements. According to the rheumatologist evaluation, 24‐h non‐invasive ambulatory blood pressure monitoring (ABPM) was also required. During this visit, specific imaging and laboratory tests were available for rheumatology assessment and a medical diagnosis of PsA was defined during this visit by the study physician.
At visit 3, patients were informed about all tests results and the need for medical follow up. Medical diagnosis of MetS, hypertension, T2DM, dyslipidemia and obesity were defined during this visit by the study physician.
Prevalence of PsA and MetS
For this study, the diagnosis of new cases of MetS was defined as the presence of three or more characteristics of the modified version of the criteria proposed by the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) and described in the first Brazilian Guidelines for Diagnosis and Treatment of Metabolic Syndrome (Table S1).24 Individuals currently using antihypertensive, lipid‐lowering or antidiabetic drugs were considered to meet the related diagnosis criterion. The new cases diagnosis of PsA was defined as the presence of 3 points or more in the Classification Criteria for Psoriatic Arthritis (CASPAR).25 The self‐reported diagnosis of MetS and PsA by patients was also considered to identify the presence of these comorbidities.
Prevalence of hypertension, T2DM, obesity and dyslipidemia
Hypertension was diagnosed/defined as any of the following: presence of disease and/or use of antihypertensive medications according to the patient's history or medical records; blood pressure of 180 × 110 mmHg or more at visit 1 and/or visit 2; or ABPM results (24‐h, >125/75 mmHg; awake, >130/85 mmHg; asleep, >110/70 mmHg).26 The diagnosis of T2DM was defined as a confirmed diagnosis of diabetes and/or use of hypoglycemic drugs, according to patient history or medical records or fasting plasma glucose of 126 mg/dL or more, or glycated hemoglobin A1c of 6.5% or more.27 Obesity was defined as a body mass index of 30 kg/m2 or more.28 Dyslipidemia was identified as the presence of already diagnosed disease and/or the use of lipid‐lowering medications, according to the patient history or medical records, considering the Brazilian Guidelines for Dyslipidemia (low‐density lipoprotein [LDL] of ≥160 mg/dL and/or triglycerides of ≥150 mg/dL and/or high‐density lipoprotein [HDL] of <40 mg/dL in men/<50 mg/dL in women or in association with LDL or triglyceride increase).24
Health‐related quality of life
The DLQI is a quality‐of‐life questionnaire specifically for patients with dermatological conditions, consisting of 10 questions concerning symptoms and feelings, daily activities, leisure, work, school, personal relationships and treatment. The total score is calculated by summing the score of each question, resulting in a maximum score of 30 and a minimum score of 0, with higher scores representing a worsening quality of life.29
The SF‐36 is a generic measure of HRQoL, composed of 36 questions grouped into eight health domains (physical functioning, role‐physical, bodily pain, general health, vitality, social functioning, role‐emotional and mental health) that contribute to the calculation of the physical component summary (PCS) and mental component summary (MCS) scores. The raw score of each dimension was derived by summing the item scores and converting the score to a value from 0 (worst possible health state) to 100 (best possible health state).30
The EQ‐5D‐3L is a generic instrument that assesses health status through five domains (mobility, self‐care, usual activities, pain/discomfort and anxiety/depression) with three response levels (no problems, some problems and extreme problems). It also includes a visual analog scale, which records respondents’ self‐rated health, according to end‐points labeled from best imaginable health state(100 points) to worst imaginable health state (0 points). A utility score is also calculated based on the dimensions scores, using the UK value set, ranging between 0 (representing death) and 1 (representing perfect health).31, 32
All HRQoL instruments applied in this study were previously translated and validated to Brazilian Portuguese.33, 34, 35
Sample size calculation
Sample size was calculated according to the study's primary objective, considering a PsA prevalence of 20.6%36 and a MetS prevalence of 17.8%.37 Samples of 238 and 225 subjects were necessary to estimate the prevalence of PsA and MetS, respectively, with a precision of 0.05 (error, ±0.05). Including a 20% dropout rate, the largest sample size estimation was a total of 298 subjects with plaque psoriasis.
Statistical analysis
Descriptive analysis was performed by estimation of measures of central tendency and dispersion for quantitative variables and frequency for qualitative variables. Logistic regression was used to build a multivariate model to assess factors associated with PsA/MetS, controlled for possible confounders and interactions. Confounders/interactions were identified from inclusion and exclusion of each covariate in the models. In this process, significant changes in respective coefficients were observed, and the possibilities of confounding or interactions were verified. All potential covariates were selected according to their importance as described in the published work. Final model variates were selected from univariate model, according to a significance level of 0.250. A significance level of α = 0.05 was used for confidence interval (CI) calculations and significance testing. Analyses were performed using Stata version MP11 (StataCorp, College Station, TX, USA) and R Project version 2.13.1 (R Foundation, Vienna, Austria).
Ethical approval
This study was approved by the independent ethics committees of each participating site: Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo – HCFMRP‐USP (no. 532.679); Hospital das Clinicas da Faculdade de Medicina da Universidade de São Paulo–HCFMUSP (no. 706.903); Faculdade de Medicina do ABC–FMABC (no. 676.619); Instituto de Dermatologia e Estética do Brasil Ltda–IDERJ (no. 694.166); Universidade Federal Fluminense – UFF (no. 783.956); Hospital Universitário Evangélico de Curitiba – HUEC (no. 725.735); Irmandade da Santa Casa de Misericórdia de Curitiba (no. 680.066); Hospital de Clínicas de Porto Alegre–HCPA (no. 692.687); and Universidade do Estado do Pará – UEPA (no. 753.414). Written approval was obtained from all respondents before participation in the research.
Results
Sociodemographic characteristics
In Table 1, several characteristics of the patient population are described. The study enrolled 293 individuals, 51.9% male, with a mean age of 52.0 (standard deviation [SD] = 12.8) years. Most patients were Caucasian/white (67.9%). Regarding employment status and education levels, 31.4% of subjects were employed and 28.3% completed high school. Of the total sample, 16.7% were current smokers, 35.8% reported alcohol consumption (of drinkers, 54.3% reported ingesting alcohol <1/week) and 33.4% were sedentary. According to the Rule of Tens, 83.3% of patients were classified as having severe PsO. In the past 12 months, 97.2% of patients reported the use of one or more drug for PsO treatment and 20.1% used phototherapy. Among patients who reported medication use, 90.2% received topical drugs, 42.1% received conventional systemic drugs and 31.6% received biologic drugs. Only 4.4% of patients had a family history of PsA and 15.7% had a family history of MetS.
Table 1.
Sociodemographic and clinical characteristics of patients with plaque‐type psoriasis in a Brazilian population (n = 293)
| Characteristics | n | % |
|---|---|---|
| Age | ||
| Mean ± SD | 52.0 ± 12.8 | – |
| Sex | ||
| Male | 152 | 51.9 |
| Female | 141 | 48.1 |
| Race | ||
| Caucasian/white | 199 | 67.9 |
| Brown | 79 | 27.0 |
| Black | 13 | 4.4 |
| Asian | 1 | 0.3 |
| Indigenous | 1 | 0.3 |
| Educational level | ||
| No education | 3 | 1.0 |
| Incomplete elementary school | 75 | 25.6 |
| Complete elementary school | 33 | 11.3 |
| Incomplete high school | 31 | 10.6 |
| Complete high school | 83 | 28.3 |
| Incomplete college and/or university degree | 21 | 7.2 |
| Complete college and/or university degree | 36 | 12.3 |
| Post‐graduate | 11 | 3.7 |
| Family history | ||
| MetS | 46 | 15.7 |
| PsA | 13 | 4.4 |
| Employment status | ||
| Employed | 92 | 31.4 |
| Retired | 87 | 29.7 |
| Autonomous worker | 66 | 22.5 |
| Unemployed | 22 | 7.5 |
| Housewife | 13 | 4.4 |
| Student | 9 | 3.1 |
| Other† | 4 | 1.4 |
| Monthly household income ($US) | ||
| Mean ± SD | 774.29 ± 687.85 | – |
| PASI | ||
| PASI score, mean ± SD | 7.3 ± 8.4 | – |
| PASI score >10 | 219 | 74.7 |
| BSA | ||
| BSA involvement %, mean ±SD | 12.7 ± 15.8 | – |
| BSA involvement >10 | 212 | 72.4 |
| DLQI score | ||
| DLQI score, mean ± SD | 6.5 ± 6.9 | – |
| DLQI score >10 | 65 | 22.2 |
| Severity of psoriasis (defined as Finlay's Rule of Tens) | ||
| Severe psoriasis (PASI score >10 and/or DLQI score >10 or BSA involved >10%) | 244 | 83.3 |
| Mild to moderate psoriasis | 49 | 16.7 |
| Treatment pattern | ||
| Phototherapy | 59 | 20.1 |
| Medicines | 285 | 97.2 |
| Biologic | 90 | 31.6 |
| Conventional systemic | 120 | 42.1 |
| Topical | 257 | 90.2 |
| Smoking | ||
| Non‐smokers | 131 | 44.7 |
| Current | 49 | 16.7 |
| Past | 113 | 38.6 |
| Length of abstinence in years (n = 113), mean ± SD | 15 ± 10.4 | – |
| No. of cigarettes per day (n = 162), mean ± SD | 19.3 ± 18.5 | – |
| “How long have you been smoking?”, years (n = 162), mean ± SD | 22.3 ± 13.9 | – |
| Alcoholism | ||
| Non‐drinkers | 188 | 64.2 |
| Drinkers | 105 | 35.8 |
| Frequency of alcohol consumption (n = 105) | ||
| <1 per week | 57 | 54.3 |
| Once per week | 20 | 19 |
| Twice per week | 16 | 15.2 |
| 3 times a week | 5 | 4.8 |
| 4 times a week | 1 | 1.0 |
| 5 times a week | 1 | 1.0 |
| 6 times a week | 1 | 1.0 |
| 7 times a week | 3 | 2.9 |
| No information | 1 | 1.0 |
| “How long have you been drinking?”, years (n = 105), mean ± SD | 28.6 ± 14 | – |
| Physical activity | ||
| Sedentary | 98 | 33.4 |
| Currently physically active | 101 | 34.5 |
| Physically active in the past | 94 | 32.1 |
| Time (years) without practicing (n = 94), mean ± SD | 7.2 ± 7.8 | – |
| Frequency of physical activity (n = 195) | ||
| Once per week | 26 | 13.3 |
| Twice per week | 39 | 20 |
| 3 times a week | 47 | 24.1 |
| 4 times a week | 15 | 7.7 |
| 5 times a week | 40 | 20.5 |
| 6 times a week | 5 | 2.6 |
| 7 times a week | 23 | 11.8 |
| “How long have you been practicing physical activity?”, years, mean ± SD | 10 ± 12.5 | – |
†Off work (n = 2) and pensioner (n = 2). BSA, body surface area; DLQI, Dermatology Life Quality Index; MetS, metabolic syndrome; PASI, Psoriasis Area and Severity Index; PsA, psoriatic arthritis; SD, standard deviation.
Prevalence of PsA, MetS and related comorbidities
The prevalence of PsA was 41.8% (95% CI, 36.0–47.6) in patients who fulfilled CASPAR (35.7%) and/or had reported a medical history of PsA (23.1%). Of the total number of patients with a PsA diagnosis, new cases accounted for 44.9% (53/118). Considering only CASPAR (n = 100), the estimated proportion of newly diagnosed cases represented 53.0% of patients diagnosed using these criteria (Table 2), and all of these patients presented with a current/personal history/family history of PsO and inflammatory articular disease. For the other CASPAR items, 86.0% had a negative test for rheumatoid factor, 79.0% had radiological evidence of juxta‐articular new bone formation, 61.0% had psoriatic nail dystrophy and 22.0% had dactylitis (current or history).
Table 2.
Prevalence of PsA and MetS among patients with plaque‐type psoriasis in a Brazilian population (n = 282)
| Comorbidity | Diagnostic categories | n | % | 95% CI |
|---|---|---|---|---|
| Psoriatic arthritis | History of PsA reported by the patient (n = 282) | 65 | 23.1 | 30.1–41.3 |
| PsA diagnosed by the study physician, CASPAR (n = 282) | 100 | 35.7 | 37.2–56.8 | |
| Previous diagnosis among CASPAR‐positive patients (n = 100) | 47 | 47.0 | 43.2–62.8 | |
| Newly diagnosed among CASPAR‐positive patients (n = 100) | 53 | 53.0 | 18.2–28.0 | |
| Final prevalence of PsA (CASPAR or history) (n = 282) | 118 | 41.8 | 36.0–47.6 | |
| Metabolic syndrome | History of MetS reported by the patient (n = 282) | 17 | 6.0 | 3.2–8.8 |
| Diagnosis confirmed by the physician (n = 282) | 137 | 48.6 | 42.8–54.4 | |
| Patients newly diagnosed in the study (n = 141) | 107 | 75.9 | 68.8–83.0 | |
| Final prevalence of MetS (physician diagnosis or history) (n = 282) | 141 | 50.0 | 44.2–55.8 |
CASPAR, Classification Criteria for Psoriatic Arthritis; CI, confidence interval; MetS, metabolic syndrome; PsA, psoriatic arthritis.
The prevalence of MetS was 50.0%; 75.9% were newly diagnosed in the study. Considering the modified version of NCEP ATP III criteria (n = 138), 88.4% had a fasting blood glucose level of 110 mg/dL or more, and/or history of T2DM and/or use of antidiabetic drugs; 81.2% had HDL of less than 40 mg/dL (males) or less than 50 mg/dL (females); 77.5% had a waist circumference of more than 102 cm (males) or more than 88 cm (females); 63.8% had triglycerides of 150 mg/dL or more; and 56.5% had blood pressure of 130 × 85 mmHg or more, and/or a history of systemic arterial hypertension and/or use of antihypertensive drugs.
As assessed by secondary outcomes, the estimated prevalence of comorbidities is shown in Figure 1. Dyslipidemia was the most prevalent comorbidity (74.5%), followed by hypertension (61.8%), obesity (52.5%) and T2DM (30.9%).
Figure 1.
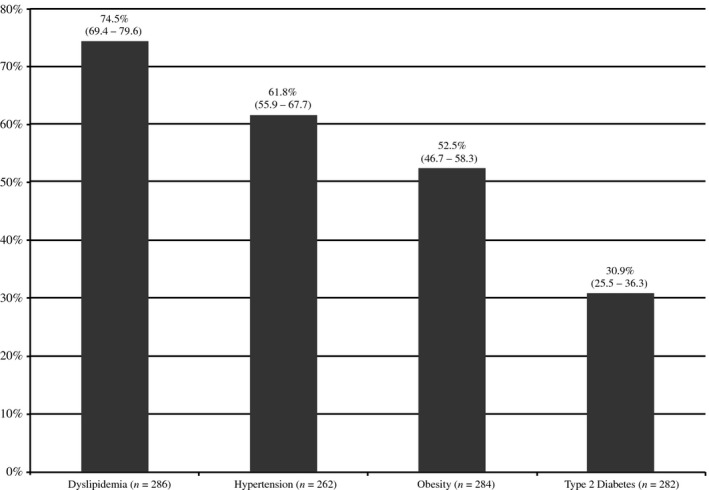
Prevalence of comorbidities assessed as secondary outcomes among patients with plaque‐type psoriasis.
Health‐related quality of life
Data regarding HRQoL are shown in Table 3. The DLQI mean score was 6.5 (SD = 6.9). According to questionnaire's dimensions, the greatest impact was noted in symptoms and feelings (mean = 2.1; SD = 1.7). Nevertheless, all dimensions presented less than half of the maximum value for each dimension, indicating impairment of patients’ HRQoL. Regarding DLQI categories, the quality of life of a majority of patients (70.2%) was affected, considering the summation of the answers that represents some effect on the patient's life (whether small, moderate, very or extreme; Fig. 2).
Table 3.
Quality‐of‐life scores on each scale, using DLQI, SF‐36 and EQ‐5D‐3L questionnaires among patients with plaque‐type psoriasis in a Brazilian population
| HRQoL measure | Dimension | Mean | SD |
|---|---|---|---|
| DLQI (n = 292) | Symptoms and feelings (maximum = 6) | 2.1 | 1.7 |
| Daily activities (maximum = 6) | 1.5 | 1.8 | |
| Leisure (maximum = 6) | 1.3 | 1.7 | |
| Personal relationships (maximum = 6) | 0.8 | 1.5 | |
| Work and school (maximum = 3) | 0.4 | 0.8 | |
| Treatment (maximum = 3) | 0.4 | 0.7 | |
| Total (maximum = 30) | 6.5 | 6.9 | |
| SF‐36 (n = 292) | Vitality (0–100) | 50.6 | 11.2 |
| General health (0–100) | 45.1 | 11.0 | |
| Role‐physical (0–100) | 44.8 | 11.8 | |
| Social functioning (0–100) | 44.4 | 12.3 | |
| Mental health (0–100) | 44.1 | 12.8 | |
| Physical functioning (0–100) | 44.0 | 11.4 | |
| Bodily pain (0–100) | 43.9 | 11.6 | |
| Role‐emotional (0–100) | 43.5 | 12.6 | |
| Physical component summary score (0–100) | 45.2 | 10.4 | |
| Mental component summary score (0–100) | 45.5 | 12.3 | |
| EQ‐5D‐3L (n = 286) | Utility score (0–1) | 0.68 | 0.27 |
| Overall value (0–100) | 72.7 | 19.7 |
DLQI, Dermatology Life Quality Index; EQ‐5D‐3L, EuroQol Five‐Dimension Questionnaire Three‐Level version; SD, standard deviation; SF‐36, 36‐Item Short Form Health Survey.
Figure 2.
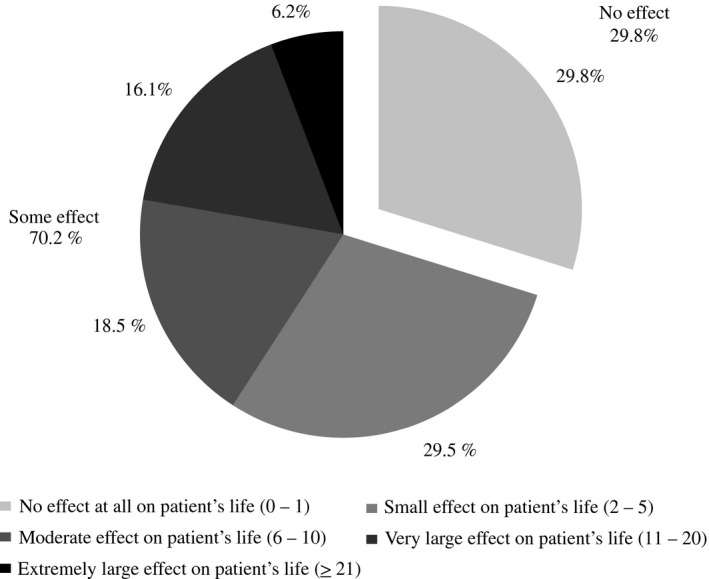
Impairment in health status according to the Dermatology Life Quality Index classification among patients with plaque psoriasis (n = 292).
According to SF‐36 results, the most impaired scales were role‐emotional (mean = 43.5; SD = 12.6) and bodily pain (mean = 43.9; SD = 11.6). For the EQ‐5D‐3L instrument, the mean utility index was 0.68 (SD = 0.27); the mean EQ‐5D‐3L visual analog scale was 72.7 (SD = 19.7). Among the five dimensions, self‐care was the least affected, with 84.6% of the responders reporting an absence of problems. The highest impairment was observed for the dimension of pain and discomfort (66.4% of patients reported to have problems, some problems or extreme). Extreme problems were more frequently reported in the dimension of anxiety and depression (9.4%; Fig. 3).
Figure 3.
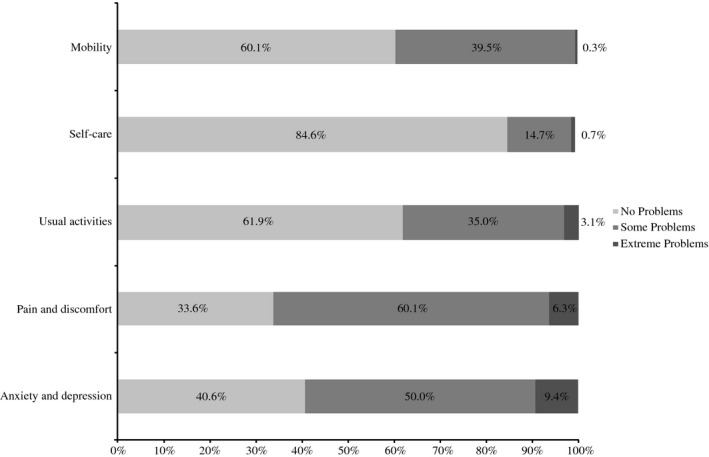
Limitations in health‐related quality of life in each dimension of the EuroQol Five‐Dimension Questionnaire Three‐Level version questionnaire among patients with plaque‐type psoriasis (n = 286).
Logistic regression models
In the present study, multivariate models were used to assess factors associated with the presence of PsA and MetS among plaque psoriasis patients (Tables S2,S3). A multivariate model for PsA (adjusted by use of topical drugs and phototherapy and frequency of alcohol consumption) showed that a greater odds of PsA diagnosis was associated with treatment with biologic drugs (odds ratio [OR] = 3.49; 95% CI, 1.91–6.38; P < 0.001) and conventional systemic medicines (OR = 2.41; 95% CI, 1.4–4.16; P = 0.002); conversely, a higher SF‐36 PCS was associated with a decreased odds of PsA diagnosis (OR = 0.96; 95% CI = 0.93–0.99; P = 0.002).
For MetS, the final model (adjusted by severe PsO, PsA, use of conventional systemic drugs, alcohol consumption, frequency of smoking and EQ‐5D‐3L) indicated that patients with a family history of MetS had a greater odds of having MetS compared with those without a family history (OR = 2.28; 95% CI, 1.35–3.87; P = 0.002); the use of phototherapy (OR = 0.51; 95% CI, 0.26–0.98; P = 0.045) and an increase in SF‐36 PCS (OR = 0.96; 95% CI, 0.93–0.99; P = 0.015) were negatively associated.
Discussion
Psoriatic arthritis and MetS are common comorbidities of patients with PsO; however, their frequency present a great variation across subpopulations. Thus, there is a need to understand the prevalence of these conditions in patients with plaque psoriasis to establish proper therapeutic management and reinforce the need for comprehensive guidelines addressing comorbidities.
This study demonstrated that the prevalence of PsA findings among Brazilian patients with PsO is in agreement with other investigations performed in Brazilian cohorts. Carneiro et al.38, 39 and Ranza et al.38, 39 reported PsA prevalence of 35.0% and 33.3%, respectively, using CASPAR. The aforementioned studies included patients with all types of PsO and both studies reported a high prevalence of plaque‐type psoriasis (66.9–78.8%). Therefore, these analyses had populations similar to the sample of the present study.
The results of our study also showed that of 118 prevalent cases of PsA, 44.9% were newly diagnosed by the study rheumatologist. This is consistent with the results reported in Ranza et al. (49.0%)39 that highlighted the frequency of undiagnosed cases. Some authors feel that the diagnosis of PsA should be facilitated by dermatologists, as underdiagnosis may lead to potentially avoidable joint damage that permanently disables and deforms some individuals.40
To our knowledge, to date, this is the first multicenter study conducted in Brazil that reports the prevalence of MetS in a population of patients with PsO. The prevalence of MetS in the present study was 50.0%. A single center study carried out by Baeta et al.41 in the southeastern region of Brazil (n = 190 patients with PsO) observed a 44.9% prevalence of MetS, similar to our study. A case–control Brazilian study investigated research questions similar to ours regarding PsO comorbidities, specifically, the frequency of hypertension and MetS. Our findings were similar to the ones described by Menegon et al.42 regarding both the proportion of patients with MetS (47.1%) and the frequency of hypertension (57.7%). The prevalence of MetS in Brazilian patients with PsO reported in the present study was far higher than in the general Brazilian population (29.6%, ranging 14.9–65.3%).43 This finding is probably indicative of the mean age of our sample (52.0 years; SD = 12.8), as older age contributes to the higher prevalence of diabetes and hypertension.
Regarding HRQoL, DLQI values are slightly lower than those reported in previous research.44, 45, 46 This may be because a significant percentage of our sample received systemic and biologic drugs, treatments that are associated with a positive impact on HRQoL. Tejada et al.47 have pointed out that PsO is the skin disease with the highest DLQI scores and, therefore, one of the most damaging dermatological illnesses, which highlights the importance of effective treatment options. The SF‐36 PCS (mean = 45.2; SD = 10.4) and MCS (mean = 45.5; SD = 12.3) central tendency measures for our cohort were worse than in the general Brazilian population (mean PCS = 49.3; 95% CI, 49.1–49.5; mean MCS = 51.1; 95% CI, 50.9–51.3).48 However, considering the high SD, the central tendency measures may vary between better and worse than measures of the general Brazilian population. The multivariate analysis showed that a high PCS is inversely associated with the presence of MetS and PsA, indicating that patients that do not have these comorbidities likely have better HRQoL related to physical aspects. The EQ‐5D‐3L utility index among our sample was 0.68 (SD = 0.27), which is in consonance with other investigations performed in patients with PsO, ranging 0.52 to 0.90.44, 49
Results from the DLQI, SF‐36 and EQ‐5D‐3L showed the impact of the disease on patients’ emotional aspects. The most affected DLQI domain in this PsO cohort was symptoms and feelings. This pattern was similarly seen in other studies performed in patients with dermatological conditions.47, 50 Similarly, role‐emotional was the most impaired SF‐36 domain in the study participants, followed by bodily pain. The mean SF‐36 scores for role‐emotional and bodily pain in our sample were 43.5 (SD = 12.6) and 43.9 (SD = 11.6), which were much lower than the mean scores for these domains in the general Brazilian population (81.7 and 76.7, respectively).48 The results of the EQ‐5D‐3L showed that the most affected dimensions of patients’ well‐being were those referring to pain and discomfort and anxiety/depression. Our results emphasize the role played by emotional aspects in the quality of life of patients with PsO, endorsing the argument that skin diseases frequently invoke strong negative emotions such as frustration and embarrassment as a reflection that the skin is responsible in large part for an individual's appearance.
The study's main limitation is the impossibility of establishing a temporal relationship between exposure and outcome due to its cross‐sectional nature; nonetheless, it still provides useful information worthy of exploring in further prospective studies. A certain degree of caution is required concerning the transferability of the results for the whole country, because Brazil is a continent‐sized country marked by considerable environmental and genetic differences, and the northeastern and mid‐west regions were not included. Furthermore, it is important to underscore that the study patients are from tertiary care centers; consequently, those subjects tend to have more severe cases, contributing to a higher prevalence of comorbidities. There are also limitations regarding the understanding of the disease scenario in other levels of care, particularly for patients presenting with milder forms of PsO.
The results of this study firmly establish that MetS and PsA are extremely prevalent among patients with plaque psoriasis. These findings emphasize the relevance of diagnosis and treatment of patients with PsO beyond the care of skin lesions. Cooperation between rheumatologists and dermatologists for the early detection of these comorbidities is crucial.
Patients with plaque psoriasis have a reduced quality of life mainly in terms of emotional aspects, which reinforces the existing data regarding feelings of stigmatization in patients with PsO. It also indicates the relevance of HRQoL measures for patients with plaque psoriasis, already incorporated in Brazilian clinical practise. Physicians should routinely use HRQoL scores to assess patient response to treatment in a more comprehensive way.
Supporting information
Table S1. Components of metabolic syndrome according to National Cholesterol Education Program Adult Treatment Panel III criteria22
Table S2. Logistic regression model for psoriatic arthritis (first model, n = 275)
Table S3. Logistic regression model for metabolic syndrome (first model, n = 261)
Acknowledgments
The design, study conduct and financial support for the study were provided by AbbVie. AbbVie participated in the interpretation of data, review and approval of the content. All the authors had access to all relevant data and participated in the writing, review and approval of this manuscript. Medical writing support and editorial support was provided by Leticia Dias, Pamela Santana and Maíra Takemoto of ANOVA ‐ Consultoria em Saúde Ltda (Rio de Janeiro/Brazil) and was funded by AbbVie.
Conflict of Interest
Dr Cacilda da Silva Souza has served as a consultant and/or investigator to AbbVie, Janssen‐Cilag and Novartis. Dr Caio César Silva de Castro has served as a speaker for Novartis and Janssen, an investigator for AbbVie and Janssen, and a consultant for Pfizer. Dr Francisca Regina Oliveira Carneiro has received consultant and speaker payments from AbbVie. Dr Jane Marcy Neffá Pinto has received sponsorship for scientific events from AbbVie, Pfizer and Leo Pharma. Dr Lincoln Helder Zambaldi Fabricio has served as speaker for Abbott/AbbVie, Bayer, Bioderma, Biolab, Boticário, Galderma, Hypermarcas, Isdin, Janssen, La Roche‐Posay, Leo Pharma, Pfizer and Stiefel/GSK; has received sponsorship for scientific events from Abbott/AbbVie, Bayer, Bioderma, Galderma, Isdin, Janssen, La Roche‐Posay, Leo Pharma, Pfizer MSD and Novartis; and has participated in advisory boards for Bayer, Janssen, La Roche‐Posay, Leo Pharma and MSD. Dr Luiza Keiko Matsuka Oyafuso is a consultant and/or investigator for AbbVie, Janssen‐Cilag and Novartis. Dr Luna Azulay‐Abulafia is/has served as a consultant and/or investigator for AbbVie, Janssen‐Cilag, Leo Pharma, Lilly, Novartis and Pfizer. Dr Ricardo Romiti is/has served as a consultant, speaker and/or investigator for AbbVie, Bioderma, Galderma, Janssen‐Cilag, Leo Pharma, Lilly, Novartis, Pfizer and UCB. Dr Tania Ferreira Cestari is/has served as a speaker, consultant and/or investigator for AbbVie, Janssen‐Cilag, Leo Pharma, La Roche‐Posay, Novartis, Pierre‐Fabre and Vichy. Cláudia Eiko Suzuki and Priscila Martin Biegun are AbbVie employees and may own AbbVie stocks or options. Luciana Schuck Guedes was an AbbVie employee during the development of this publication.
References
- 1. Meier M, Sheth PB. Clinical spectrum and severity of psoriasis. Curr Probl Dermatol 2009. Jan; 38: 1–20. [DOI] [PubMed] [Google Scholar]
- 2. Departamento de Ciência e Tecnologia, Ministerio da Saúde, Brasil . Secretaria de Ciência Tecnologia e Insumos Estratégicos. Protocolo Clínico e diretrizes terapêuticas da psoríase. 2013.
- 3. Pathirana D, Ormerod AD, Saiag P et al European S3‐guidelines on the systemic treatment of psoriasis vulgaris. J Eur Acad Dermatol Venereol 2009. Oct; 23(Suppl 2): 1–70. [DOI] [PubMed] [Google Scholar]
- 4. Chandran V, Raychaudhuri SP. Geoepidemiology and environmental factors of psoriasis and psoriatic arthritis. J Autoimmun 2010. May; 34(3): J314–J321. [DOI] [PubMed] [Google Scholar]
- 5. Gudjonsson JE, Elder JT. Psoriasis: epidemiology. Clin Dermatol 2007; 25(6): 535–546. [DOI] [PubMed] [Google Scholar]
- 6. Romiti R, Amone M, Menter A, Miot HA. Prevalence of psoriasis in Brazil ‐ a geographical survey. Int J Dermatol 2017; 56(8): e167–e168. [DOI] [PubMed] [Google Scholar]
- 7. Gottlieb AB, Chao C, Dann F. Psoriasis comorbidities. J Dermatol Treat 2008. Jan 12; 19(1): 5–21. [DOI] [PubMed] [Google Scholar]
- 8. Oliveira Mde FSP, Rocha Bde O, Duarte GV. Psoriasis: classical and emerging comorbidities. An Bras Dermatol 2015; 90(1): 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mrowietz U, Elder JT, Barker J. The importance of disease associations and concomitant therapy for the long‐term management of psoriasis patients. Arch Dermatol Res 2006. Dec; 298(7): 309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meyer N, Paul C, Feneron D et al Psoriasis: an epidemiological evaluation of disease burden in 590 patients. J Eur Acad Dermatol Venereol 2010. Sep; 24(9): 1075–1082. [DOI] [PubMed] [Google Scholar]
- 11. Al‐Mutairi N, Al‐Farag S, Al‐Mutairi A, Al‐Shiltawy M. Comorbidities associated with psoriasis: an experience from the Middle East. J Dermatol 2010. Feb; 37(2): 146–155. [DOI] [PubMed] [Google Scholar]
- 12. Gisondi P, Tessari G, Conti A et al Prevalence of metabolic syndrome in patients with psoriasis: a hospital‐based case‐control study. Br J Dermatol 2007. Jul; 157(1): 68–73. [DOI] [PubMed] [Google Scholar]
- 13. Chen Y‐J, Wu C‐Y, Shen J‐L et al Psoriasis independently associated with hyperleptinemia contributing to metabolic syndrome. Arch Dermatol 2008. Dec; 144(12): 1571–1575. [DOI] [PubMed] [Google Scholar]
- 14. Augustin M, Reich K, Glaeske G, Schaefer I, Radtke M. Co‐morbidity and age‐related prevalence of psoriasis: analysis of health insurance data in Germany. Acta Derm Venereol 2010. Mar; 90(2): 147–151. [DOI] [PubMed] [Google Scholar]
- 15. Parisi R, Symmons DPM, Griffiths CEM, Ashcroft DM. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 2013. Feb; 133(2): 377–385. [DOI] [PubMed] [Google Scholar]
- 16. Praveenkumar U, Ganguly S, Ray L, Nanda SK, Kuruvila S. Prevalence of metabolic syndrome in psoriasis patients and its relation to disease duration: a hospital based case‐control study. J Clin Diagn Res 2016; 10(2): WC01–WC05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Prey S, Paul C, Bronsard V et al Assessment of risk of psoriatic arthritis in patients with plaque psoriasis: a systematic review of the literature. J Eur Acad Dermatol Venereol 2010. Apr; 24(Suppl 2): 31–35. [DOI] [PubMed] [Google Scholar]
- 18. Sociedade Brasileira de Dermatologia . Consenso Brasileiro de Psoríase 2012, 2nd edn Rio de Janeiro: Sociedade Brasileira de Dermatologia, 2012. [Google Scholar]
- 19. Langan SM, Seminara NM, Shin DB et al Prevalence of metabolic syndrome in patients with psoriasis: a population‐based study in the United Kingdom. J Invest Dermatol 2012. Mar; 132(3 Pt 1): 556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parodi A, Aste N, Calvieri C et al Metabolic syndrome prevalence in psoriasis: a cross‐sectional study in the Italian population. Am J Clin Dermatol 2014. Aug; 15(4): 371–377. [DOI] [PubMed] [Google Scholar]
- 21. Haroon M, Kirby B, FitzGerald O. High prevalence of psoriatic arthritis in patients with severe psoriasis with suboptimal performance of screening questionnaires. Ann Rheum Dis 2013. May; 72(5): 736–740. [DOI] [PubMed] [Google Scholar]
- 22. Phan C, Sigal M‐L, Lhafa M et al Metabolic comorbidities and hypertension in psoriasis patients in France. Comparisons with French national databases. Ann Dermatol Vénéréol 2016; 143(4): 264–274. [DOI] [PubMed] [Google Scholar]
- 23. Finlay AY. Current severe psoriasis and the rule of tens. Br J Dermatol 2005; 152(5): 861–867. [DOI] [PubMed] [Google Scholar]
- 24. Sociedade Brasileira de Cardiologia . IV Diretriz Brasileira sobre dislipidemias e prevenção da aterosclerose departamento de aterosclerose da sociedade Brasileira de cardiologia. Arq Bras Cardiol. 2007;88:2–19. [DOI] [PubMed] [Google Scholar]
- 25. Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006. Aug; 54(8): 2665–2673. [DOI] [PubMed] [Google Scholar]
- 26. Nobre F, Andrade JP, Sociedade Brasileira de Cardiologia . VI diretrizes Brasileiras de hipertensão. Arq Bras Cardiol 2010;95:(Suppl 1):1–51. [PubMed] [Google Scholar]
- 27. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diab Care. 2012;35(Suppl 1):S64–S71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Associação Brasileira para o Estudo da Obesidade e da Síndrome Metabólica (Abeso) . Diretrizes Brasileiras de Obesidade 2009‐2010. 3rd edn São Paulo: Abeso, 2009; 11–83. [Google Scholar]
- 29. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol 1994. May; 19(3): 210–216. [DOI] [PubMed] [Google Scholar]
- 30. Saris‐Baglama RN, Dewey CJ, Chisholm GB et al QualityMetric Health Outcomes™ Scoring Software 4.0‐User's Guide. Lincoln: QualityMetric Incorporated, 2010; 138. [Google Scholar]
- 31. The EuroQol Group . EQ‐D User Guide. 2015.
- 32. Dolan P. Modeling valuations for EuroQol health states valuations modeling. Med Care 1997; 35(11): 1095–1108. [DOI] [PubMed] [Google Scholar]
- 33. Martins GA, Arruda L, Mugnaini ASB. Validação de questionários de avaliação da qualidade de vida em pacientes de psoríase. An Bras Dermatol 2004. Oct; 79(5): 521–535. [Google Scholar]
- 34. Pinto EB, Maso I, Vilela RNR, Santos LC, Oliveira‐Filho J. Validation of the EuroQol quality of life questionnaire on stroke victims. Arq Neuropsiquiatr 2011; 69(2b): 320–323. [DOI] [PubMed] [Google Scholar]
- 35. Soárez PC, Kowalski CCG, Ferraz MBCR. Tradução para português brasileiro e validação de um questionário de avaliação de produtividade. Rev Panam Salud Publica 2007; 22(1): 21–28. [DOI] [PubMed] [Google Scholar]
- 36. Reich K, Kruger K, Mössner R, Augustin M. Epidemiology and clinical pattern of psoriatic arthritis in Germany: a prospective interdisciplinary epidemiological study of 1511 patients with plaque‐type psoriasis. Br J Dermatol 2009; 160(5): 1040–1047. [DOI] [PubMed] [Google Scholar]
- 37. Choi WJ, Park EJ, Kwon IH, Kim KH, Kim KJ. Association between psoriasis and cardiovascular risk factors in Korean patients. Ann Dermatol 2010. Aug; 22(3): 300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carneiro JN, de Paula AP, Martins GA. Psoriatic arthritis in patients with psoriasis: evaluation of clinical and epidemiological features in 133 patients followed at the University Hospital of Brasília. An Bras Dermatol 2012. Aug; 87(4): 539–544. [DOI] [PubMed] [Google Scholar]
- 39. Ranza R, Carneiro S, Qureshi AA et al Prevalence of psoriatic arthritis in a large cohort of brazilian patients with psoriasis. J Rheumatol 2015. May; 42(5): 829–834. [DOI] [PubMed] [Google Scholar]
- 40. Spelman L, Su JC, Fernandez‐Peñas P et al Frequency of undiagnosed psoriatic arthritis among psoriasis patients in Australian dermatology practice. J Eur Acad Dermatol Venereol 2015. Nov; 29(11): 2184–2191. [DOI] [PubMed] [Google Scholar]
- 41. Baeta IGR, Bittencourt FV, Gontijo B, Goulart EMA. Comorbidities and cardiovascular risk factors in patients with psoriasis. An Bras Dermatol 2014. Sep; 89(5): 735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Menegon DB, Pereira AG, Camerin AC, Cestari T. Psoriasis and comorbidities in a southern Brazilian population: a case‐control study. Int J Dermatol 2014. Nov; 53(11): e518–e525. [DOI] [PubMed] [Google Scholar]
- 43. de Carvalho Vidigal F, Bressan J, Babio N, Salas‐Salvadó J. Prevalence of metabolic syndrome in Brazilian adults: a systematic review. BMC Public Health 2013. Jan; 13: 1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Balogh O, Brodszky V, Gulácsi L et al Cost‐of‐illness in patients with moderate to severe psoriasis: a cross‐sectional survey in Hungarian dermatological centres. Eur J Health Econ 2014; 15(Suppl 1): S101–S109. [DOI] [PubMed] [Google Scholar]
- 45. Moradi M, Rencz F, Brodszky V, Moradi A, Balogh O, Gulácsi L. Health status and quality of life in patients with psoriasis: an Iranian cross‐sectional survey. Arch Iran Med 2015; 18(3): 153–159. [PubMed] [Google Scholar]
- 46. Sojević Timotijević Z, Janković S, Trajković G et al Identification of psoriatic patients at risk of high quality of life impairment. J Dermatol 2013; 40(10): 797–804. [DOI] [PubMed] [Google Scholar]
- 47. Tejada Cdos S, Almeida HL Jr, Tejada VF, Mendoza‐Sassi RA, Figueiredo PN. Impact on the quality of life of dermatological patients in southern Brazil. An Bras Dermatol 2011; 86(6): 1113–1121. [DOI] [PubMed] [Google Scholar]
- 48. Laguardia J, Campos MR, Travassos C, Najar AL, dos Anjos LA, Vasconcellos MM. Brazilian normative data for the Short Form 36 questionnaire, version 2. Rev Bras Epidemiol 2013; 16(4): 889–897. [DOI] [PubMed] [Google Scholar]
- 49. Møller AH, Erntoft S, Vinding GR, Jemec GB. A systematic literature review to compare quality of life in psoriasis with other chronic diseases using EQ‐5D‐derived utility values. Patient Relat Outcome Meas 2015; 6: 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lau MYZ, Burgess JA, Nixon R, Dharmage SC, Matheson MC. A review of the impact of occupational contact dermatitis on quality of life. J Allergy 2011; 2011: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Components of metabolic syndrome according to National Cholesterol Education Program Adult Treatment Panel III criteria22
Table S2. Logistic regression model for psoriatic arthritis (first model, n = 275)
Table S3. Logistic regression model for metabolic syndrome (first model, n = 261)


