Abstract
To identify the value of predictors of poor prognosis of elderly patients with rectal cancer who underwent surgery, we investigated the relations between albumin to globulin ratio (AGR) and clinicopathological findings.
We conducted a retrospective cohort study of clinicopathological characteristics (general status, pathological features of tumors, preoperative laboratory data, disease free, and overall survival) for elderly patients with stage I-III rectal cancer. The AGR is calculated as albumin/(total protein − albumin).
According to the optimal cut-off point of AGR (1.43), the enrolled patients were divided into low AGR (n = 83) and high AGR (n = 136) groups. Meanwhile, age, hemoglobin, tumor size, and differentiation degree were the independent risk factors of low preoperative AGR value. Compared to patients with high AGR, those with low AGR were related to worse disease-free survival (DFS) (P = .0008) and overall survival (OS) (P = .0003). Moreover, in multivariate analysis, low AGR and poor TNM stage were the independent predictor of poor DFS and OS. Finally, the nomograms illustrated the effect of prognostic factors on DFS and OS.
Preoperative AGR has a significant prognostic value and was identified as an independent predictor of DFS and OS in elderly rectal cancer patients.
Keywords: albumin to globulin ratio, elderly patients, prognostic value, rectal cancer
1. Introduction
Colorectal cancer is the third site of malignant tumors worldwide.[1] Compared with colon cancer, the incidence of rectal cancer increases with age and slightly decrease after 80 years.[2,3] Thus, rectal cancer is the most common tumors in the population of elderly. These patients were frequently associated with more comorbidities, poorer general conditions, and shorter life expectancy. Therefore, whether it is safe or beneficial to perform radical curative procedure for elderly patients is under controversy. As a result, the identification of a reliable biomarker which can precisely predict the prognosis of elderly patients with rectal cancer is very imperative.
The total protein (TP) is composed of albumin (ALB) and globulin (GLB). Albumin to globulin ratio (AGR) is calculated as AGR = ALB/(TP − ALB).[4] Depending on different physical conditions, patients with the same TP could have different composition of ALB and GLB. In 1917, Hurwitz et al described the relationship between AGR and experimental intoxication and infection conditions in animals.[5] This is the first practical application of AGR. Nowadays, low AGR is proved to be a significant predictor of soon recurrence and poor survival status of several types of cancer.[6–9] In colorectal cancer patients, several studies reported that low preoperative AGR is closely associated with poor long-term survival.[10,11] However, few randomized controlled trials have focused on elderly patients. Consequently, the relation between AGR and elderly patient with rectal cancer is not quite clear to date.
The purpose of this study is to investigate the association between AGR and long-term survival, including disease-free survival (DFS) and overall survival (OS) in elderly patients with rectal cancer who underwent surgery.
2. Methods
2.1. Patients and methods
This is a retrospective cohort study of elderly patients (>65 years) with rectal cancer who underwent surgery at our institution between January 1st, 2010 and January 1st, 2015. The exclusion criteria included: patients younger than 65 years; those who had pre-existing liver diseases, other malignancies, and inflammatory diseases, including autoimmune disorder and infection; those who could not tolerate surgery; complete follow-up data were not unavailable. A total of 219 patients matched the inclusion and exclusion criteria.
The TNM classification of union for International Cancer Control (Eighth Edition) was used to stage the tumor in each patient.[12] All patients were followed up after surgery until death or January 1st, 2018, using our standard protocol every 3 months for the first year after surgery, every 4 months for the second year and half a year for the rest of the time. The protocol included tumor marker, colonoscopy examinations, abdominal ultrasonography, abdomen, and chest CT.
The data regarding patients’ demographic characteristics, comorbidities, preoperative laboratory data, pathology (tumor size, cell differentiation, lymph node metastasis, neurovascular invasion, and tumor staging), and surgical treatment were collected from the hospital's database by using electronic medical record software. The follow-up survey data were collected from outpatient record, including DFS and OS.
The receiver operating characteristics (ROC) curve was used to determine the optimal cut-off point for predicting the recurrence of cancer. The predictive value of AGR was 1.43 with 71.7% of the area under the ROC curve. According to this cut-off point, patients enrolled were divided into low and high AGR group.
This study was conducted in accordance with guidelines of the 1975 Declaration of Helsinki. All patients obtained informed consent. This study and protocol were designed with permission by our institutional review board.
2.2. Statistical analysis
For comparison between low and high AGR groups, independent t test was used to analyze the continuous variables. Categorical variables are presented as frequencies and percentages and were analyzed by using chi-square or 2-tailed Fisher exact tests. The binary logistic regression analysis was used to find independent risk factors of the low preoperative AGR value. The DFS was defined as the date from surgery to the date of cancer recurrence or death due to the disease progression. The OS was measured from the date of surgery to the date of the death resulting from any cause or the follow-up deadline. Survival rates were analyzed using the Kaplan–Meier methods and compared using the log-rank test. The hazard ratio (HR) of recurrence and death was calculated by using Cox's proportional hazards models. Nomograms were performed to illustrate the effect of prognostic factors on DFS and OS. The risk score, based on Cox regression coefficients, was a combination of the values of independent prognostic factors calculated by their respective Cox regression analysis. A result was defined statistically significant with P < .05. All statistical analyses were performed using Statistical Package for Social Science version 22.0 (IBM Corp, Armonk, NY). Nomograms were performed using R software (version 3.5.2; www.r-project.org).
3. Result
3.1. Characteristics of entire cohort
This study enrolled 219 patients, including 135 (61.6%) males and 84 (38.4%) females aged 65 to 93 years (median age of 74 years). The median follow-up time was 48.89 ± 19.15 months. Among them, 30 patients had stage I (13.7%), 81 had stage II (37.0%), and 108 had stage III (49.3%) rectal cancer. During the follow-up, 56 patients were dead.
3.2. Association and difference between low and high AGR group
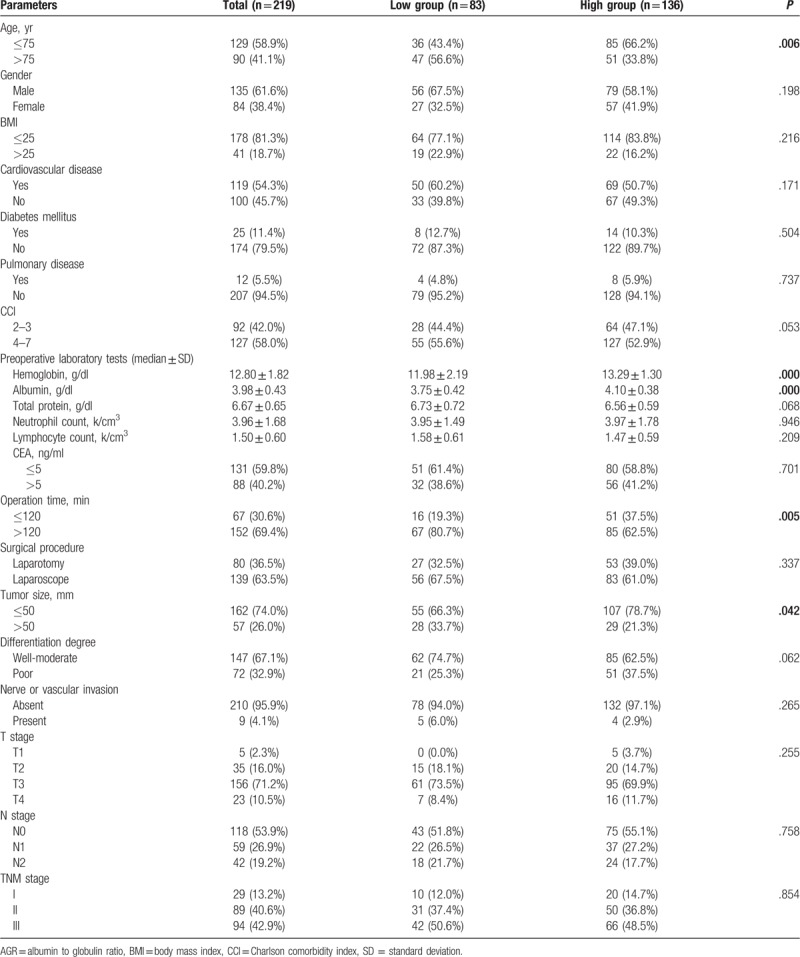
The whole group was divided into low AGR (n = 83) and high AGR (n = 136) groups according to the cut-off point of AGR (1.43). Age, preoperative hemoglobin, ALB, operation time, and tumor size were obtained significantly different between the 2 groups (Table 1).
Table 1.
Clinic characteristics between low (AGR ≤1.43) and high AGR (AGR >1.43) group.
3.3. The independent risk factors of preoperative low AGR value
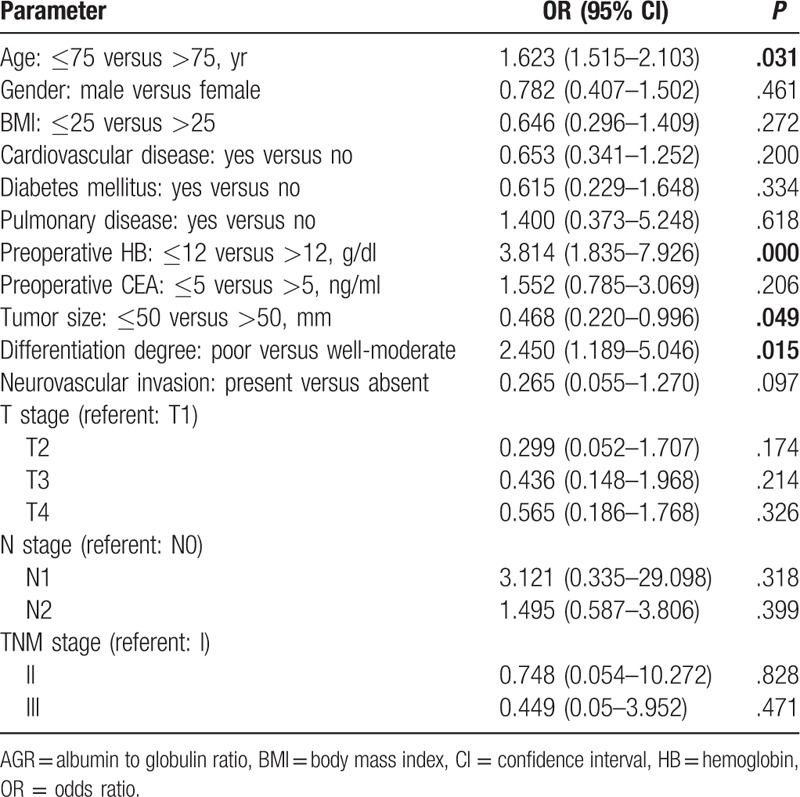
The binary logistic regression analysis revealed age, hemoglobin, tumor size, and differentiation degree were independent risk factors of low preoperative AGR value. Patients who were older than 75 years (odds ratio [OR] = 1.623; 95% confidence interval [CI]: 1.515, 2.103; P = .031) and with hemoglobin less than 12 g/dl (OR = 3.814; 95% CI: 1.835, 7.926; P = .000), tumor size larger than 50 mm (OR = 0.468; 95% CI: 0.220, 0.996; P = .049), and poor differentiation degree (OR = 2.450; 95% CI: 1.189, 5.046; P = .015) had a higher incidence of low AGR value compared of others (Table 2).
Table 2.
Binary logistic regression analysis of low AGR (AGR ≤ 1.43) associated risk factors.
3.4. Survival analysis (DFS and OS) according to the preoperative AGR value
The analysis of DFS and OS of elderly rectal cancer patients showed DFS (Fig. 1A) and OS (Fig. 1B) were significantly worse in patients with low AGR than others (DFS, P = .0008; OS, P = .0003).
Figure 1.
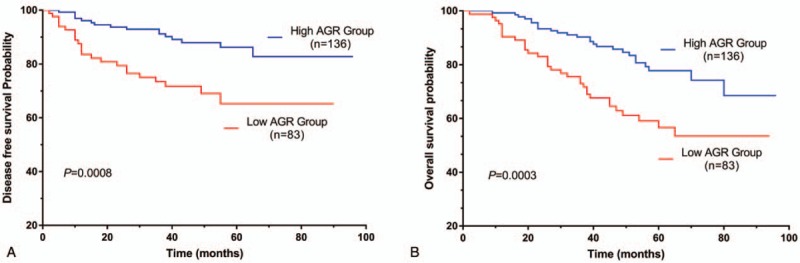
Kaplan–Meier survival curve of elderly patients with rectal cancer. K–M curve for DFS (A); K–M curve for OS (B). AGR = albumin to globulin ratio, DFS = disease-free survival, K–M = Kaplan–Meier, OS = overall survival.
3.5. Identified independent prognostic factors for DFS and OS
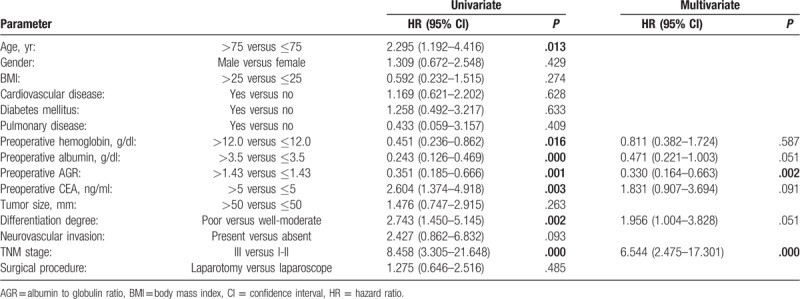
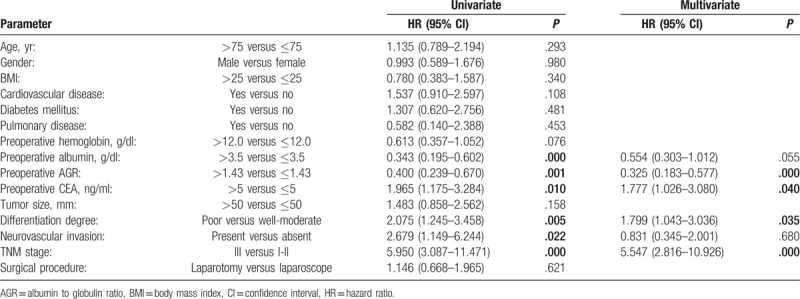
Univariate analysis of DFS showed that preoperative low hemoglobin, low ALB, low AGR, high CEA, and poor TNM stage were associated with earlier cancer recurrence. Multivariate analysis suggested that low AGR (HR = 0.330; 95% CI: 0.164, 0.663; P = .002) and poor TNM stage (HR = 6.544; 95% CI: 2.475, 17.301; P = .000) were the independent predictors (Table 3).
Table 3.
Cox proportional hazard model analysis of predictors of disease-free survival (DFS) of elderly patients with rectal cancer.
In the univariate analysis of OS, preoperative low ALB, low AGR, high CEA, present of neurovascular invasion, poor differentiation degree, and TNM stage were significantly associated with poor prognosis. Multivariate analysis revealed low AGR (HR = 0.325; 95% CI: 0.183, 0.5779; P = .000), high CEA (HR = 1.777; 95% CI: 1.026, 3.080; P = .040), poor differentiation degree (HR = 1.799; 95% CI: 1.043, 3.036; P = .035), and poor TNM stage (HR = 5.547; 95% CI: 2.816, 10.926; P = .000) were independent predictors of OS (Table 4).
Table 4.
Cox proportional hazard model analysis of predictors of overall survival (OS) of elderly patients with rectal cancer.
Nomograms were performed to illustrate the effect of prognostic factors on DFS (Fig. 2) and OS (Fig. 3) according to the Cox regression coefficients.
Figure 2.
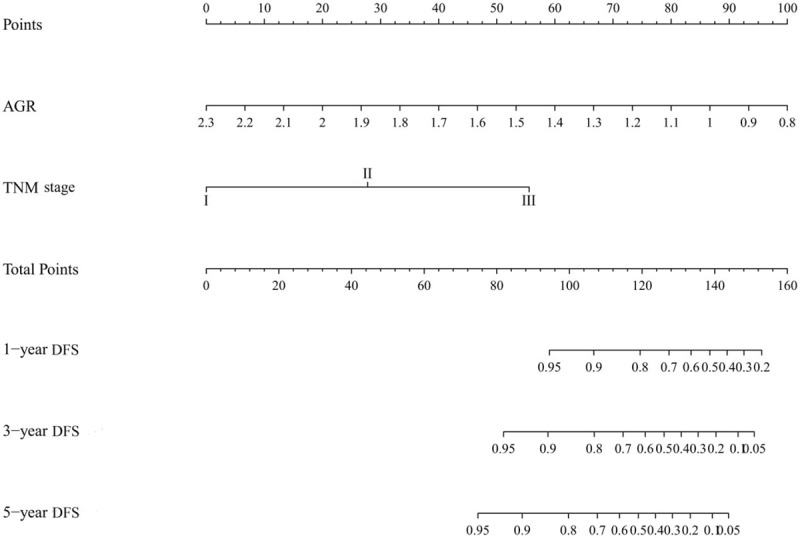
Nomogram for predicting DFS in elderly patients with rectal cancer. AGR = albumin to globulin ratio, DFS = disease-free survival.
Figure 3.
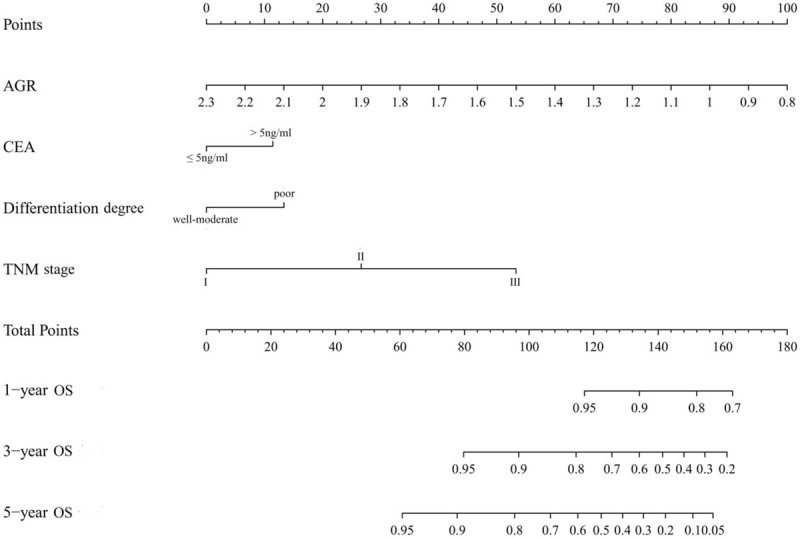
Nomogram for predicting OS in elderly patients with rectal cancer. AGR = albumin to globulin ratio, OS = overall survival.
4. Discussion
In the present study, our main finding is that the low AGR is a significantly independent predictor of early recurrence and poor prognosis in elderly rectal cancer patients. As observed, patients with low AGR were older and had hypohemoglobinemia, hypoalbuminemia, large tumor size, and advanced tumor stages. Poor nutrition status and tumor pathological features were independent risk factors of low AGR.
Systemic chronic inflammation is closely associated with tumor development, proliferation, metastasis, and poor prognosis in various types of cancer.[13,14] The mechanism of systemic inflammation may be the chronic oxidative stress and general oxygen free radicals,[15] which can be evaluated by white blood cell count, neutrophil count, c-reactive protein (CRP), and so on.
ALB is the most abundant serum protein which is mainly produced by hepatic cell. Hypoalbuminemia often occurs in hepatic dysfunction, malnutrition, and systemic inflammation. There exists close interactions between ALB and tumors. Low ALB level is often associated with poor prognosis in several types of digestive cancer, including esophageal cancer,[16] gastric cancer,[17] colon cancer,[18] pancreatic cancer,[19] and hepatic cancer.[20] First, patients with gastrointestinal cancer often suffer from poor absorption, which results in hypoalbuminemia.[21] Second, the tumor can cause chronic inflammation which will produce some cytokines, such as interleukin (IL)-1β, IL-6, and tumor necrosis factor. These cytokines promote the inhibition of ALB synthesis and the enhancement of capillary permeability, which will induce the loss of ALB.[22] In addition, several anticancer mechanisms of circulating ALB, including its antioxidant function, have been observed in previous studies.[23,24]
In contrast, GLB, the carrier of sex hormones, is thought to reflect most pro-inflammation protein, such as complement components, immunoglobins, CRP, ILs, and tumor necrosis factors.[25] Previous studies have shown that serum globin level is an independent predictor of long-term mortality in several kinds of malignancies.[26–28]
The AGR is composed of these 2 major elements of serum protein and affected by patients’ nutritional status and systemic inflammation. Emerging evidences indicate that pretreatment AGR is potential prognostic biomarker for several kinds of cancer.[29] To examine the relevance between AGR and prognosis in patients with colorectal cancer, Azab et al have enrolled 534 patients into their study. The result showed AGR was independent predictor of long-term survival in patients with colorectal cancer regardless of value of pretreatment ALB.[10] In another study, Fujikawa et al also found that preoperative AGR was an independent predictor of recurrence and poor prognosis of patients with colon cancer patients.[11] These studies have only focused on the prognosis in general population with colorectal cancer. They have failed to consider the elderly are the main part of rectal cancer patients, because the incidence of rectal cancer increases with age and slightly decrease after 80 years.[2,3] Our major finding fills the gap in time.
The NCCN guidelines suggested patients with stage I-II rectal cancer who underwent surgery with high-risk factors such as positive margin, lymphatic and vascular invasion, poorly differentiated histology, obstruction, perforation, and <13 lymph nodes in the surgical specimen should have postoperative adjuvant chemotherapy.[30] By comparison, all patients with lymph node metastasis should receive postoperative adjuvant chemotherapy. The standard treatment is capecitabine/oxaliplatin regimen or folinic acid/fluorouracil/oxaliplatin regimen.[30] However, not all elderly patients are suitable for adjuvant chemotherapy, because their condition is much more complicated than young patients, such as more varied comorbidities, poorer nutrition condition and recovery capability, shorter life expectancy and worse tolerance of adverse effect. In our present study, these risk factors were identified using univariate and multivariate analysis. The result revealed that AGR was more accurate than other factors in predicting cancer recurrence and mortality. Therefore, preoperative AGR may be a promising and convenient biomarker, which can help physician to decide who really need radical curative therapy.
AGR is a ratio combined with ALB and GLB, which is less vulnerable to the measurement variability compared to the absolute value like ALB. Then, the variation of AGR is the result of the interaction both of ALB and GLB. AGR has a high tolerance because the false positive and negative rate is low. Besides, AGR has already been given in the serum biochemical test in most hospitals, so it can be measured easily at a low cost. Therefore, AGR can be widely applied in clinical practice.
There are some limitations of our study. First, this is a retrospective study based on 1 single center. Second, the concentrations of GLB were calculated through formula (GLB = TP − ALB). Data of GLB value on direct laboratory measurement is not available in our hospital. Third, some other nutrition and inflammation biomarkers are not included, such as pre-ALB, CRP and cytokine.
In conclusion, Preoperative low AGR is a significantly independent predictor of early recurrence and poor survival in elderly patients with rectal cancer. Poor nutrition status and tumor pathological features may be the high-risk factors of low AGR. These findings facilitate the best choice of treatment in the elderly population with rectal cancer.
Acknowledgments
The authors gratefully acknowledge all of the investigators for their contributions to this trial.
Author contributions
Yixin Xu contributed to the study design and drafted the manuscript. Xuezhong Xu worked on the study design and data analysis. Nianyuan Ye and Cheng Xi were involved in the data collection and extraction. Yibo Wang revised the manuscript. All authors have read and approved the final manuscript.
Data curation: Cheng Xi.
Formal analysis: Xuezhong Xu.
Investigation: Nianyuan Ye.
Methodology: Cheng Xi.
Resources: Nianyuan Ye.
Software: Xuezhong Xu, Nianyuan Ye.
Writing – original draft: Yixin Xu.
Writing – review and editing: Yibo Wang.
Footnotes
Abbreviations: AGR = albumin to globulin ratio, ALB = albumin, BMI = body mass index, DFS = disease-free survival, GLB = globulin, HR = hazard ratio, OS = overall survival, ROC = receiver operating characteristics, TP = total protein.
This study was approved by the ethics committee of Wujin Hospital Affiliated to Jiangsu University. Written informed consent was obtained from all participants.
This work was supported by the Science and Technology Development Fund of Wujin District, Changzhou (No. WS201515).
The authors declare that they have no competing interests.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Arnold M, Sierra MS, Laversanne M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017;66:683–91. [DOI] [PubMed] [Google Scholar]
- [3].Rutten HJ, den Dulk M, Lemmens VE, et al. Controversies of total mesorectal excision for rectal cancer in elderly patients. Lancet Oncol 2008;9:494–501. [DOI] [PubMed] [Google Scholar]
- [4].Beamer N, Coull BM, Sexton G, et al. Fibrinogen and the albumin-globulin ratio in recurrent stroke. Stroke 1993;24:1133–9. [DOI] [PubMed] [Google Scholar]
- [5].Hurwitz SH, Whipple GH. Studies on the blood proteins: II. The albumin-globulin ratio in experimental intoxications and infections. J Exp Med 1917;25:231–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhou T, He X, Fang W, et al. Pretreatment albumin/globulin ratio predicts the prognosis for small-cell lung cancer. Medicine (Baltimore) 2016;95:e3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Deng Y, Pang Q, Miao RC, et al. Prognostic significance of pretreatment albumin/globulin ratio in patients with hepatocellular carcinoma. Onco Targets Ther 2016;9:5317–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Toiyama Y, Yasuda H, Ohi M, et al. Clinical impact of preoperative albumin to globulin ratio in gastric cancer patients with curative intent. Am J Surg 2017;213:120–6. [DOI] [PubMed] [Google Scholar]
- [9].Liu J, Dai Y, Zhou F, et al. The prognostic role of preoperative serum albumin/globulin ratio in patients with bladder urothelial carcinoma undergoing radical cystectomy. Urol Oncol 2016;34:484.e1–8. [DOI] [PubMed] [Google Scholar]
- [10].Azab B, Kedia S, Shah N, et al. The value of the pretreatment albumin/globulin ratio in predicting the long-term survival in colorectal cancer. Int J Colorectal Dis 2013;28:1629–36. [DOI] [PubMed] [Google Scholar]
- [11].Fujikawa H, Toiyama Y, Inoue Y, et al. Prognostic impact of preoperative albumin-to-globulin ratio in patients with colon cancer undergoing surgery with curative intent. Anticancer Res 2017;37:1335–42. [DOI] [PubMed] [Google Scholar]
- [12].Brierley J, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. Eighth edition. ed Chichester, West Sussex, UK; Hoboken, NJ: John Wiley & Sons, Inc.; 2017. [Google Scholar]
- [13].Coffelt SB, de Visser KE. Cancer: inflammation lights the way to metastasis. Nature 2014;507:48–9. [DOI] [PubMed] [Google Scholar]
- [14].Fox P, Hudson M, Brown C, et al. Markers of systemic inflammation predict survival in patients with advanced renal cell cancer. Br J Cancer 2013;109:147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Szkandera J, Pichler M, Absenger G, et al. The elevated preoperative platelet to lymphocyte ratio predicts decreased time to recurrence in colon cancer patients. Am J Surg 2014;208:210–4. [DOI] [PubMed] [Google Scholar]
- [16].Oki S, Toiyama Y, Okugawa Y, et al. Clinical burden of preoperative albumin-globulin ratio in esophageal cancer patients. Am J Surg 2017;214:891–8. [DOI] [PubMed] [Google Scholar]
- [17].Yamashita K, Ushiku H, Katada N, et al. Reduced preoperative serum albumin and absence of peritoneal dissemination may be predictive factors for long-term survival with advanced gastric cancer with positive cytology test. Eur J Surg Oncol 2015;41:1324–32. [DOI] [PubMed] [Google Scholar]
- [18].Lai CC, You JF, Yeh CY, et al. Low preoperative serum albumin in colon cancer: a risk factor for poor outcome. Int J Colorectal Dis 2011;26:473–81. [DOI] [PubMed] [Google Scholar]
- [19].Pant S, Martin LK, Geyer S, et al. Baseline serum albumin is a predictive biomarker for patients with advanced pancreatic cancer treated with bevacizumab: a pooled analysis of 7 prospective trials of gemcitabine-based therapy with or without bevacizumab. Cancer 2014;120:1780–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yang J, Wang WT, Yan LN, et al. Alternatives to albumin administration in hepatocellular carcinoma patients undergoing hepatectomy: an open, randomized clinical trial of efficacy and safety. Chin Med J (Engl) 2011;124:1458–64. [PubMed] [Google Scholar]
- [21].Alkan A, Koksoy EB, Utkan G. Albumin to globulin ratio, a predictor or a misleader? Ann Oncol 2015;26:443–4. [DOI] [PubMed] [Google Scholar]
- [22].Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J 2010;9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Roche M, Rondeau P, Singh NR, et al. The antioxidant properties of serum albumin. FEBS Lett 2008;582:1783–7. [DOI] [PubMed] [Google Scholar]
- [24].Seaton K. Albumin concentration controls cancer. J Natl Med Assoc 2001;93:490–3. [PMC free article] [PubMed] [Google Scholar]
- [25].Du XJ, Tang LL, Mao YP, et al. The pretreatment albumin to globulin ratio has predictive value for long-term mortality in nasopharyngeal carcinoma. PLoS One 2014;9:e94473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fortunati N, Catalano MG, Boccuzzi G, et al. Sex hormone-binding globulin (SHBG), estradiol and breast cancer. Mol Cell Endocrinol 2010;316:86–92. [DOI] [PubMed] [Google Scholar]
- [27].Berndt SI, Chatterjee N, Huang WY, et al. Variant in sex hormone-binding globulin gene and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 2007;16:165–8. [DOI] [PubMed] [Google Scholar]
- [28].Qu X, Pang Z, Yi W, et al. High percentage of alpha1-globulin in serum protein is associated with unfavorable prognosis in non-small cell lung cancer. Med Oncol 2014;31:238. [DOI] [PubMed] [Google Scholar]
- [29].Suh B, Park S, Shin DW, et al. Low albumin-to-globulin ratio associated with cancer incidence and mortality in generally healthy adults. Ann Oncol 2014;25:2260–6. [DOI] [PubMed] [Google Scholar]
- [30].Benson AB, 3rd, Venook AP, Al-Hawary MM, et al. Rectal cancer, version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018;16:874–901. [DOI] [PMC free article] [PubMed] [Google Scholar]


