Abstract
Background
Increased asymmetrical dimethylarginine (ADMA) and NT pro‐BNP concentrations have been associated with mortality in patients with cardiovascular (CV) disease and the general population. The use of these prognostic markers in an older population is not established yet. The aim of the present study was to investigate the prognostic value of age, sex, BMI, co‐medication and CV laboratory risk markers in geriatric care patients.
Materials and methods
In this prospective observational single‐centre cohort study data of long‐term geriatric care patients were collected. Blood samples were collected between 14.09.2009 and 16.12.2009, and mortality was recorded up to 90 months. ADMA, its symmetric isomer SDMA, L‐arginine, NT pro‐BNP and CRP were determined at study entry. Simple associations of risk factors for survival period were explored by Spearman correlation coefficient. Significant univariate predictors for survival period were used in the Cox proportional hazard model.
Results
A total of 481 patients were screened, and data from 449 patients were analysed. A total of 381 patients died during the observation period. Full data sets from 344 patients were used for Cox regression analysis. Male sex, older age, lower BMI, use of neuroleptic medicine, peripheral artery disease, and elevated plasma concentrations of ADMA, NT pro‐BNP, and CRP were significant predictors of mortality.
Conclusion
The concentration of ADMA and NT pro‐BNP may be used as an early risk marker for overall mortality in geriatric care. Neuroleptic medicine is associated with increased mortality in this population.
Keywords: ADMA, geriatric care, overall mortality, risk markers
1. INTRODUCTION
The use of predictive markers in the ageing population at risk is getting more important. Older patients represent a vulnerable population group with a particularly high prevalence of co‐morbidities and mortality.1 Cardiovascular (CV) disease is the leading cause of death and disability among these patients; however, robust biomarkers are not generally established.
Plasma asymmetric dimethylarginine (ADMA) is an endogenous inhibitor of nitric oxide (NO). ADMA and its symmetric isomer SDMA are novel predictors for CV disease, chronic kidney disease and mortality.2 N‐terminal pro‐brain natriuretic peptide (NT pro‐BNP) provides prognostic information for CV events and mortality in the older patients.3 C‐reactive protein (CRP) is a sensitive acute phase reactant and is used as prognostic marker in patients with CV disease.4, 5 These cardiac risk markers as well as body mass index (BMI) have emerged as promising tools for risk estimate of older patients,6, 7 but have not been established in geriatric care.
Since limited trial data are available for the combined use of CV risk markers in an older population, we aimed to investigate the prognostic value of age, sex, BMI, co‐medication and CV laboratory risk markers in long‐term geriatric care patients aged ≥65 years.
2. MATERIALS AND METHODS
The study protocol was approved by the Ethics Committee of the Medical University of Vienna (EK 511‐2008) and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained before study entry from all patients or their legal representatives, respectively.
2.1. Study protocol
In this prospective observational single‐centre cohort study all long‐term geriatric care residents of the Haus der Barmherzigkeit Vienna, Austria were screened for eligibility between 14.09.2009 and 16.12.2009. All patients who were hospitalized for at least 1 month in geriatric care were included. Patients with symptomatic heart failure were excluded. The observation period was defined with a maximum of 90 months and mortality was identified from the public register of death certificates. Demographic data including age, sex, admission diagnosis, height and weight were collected. ADMA, SDMA, L‐arginine, NT pro‐BNP and CRP were determined at study entry from leftovers of routine venous blood samples. Plasma was separated after centrifugation and stored at −80°C until batch analysis.
2.2. Laboratory assays
Quantification of arginines was performed by high‐performance liquid chromatography (HPLC) as described previously.8 The coefficients of variation for inter‐ and intra‐assay variations are <3% for all analyses. The detection limit for (methyl‐) arginines is 0.04 μmol/L. NT pro‐BNP measurements were performed according to standard procedures using an assay by Roche Diagnostics (Eleccsys® NT pro‐BNP, Cobas, Rotkreuz, Switzerland). The analytical sensitivity of the kit is 0.063 ng/mL, assay range 0.31‐10 ng/mL, and the intra‐assay CV is 5.5%. Serum levels of CRP were quantified using a Human Solid Phase Sandwich ELISA from R&D Systems (Wiesbaden, Germany) with a lower limit of quantification of 0.1 mg/dL.
2.3. Statistical analysis
Metric variables are expressed as mean, and standard deviation (SD). Simple associations of risk factors for survival period were explored by Spearman correlation coefficient. Only data from patients with full set of laboratory CV risk parameters were used. Significant univariate predictors for survival period were used in the Cox proportional hazard model. In this model, significant variables were determined by backward selection. In all analyses a P‐value of <0.05 was considered significant. All statistical calculations were performed using SPSS Version 19.0 (SPSS Inc., Chicago, IL, USA).
3. RESULTS
In total, 481 patients were screened for eligibility and data from 449 patients aged between 65 and 105 years were available for analysis (Figures 1 and 2). Baseline characteristics are presented in Tables 1 and 2. A total of 381 patients died during the observation period of 90 months. The cumulative survival of female and male patients is presented in Figure 3.
Figure 1.
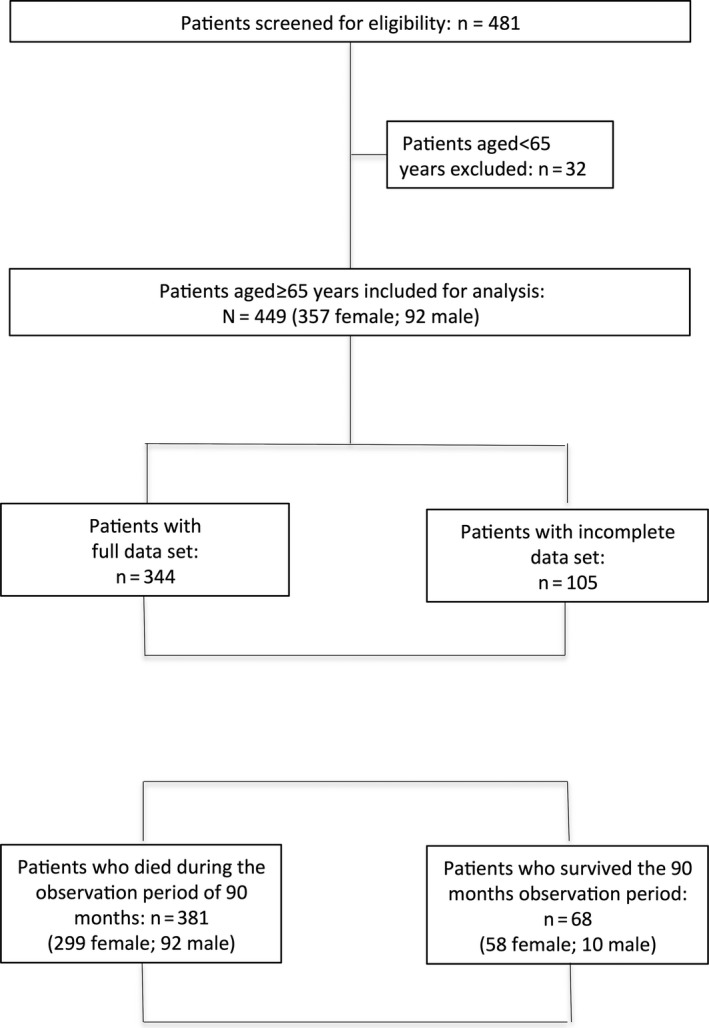
Flow chart of study population
Figure 2.
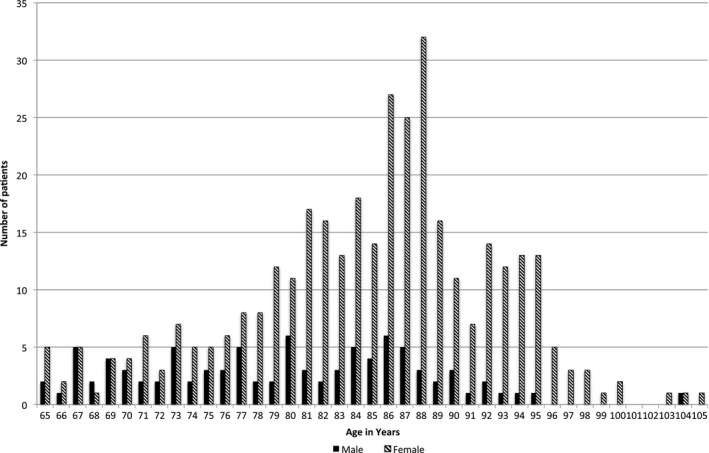
Age distribution of patients at study entry
Table 1.
Baseline characteristics
| Female/male | 357/92 |
| Age years (mean ± SD, n = 449) | 84 ± 8 |
| BMI kg/m2 (mean ± SD, n = 444) | 24.0 ± 8.0 |
| Number of patients (%) | |
|---|---|
| Atherosclerotic co‐morbidity (n = 449) | |
| Coronary artery disease | 320 (71%) |
| Peripheral artery disease | 409 (91%) |
| History of stroke | 335 (75%) |
| Co‐medication (n = 449) | |
| Antidiabetic medicines | 337 (75%) |
| ACE‐inhibitors | 289 (64%) |
| Beta‐blockers | 378 (84%) |
| Calcium antagonists | 381 (85%) |
| Loop diuretics | 357 (79%) |
| Other diuretics | 426 (93%) |
| Proton pump inhibitors | 183 (41%) |
| Anticoagulation therapy | 163 (36%) |
| Selective serotonin re‐uptake inhibitors | 264 (59%) |
| Other antidepressants | 271 (60%) |
| Anxiolytic and hypnotic medicines | 308 (69%) |
| Neuroleptic medicine | 262 (58%) |
| HMG‐CoA‐Reduktase‐Inhibitor (n = 316) | 70 (22%) |
| Antiplatelet therapy (n = 316) | 166 (53%) |
Table 2.
Laboratory CV risk parameters. Data presented as mean and standard deviation
| Female | Male | |
|---|---|---|
| L‐arginine (μmol/L)a | 66.0 ± 19.6 | 65.0 ± 18.9 |
| ADMA (μmol/L)a | 0.8 ± 0.2 | 0.7 ± 0.2 |
| SDMA (μmol/L)a | 1.4 ± 0.5 | 1.2 ± 0.4 |
| NT pro‐BNP (pg/mL)a | 235 ± 258 | 233 ± 415 |
| CRP (mg/dL)a | 1.1 ± 2.2 | 1.2 ± 1.5 |
| Erythrocyte count (T/L)b | 4.1 ± 0.6 | 4.3 ± 0.6 |
| Haemoglobin (g/dL)b | 11.9 ± 1.5 | 12.9 ± 1.7 |
| Creatinine (mg/dL)b | 1.0 ± 0.5 | 1.1 ± 0.7 |
| Total protein (g/dL)c | 6.8 ± 4.2 | 6.7 ± 0.8 |
n = 344 (female n = 271, male n = 73).
n = 358 (female n = 287, male n = 71).
n = 314(female n = 248, male n = 66).
Figure 3.
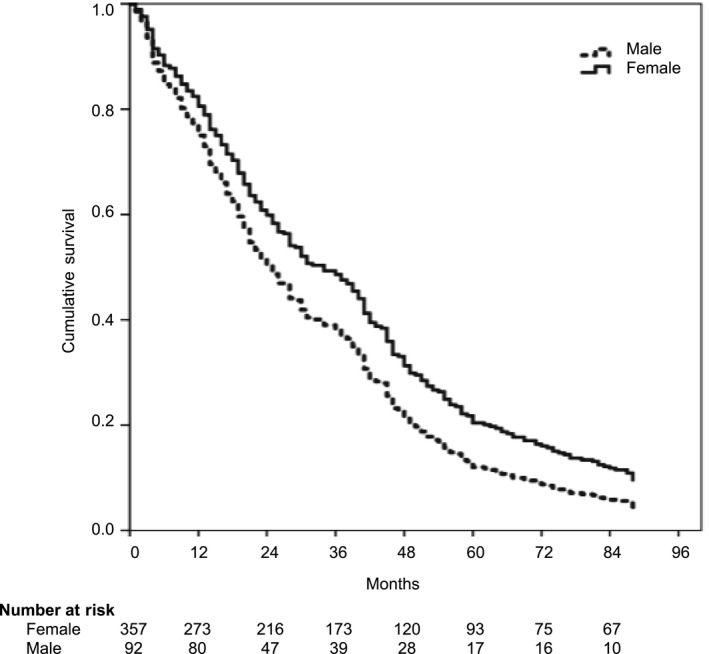
Cumulative survival of female and male patients
3.1. Association of age, sex, BMI, and CV risk parameters with mortality
Full data sets including laboratory assessments, diagnosis and treatment from 344 patients (271 female, 73 male patients) were used for the Cox regression analysis. Male gender [P < 0.05; 95%CI 1.1‐2.1], older age [P < 0.001; 95%CI 1.0‐1.1], lower BMI [P < 0.05; 95%CI 0.9‐1.0], elevated plasma concentrations of ADMA [P < 0.05; 95%CI 1.0‐3.5], and NT pro‐BNP [P < 0.001; 95%CI 1.0‐1.0], and CRP [P < 0.001; 95%CI 1.1‐1.2%] were significant predictors for overall mortality. SDMA [P = 0.820; 95%CI 0.8‐1.4] and L‐arginine [P = 0.19; 95%CI 1.0‐1.0] were not associated with overall mortality.
3.2. Association of medication and co‐morbidity with mortality
Neuroleptic medicine was a significant predictor [P < 0.05; 95%CI 1.0‐1.6] for mortality. Peripheral artery disease was significantly associated with mortality [P < 0.05; 95%CI 1.1‐2.6]. There was no correlation between other medications or co‐morbidities with mortality.
4. DISCUSSION
This prospective observational longitudinal cohort study reveals two major findings from long‐term geriatric care patients. Our first major finding indicates that increased CV risk markers including ADMA, NT pro‐BNP, CRP and BMI are associated with mortality in this older population. Mean plasma concentrations of ADMA and SDMA were higher than those of patients with diabetes, COPD or cardiovascular disease using the same analytical method. Concentrations of L‐arginin, which are influenced by protein intake, were lower in the elderly patients under study compared with our previous observation in other group of patients.9, 10, 11, 12, 13, 14, 15, 16, 17, 18
The statistical correlation between increased ADMA plasma concentration and mortality in older patients is in agreement with the Framingham heart study and a previous study conducted in older patients in Italy.2, 6 ADMA is an endogenous inhibitor of NO synthase and affects endothelial function, blood pressure and vascular remodelling by reducing NO production.19 Increased ADMA plasma concentration has been shown to be associated with increased overall mortality in the general population.2, 20 Furthermore, there is an association between elevated ADMA plasma concentration and increased risk of CV events,16 and disease progression in patients with diabetes and kidney disease.12, 21
L‐arginine is the substrate of the NO synthase. In the present study, we could not demonstrate a predictive role of L‐arginine plasma concentration on patients survival. Furthermore, in contrast to previous studies, we could not detect a relationship between L‐arginine and ADMA.6, 22 One should note that the association between L‐arginine as NO‐synthase substrate and the endogenous antagonist of ADMA was not related to CV mortality.6 The absence of this association has also been reported in a previous longitudinal population‐based cohort study conducted in patients aged above 65 years.2
Elevated NT pro‐BNP plasma concentration was also associated with mortality in the older population under study. This is in agreement with a previous data reporting the predictive value of NT pro‐BNP for long‐ and short‐term mortality in older patients with CV diseases.23 However, the use of NT pro‐BNP in older patients has been discussed controversially, due to the correlation between natriuretic peptide plasma concentration, gender and age.24, 25 In contrast, the present study shows the independent predictive value of elevated NT pro‐BNP plasma concentration for mortality, regardless of gender and age.
Furthermore, our results confirm the prognostic role of elevated CRP plasma concentrations in this cohort of patients. The association between CRP plasma concentration and survival is well established in CV diseases.26 Elevated plasma CRP is also associated with increased mortality in patients with type II diabetes,27 cancer28 and also in the general population.26 Our results are consistent with previous studies, which reported an increased mortality in patients with elevated plasma CRP and low BMI.29
Low BMI was also associated with increased mortality in our observation. This is in agreement with the Health ABC study, which showed an increased mortality and mobility disability risk with weight loss in older patients.30 There are different approaches to explain the role of BMI in patients outcome and its association with mortality, especially in older population.31, 32 Previous studies have reported an inverse association between BMI and NT pro‐BNP plasma concentration.33, 34, 35 Female sex and waist circumference have been revealed as dependent variables for NT pro‐BNP plasma concentrations.36 In the present study, we did not confirm this correlation between BMI and NT pro‐BNP, despite the large numbers of female patients in our cohort. The correlation between BMI and NT pro‐BNP plasma concentration seems to be rather complex and requires further investigation.
Our second major finding is that use of neuroleptic medicine is an independent predictor for mortality in the elderly. Neuroleptic medications are used for the treatment of schizophrenia, psychotic disorders, and in some cases there is an off‐label use for treatment of behavioural and psychological symptoms of dementia in nursing homes.37, 38 The US Food and Drug Administration as well as the European and national drug agencies have raised concerns about side effects in the older population, especially in patients with dementia.39, 40 A recent study recommends to avoid neuroleptic medicines in an older population.41 In the present cohort, over 50% of patients received neuroleptic medicines, and this number seems to be very high, given the fact that all these patients received these medication under continued medical supervision.
Increased mortality has been reported in patients with CV diseases including arrhythmia, venous thromboembolism and stroke due to drug‐drug interaction between neuroleptic, antipsychotic and CV medicines.41, 42 In the present study, over 80% of our patients received concomitant beta‐blocker and calcium antagonists, and over 60% psychotropic medicine. We cannot exclude that this co‐medication has an impact on the increased mortality in users of neuroleptic medicine. Around 70% and 90% of elderly patients had a diagnosis of coronary artery disease and peripheral artery disease, respectively. Of note, only a small number of patients received statin therapy.
4.1. Limitation
The present study had a prospective design and included a large and well‐characterized older population, which was observed for the occurrence of death for a maximum of 90 months. However, our study has several limitations. The first limitation is that CV risk parameters were measured at study entry, but not during the observational period. Possible changes in biomarkers related to disease progression are therefore not accounted for in our prognostic model. Another limitation is that severity of dementia diagnosis in this cohort of patients was not available.
This prospective observational longitudinal cohort study demonstrates that the cardiovascular risk markers ADMA, NT pro‐BNP and CRP are associated with overall mortality in geriatric care patients. Chronic use of neuroleptic medicine use is associated with mortality in older population.
CONFLICT OF INTERESTS
All authors have no personal or financial conflict of interests to declare.
AUTHOR′ CONTRIBUTION
Safoura Sheikh Rezaei: analysed data, wrote paper Stefan Weisshaar: performed research/study, collected data. Brigitte Litschauer: collected data, analysed data. Ghazaleh Gouya: designed study, collected data. Gerald Ohrenberger: designed study, collected data. Michael Wolzt: designed study, revised the manuscript.
Sheikh Rezaei S, Weisshaar S, Litschauer B, Gouya G, Ohrenberger G, Wolzt M. ADMA and NT pro‐BNP are associated with overall mortality in elderly. Eur J Clin Invest. 2019;49:e13041 10.1111/eci.13041
REFERENCES
- 1. Shah SM, Carey IM, Harris T, DeWilde S, Cook DG. Mortality in older care home residents in England and Wales. Age Ageing. 2013;42(2):209‐215. [DOI] [PubMed] [Google Scholar]
- 2. Boger RH, Sullivan LM, Schwedhelm E, et al. Plasma asymmetric dimethylarginine and incidence of cardiovascular disease and death in the community. Circulation. 2009;119(12):1592‐1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kistorp C, Raymond I, Pedersen F, Gustafsson F, Faber J, Hildebrandt P. N‐terminal pro‐brain natriuretic peptide, C‐reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. JAMA. 2005;293(13):1609‐1616. [DOI] [PubMed] [Google Scholar]
- 4. Lloyd‐Jones DM, Liu K, Tian L, Greenland P. Narrative review: assessment of C‐reactive protein in risk prediction for cardiovascular disease. Ann Intern Med. 2006;145(1):35‐42. [DOI] [PubMed] [Google Scholar]
- 5. Cozlea DL, Farcas DM, Nagy A, et al. The impact of C reactive protein on global cardiovascular risk on patients with coronary artery disease. Curr Health Sci J. 2013;39(4):225‐231. [PMC free article] [PubMed] [Google Scholar]
- 6. Pizzarelli F, Maas R, Dattolo P, et al. Asymmetric dimethylarginine predicts survival in the elderly. Age (Dordr). 2013;35(6):2465‐2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. He J, McGee D, Niu X, Choi W. Examining the dynamic association of BMI and mortality in the Framingham Heart Study. Int J Environ Res Public Health. 2009;6(12):3115‐3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mittermayer F, Namiranian K, Pleiner J, Schaller G, Wolzt M. Acute Escherichia coli endotoxaemia decreases the plasma l‐arginine/asymmetrical dimethylarginine ratio in humans. Clin Sci (Lond). 2004;106(6):577‐581. [DOI] [PubMed] [Google Scholar]
- 9. Mittermayer F, Schaller G, Pleiner J, et al. Rosiglitazone prevents free fatty acid‐induced vascular endothelial dysfunction. J Clin Endocrinol Metab. 2007;92(7):2574‐2580. [DOI] [PubMed] [Google Scholar]
- 10. Mittermayer F, Krzyzanowska K, Exner M, et al. Asymmetric dimethylarginine predicts major adverse cardiovascular events in patients with advanced peripheral artery disease. Arterioscler Thromb Vasc Biol. 2006;26(11):2536‐2540. [DOI] [PubMed] [Google Scholar]
- 11. Mittermayer F, Kautzky‐Willer A, Winzer C, et al. Elevated concentrations of asymmetric dimethylarginine are associated with deterioration of glucose tolerance in women with previous gestational diabetes mellitus. J Intern Med. 2007;261(4):392‐398. [DOI] [PubMed] [Google Scholar]
- 12. Krzyzanowska K, Mittermayer F, Wolzt M, Schernthaner G. Asymmetric dimethylarginine predicts cardiovascular events in patients with type 2 diabetes. Diabetes Care. 2007;30(7):1834‐1839. [DOI] [PubMed] [Google Scholar]
- 13. Krzyzanowska K, Mittermayer F, Schernthaner GH, et al. Renal function but not asymmetric dimethylarginine is independently associated with retinopathy in type 2 diabetes. Cardiol Res Pract. 2011;2011:260191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heutling D, Schulz H, Nickel I, et al. Asymmetrical dimethylarginine, inflammatory and metabolic parameters in women with polycystic ovary syndrome before and after metformin treatment. J Clin Endocrinol Metab. 2008;93(1):82‐90. [DOI] [PubMed] [Google Scholar]
- 15. Duckelmann C, Mittermayer F, Haider DG, Altenberger J, Wolzt M. Plasma asymmetric dimethylarginine and cardiovascular events in patients with acute decompensated heart failure. Transl Res. 2008;152(1):24‐30. [DOI] [PubMed] [Google Scholar]
- 16. Duckelmann C, Mittermayer F, Haider DG, Altenberger J, Eichinger J, Wolzt M. Asymmetric dimethylarginine enhances cardiovascular risk prediction in patients with chronic heart failure. Arterioscler Thromb Vasc Biol. 2007;27(9):2037‐2042. [DOI] [PubMed] [Google Scholar]
- 17. Urban MH, Eickhoff P, Funk GC, Burghuber OC, Wolzt M, Valipour A. Increased brachial intima‐media thickness is associated with circulating levels of asymmetric dimethylarginine in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:169‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Skoro‐Sajer N, Mittermayer F, Panzenboeck A, et al. Asymmetric dimethylarginine is increased in chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2007;176(11):1154‐1160. [DOI] [PubMed] [Google Scholar]
- 19. Zhou S, Zhu Q, Li X, et al. Asymmetric dimethylarginine and all‐cause mortality: a systematic review and meta‐analysis. Sci Rep. 2017;7:44692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leong T, Zylberstein D, Graham I, et al. Asymmetric dimethylarginine independently predicts fatal and nonfatal myocardial infarction and stroke in women: 24‐year follow‐up of the population study of women in Gothenburg. Arterioscler Thromb Vasc Biol. 2008;28(5):961‐967. [DOI] [PubMed] [Google Scholar]
- 21. Zoccali C, Bode‐Boger S, Mallamaci F, et al. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end‐stage renal disease: a prospective study. Lancet (London, England). 2001;358(9299):2113‐2117. [DOI] [PubMed] [Google Scholar]
- 22. Perticone F, Sciacqua A, Maio R, et al. Asymmetric dimethylarginine, L‐arginine, and endothelial dysfunction in essential hypertension. J Am Coll Cardiol. 2005;46(3):518‐523. [DOI] [PubMed] [Google Scholar]
- 23. Passantino A, Guida P, Lagioia R, et al. Predictors of Long‐Term Mortality in Older Patients Hospitalized for Acutely Decompensated Heart Failure: clinical Relevance of Natriuretic Peptides. J Am Geriatr Soc. 2017;65(4):822‐826. [DOI] [PubMed] [Google Scholar]
- 24. Collerton J, Kingston A, Yousaf F, et al. Utility of NT‐proBNP as a rule‐out test for left ventricular dysfunction in very old people with limiting dyspnoea: the Newcastle 85 + Study. BMC Cardiovasc Disord. 2014;14:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raymond I, Groenning BA, Hildebrandt PR, et al. The influence of age, sex and other variables on the plasma level of N‐terminal pro brain natriuretic peptide in a large sample of the general population. Heart. 2003;89(7):745‐751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaptoge S, Di Angelantonio E, Lowe G, et al. C‐reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta‐analysis. Lancet (London, England). 2010;375(9709):132‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lowe G, Woodward M, Hillis G, et al. Circulating inflammatory markers and the risk of vascular complications and mortality in people with type 2 diabetes and cardiovascular disease or risk factors: the ADVANCE study. Diabetes. 2014;63(3):1115‐1123. [DOI] [PubMed] [Google Scholar]
- 28. Singh‐Manoux A, Shipley MJ, Bell JA, Canonico M, Elbaz A, Kivimaki M. Association between inflammatory biomarkers and all‐cause, cardiovascular and cancer‐related mortality. CMAJ 2017;189(10):E384‐e390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arnold AM, Newman AB, Cushman M, Ding J, Kritchevsky S. Body weight dynamics and their association with physical function and mortality in older adults: the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2010;65(1):63‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murphy RA. Weight Change, body composition and risk of mobility disability and mortality in older adults: a population based cohort study. J Am Geriatr Soc. 2014;62(8):1476‐1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Losonczy KG, Harris TB, Cornoni‐Huntley J, et al. Does weight loss from middle age to old age explain the inverse weight mortality relation in old age? Am J Epidemiol. 1995;141(4):312‐321. [DOI] [PubMed] [Google Scholar]
- 32. Peter RS, Keller F, Klenk J, Concin H, Nagel G. Body mass trajectories, diabetes mellitus, and mortality in a large cohort of Austrian adults. Medicine. 2016;95(49):e5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang TJ, Larson MG, Levy D, et al. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109(5):594‐600. [DOI] [PubMed] [Google Scholar]
- 34. Das SR, Drazner MH, Dries DL, et al. Impact of body mass and body composition on circulating levels of natriuretic peptides: results from the Dallas Heart Study. Circulation. 2005;112(14):2163‐2168. [DOI] [PubMed] [Google Scholar]
- 35. Frankenstein L, Remppis A, Nelles M, et al. Relation of N‐terminal pro‐brain natriuretic peptide levels and their prognostic power in chronic stable heart failure to obesity status. Eur Heart J. 2008;29(21):2634‐2640. [DOI] [PubMed] [Google Scholar]
- 36. Suthahar N, Meijers WC, Ho JE, et al. Sex‐specific associations of obesity and N‐terminal pro‐B‐type natriuretic peptide levels in the general population. Eur J Heart Fail. 2018;20(8):1205‐1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Madhusoodanan S, Ting MB. Pharmacological management of behavioral symptoms associated with dementia. World J Psychiatry. 2014;4(4):72‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schulze J, van den Bussche H, Glaeske G, Kaduszkiewicz H, Wiese B, Hoffmann F. Impact of safety warnings on antipsychotic prescriptions in dementia: nothing has changed but the years and the substances. Eur Neuropsychopharmacol. 2013;23(9):1034‐1042. [DOI] [PubMed] [Google Scholar]
- 39. Renom‐Guiteras A, Meyer G, Thürmann PA. The EU(7)‐PIM list: a list of potentially inappropriate medications for older people consented by experts from seven European countries. Eur J Clin Pharmacol. 2015;71(7):861‐875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cioltan H, Alshehri S, Howe C, et al. Variation in use of antipsychotic medications in nursing homes in the United States: A systematic review. BMC geriatri. 2017;17(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liperoti R, Sganga F, Landi F, et al. Antipsychotic Drug Interactions and Mortality Among Nursing Home Residents With Cognitive Impairment. J Clin Psychiatry. 2017;78(1):e76‐e82. [DOI] [PubMed] [Google Scholar]
- 42. Barbui C, Conti V, Cipriani A. Antipsychotic drug exposure and risk of venous thromboembolism: a systematic review and meta‐analysis of observational studies. Drug Saf. 2014;37(2):79‐90. [DOI] [PubMed] [Google Scholar]


