Abstract
Background
Bioimpedance spectroscopy (BIS) has enabled the early identification of breast cancer‐related lymphedema. In this study, differences in health service metrics and in the incidence of breast cancer‐related lymphedema are evaluated in an early surveillance model of care compared with a traditional referral model of care.
Methods
In a retrospective analysis of data from 753 women who underwent BIS measures between January 1, 2007 and December 31, 2016, 188 women were assigned to the “early surveillance” group if they began lymphedema monitoring presurgery (n = 121) or within 90 days postsurgery (n = 67), and 285 women were assigned to the “traditional referral” group if they began monitoring after 90 days postsurgery. Health service metrics were calculated as the time to the first BIS measure after 90 days postsurgery, the median follow‐up, and the number of health care visits. Lymphedema was diagnosed based on BIS measures.
Results
Women in the early surveillance group received lymphedema care significantly earlier than those in the traditional referral group. However, there was no difference in the number of visits per year to the clinic between groups. Significantly more women in the traditional referral group were diagnosed with clinical lymphedema (stage I‐III, 39 % vs 14%; P < .001) and with greater severity (stage II‐III, 24%) compared with those in the early surveillance group (4%).
Conclusions
The current findings support the adoption of an early prospective surveillance model of care using BIS for the early detection and management of breast cancer–related lymphedema.
Keywords: bioimpedance spectroscopy (BIS), breast cancer‐related lymphedema (BCRL), lymphedema, prospective surveillance, screening
Short abstract
The differences in health service metrics and in the incidence of breast cancer–related lymphedema are evaluated in an early surveillance model of care compared with a traditional referral model of care. The findings support the adoption of an early prospective surveillance model of care using bioimpedance spectroscopy for the early detection and management of breast cancer–related lymphedema.
Introduction
It is estimated that breast cancer‐related lymphedema (BCRL) affects 21% of patients who have breast cancer1 and results in substantial physical,2, 3 functional,2, 3, 4 psychosocial,5, 6, 7, 8 and financial9, 10 burden. Clinical guidelines and position statements from the United States, the United Kingdom, and Australia11, 12, 13, 14 advise that there is a need to develop early detection and intervention programs.15, 16, 17
A prospective surveillance model of care for women with breast cancer involves education, support, empowerment, monitoring, and management of the physical and psychological side effects of treatment. Because up to 80% of patients with breast cancer will attain full life expectancy, chronic treatment‐related morbidity should be minimized.18 Stout and colleagues proposed a prospective surveillance model of care for breast cancer rehabilitation that includes lymphedema surveillance and early intervention.18
A systematic review by Shah and colleagues16 indicated that newer diagnostic modalities like bioimpedance spectroscopy (BIS) have increased sensitivity, which allows for the earlier detection of BCRL.19, 20 It has been reported that the detection of subclinical lymphedema through surveillance and early intervention reduces progression to clinical lymphedema.15, 17 For example, Soran and colleagues17 monitored 180 women who were at high risk of lymphedema using regular BIS, and those who were diagnosed with subclinical BCRL underwent short‐term physical therapy, education, and were prescribed compression sleeves. In that study, subclinical BCRL occurred in 33% of patients but progressed to clinical BCRL in only 4%.
Unlike other methods of clinical lymphedema assessment using volumetric measures, such as water displacement, perometry, or limb circumference, BIS is capable of detecting subclinical lymphedema.21 BIS directly measures the extracellular fluid that is characteristic of early lymphedema.17, 21, 22, 23, 24, 25, 26 When fluid accumulates and remains in the affected limb, inflammatory and hemodynamic changes increase in severity,27 and early intervention may prevent or delay progression. Indeed, Whitworth and colleagues28, 29 observed that, of 93 high‐risk patients who underwent axillary lymph node dissection and were managed with prospective surveillance, only 3% required additional therapies or had evidence of chronic BCRL over a median 2 years of follow‐up. Similarly, Kilgore and colleagues30 reported that only 6% of 146 patients developed chronic lymphedema after early intervention, supporting the observations of Whitworth and colleagues.
With the introduction of BIS in Australia in the early 2000s, our private lymphedema clinic adopted a prospective surveillance model of care and has collected data for over a decade. Therefore, we are well situated to retrospectively examine the difference between an “early surveillance” versus “traditional referral‐based” model of care in BCRL management. All women received BIS assessment from the time of their initial consultation. This study did not test the efficacy of whether early surveillance prevents the development of lymphedema; rather, we were interested in exploring the differences between the 2 models of care over time in relation to the following metrics:
The difference in time to first measure of lymphedema beyond 90 days postsurgery and duration of follow‐up;
The difference in health system use;
Differences in the incidence of and severity of lymphedema for those diagnosed with lymphedema; and
Difference in the evolution of BIS measurement over time for those diagnosed with lymphedema.
These 4 metrics were chosen because they are most relevant to health system use and severity of disease. We wanted to ascertain whether there was a difference in care use and the incidence and severity of lymphedema for the 2 models of care. We hypothesized that early surveillance would result in both less incidence and less severity of lymphedema. Differences between the models of care are important not only from a research perspective but also from a clinical and health services standpoint.
Materials and Methods
Design
For this retrospective cohort study, we used prospectively collected data from 753 women who attended our clinic between January 1, 2007 and December 31, 2016. Baseline data were sourced from electronic medical records and self‐report. The Macquarie University Human Research Ethics Committee provided ethical approval (reference no. 5201500844). This study is reported according to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) checklist.31
Participants
Patients were included if they were women, aged ≥18 years, diagnosed with unilateral breast cancer, and had undergone BIS. Exclusions included neoadjuvant chemotherapy, bilateral lymph node surgery, metastatic breast cancer or recurrent disease, and contraindications for BIS measurement (ie, pregnancy, pacemaker or electronic implantable device). Records were screened by 2 research assistants to determine eligibility.
We defined the early surveillance group as women who were assessed before their surgery for breast cancer or soon after (within 90 days) and were routinely referred from a multidisciplinary breast cancer team. The traditional referral group was defined as women who were assessed more than 90 days postsurgery, who typically were referred from external health centers. Women in both groups received lymphedema education and monitoring using BIS, clinical management of potential breast cancer complications (eg, scarring, cording, or swelling), as well as exercises and psychosocial support.
Outcome Measures
Timing of BIS measure, follow‐up duration, and health system use
We recorded the time of the first BIS measure from 90 days postsurgery in days to ascertain differences between the groups in the timing of access to health care as well as the median follow‐up duration, defined as the period between the first and last BIS measurements. We also calculated the total number of visits per year to ascertain health care utilization.
Incidence and severity of lymphedema
BIS measurements were taken in a supine position using the ImpediMed L‐Dex® U400 device (ImpediMed, Brisbane, Australia). Lymphedema was diagnosed if BIS measures had increased by >10 L‐Dex units from a woman’s presurgical baseline or had exceeded the normative value of +10 L‐Dex units or were maintained below these levels only by ongoing compression therapy. International Society of Lymphology stage (from 0 to III) at diagnosis was recorded.32 Transient swelling within 90 days of surgery or within 270 days of commencing taxane‐based chemotherapy was not defined as lymphedema.
Progression of BIS values over time
Repeated measures, mixed‐effects models were created to evaluate progression in BIS values over time among women who were diagnosed with lymphedema for the 2 groups up to 5 years from surgery. Random intercepts were applied at the subject level, and random slopes with unstructured covariance matrices were used to consider the correlated results of repeated‐measure data. Only women who had 2 or more BIS measures were included.
Data Analysis
Descriptive statistics were used to describe the baseline characteristics of the sample by treatment group, with 2‐sample t tests and chi‐square tests used to investigate significant differences. Nonparametric, Wilcoxon rank‐sum tests were carried out on ordinal and continuous variables because of the non‐normal distribution of the data overall and within study groups. Stata software (version 14; StataCorp LLC, College Station, TX) was used for all statistical analyses.
Results
Characteristics of Participants
Eligible women (n = 473) were categorized into 2 groups. The early surveillance group (n = 188) was made up of those whose surveillance and intervention commenced presurgery (n = 121) or within 90 days postsurgery (n = 67) and continued for at least 90 days thereafter (Fig. 1). The traditional referral group included 285 participants. The cohort’s baseline demographic and intervention characteristics are summarized in Table 1.
Figure 1.
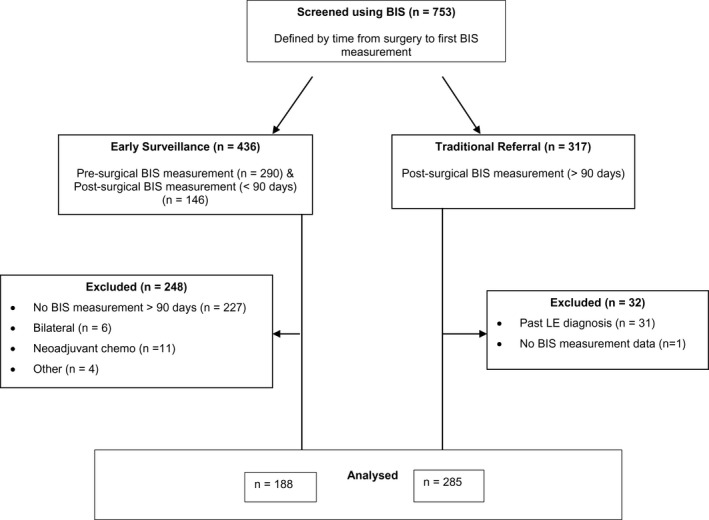
The study design and the flow of participants through the study are illustrated. BIS indicates bioimpedance spectroscopy; LE, lymphedema.
Table 1.
Baseline Characteristics of Participants
| Characteristic | No. of Participants (%) | P a | ||
|---|---|---|---|---|
| All, n = 473 | Early Surveillance Group, n = 188 | Traditional Referral Group, n = 285 | ||
| Age: Mean ± SD, y | 55 ± 11 | 54 ± 12 | 56 ± 11 | <.05 |
| Arm at risk | ||||
| Right | 216 (46) | 84 (45) | 132 (46) | .621 |
| Left | 257 (54) | 103 (55) | 154 (54) | |
| Sentinel lymph nodes dissectedb | ||||
| No | 217 (46) | 55 (29) | 162 (57) | <.001 |
| Yes | 256 (54) | 133 (71) | 123 (43) | |
| Axillary lymph nodes dissectedb | ||||
| No | 173 (37) | 68 (36) | 105 (37) | .799 |
| Yes | 301 (64) | 121 (64) | 180 (63) | |
| Medical intervention | n = 186 | n = 94 | ||
| Nil adjuvant | 31 (11) | 19 (10) | 12 (13) | <.001 |
| RT onlyc | 47 (17) | 34 (18) | 13 (14) | |
| CT only, without taxane | 16 (6) | 11 (6) | 5 (5) | |
| CT only, with taxane | 25 (9) | 22 (12) | 3 (3) | |
| RT + CT, without taxanec | 161 (58) | 100 (53) | 61 (65) | |
| RT + CT, with taxanec | 95 (34) | 73 (39) | 21 (22) | |
Abbreviations: CT, chemotherapy; RT, radiotherapy.
P values were determined with 2‐sample t test or a chi‐square test.
Values for this characteristic were based on those who had a date of procedure recorded; it was assumed that all those without a date did not undergo dissection.
Specific data on radiation fields were not available from therapy clinical files.
Time to First Measure of Lymphedema Beyond 90 Days Postsurgery
The first BIS measurement (from 90 days postsurgery) was taken significantly sooner for the early surveillance group compared with the traditional referral group (Wilcoxon P < .001). The median first BIS measurement (90 days postsurgery) in the early surveillance group was obtained approximately 3 months postsurgery (Table 2). This was 1.8 years sooner than that reported in the traditional referral group.
Table 2.
Time to First Bioimpedance Spectroscopy Measure and Health System Use
| Outcome | Median (IQR) | P a | |
|---|---|---|---|
| Early Surveillance Group, n = 188 | Traditional Referral Group, n = 285 | ||
| Time to first BIS measurement, y | 0.34 (0.28‐0.51) | 2.15 (0.97‐5.41) | <.001 |
| Follow‐up duration, y | 0.74 (0.12‐2.17) | 0.17 (0.0‐1.5) | <.001 |
| n = 108 | n = 108 | ||
| Health system use: No. of visits/yb | 4.1 (2.9‐6.0) | 3.9 (2.5‐5.9) | .238 |
Abbreviations: BIS, bioimpedance spectroscopy; IQR, interquartile range.
P values were determined with a nonparametric Wilcoxon rank‐sum test.
Health system use was measured only among 108 women in each group who attended clinic for ≥6 months.
Follow‐Up Duration
Given the difference in the time to first BIS measure, the follow‐up duration was significantly longer for the early surveillance group than for the traditional referral group (8 vs 2 months). Therefore, most women who received early surveillance completed intervention before most women in the traditional referral group sought treatment. Irrespective of group, women who were diagnosed with lymphedema had a longer median follow‐up than those without lymphedema (Table 2).
Health System Use
For those who attended surveillance for over 6 months (n = 216), the median number of health visits per year for both groups was 4 visits, which was not significantly different (Table 2).
Severity of Lymphedema at Diagnosis of Lymphedema
More women in the traditional referral group (39%) were diagnosed with clinical lymphedema (stage I, II or III) compared with those in the early surveillance group (14%; P < .001) (Table 3). In addition, more women in the early surveillance group (10%) were diagnosed with subclinical (stage 0) lymphedema compared with those in the traditional referral group (1%), and more women in the traditional referral group (24%) had moderate‐to‐severe lymphedema (stage II or III) compared with those in the early surveillance group (4%) (Table 3).
Table 3.
Lymphedema Stage at Diagnosis by Patient Group
| Outcome | No. of Women (%) | P a | |
|---|---|---|---|
| Early Surveillance Group, n = 188 | Traditional Referral Group, n = 285 | ||
| Stage of lymphedema | |||
| No lymphedema | 142 (76) | 173 (61) | <.001 |
| Stage 0 | 19 (10) | 3 (1) | |
| Stage I | 19 (10) | 43 (15) | |
| Stage II | 8 (4) | 53 (19) | |
| Stage III | 0 (0) | 13 (5) | |
P values were determined with a nonparametric Wilcoxon rank‐sum test.
Progression of Lymphedema Over Time
Among women who were diagnosed with lymphedema, the repeated‐measures model was used to predict a mean BIS of 16.1 L‐Dex units (95% confidence interval [CI], 11.5‐20.8 L‐Dex units) at 90 days postsurgery for the early surveillance group versus 18.3 L‐Dex units (95% CI, 13.2‐23.4 L‐Dex units) for the traditional referral group (Fig. 2). There was some evidence to suggest an increase in L‐Dex scores over time for the traditional referral group, with an average increase of 2.3 L‐Dex units per year (95% CI, −0.2 to 4.8 L‐Dex units per year), which approached statistical significance (P = .067) (Table 4). In contrast, the early surveillance group had an average increase of only 1.6 L‐Dex units per year (95% CI, −1.0 to 4.1 L‐Dex units per year) that was not significant (P = .232). The difference in slope between the early surveillance and traditional referral groups was not significant (P = .666).
Figure 2.
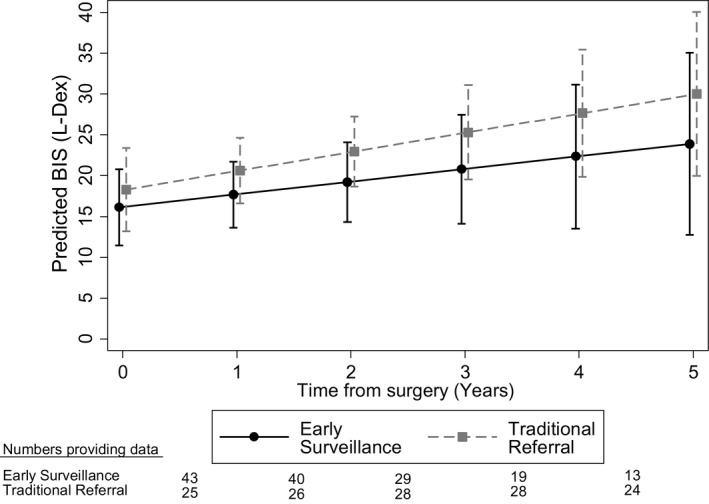
The predicted progression of the L‐Dex score is illustrated among patients who had lymphedema along with model parameters. BIS indicates bioimpedance spectroscopy.
Table 4.
Predicted Progression of L‐Dex Score in Patients With Lymphedema along with model parameter
| Model Output | L‐Dex Score Estimate | SE | 95% CI | P a |
|---|---|---|---|---|
| Predicted mean at 90 days postsurgery | ||||
| Early surveillance intercept | 16.1 | 2.4 | 11.5‐20.8 | <.000 |
| Traditional referral intercept | 18.3 | 2.6 | 13.2‐23.4 | <.000 |
| Progression over time | ||||
| Early surveillance slope | 1.6 | 1.3 | −1.0‐4.1 | .232 |
| Traditional referral slope | 2.3 | 1.3 | −0.2‐4.8 | .067 |
| Difference between slopes: Interaction | 0.8 | 1.8 | −2.8‐4.4 | .666 |
Abbreviations: CI, confidence interval; SE, standard error.
P values were tested if the estimate differed statistically from zero.
Discussion
The current results indicate that women who underwent early surveillance received lymphedema care almost 2 years earlier than women in the traditional referral group without any difference in number of visits to the lymphedema clinic. The early surveillance group had a significantly lower incidence of clinical lymphedema than the traditional referral group, and those who were diagnosed had significantly less severe lymphedema. For women who were diagnosed with lymphedema, BIS scores increased slowly over time, but the rate of increase was less for patients who underwent early surveillance.
Women undergoing early surveillance were monitored for lymphedema at a much earlier time after surgery than those in the traditional referral group. Before prospective early surveillance was practiced, the traditional referral model of care relied on a clinically apparent, visible limb swelling, for which the patient would seek care. This approach often resulted in missed or delayed diagnoses and a protracted time line for intervention.33 Ramos and colleagues34 reported that a greater volume in the arm required more intensive, complex decongestive treatment to achieve better outcomes. They advised early referral for lymphedema treatment in an era when BIS was not routinely used for the early detection of subclinical lymphedema.
The evidence supports using BIS to diagnose subclinical lymphedema to allow early intervention. For example, Soran and colleagues17 detected subclinical lymphedema in 33% of participants who were regularly monitored using BIS. These individuals were provided with intervention, including physical therapy, compression garments, and education, and the authors observed that only 4% progressed to clinical lymphedema. Similarly, Whitworth and colleagues28, 29 also reported regular BIS monitoring and early intervention and observed that only 3% of 93 patients progressed from subclinical to clinical lymphedema. Iyigun and colleagues35 reported that the detection of subclinical lymphedema in 22% of their cohort led to only 14% progressing to clinical lymphedema; and Kilgore and colleagues30 also observed that only 6% of 146 high‐risk patients developed persistent BCRL after early intervention. In our study, we noted that 10% of women who were diagnosed with lymphedema in the early surveillance group were diagnosed with subclinical lymphedema (stage 0) compared with only 1% of those in the traditional referral group, allowing for a greater proportion of women in the early surveillance group to access early intervention to prevent progression to clinical lymphedema.
We observed that the incidence of clinical lymphedema (stage I‐III) differed significantly between the 2 groups (39% vs 14% in the traditional referral and early surveillance groups, respectively). Our traditional referral group incidence was similar to that reported by Soran and colleagues,17 who observed that 36% of women in a control group were diagnosed with clinical lymphedema. Yang and colleagues also compared a surveillance group with a historical control group and reported a 5‐year cumulative incidence of lymphedema at any stage of 32% in the surveillance group compared with 46% in the historical control group.38 In the current study, fewer women in the early surveillance group were diagnosed with moderate or severe lymphedema (stage II‐III, 4% vs 24%) compared with women in the traditional referral group.
In terms of health care use, there was no significant difference in the number of visits per year to the lymphedema clinic between groups. Although there were no fewer clinic visits in the early surveillance group, the actual cost of their intervention may have been lower than that for the traditional referral group. Although no data were collected specifically on the time and cost of clinic visits for women attending the clinic for each group, generally, women without a diagnosis of lymphedema were attending for education and monitoring and were scheduled for the less expensive 30‐minute (AUD$110), versus 60‐minute (AUD$150), consultation sessions for the treatment of diagnosed lymphedema. Women with BCRL required more intensive lymphatic drainage massage and compression therapy, which included more costly consumable products, such as bandages, pneumatic compression pumps, custom‐made garments, and possible costs of antibiotics and inpatient hospital care for those requiring intravenous antibiotics for cellulitis. We previously examined the financial cost of BCRL and observed that 80% of women in the study reported that having lymphedema affected them financially with significant out‐of‐pocket expenses, which increased with lymphedema severity.10 Shih and colleagues36 demonstrated that women with BCRL had a greater risk of infections and incurred significantly higher medical costs for their lymphedema care compared with women not diagnosed with clinical lymphedema and recommended reduction and prevention strategies, supporting an early surveillance model of care. Furthermore, Stout and colleagues18 compared a prospective surveillance model with a traditional model of impairment‐based care and examined direct treatment costs associated with each program. Those authors estimated that the cost of a prospective surveillance model of care for BCRL per patient per year was a significant 20% of the cost of managing lymphedema using the traditional referral‐based model.
There are strengths and limitations of this study. The main strength is the volume of data available for analysis collected in the same clinic using the same method over a decade of practice. The data were recorded routinely as part of normal practice by 1 therapist. Women in the early surveillance group were receiving this package of lymphedema care even before adoption of the Australasian Lymphology Association’s statement advocating regular monitoring for the early detection of BCRL. Second, the number of women and baseline characteristics for both groups were very similar, and these women were at greater risk for developing clinical lymphedema.
In terms of limitations, the 2 groups were not randomly assigned. Although both groups were similar, the number of women who never developed lymphedema is likely under‐represented in the traditional referral group because, historically, they only sought treatment when they had developed a clinical symptom or need. Although a larger proportion of women in the early surveillance group had been prescribed taxane‐based chemotherapy, this group had a lower incidence of clinical lymphedema at diagnosis compared with the traditional referral group.
It is accurate that there was no statistically significant difference in progression between the 2 groups; however, the slopes of disease progression differed over time. Herein, we reiterate an important section from our results in relation to the progression of BIS measurement. There was some evidence to suggest that, among women in the traditional referral group, there was an increase in L‐Dex scores over time of up to 2.3 L‐Dex units per year, on average, that approached statistical significance (P = .067) (Table 4). In contrast, in the early surveillance group, the average increase was only 1.6 L‐Dex units per year (P = .232). Although there was not a statistically significant difference in the slopes between the 2 groups, the 5‐year progression is noteworthy and reflects a clinically important effect. For instance, the traditional referral group gets more severe lymphedema sooner and increases the risk of unintended health outcomes like cellulitis. In contrast, the early surveillance group does progress, but more slowly. We note that the population estimates from our study sample are conservative and could be affected in part by sample size (Fig. 2, Table 4), resulting in larger expected confidence intervals.
Furthermore, no data are available for women who discontinued visits to the lymphedema clinic in either group. Women may have discontinued visits for a variety of reasons, including positive health outcomes, a low risk of developing lymphedema, and costs.
The prevention of progression from subclinical to clinical lymphedema remains important for breast cancer survivors. Although our study supports early surveillance and intervention using BIS, recent literature suggests that earlier detection may be even more beneficial using a lower threshold of a 6.5 rather than a 10 L‐Dex–unit change for the detection of subclinical lymphedema.28, 37, 39
Current practice in Australia requires that “at‐risk” women regularly attend clinics to be monitored for lymphedema. Typically, this occurs on a 3‐month to 6‐month cycle, depending on the individuals’ risk of lymphedema. It is proposed that future research could explore the concept of “home monitoring” using BIS with education and support for the woman to be able to receive immediate and more frequent feedback and potentially earlier intervention, if required.
Conclusions
Scholars and guidelines11, 12, 13, 14 have advocated for the routine implementation of early lymphedema surveillance and intervention after breast cancer treatment. Regular clinic visits to monitor extracellular fluid present an opportunity for therapists to provide risk‐management education, psychological support, physical rehabilitation, empowerment, and survivorship care. The findings from the current study support the use of BIS as part of an early prospective surveillance model of care that results in significantly earlier detection of lymphedema over time. Furthermore, the earlier detection of lymphedema will lead to lower health care costs if it results in the effective management of symptoms and prevents progression to severe clinical lymphedema.
Funding Support
This research was financially supported by a grant from ImpediMed Limited (Brisbane, Australia) for data collection and analysis to the Australian Lymphoedema Education, Research, and Treatment (ALERT) Program, Macquarie University.
Conflict of Interest Disclosures
Louise A. Koelmeyer has acted as an Education Consultant to ImpediMed Limited outside the submitted work. Robert J. Borotkanics and Philip Prah were subcontracted by the ALERT Program at Macquarie University to assist in completing data analyses. The remaining authors made no disclosures.
Author Contributions
Louise A. Koelmeyer: Conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, writing–original draft, and writing–review and editing. Robert J. Borotkanics: Data curation, formal analysis, writing–original draft, and writing–review and editing. Jessica Alcorso: Conceptualization, data curation, methodology, project administration, writing–original draft, and writing–review and editing. Philip Prah: Data curation, formal analysis, writing–original draft, and writing–review and editing. Caleb J. Winch: Conceptualization, data curation, methodology, project administration, writing–original draft, and writing–review and editing. Kristine Nakhel: Data curation, methodology, and writing–review and editing. Catherine M. Dean: Supervision, writing–original draft, and writing–review and editing. John Boyages: Conceptualization, data curation, methodology, supervision, writing–original draft, and writing–review and editing.
The last 2 authors contributed equally to this work.
We thank Louise Ada for editorial assistance.
References
- 1. DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta‐analysis. Lancet Oncol. 2013;14:500‐515. [DOI] [PubMed] [Google Scholar]
- 2. Chachaj A, Malyszczak K, Pyszel K, et al. Physical and psychological impairments of women with upper limb lymphedema following breast cancer treatment. Psychooncology. 2010;19:299‐305. [DOI] [PubMed] [Google Scholar]
- 3. Hayes SC, Janda M, Cornish B, Battistutta D, Newman B. Lymphedema after breast cancer: incidence, risk factors, and effect on upper body function. J Clin Oncol. 2008;26:3536‐3542. [DOI] [PubMed] [Google Scholar]
- 4. Voogd AC, Ververs JM, Vingerhoets AJ, Roumen RM, Coebergh JW, Crommelin MA. Lymphoedema and reduced shoulder function as indicators of quality of life after axillary lymph node dissection for invasive breast cancer. Br J Surg. 2003;90:76‐81. [DOI] [PubMed] [Google Scholar]
- 5. Fu MR, Ridner SH, Hu SH, Stewart BR, Cormier JN, Armer JM. Psychosocial impact of lymphedema: a systematic review of literature from 2004 to 2011. Psychooncology. 2013;22:1466‐1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Winch CJ, Sherman KA, Koelmeyer LA, Smith KM, Mackie H, Boyages J. Sexual concerns of women diagnosed with breast cancer‐related lymphedema. Support Care Cancer. 2015;23:3481‐3491. [DOI] [PubMed] [Google Scholar]
- 7. Burckhardt M, Belzner M, Berg A, Fleischer S. Living with breast cancer‐related lymphedema: a synthesis of qualitative research. Oncol Nurs Forum. 2014;41:E220‐E237. [DOI] [PubMed] [Google Scholar]
- 8. Alcorso J, Sherman KA. Factors associated with psychological distress in women with breast cancer‐related lymphoedema. Psychooncology. 2016;25:865‐872. [DOI] [PubMed] [Google Scholar]
- 9. Boyages J, Kalfa S, Xu Y, et al. Worse and worse off: the impact of lymphedema on work and career after breast cancer [serial online]. Springerplus. 2016;5:657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boyages J, Xu Y, Kalfa S, et al. Financial cost of lymphedema borne by women with breast cancer. Psychooncology. 2016;26:849‐855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National Lymphedema Network . Position Statement of the National Lymphedema Network: The Diagnosis and Treatment of Lymphedema. New York: National Lymphedema Network; 2011. [Google Scholar]
- 12. Gradishar WJ, Anderson BO, Blair SL, et al. Breast cancer version 3.2014. J Natl Compr Canc Netw. 2014;12:542‐590. [DOI] [PubMed] [Google Scholar]
- 13. Australasian Lymphology Association . Position Statement: Monitoring for the Early Detection of Breast Cancer Related Lymphoedema. Beaumaris, Victoria, Australia: Australasian Lymphology Association; 2012. [Google Scholar]
- 14. Clinical Resource Efficiency Support Team . Guidelines for the Diagnosis, Assessment and Management of Lymphoedema. Belfast, Northern Ireland: Clinical Resource Efficiency Support Team; 2008. [Google Scholar]
- 15. Stout Gergich NL, Pfalzer LA, McGarvey C, Springer B, Gerber LH, Soballe P. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer. 2008;112:2809‐2819. [DOI] [PubMed] [Google Scholar]
- 16. Shah C, Arthur DW, Wazer D, Khan A, Ridner S, Vicini F. The impact of early detection and intervention of breast cancer‐related lymphedema: a systematic review. Cancer Med. 2016;5:1154‐1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Soran A, Ozmen T, McGuire KP, et al. The importance of detection of subclinical lymphedema for the prevention of breast cancer‐related clinical lymphedema after axillary lymph node dissection; a prospective observational study. Lymphat Res Biol. 2014;12:289‐294. [DOI] [PubMed] [Google Scholar]
- 18. Stout NL, Pfalzer LA, Springer B, et al. Breast cancer‐related lymphedema: comparing direct costs of a prospective surveillance model and a traditional model of care. Phys Ther. 2012;92:152‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cornish BH, Chapman M, Hirst C, et al. Early diagnosis of lymphedema using multiple frequency bioimpedance. Lymphology. 2001;34:2‐11. [PubMed] [Google Scholar]
- 20. Shah C, Vicini F, Beitsch P, et al. The use of bioimpedance spectroscopy to monitor therapeutic intervention in patients treated for breast cancer related lymphedema. Lymphology. 2013;46:184‐192. [PubMed] [Google Scholar]
- 21. Ward LC. Bioelectrical impedance analysis: proven utility in lymphedema risk assessment and therapeutic monitoring. Lymphat Res Biol. 2006;4:51–56. [DOI] [PubMed] [Google Scholar]
- 22. Cornish B. Bioimpedance analysis: scientific background. Lymphat Res Biol. 2006;4:47‐50. [DOI] [PubMed] [Google Scholar]
- 23. Ward LC, Bunce IH, Cornish BH, Mirolo BR, Thomas BJ, Jones LC. Multi‐frequency bioelectrical impedance augments the diagnosis and management of lymphoedema in post‐mastectomy patients. Eur J Clin Invest. 1992;22:751‐754. [DOI] [PubMed] [Google Scholar]
- 24. Cornish BH, Bunce IH, Ward LC, Jones LC, Thomas BJ. Bioelectrical impedance for monitoring the efficacy of lymphoedema treatment programmes. Breast Cancer Res Treat. 1996;38:169‐176. [DOI] [PubMed] [Google Scholar]
- 25. Cornish BH, Ward LC, Thomas BJ, Bunce IH. Quantification of lymphoedema using multi‐frequency bioimpedance. Appl Radiat Isot. 1998;49:651‐652. [DOI] [PubMed] [Google Scholar]
- 26. Laidley A, Anglin B. The impact of L‐Dex measurements in assessing breast cancer‐related lymphedema as part of routine clinical practice. Front Oncol. 2016;6:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ridner SH. Pathophysiology of lymphedema. Semin Oncol Nurs. 2013;29:4‐11. [DOI] [PubMed] [Google Scholar]
- 28. Whitworth PW, Shah C, Vicini F, Cooper A. Preventing breast cancer‐related lymphedema in high‐risk patients: the impact of a structured surveillance protocol using bioimpedance spectroscopy [serial online]. Front Oncol. 2018;8:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Whitworth P, Cooper A. Reducing chronic breast cancer‐related lymphedema utilizing a program of prospective surveillance with bioimpedance spectroscopy. Breast J. 2018;24:62‐65. [DOI] [PubMed] [Google Scholar]
- 30. Kilgore LJ, Korentager SS, Hangge AN, et al. Reducing breast cancer‐related lymphedema (BCRL) through prospective surveillance monitoring using bioimpedance spectroscopy (BIS) and patient directed self‐interventions. Ann Surg Oncol. 2018;25:2948‐2952. [DOI] [PubMed] [Google Scholar]
- 31. Gallo V, Egger M, McCormack V, et al. Strengthening the Reporting of Observational Studies in Epidemiology‐Molecular Epidemiology (STROBE‐ME): an extension of the STROBE statement [serial online]. PLoS Medicine. 2011;8:e1001117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. International Society of Lymphology . The diagnosis and treatment of peripheral lymphedema: 2013 Consensus Document of the International Society of Lymphology. Lymphology. 2013;46:1‐11. [PubMed] [Google Scholar]
- 33. Bland KL, Perczyk R, Du W, et al. Can a practicing surgeon detect early lymphedema reliably? Am J Surg. 2003;186:509‐513. [DOI] [PubMed] [Google Scholar]
- 34. Ramos SM, O'Donnell LS, Knight G. Edema volume, not timing, is the key to success in lymphedema treatment. Am J Surg. 1999;178:311‐315. [DOI] [PubMed] [Google Scholar]
- 35. Erdogan Iyigun Z, Selamoglu D, Alco G, et al. Bioelectrical impedance for detecting and monitoring lymphedema in patients with breast cancer. Preliminary results of the florence nightingale breast study group. Lymphat Res Biol. 2014. [DOI] [PubMed] [Google Scholar]
- 36. Shih YC, Xu Y, Cormier JN, et al. Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: a 2-year follow-up study. J Clin Oncol. 2009. [DOI] [PubMed] [Google Scholar]
- 37. Fu MR, Cleland CM, Guth AA, et al. L‐Dex ratio in detecting breast cancer‐related lymphedema: reliability, sensitivity, and specificity. Lymphology. 2013;46:85‐96. [PMC free article] [PubMed] [Google Scholar]
- 38. Yang EJ, Ahn S, Kim EK, et al. Use of a prospective surveillance model to prevent breast cancer treatment‐related lymphedema: a single‐center experience. Breast Cancer Res Treat. 2016;160:269‐276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ridner SH, Dietrich MS, Spotanski K, et al. A prospective study of L‐Dex values in breast cancer patients pretreatment and through 12 months postoperatively. Lymphat Res Biol. 2018;16:435‐441. [DOI] [PMC free article] [PubMed] [Google Scholar]


