Abstract
Aim
To characterize the pharmacokinetic and pharmacodynamic properties of dasiglucagon, a novel, stable and liquid formulated glucagon analogue, during hypoglycaemic and euglycaemic conditions in adult patients with type 1 diabetes mellitus.
Research Design and Methods
In this randomized double‐blind trial, 17 patients received four single subcutaneous doses (0.03, 0.08, 0.2 and 0.6 mg) of dasiglucagon (4 mg/mL formulation) under euglycaemic (plasma glucose [PG] 5.6 mmol/L [100 mg/dL]) or hypoglycaemic (PG 3.1‐3.7 mmol/L [56‐66 mg/dL]) conditions. For comparison, three doses (0.03, 0.08 and 0.2 mg) of a commercial glucagon formulation (Eli Lilly) were investigated at euglycaemia.
Results
Dasiglucagon led to a dose‐dependent and rapid increase in PG levels across all doses tested (mean increases 30 minutes post‐dosing of 2.2 to 4.4 mmol/L [39‐80 mg/dL] from euglycaemia and 1.3 to 5.2 mmol/L [24‐94 mg/dL] from hypoglycaemia), which was higher than the rises elicited by similar doses of commercial glucagon (1.7‐3.9 mmol/L [30‐71 mg/dL]). The median time (range) to an increase in PG of >1.1 mmol/L (20 mg/dL) was <20 (18‐19.5) minutes with 0.03 mg dasiglucagon and, with higher doses, the median times ranged from 9 to 15 minutes (commercial glucagon 13‐14 minutes). In hypoglycaemia, 0.03 and 0.08 mg dasiglucagon re‐established normoglycaemia (PG ≥3.9 mmol/L [70 mg/dL]) within median times of 14 and 10 minutes, respectively. Nausea and vomiting occurred more frequently with dasiglucagon than with commercial glucagon at identical doses which might be attributable to dasiglucagon's higher potency.
Conclusion
Dasiglucagon rapidly increased PG at doses of 0.03 to 0.6 mg in a dose‐dependent manner and, therefore, is a good candidate for use in dual‐hormone artificial pancreas systems.
Keywords: glucagon, hypoglycaemia, pharmacodynamics, pharmacokinetics, type 1 diabetes
1. INTRODUCTION
Achieving tight glycaemic control without severe hypoglycaemia still presents a complex challenge in insulin‐treated diabetes.1 Closed‐loop artificial pancreas systems hold promise for reducing the burden of diabetes self‐management, but there is still potential for improvement with regard to avoidance of both hypoglycaemia and hyperglycaemia.2 One option for further improvement might be dual‐hormone artificial pancreas (DHAP) systems which, in addition to insulin, use glucagon to counteract the effect of excessive insulin.3 The administration of mini‐doses of glucagon in a DHAP system has been shown to be more effective in preventing hypoglycaemia than the suspension of insulin delivery alone, particularly in situations of rapidly decreasing glucose levels, such as late postprandial hypoglycaemia or physical activity.4, 5, 6, 7, 8, 9 As currently available glucagon formulations show limited stability, daily glucagon renewal is required, making the use of DHAP systems cumbersome.10, 11, 12 Because of this practical limitation, mainly short‐term efficacy and safety have been demonstrated to date.13, 14, 15, 16, 17, 18
Dasiglucagon is a novel, stable glucagon analogue in a ready‐to‐use aqueous solution with in‐use stability data for at least 7 days at body temperature, making it a promising candidate for long‐term use in DHAP systems.
Dasiglucagon (1 mg/mL) was well tolerated and showed a dose‐dependent and rapid increase in plasma concentrations, with a similar early, but longer‐lasting and greater total plasma glucose (PG) increase from hypoglycaemia compared to a commercially available glucagon formulation. With these characteristics, dasiglucagon also has the potential to become an effective and reliable rescue treatment for severe hypoglycaemia in a ready‐to‐use formulation.19
In this recent trial we characterized the pharmacokinetic (PK) and pharmacodynamic (PD) properties of a more highly concentrated formulation of dasiglucagon (4 mg/mL) intended for use in DHAP systems, in both insulin‐induced euglycaemia and hypoglycaemia in patients with type 1 diabetes mellitus (T1DM). Dasiglucagon was used over a wide range of small doses (0.03, 0.08, 0.2 mg) up to a full dose of 0.6 mg, which showed similar effects to those of 1‐mg doses of existing glucagon formulations in the previous trial.19 For comparison, freshly reconstituted commercially available glucagon (Eli Lilly, Indianapolis, Indiana; hereafter referred to as “commercial glucagon”) was used at the same small doses (0.03, 0.08, 0.2 mg) in euglycaemia in order to draw on prior experience from DHAP systems using this freshly reconstituted commercial glucagon.
2. MATERIALS AND METHODS
2.1. Trial design
This was a single‐centre (Profil, Neuss, Germany), randomized, four‐period, crossover, double‐blind phase II trial in patients with T1DM. Each of the four dosing periods (visits 2‐5) consisted of two consecutive administration days, where two dosings were performed at euglycaemia on the first day (day 1) and one additional dosing at hypoglycaemia on the second day (day 2). Patients received single subcutaneous administrations of dasiglucagon (4 mg/mL, aqueous formulation with preservatives [m‐cresol]; Zealand Pharma A/S, Copenhagen, Denmark) and lyophilized commercial glucagon (1 mg/mL for reconstitution; Eli Lilly) at doses of 0.03, 0.08 or 0.2 mg at euglycaemic conditions on day 1 (the two injections were separated by at least 5 hours). The order of the three lowest dose levels as well as the order of the treatment sequence, dasiglucagon vs glucagon, were randomized (of eight possible treatment sequences, six were randomly chosen). The same dose level of dasiglucagon was administered under hypoglycaemic conditions on day 2 in the morning. At the last dosing period (visit 5), all patients received 0.6 mg dasiglucagon at euglycaemic baseline conditions on day 1 and at hypoglycaemia on day 2; thus, each patient received 11 dosings during this trial (the dosing periods design is shown in Figure S1, Supporting Information).
The trial was approved by the local ethics committee and health authorities in accordance with the Declaration of Helsinki and International Conference on Harmonization's guidelines on Good Clinical Practice. Before any trial‐related activities were initiated, written informed consent was obtained from all participants. The trial was registered at ClinicalTrials.gov (trial identifier: NCT02916251).
2.2. Participants
Adults eligible for inclusion were aged between ≥18 and ≤64 years, diagnosed with T1DM20 and treated with a stable insulin regimen using a continuous subcutaneous insulin infusion (CSII) pump for at least 1 month prior to screening. Participants were required to have a glycated haemoglobin (HbA1c) level ≤69.4 mmol/mol (8.5%) and body weight between ≥60 and ≤90 kg. Patients were excluded if they had clinically significant concomitant diseases or clinically significant abnormal values in laboratory screening tests or were currently being treated with any drugs which may have interfered with glucose metabolism.
2.3. Procedures
The trial consisted of a screening visit, the four dosing periods separated by 3 to 7 days of washout, and a follow‐up visit. Patients maintained their usual basal insulin infusion rate in their CSII pump and attended the clinical site in a fasting state in the morning, where their CSII pump infusion was stopped and a variable intravenous soluble insulin infusion was started. PG levels were lowered to a euglycaemic target level of 5.6 ± 0.1 mmol/L (100 ± 2 mg/dL). Insulin infusion rates were different among individuals but were kept constant for all dosing visits of each individual (Table S6, Supporting Information). On day 1, the same run‐in strategy was used to re‐establish euglycaemia before the second dosing. After completion of the second dosing procedure, the patient received a standardized carbohydrate‐rich dinner and stayed at the site. On day 2, intravenous insulin was used to establish a hypoglycaemic PG range of 3.1 to 3.7 mmol/L (56‐66 mg/dL). The basal insulin infusion rate was identical for all dosing visits of each individual at hypoglycaemia (Table S6, Supporting Information). To maintain double‐blinding, the respective dose/volume was transferred from the dasiglucagon or the commercial glucagon vial (reconstituted solution) into disposable syringes by trained personnel not otherwise involved in trial procedures. Both trial products were administered by subcutaneous injection into a lifted skinfold of the abdominal wall around the umbilicus.
The PK and PD effects of the study drugs were assessed over 240 minutes after each dosing, with frequent plasma sampling for the determination of dasiglucagon/glucagon and PG concentrations.
Safety assessments included adverse events, hypoglycaemic episodes, local tolerability at the injection site, vital signs, laboratory safety variables and antidrug antibody measurements (detailed description in Text S1, Supporting Information).
2.4. Assessments
Plasma concentrations of dasiglucagon were determined with a validated assay using liquid chromatography with tandem mass spectrometry. The lower limit of quantification was 10.0 pmol/L. Commercial glucagon concentration was determined using a validated radioimmunoassay (Eurodiagnostica AB, Malmö, Sweden), performed on a 2470 Wizard Automatic Gamma Counter (Perkin Elmer, Waltham, MA, USA) with a lower limit of quantification of 4.7 pmol/L.
An on‐site laboratory glucose analyser (Super GL, Glucose Analyser; Dr. Müller Gerätebau GmbH, Freital, Germany) was used to determine PG levels and for calculation of PD endpoints. IgG‐ and IgM‐dasiglucagon and IgG‐ and IgM‐glucagon antibodies were measured with a validated ELISA (YBS, York, UK; assay sensitivity for anti‐dasiglucagon and anti‐glucagon concentrations: 13600 and 11800 ng/L, respectively) and a cell‐based validated neutralizing antibody assay (BioAgilytix Laboratories, Durham, NC, USA) was used for the detection of neutralizing antibodies to dasiglucagon and commercial glucagon.
2.5. Endpoints
To evaluate early PK and PD effects, the areas under the curve (AUCs) in the first 30 minutes post‐dosing were analysed for plasma dasiglucagon and commercial glucagon concentrations (AUC0‐30min) and PG concentrations (area under the effect curve, AUE0‐30min). Likewise, PG excursions at 30 minutes (CE3 0min) post‐dose as well as time to half maximum concentration effect (t50%CE, early) were analysed. Primary PK endpoints comprised the total (AUC0‐240min) and maximum (Cmax) plasma dasiglucagon and commercial glucagon concentrations, as well as time to maximum concentration (tmax). To correct for endogenous glucagon concentrations, all commercial glucagon concentrations were baseline‐adjusted. Other PK measures were analysed using total concentrations to infinity (AUC0‐inf). Further analysis referred to terminal elimination rate constant (λz) which allowed the determination of terminal plasma elimination half‐life (t½), total body clearance, volume of distribution, and mean residence time. Primary PD effects were analysed using total PG area under the effect curves (AUE0‐240min), maximum PG excursions (CE) and time to maximum excursions (tCEmax). Key secondary PD endpoints were time to reach a PG increase of ≥1.1 mmol/L (20 mg/dL; TPG_increase_≥1.1 mmol/L) and percentage of patients who reached a PG increase of ≥1.1 mmol/L within 30 minutes post‐dose. In addition, time to reach PG concentration of ≥3.9 mmol/L (70 mg/dL; TPG_≥3.9 mmol/L) was determined for the hypoglycaemic conditions.
2.6. Statistical analyses
No formal sample size calculation was performed for the present exploratory study. A sample size of 17 randomized patients was considered to be sufficient to describe the PK and PD characteristics of dasiglucagon and commercial glucagon.
The PK/PD endpoints were log‐transformed and comparisons were made between dasiglucagon/commercial glucagon and between hypoglycaemic/euglycaemic conditions by using a linear model ANOVA, with treatment as well as glycaemic state, dose, their interaction, period, sequence and patient‐within‐sequence as fixed effects. Least squares means of treatments, for each glycaemic state and doses as well as the differences of the means were calculated and 90% confidence intervals (CIs) were estimated and back‐transformed (exponentially transformed) in order to find the estimated ratios and CIs of responses. As tmax and tCEmax were neither normally nor log‐normally distributed, point estimates for median differences between treatments and corresponding 90% CIs were calculated according to Hodges and Lehmann, and comparisons were made using the Wilcoxon signed rank test.
Dose proportionality of Cmax and the AUCs of dasiglucagon were analysed using a power model by means of a regression analysis with the log‐transformed endpoint as response and log‐dose as a fixed effect. The estimated slope of the regression was calculated. If 1 was included in the 95% CI, dose proportionality was assumed.
All statistical analyses were performed using the SAS® System for Windows, version 9.4 (SAS Institute Inc., Cary, North Carolina). PK characteristics were calculated using WinNonlin, version 7.0 (Certara, Princeton, New Jersey).
3. RESULTS
3.1. Patient disposition and characteristics
After finalization of the latest protocol version, 17 patients with T1DM were randomized and treated with trial products. All patients completed the trial and were included in the PK and PD analysis. Four profiles (two with 0.2 mg commercial glucagon, one with 0.03 mg dasiglucagon and one with 0.2 mg dasiglucagon) were excluded from the PK/PD analysis at the blinded data review meeting before database lock because of the use of a wrong PK assay, assumed insufficient dosing or other important protocol deviations. The final analysis set comprised 184 PK and 183 PD profiles.
In the first six patients included in the study, higher intravenous insulin infusion rates had been used, which led to high hypoglycaemia rates; therefore, the protocol was changed and these first six patients were withdrawn and excluded from data analysis. All 23 patients exposed to the trial drug were included in the safety analysis (Table S1, Supporting Information).
3.2. PK results
The PK profile of dasiglucagon was characterized by a rapid increase with similar exposure to commercial glucagon in the first 15 minutes (Figure 1). Dasiglucagon reached maximum concentrations later than commercial glucagon (30 vs 15 minutes, based on medians over all doses). After tmax, dasiglucagon concentration decreased, with a t1/2 of ~0.4 to 0.7 hours compared with a t1/2 of 0.25 hours with commercial glucagon (Table 1 and Figure 1), resulting in substantially (ie, 1.4‐ to 4‐fold) higher values for dasiglucagon for AUC0‐30min, AUC0‐240min, AUC0‐inf and Cmax at euglycaemic conditions (Table 1 and Table S2, Supporting Information). No substantial differences were observed for dasiglucagon PK characteristics between euglycaemic and hypoglycaemic conditions.
Figure 1.
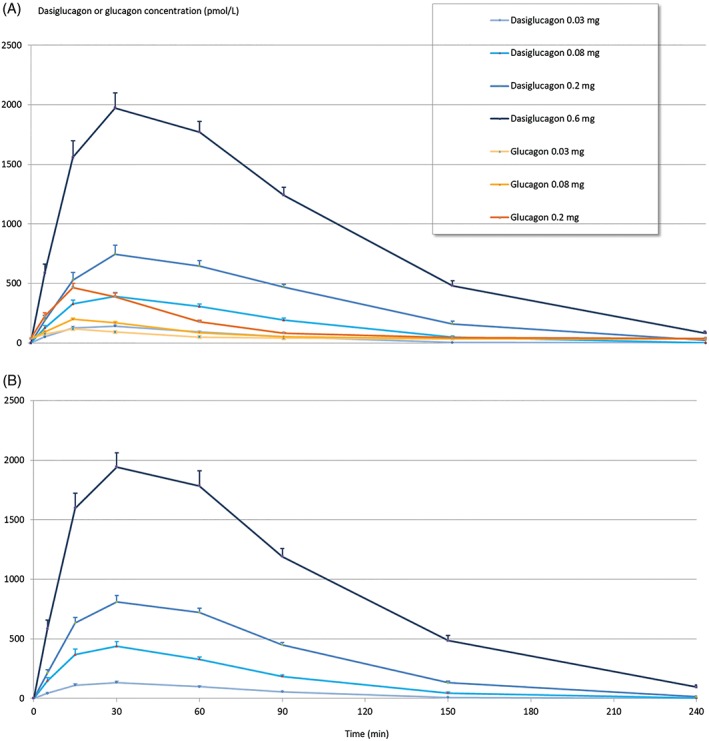
Pharmacokinetic profiles. Mean plasma concentration profiles and SEM at euglycaemia (A) and at hypoglycaemia (B) after single subcutaneous doses of dasiglucagon and commercial glucagon (Eli Lilly)
Table 1.
Pharmacokinetic data
| Euglycaemia | Hypoglycaemia | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dasiglucagona | Glucagon | Dasiglucagona | Glucagon | Dasiglucagona | Glucagon | Dasiglucagona | Dasiglucagona | ||||
| Dose, mg | 0.03 | 0.03 | 0.08 | 0.08 | 0.2 | 0.2 | 0.6 | 0.03 | 0.08 | 0.2 | 0.6 |
| N | 16 | 17 | 17 | 17 | 16 | 16 | 17 | 17 | 17 | 17 | 17 |
| AUC 0‐240min [pmol*h/L] | 174 (43.7) | 54.3 (21.2) | 562 (131) | 133 (35.0) | 1280 (252) | 365 (112) | 3610 (594) | 174 (43.1) | 602 (129) | 1290 (211) | 3560 (723) |
| AUC 0‐30min [pmol*h/L] | 50.8 (18.8) | 28.7 (12.6) | 133 (53.5) | 58.0 (15.9) | 225 (98.8) | 157 (54.0) | 645 (206) | 45.2 (17.6) | 148 (70.7) | 260 (77.0) | 638 (197) |
| C max [pmol/L] | 149 (50.1) | 81.9 (35.0) | 407 (129) | 166 (48.5) | 775 (258) | 438 (132) | 2030 (525) | 137 (46.1) | 453 (176) | 831 (189) | 2010 (523) |
| t max [h] | 0.5 (0.25‐1) | 0.25 (0.08‐0.5) | 0.5 (0.25‐1.5) | 0.25 (0.25‐0.5) | 0.5 (0.5‐1.5) | 0.25 (0.25‐0.5) | 0.5 (0.25‐1) | 0.5 (0.25‐1) | 0.5 (0.25‐1) | 0.5 (0.25‐1) | 0.5 (0.25‐1) |
| N | 14 | 13 | 13 | 15 | 14 | 16 | 17 | 14 | 16 | 17 | 17 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC 0‐inf [pmol*h/L] | 181 (44.5) | 55.7 (20.8) | 604 (145) | 138 (33.3) | 1350 (253) | 366 (112) | 3710 (585) | 182 (45.9) | 622 (133) | 1350 (240) | 3680 (730) |
| t 1/2 [h] | 0.381 (0.065) | 0.310 (0.166) | 0.474 (0.201) | 0.328 (0.092) | 0.565 (0.165) | 0.393 (0.078) | 0.626 (0.189) | 0.447 (0.138) | 0.472 (0.154) | 0.547 (0.136) | 0.668 (0.171) |
| CL/f [L/h] | 51.8 (11.7) | 177 (70.1) | 41.3 (9.53) | 176 (41.3) | 45.4 (8.71) | 172 (55.7) | 49.1 (7.79) | 52.6 (16.7) | 39.8 (8.50) | 45.2 (7.88) | 50.0 (9.38) |
Abbreviations: AUC0‐30min, AUC0‐240min and AUC0‐inf, area under the plasma concentration (PK) curve above baseline from 0 to 30 minutes, 0 to 240 minutes and 0 to infinity, respectively; Cmax, maximum plasma concentration; tmax, time to maximum plasma concentration; t1/2, terminal elimination half‐life; CL/f, total body clearance.
As the glucagon assay captures exogenous administered glucagon (commercial glucagon; Eli Lilly) and endogenous glucagon, AUC0‐30min, AUC0‐240min and Cmax are shown calculated from baseline‐adjusted data, whereas AUC0‐inf, t1/2 and CL/f are displayed as calculated from baseline‐adjusted and truncated commercial glucagon profiles with a cut‐off at 2.5 hours. Terminal elimination rate constant (λz)‐dependent parameters were not calculated in case of a regression fit <0.7. Data are arithmetic means (SD) or medians (range) in case of tmax.
Registered international non‐proprietary name.
Dasiglucagon met the dose proportionality criteria for AUC0‐240 and AUC0‐inf. For Cmax and AUC0‐30 the upper limit of the 95% CI was slightly below 1, which may be attributed to a relatively high early plasma exposure per mg in the lower dose groups (as indicated by an increased AUC0‐30min/AUC0‐240min ratio) compared with the higher dose groups (Table S3, Supporting Information).
3.3. PD results
The PD responses after dasiglucagon were characterized by a dose‐dependent and rapid increase in PG concentration, reaching maximal PG concentrations ~50 to 90 minutes after administration, both in euglycaemic and hypoglycaemic conditions. The overall glucodynamic response (AUE0‐240min) after dasiglucagon administration was 2‐ to 4‐fold higher than that of equal doses of commercial glucagon administration; whereas differences in CEmax were 1.47‐ to 1.85‐fold higher (Table 2 and Table S2, Supporting Information).
Table 2.
Pharmacodynamic data
| Euglycaemia | Hypoglycaemia | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dasiglucagona | Glucagon | Dasiglucagona | Glucagon | Dasiglucagona | Glucagon | Dasiglucagona | Dasiglucagona | ||||
| Dose, mg | 0.03 | 0.03 | 0.08 | 0.08 | 0.2 | 0.2 | 0.6 | 0.03 | 0.08 | 0.2 | 0.6 |
| N | 16 | 17 | 17 | 17 | 16 | 15 | 17 | 17 | 17 | 17 | 17 |
| AUE 0‐240min [mg*h/dL] | 105 (87.9) | 49.7 (53.3) | 242 (149) | 112 (102) | 386 (146) | 179 (115) | 479 (159) | 70.6 (100) | 249 (201) | 406 (175) | 535 (187) |
| AUE 0‐30min [mg*h/dL] | 7.59 (4.92) | 6.90 (5.34) | 11.5 (5.43) | 10.5 (6.24) | 15.4 (6.04) | 14.5 (6.76) | 18.3 (5.25) | 4.60 (5.24) | 14.3 (10.1) | 19.0 (6.43) | 21.9 (5.68) |
| CE max [mg/dL] | 54.0 (32.9) | 32.6 (25.2) | 101 (44.4) | 59.5 (36.3) | 139 (42.7) | 86.1 (38.2) | 165 (46.1) | 37.9 (38.9) | 106 (61.1) | 154 (48.4) | 192 (54.1) |
| CE 30min [mg/dL] | 38.7 (20.9) | 30.1 (22.7) | 55.9 (22.1) | 48.6 (27.0) | 72.5 (23.8) | 70.6 (32.8) | 79.8 (23.4) | 23.7 (24.9) | 63.9 (38.6) | 86.8 (27.2) | 94.2 (21.9) |
| tCE max [min] | 50.0 (0‐90) | 30.0 (0‐70) | 70.0 (50‐150) | 50.0 (0‐70) | 90.0 (70‐150) | 50.0 (30‐90) | 90.0 (70‐240) | 50.0 (0‐90) | 70.0 (50‐90) | 90.0 (70‐150) | 90.0 (70‐150) |
| N | 14 | 10 | 17 | 14 | 16 | 15 | 17 | 10 | 17 | 17 | 17 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| tPG increase ≥ 20 mg/dL [min] | 18.0 (11‐40) | 13.5 (11‐23) | 15.0 (9‐26) | 13.5 (8‐25) | 12.5 (7‐23) | 13.0 (8‐23) | 10.0 (8‐14) | 19.5 (12‐42) | 14.0 (7‐48) | 11.0 (8‐20) | 9.00 (8‐14) |
| N | 13 | 10 | 17 | 14 | 16 | 15 | 17 | 8 | 14 | 17 | 17 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| %PG increase ≥ 20 mg/dL [%] | 81.3 | 8.8 | 100.0 | 82.4 | 100.0 | 100.0 | 100.0 | 47.1 | 82.4 | 100.0 | 100.0 |
| N | 13 | 17 | 17 | 17 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| tPG ≥70 mg/dL [min] | NA | NA | NA | NA | NA | NA | NA | 14.0 (5‐37) | 10.0 (2‐27) | 6.0 (5‐11) | 6.0 (2‐8) |
Abbreviations: AUE0‐30min and AUE0‐240min, area under the plasma glucose excursions above baseline from 0 to 30 minutes and 0 to 240 minutes; CE30min, plasma glucose excursion at 30 minutes; CE, maximum plasma glucose excursion; tmax, time to maximum plasma glucose excursion; % of patients achieving a plasma glucose increase of ≥1.1 mmol/L within 30 minutes after dosing; time to increase in plasma glucose levels ≥1.1 mmol/L; time to reach plasma glucose levels ≥3.9 mmol/L.
Data are arithmetic means (SD) or medians (range) in case of tCEmax, tPGincrease ≥ 20mg/dL, and tPG≥70mg/dL with the number of observations (N).
Registered international non‐proprietary name.
At similar (mg) doses, the glucodynamic responses during the first 30 minutes (AUE0‐30min) were higher after dasiglucagon than after commercial glucagon administration. In line with the PK results, time to maximum effect or half maximum of concentration effect were reached later with dasiglucagon (Table 2 and Table S2, Supporting Information).
The median time (range) to increase PG by at least 1.1 mmol/L was below 20 (18‐19.5) minutes at both euglycaemia and hypoglycaemia with 0.03 mg dasiglucagon and ranged from 9 to 15 minutes with the higher doses. Hypoglycaemia was corrected to PG ≥3.9 mmol/L within median times of 14 and 10 minutes with 0.03 and 0.08 mg dasiglucagon, respectively, and within 6 minutes with 0.2 mg dasiglucagon. All patients (100%) achieved a PG level of at least 3.9 mmol/L within 30 minutes post‐dose at dose levels of 0.08 mg and above. For the lowest dose level (0.03 mg) this was achieved by the majority of patients (70.6%; Table 2 and Figure 2).
Figure 2.
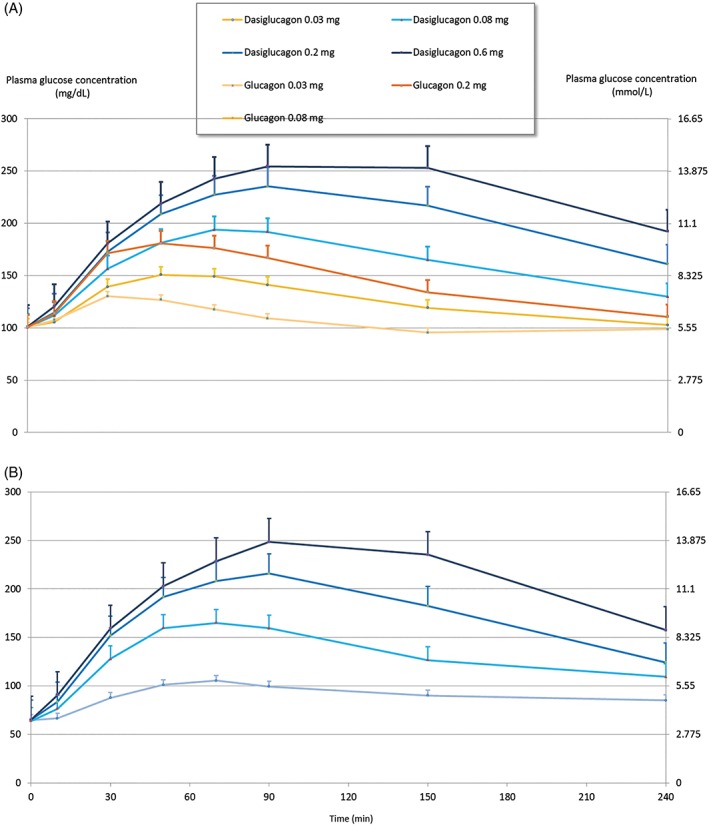
Pharmacodynamic profiles. Mean plasma glucose concentration profiles and SEM at euglycaemia (A) and at hypoglycaemia (B) after single subcutaneous doses of dasiglucagon and commercial glucagon (Eli Lilly)
3.4. Safety
All doses of dasiglucagon were safe and well tolerated. As expected, both commercial glucagon and dasiglucagon showed mainly gastrointestinal side effects (nausea and vomiting) which were more frequent with higher dosing. Nausea and vomiting were more frequent with dasiglucagon than with commercial glucagon when comparing the same dose levels in terms of mg administered. At the lowest dose levels, nausea was observed in 5% to 10% of patients and vomiting in 0% to 5% of patients. At the highest dose levels, up to 50% of patients experienced nausea and up to 25% experienced vomiting. Other less frequent non‐hypoglycaemic treatment emergent adverse events were equally distributed across treatment and dose levels. Injection site reactions were only observed with commercial glucagon. All seven injection site reaction events observed were mild and transient, with only one reaction lasting >30 minutes (Table S4, Supporting Information).
A total of 18 hypoglycaemic events (in 17 completers) occurred with dasiglucagon [over the range of small doses (0.03‐0.2 mg)] compared with 26 hypoglycaemic events with commercial glucagon, in particular with the lower doses (Table S4). The median start time of intravenous glucose infusion post‐dosing to treat hypoglycaemia ranged from 2.4 to 3.8 hours for dasiglucagon versus 2.2 to 3.2 hours for commercial glucagon (post hoc analysis; Table S5, Supporting Information). No serious adverse events occurred, and all adverse events were either of moderate or mild intensity.
Positive antidrug antibody and neutralizing antidrug antibody titres towards commercial glucagon and dasiglucagon were observed in one patient at the follow‐up visit, after this patient had received a total of three commercial glucagon and eight dasiglucagon administrations. These antidrug antibody titres disappeared when reassessed 7 months after the last dosing.
4. DISCUSSION
The present study showed that a stable aqueous formulation of dasiglucagon 4 mg/mL provided dose‐dependent and rapid increases in PG concentrations in both hypoglycaemic and euglycaemic baseline conditions across all doses tested (0.03‐0.6 mg) in adults with T1DM. Importantly, at the same small doses (0.03‐0.2 mg) the early glucodynamic responses indicated a rapid increase, which occurred earlier and was higher compared with commercial glucagon at the same dose levels. It should be noted that time to maximum glucose excursion (tmax) was slightly longer for dasiglucagon, whereas overall glucose response (AUE240min) showed a longer‐lasting and greater maximum effect (CEmax) compared with commercial glucagon. These findings showed that dasiglucagon had titratable and clinically relevant PD responses, enabling, even at small doses, future use in DHAP systems. It could be hypothesized that the higher overall glycaemic response with dasiglucagon versus commercial glucagon could reduce the incidence of recurrent hypoglycaemia, further supporting its suitability for use in DHAP systems. Glucagon doses needed in artificial pancreas settings are aimed at preventing hypoglycaemia and therefore are typically much smaller than those used for rescue treatment of severe hypoglycaemia. Previously published data showed that native glucagon boluses as small as 100 to 300 μg potently raised PG by 2.2 to 4.8 mmol/L (40‐86 mg/dL) from insulin‐induced mild hypoglycaemia.21 We previously found that dasiglucagon showed similar early PD responses to those of GlucaGen® after insulin‐induced hypoglycaemia at corresponding rescue size doses and that higher exposure of dasiglucagon was reflected by a higher glucose response (AUE).19 In line with this, the present study showed similar characteristics of dasiglucagon (4 mg/mL) to those of the previously tested 1‐mg/mL formulation.
The higher bioavailability of dasiglucagon compared with commercial glucagon has also been observed in non‐clinical data22 and may be attributable to the tendency for glucagon to fibrillate, also after it is injected into the subcutaneous compartment, thereby leading to less glucagon being absorbed.
A feasibility trial has been performed with dasiglucagon delivered by a DHAP system (as developed by Beta Bionics, Boston, Massachusetts) and results showed glycaemic responses similar to those of native glucagon during 8‐hour test conditions using high insulin exposures under fasting conditions, combined with structured exercise.23 In that study, both dasiglucagon and native glucagon were effectively able to counteract hypoglycaemia under the challenging conditions.
A major barrier to the development of DHAP systems has been the unstable nature of native glucagon. Currently available glucagon formulations start degrading and form fibrils shortly after reconstitution, meaning that glucagon has to be replaced every day24, 25 to avoid a loss in glucagon efficacy or pump occlusions attributable to fibrils.26, 27 This has led to the search for stable liquid glucagon formulations not requiring reconstitution.28, 29, 30, 31 Until now, “in‐use” drug stability and compatibility in an infusion‐pump (G‐Pump) has been shown for dimethylsulfoxid (DMSO) glucagon, which demonstrated sufficient efficacy at all three doses tested (0.3, 1.2 and 2.0 μg/kg) compared with GlucaGen; however, DMSO glucagon was associated with significantly more erythema and pain at the infusion site compared with GlucaGen. The majority of reactions were mild or moderate, but may limit the clinical utility of DMSO glucagon.30 Furthermore, initial preclinical reports indicate that the BioChaperone technology allows the formulation of stable ready‐to‐use liquid formulations of human glucagon suited for rescue therapy of severe hypoglycaemia.31, 32 Recent clinical data showed safety and efficacy of two BioChaperone formulations, with only slightly slower effects than GlucaGen in people with T1DM.33
Glucagon concentrations in short‐term DHAP studies rarely exceeded the physiological fasting ranges and there were no major safety concerns.34 The small doses of dasiglucagon tested in the present study showed very good tolerability and did not raise any significant safety concerns. The higher incidence of gastrointestinal side effects observed with dasiglucagon compared with glucagon at comparable milligram doses might be caused by a higher potency of the administered dasiglucagon doses. Integrating glucagon into a DHAP system may further protect against hypoglycaemia, help achieve near‐physiological glycaemic control and improve quality of life in people with diabetes mellitus; however, extended monitoring of long‐term dasiglucagon use in DHAP systems will be needed. The safety profile of long‐term use of dasiglucagon in animals supports human testing of dasiglucagon in DHAP systems.35 So far, the clinical trial data have shown that dasiglucagon confers a low risk of immunogenic reactions. In the present study, one sample with transient low‐titre positive antidrug antibody to commercial glucagon and dasiglucagon was detected. As a result of the crossover trial design, the low‐titre antidrug antibody induction could not be associated with a specific treatment. As no apparent clinical effects on PK/PD characteristics or adverse events were observed, the clinical relevance of this finding remains questionable.
A strength of the present study is the inclusion of patients with T1DM, who are the most sensitive and relevant target population for hypoglycaemia rescue/prevention and DHAP therapy. Patients with T1DM were selected to avoid the confounding influence of endogenous insulin and to reduce the effect of counter‐regulation to hypoglycaemia, which might be more pronounced in healthy patients with a fully intact endogenous glucagon production.3, 36, 37 A controlled setting was needed to establish comparable baseline conditions across different doses and treatments as well as for different glycaemic states, a pre‐requisite for valid comparisons. By creating different glycaemic states, the design was not intended to reproduce “real‐life” conditions that most often comprise insulin dosing errors, strenuous exercise, and/or alcohol consumption.38 High prevailing insulin concentrations (in hypoglycaemic settings) may prevent very low doses of glucagon (such as the 0.03‐mg dose in the present study) from significantly increasing glucose output and may attenuate the early glucodynamic response relative to the response at euglycaemic conditions and relative to the responses at higher doses39 (Table 2); however, at higher doses of dasiglucagon, the insulin on board apparently did not impair the glucagon‐induced increase in PG level. This trial design confirmed that a stable dose‐dependent PG response, independent of different glycaemic baseline conditions, could be demonstrated for dasiglucagon, as already described for native glucagon.40 In the previous trial, dasiglucagon doses from 0.1 to 1 mg were used and a linear PK dose–response relationship was demonstrated.19 There was an indication of a sigmoidal dose–response curve for the PD profile which was mainly attributable to a lower response with the 1‐mg dose, whereas the 0.6‐mg dose was only slightly below the expected linear response. For the recent trial, we observed a higher slope of the dose–response relationship for dasiglucagon compared with commercial glucagon for PK and PD variables (PK‐AUC[0‐240min] was in the range of 3‐ to 4‐fold, whereas this ratio was ~2‐fold for PD‐AUE[0‐240min]). The use of two different analytical methods for GlucaGen (radioimmunoassay) and dasiglucagon liquid chromatography mass spectrometry (LCMS) might be deemed a limitation of the present study. Nevertheless, differences in PK characteristics might be expected when two different molecular entities are compared, and the use of a specific assay for dasiglucagon could also be regarded as an advantage because any interference with endogenous glucagon was avoided. Overall, the PD dose–response relationship is the important variable for patient treatment.
The small sample size could be regarded as a limitation of the study; however, a crossover design was chosen in order to reduce variability and increase the power as each patient served as their own control. The chosen sample size in combination with the crossover design (thereby excluding inter‐individual confounders)38 was still sufficient to demonstrate small differences in early exposure and glucose response.
In conclusion, the present study showed that dasiglucagon in a stable liquid formulation of 4 mg/mL had PK and PD properties suitable for use in a DHAP device, with a higher bioavailability than native glucagon. Dasiglucagon quickly and effectively increases PG concentrations from euglycaemic and hypoglycaemic baseline conditions in a dose‐dependent fashion, with clinically relevant effects already observed with small doses. Further clinical trials to evaluate use of dasiglucagon in an automated DHAP system are underway.
CONFLICTS OF INTEREST
U.H., D.L. and B.K. declare no conflicts of interest. T.H. is a shareholder of Profil, which received research funds from Adocia, Biocon, Dance Pharmaceuticals, Eli Lilly, Johnson&Johnson, Julphar, Medimmune, Mylan, Nordic Bioscience, Novo Nordisk, Poxel, Roche Diagnostics, Saniona, Sanofi, Senseonics, SkyePharma and Zealand Pharma. In addition, T.H. is a member of advisory panels for Novo Nordisk and received speaker honoraria and travel grants from Eli Lilly, and Novo Nordisk and Sanofi. M.B. Olsen and U.M. are employees of Zealand Pharma A/S.
Author contributions
U.H. contributed to study design, research data, wrote the manuscript, and reviewed and edited the manuscript. T.H., M.B.O. and U.M. contributed to study design, research data, and reviewed and edited the manuscript. D.L. and B.K. contributed to research data, and reviewed and edited the manuscript. B.K. performed the statistical analysis. U.H. and T.H. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of data analysis. All authors reviewed the manuscript and approved it for submission.
Prior presentation
Parts of this trial were presented as an oral abstract presentation at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018 (102‐OR).
Supporting information
Table S1. Demographic and baseline characteristics (safety analysis set).
Table S2. ANOVA of Pharmacokinetic/pharmacodynamic endpoints at euglycemia.
Table S3. Dose proportionality.
Table S4. Treatment‐emergent adverse events (TEAEs).
Table S5. Post hoc summary of start time of IV Glucose Infusion[h] postdose due to hypoglycaemia completers (N = 17),
Table S6. Insulin concentration AUCins,0‐240min [mU*h/L].
Figure S1. Trial design.
Text S1. Description.
ACKNOWLEDGMENTS
This study was funded by Zealand Pharma, A/S, Denmark.
Hövelmann U, Olsen MB, Mouritzen U, Lamers D, Kronshage B, Heise T. Low doses of dasiglucagon consistently increase plasma glucose levels from hypoglycaemia and euglycaemia in people with type 1 diabetes mellitus. Diabetes Obes Metab. 2019;21:601–610. 10.1111/dom.13562
Dasiglucagon: Registered international non‐proprietary name.
Funding information This study was sponsored by Zealand Pharma A/S, Denmark.
REFERENCES
- 1. Nathan DM, Group DER . The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37:9‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weisman A, Bai JW, Cardinez M, Kramer CK, Perkins BA. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta‐analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5:501‐512. [DOI] [PubMed] [Google Scholar]
- 3. Taborsky GJ Jr. The physiology of glucagon. J Diabetes Sci Technol. 2010;4:1338‐1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jacobs PG, El Youssef J, Reddy R, et al. Randomized trial of a dual‐hormone artificial pancreas with dosing adjustment during exercise compared with no adjustment and sensor‐augmented pump therapy. Diabetes Obes Metab. 2016;18:1110‐1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Taleb N, Emami A, Suppere C, et al. Efficacy of single‐hormone and dual‐hormone artificial pancreas during continuous and interval exercise in adult patients with type 1 diabetes randomised controlled crossover trial. Diabetologia. 2016;59:2561‐2571. [DOI] [PubMed] [Google Scholar]
- 6. El‐Khatib FH, Balliro C, Hillard MA, et al. Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicentre randomised crossover trial. Lancet. 2017;389:369‐380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haidar A, Smaoui MR, Legault L, Rabasa‐Lhoret R. The role of glucagon in the artificial pancreas. Lancet Diabetes Endocrinol. 2016;4:476‐479. [DOI] [PubMed] [Google Scholar]
- 8. Castle JR, El Youssef J, Wilson LM, et al. Randomized outpatient trial of single‐ and dual‐hormone closed‐loop systems that adapt to exercise using wearable sensors. Diabetes Care. 2018;41:1471‐1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rickels MR, DuBose SN, Toschi E, et al. Mini‐dose glucagon as a novel approach to prevent exercise‐induced hypoglycaemia in type 1 diabetes. Diabetes Care. 2018;41:1909‐1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taleb N, Coriati A, Khazzaka C, Bayonne J, Messier V, Rabasa‐Lhoret R. Stability of commercially available glucagon formulation for dual‐hormone artificial pancreas clinical use. Diabetes Technol Ther. 2017;19:589‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Castle JR, DeVries JH, Kovatchev B. Future of automated insulin delivery systems. Diabetes Technol Ther. 2017;19:S67‐S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pedersen JS. The nature of amyloid‐like glucagon fibrils. J Diabetes Sci Technol. 2010;4:1357‐1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Russell SJ, El‐Khatib FH, Sinha M, et al. Outpatient glycaemic control with a bionic pancreas in type 1 diabetes. N Engl J Med. 2014;371:313‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Russell SJ, Hillard MA, Balliro C, et al. Day and night glycaemic control with a bionic pancreas versus conventional insulin pump therapy in preadolescent children with type 1 diabetes: a randomised crossover trial. Lancet Diabetes Endocrinol. 2016;4:233‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haidar A, Legault L, Matteau‐Pelletier L, et al. Outpatient overnight glucose control with dual‐hormone artificial pancreas, single‐hormone artificial pancreas, or conventional insulin pump therapy in children and adolescents with type 1 diabetes: an open‐label, randomised controlled trial. Lancet Diabetes Endocrinol. 2015;3:595‐604. [DOI] [PubMed] [Google Scholar]
- 16. Haidar A, Messier V, Legault L, Ladouceur M, Rabasa‐Lhoret R. Outpatient 60‐hour day‐and‐night glucose control with dual‐hormone artificial pancreas, single‐hormone artificial pancreas, or sensor‐augmented pump therapy in adults with type 1 diabetes: An open‐label, randomised, crossover, controlled trial. Diabetes Obes Metab. 2017;19:713‐720. [DOI] [PubMed] [Google Scholar]
- 17. Haidar A, Legault L, Messier V, Mitre TM, Leroux C, Rabasa‐Lhoret R. Comparison of dual‐hormone artificial pancreas, single‐hormone artificial pancreas, and conventional insulin pump therapy for glycaemic control in patients with type 1 diabetes: an open‐label randomised controlled crossover trial. Lancet Diabetes Endocrinol. 2015;3:17‐26. [DOI] [PubMed] [Google Scholar]
- 18. Peters TM, Haidar A. Dual‐hormone artificial pancreas: benefits and limitations compared with single‐hormone systems. Diabet Med. 2018;35:450‐459. [DOI] [PubMed] [Google Scholar]
- 19. Hövelmann U, Bysted BV, Mouritzen U, et al. Pharmacokinetic and pharmacodynamic characteristics of dasiglucagon, a novel soluble and stable glucagon analog. Diabetes Care. 2018;41:531‐537. [DOI] [PubMed] [Google Scholar]
- 20. American Diabetes Association (ADA) . 2. Classification and diagnosis of diabetes. Standards of medical care in diabetes ‐ 2018. Diabetes Care. 2018;41(suppl 1):S13‐S27. [DOI] [PubMed] [Google Scholar]
- 21. Ranjan A, Schmidt S, Madsbad S, Holst JJ, Norgaard K. Effects of subcutaneous, low‐dose glucagon on insulin‐induced mild hypoglycaemia in patients with insulin pump treated type 1 diabetes. Diabetes Obes Metab. 2016;18:410‐418. [DOI] [PubMed] [Google Scholar]
- 22. Onoue S, Ohshima K, Debari K, et al. Mishandling of the therapeutic peptide glucagon generates cytotoxic amyloidogenic fibrils. Pharm Res. 2004;21:1274‐1283. [DOI] [PubMed] [Google Scholar]
- 23. Jafri R, Maheno M, Balliro C, et al. The stable glucagon analog dasiglucagon is well‐tolerated and as effective as recombinant human glucagon when delivered by the bionic pancreas in response to insulin excess. Diabetes Technol Ther (ATTD Abstracts). 2018;20:A‐13, Abstract 028. [Google Scholar]
- 24.Novo Nordisk Limited. Summary of product characteristics. GlucaGen Hypokit 1 mg. 2015. http://www.medicines.org.uk/emc/medicine/4258/SPC/GlucaGen+Hypokit+1+mg/.pdf. Accessed February 3, 2018.
- 25.Eli Lilly and Company. Information for the Physician. Glucagon for Injection. 2017. http://pi.lilly.com/us/rglucagon-pi.pdf. Accessed February 5, 2018.
- 26. Blauw H, van Bon AC, Koops R, DeVries JH, on behalf of PCDIAB Consortium . Performance and safety of an integrated bihormonal artificial pancreas for fully automated glucose control at home. Diabetes Obes Metab. 2016;18:671‐677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Van Bon AC, Luijf YM, Koebrugge R, Koops R, Hoekstra JB, DeVries JH. Feasibility of a portable bihormonal closed‐loop system to control glucose excursions at home under free‐living conditions for 48 hours. Diabetes Technol Ther. 2014;16:131‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Newswanger B, Ammons S, Phadnis N, et al. Development of a highly stable, nonaqueous glucagon formulation for delivery via infusion pump systems. J Diabetes Sci Technol. 2015;9:24‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pohl R, Li M, Krasner A, De Souza E. Development of stable liquid glucagon formulations for use in artificial pancreas. J Diabetes Sci Technol. 2015;9:8‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Castle JR, Youssef JE, Branigan D, et al. Comparative pharmacokinetic/pharmacodynamic study of liquid stable glucagon versus lyophilized glucagon in type 1 diabetes subjects. J Diabetes Sci Technol. 2016;10:1101‐1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Soula O, Duracher D, Budin G, et al. BioChaperone technology enables rhGlucagon aqueous formulation for use in rescue and dual‐hormone artificial pancreas (DHAP). Diabetes. 2017;66:A307. [Google Scholar]
- 32. Meiffren G, Teng S, Ranson A, et al. Preclinical efficacy of a stable aqueous formulation of human glucagon with BioChaperone technology (BC GLU). Diabetes. 2017;66:A306. [Google Scholar]
- 33. Gletzer S, Hovelmann U, Teng S, et al. BioChaperone Glucagon (BCG), a stable ready‐to‐use liquid glucagon formulation, is well tolerated and quickly restores euglycaemia after insulin‐induced hypoglycaemia. Diabetes. 2018;67(suppl 1):305‐OR. [Google Scholar]
- 34. Taleb N, Haidar A, Messier V, Gingras V, Legault L, Rabasa‐Lhoret R. Glucagon in artificial pancreas systems: potential benefits and safety profile of future chronic use. Diabetes Obes Metab. 2017;19:13‐23. [DOI] [PubMed] [Google Scholar]
- 35. Castle JR, Elander M, O'Halloran SA. Long‐term safety and tolerability of dasiglucagon, a stable‐in‐solution glucagon analog. Diabetes. 2018;67(suppl 1):1230‐P. [DOI] [PubMed] [Google Scholar]
- 36. Lecavalier L, Bolli G, Cryer P, Gerich J. Contributions of gluconeogenesis and glycogenolysis during glucose counterregulation in normal humans. Am J Physiol. 1989;256:E844‐E851. [DOI] [PubMed] [Google Scholar]
- 37. Kishore P, Gabriely I, Cui MH, et al. Role of hepatic glycogen breakdown in defective counterregulation of hypoglycaemia in intensively treated type 1 diabetes. Diabetes. 2006;55:659‐666. [DOI] [PubMed] [Google Scholar]
- 38. Choudhary P, Rickels MR, Senior PA, et al. Evidence‐informed clinical practice recommendations for treatment of type 1 diabetes complicated by problematic hypoglycaemia. Diabetes Care. 2015;38:1016‐1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. El Youssef J, Castle JR, Bakhtiani PA, et al. Quantification of the glycaemic response to microdoses of subcutaneous glucagon at varying insulin levels. Diabetes Care. 2014;37:3054‐3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Blauw H, Wendl I, DeVries JH, Heise T, Jax T, on behalf of PCDIAB consortium . Pharmacokinetics and pharmacodynamics of various glucagon dosages at different blood glucose levels. Diabetes Obes Metab. 2016;18:34‐39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Demographic and baseline characteristics (safety analysis set).
Table S2. ANOVA of Pharmacokinetic/pharmacodynamic endpoints at euglycemia.
Table S3. Dose proportionality.
Table S4. Treatment‐emergent adverse events (TEAEs).
Table S5. Post hoc summary of start time of IV Glucose Infusion[h] postdose due to hypoglycaemia completers (N = 17),
Table S6. Insulin concentration AUCins,0‐240min [mU*h/L].
Figure S1. Trial design.
Text S1. Description.


