Abstract
Background
Insect‐bite hypersensitivity (IBH) in horses is a chronic allergic dermatitis caused by insect bites. Horses suffer from pruritic skin lesions, caused by type‐I/type‐IV allergic reactions accompanied by prominent eosinophil infiltration into the skin. Interleukin‐5 (IL‐5) is the key cytokine for eosinophils and we have previously shown that targeting IL‐5 by vaccination reduces disease symptoms in horses.
Objective
Here, we analyzed the potential for long‐term therapy by assessing a second follow‐up year of the previously published study.
Methods
The vaccine consisted of equine IL‐5 (eIL‐5) covalently linked to a cucumber mosaic virus‐like particle (VLP) containing a universal T cell epitope (CuMVTT) using a semi‐crossover design to follow vaccinated horses during a second treatment season. Thirty Icelandic horses were immunized with 300 μg of eIL‐5‐CuMVTT without adjuvant.
Results
The vaccine was well tolerated and did not reveal any safety concerns throughout the study. Upon vaccination, all horses developed reversible anti‐eIL‐5 auto‐antibody titers. The mean course of eosinophil levels was reduced compared to placebo treatment leading to significant reduction of clinical lesion scores. Horses in their second vaccination year showed a more pronounced improvement of disease symptoms when compared to first treatment year, most likely due to more stable antibody titers induced by a single booster injection. Hence, responses could be maintained over two seasons and the horses remained protected against disease symptoms.
Conclusion
Yearly vaccination against IL‐5 may be a long‐term solution for the treatment of IBH and other eosinophil‐mediated diseases in horses and other species including humans.
Keywords: allergic dermatitis, eosinophils, vaccination
Abbreviations
- CuMV
cucumber mosaic virus‐derived virus‐like particles
- CuMVTT
CuMV containing a tetanus toxoid universal T cell epitope tt830‐843
- eIL‐5
equine IL‐5
- IBH
insect‐bite hypersensitivity
- ISI
insect‐bite hypersensitivity severity index
- VLP
virus‐like particle
1. INTRODUCTION
Insect‐bite hypersensitivity (IBH) in horses is caused by an allergy against insect bites, more specifically against Culicoides spp. It is a severe and chronic disease affecting a large number of horses worldwide. Although IBH is the best characterized allergic dermatitis in horses, effective treatment is still lacking.1, 2, 3, 4, 5, 6, 7, 8 We recently proposed a new therapeutic vaccination targeting eosinophils by active vaccination against IL‐5.9 Host‐made auto‐antibodies induced by active vaccination show overall similar advantages and safety profiles as monoclonal antibodies (mAb) administered via passive vaccination. A major drawback of mAbs is the potential induction of anti‐mAb antibodies and their relative short half‐life, thus limiting its clinical long‐term use. On the other hand, a specific concern of therapeutic vaccines is the potential irreversibility of the antibody responses, potentially causing lifelong blockage of target self‐molecules.10 The most prominent therapeutic vaccine in veterinary use is the anti‐boar vaccine Improvac®, targeting gonadotropin‐releasing hormone (GnRH).11 A similar immunocontraceptive vaccine was developed for horses, known as GonaCon‐Equine™,12 registered in the US for female wild/feral horses and burros. For human use, a number of anti‐self vaccines are in preclinical and clinical development mostly targeting cytokines in inflammatory conditions and personalized anti‐cancer vaccines.13, 14
Originally described as an IgE‐dependent type‐I allergy, it has recently emerged that IBH also shows characteristics of a delayed‐type hypersensitivity (DTH) allergic response. Eosinophils may be a common denominator, as they can play a key role in both allergic reactions. Indeed, allergic lesions are characterized by strong eosinophilic inflammation9, 15 and it has previously been suggested that eosinophils strongly contribute to IBH disease pathology.16 This notion is supported by the fact that increased expression of IL‐4, IL‐5, and IL‐13 mRNA is found in acute lesions. IL‐5 is the key cytokine for the development, survival, and activation of eosinophils. In addition, established lesions show enhanced levels of mRNA encoding the chemokines CCL11 (Eotaxin‐1) and CCL2 (monocyte chemotactic protein 1 (MCP1)).17 Eotaxin‐1 is a potent eosinophil chemoattractant and binds to the CCR3 receptor. Furthermore, Eotaxin‐1 is involved in eosinopoiesis and cooperates with IL‐5 at inducing blood eosinophilia.18, 19 Together with IL‐5, Eotaxin‐1 stimulates eosinophils to migrate from blood to tissue19 and locally produce a large number of pro‐inflammatory and toxic mediators.17, 20 We recently found that eosinophils are not only upregulated locally within lesions but also systemically in blood and that these blood eosinophil levels strongly correlate with disease severity.9 Hence, blood eosinophilia might be a new and easy‐to‐measure diagnostic disease activity marker of IBH and related diseases.
In order to target eosinophils, we developed a therapeutic vaccine targeting equine IL‐5 (eIL‐5). IL‐5 is a classical Th2 cytokine and, as discussed above, is the master regulator for eosinophil‐mediated inflammation.18, 21, 22 Hence, IL‐5 is known to be an eosinophil lineage‐specific cytokine, thereby having limited effects on other lineages.23 In contrast to the veterinary field, IL‐5 blocking agents are already well known for use in human hyper‐eosinophilic conditions such as eosinophilic asthma. Three monoclonal antibodies, two blocking IL‐5 directly (Reslizumab and Mepolizumab), and one blocking the IL‐5Rα (Benralizumab) received market authorization over the past two years. All IL‐5 pathway interfering antibodies and in particular both anti‐IL‐5 antibodies have a very good safety profile collected over the past 10 years of clinical testing and use.24
In order to induce IL‐5‐specific neutralizing antibodies, we have developed a virus‐like particle (VLP)‐based IL‐5 vaccine, which can overcome B cell unresponsiveness.9, 25 Our previously published study shows that vaccination of horses resulted in high levels of IL‐5‐specific antibodies and statistically significant reductions of lesion scores in vaccinated IBH‐affected horses when compared to placebo‐treated IBH‐affected horses.9 Here, we document a follow‐up study subsequent to the placebo‐controlled double‐blind study of the 1st year. The current study was performed in the 2nd year with a semi‐crossover design. Three main questions were addressed. (a) Assess the impact of reducing basic vaccination injections in the first treatment year from five to three injections and in the second treatment year to a single booster injection at the beginning of the season; (b) compare eosinophil levels in previously placebo‐treated horses and first year vaccination of the same horses; (c) investigate long‐term treatment potential. We report that basic vaccination consisting of three injections with eIL‐5‐CuMVTT reduces eosinophil levels in blood. Furthermore, a single booster may be suitable for long‐term management of IBH and other eosinophil‐mediated diseases in horses. This suggests that comparable vaccines may be developed for other species including eosinophilic asthma in humans.
2. MATERIALS & METHODS
2.1. Horses & clinical study design
All study horses were Icelandic horses and were privately held by their owners. All clinical studies had been approved by the respective cantonal veterinary authorities. All horse owners signed informed consent. The clinical study was a semi‐crossover follow‐up of a double‐blind placebo‐controlled randomized trial, in which all horses received vaccine, either as a first year treatment (following placebo treatment) or as a second year treatment. Thirty‐four Icelandic horses had been recruited to the two‐year placebo‐controlled double‐blind randomized clinical study that has been described previously9: The study consisted of 2 years, an observational year (“Pre‐season”) and a treatment year (“1st year”). Thirty horses were then recruited to the here‐described semi‐crossover follow‐up year (“2nd year”) when all horses received the eIL‐5‐CuMVTT vaccine. Thirteen horses previously treated with placebo received a basic vaccination regimen consisting of two vaccinations in January (week 0) and February (week 4) and one booster immunization in June (week 19; Group 1); and 17 previously immunized horses received a single booster immunization at season start in March (week 6; Group 2; Figure S1). Of note, three horses of the previously immunized Group 2 received a second booster because of low anti‐IL‐5 titers. In order to reduce the number of injections during basic vaccination year, we now altered injection frequency and regimen in Group 1 horses to three vaccinations in weeks 0, 4, and 19, in contrast to the previous five injections in weeks 0, 4, 8, 12, and 19 in 1st year treatment of Group 2 horses.9 Vaccine was administered subcutaneously without the presence of adjuvants. In the observational year (“Pre‐season”), the eczema lesions of all horses had been recorded monthly (after study recruitment) and blood eosinophil levels and a serum IgE against insects had been determined at a single time point. In both treatment years (“1st year” and “2nd year”) before vaccination and at the end of each study year, health status in blood and parasite levels in stool of all horses were determined. During the treatment years, all horses were bled monthly in order to monitor antibody titers and eosinophil levels. Additionally, eczema lesions were scored at least monthly.
2.2. IBH lesion scoring
Described in Ref. 9.
2.3. Blood withdrawal
Blood was collected from V. jugularis at the intersection of the proximal to median third of the neck.
2.4. Blood analysis by IDEXX Diavet and production of horse sera and plasma
Blood was collected into tubes provided by IDEXX Diavet (Switzerland). Differential blood analysis was done using fresh EDTA blood, measured by IDEXX Diavet.
2.5. Anti‐CuMVTT and anti‐IL‐5 antibody titer determination
Described in Ref. 9.
2.6. Cloning, expression and purification of recombinant eIL‐5
Described in Ref. 9.
2.7. Coupling of eIL‐5 to CuMVTT
Described in Ref. 9. Briefly, CuMVTT VLP reacted with a 10‐fold molar excess of the heterobifunctional cross‐linker succinimidyl‐6(β‐maleimidopropionamido)hexanoate (SMPH) in 20 mmol/L NaP/2 mmol/L EDTA, pH 7.5 at 25°C (Pierce). Unreacted cross‐linker was removed by passage over a PD‐10 desalting column (GE Healthcare). The recombinant, purified and refolded eIL‐5‐C‐His, was reduced for 1 hour with an equimolar amount of tri(2‐carboxyethyl)phosphine hydrochloride (TCEP) in 20 mmol/L NaP/2 mmol/L EDTA, pH 7.5 to reduce the cysteine residue contained in the linker. The reduced eIL‐5‐C‐His was then mixed with the derivatized CuMVTT VLPs at a molar ratio of 2:1 (monomer IL‐5:monomer VLP) and co‐incubated for 4 hours at 22°C in 20 mmol/L NaP/2 mmol/L EDTA, pH 7.5 to allow cross‐linking. Vaccine was purified on a HiLoad 26/600 Superdex 75 prep grade (GE Healthcare) with 20 mmol/L NaP/2 mmol/L EDTA, pH 7.5 in order to remove free unbound eIL‐5. Protein concentration was determined by Bradford assay to BSA standard.
2.8. SDS‐PAGE & Coomassie staining
Described in Ref. 9.
2.9. Western blot
Described in Ref. 9.
2.10. Vaccine administration, immunization regimen
In order to generate self‐reactive antibodies to equine IL‐5, horses were injected subcutaneously with 300 μg of eIL5‐C‐His‐CuMVTT VLP in 1000 μL of 20 mmol/L NaP/2 mmol/L EDTA, pH 7.5 without additional adjuvants. Horses, that had received vaccine in the previous season (Group 2), received a single booster at the beginning of March (n = 17), whereof three horses received an additional booster in June (week 19). Horses, that had received placebo in the previous season (Group 1), received a prime‐boost vaccination in weeks 0 (January), 4 (February) and a booster in week 19 (June; n = 13).
2.11. Linear epitope mapping
Linear epitope mapping using serum of three different eIL‐5‐VLP vaccinated horses was performed by PEPperPRINT GmbH, Heidelberg, Germany. Negative control was naive serum prior to vaccination.
2.12. Model structure equine IL‐5 dimer
The monomeric equine IL‐5 was plotted onto the dimeric human IL‐5 crystal structure using Pymol. The monomeric eIL‐5 structure was calculated by Kallber et al.26
2.13. Model structures for IL‐5 and IL‐5Rα
Structure of human IL‐5 dimer binding to its human IL‐5Rα27 was used as a template for the generation of a model structure of equine IL‐5 dimer binding to the human IL‐5Rα by Pymol. Human and equine IL‐5 dimer was illustrated including epitope of Reslizumab (Ref. 28) binding site (yellow) and major epitope site of equine IL‐5 (magenta) resulting from linear epitope mapping by PEPperPRINT.
2.14. Parasitic presence
Described in Ref. 9. Worm parasites were distinguished for nematode (roundworms), cestoda oocysts (tapeworms), and tapeworm eggs/body parts. All horse owners maintained their individual anti‐helminthic treatment regimen over all seasons, depending on the individual helminth concept (according to recommendations: either helminth monitoring with a single dewormer/y or three dewormers/y).
2.15. Statistics
All graphs comparing vaccinated horses vs placebo horses show mean and standard error of mean (SEM). Statistical analysis was performed by paired, one‐tailed non‐parametric Wilcoxon test. Considered to be statistically significant were P‐values lower than 0.05: *P < 0.05; **P < 0.01; ***P < 0.001.
3. RESULTS
3.1. Vaccine preparation
Recombinant eIL‐5 with a C‐terminal linker containing a free cysteine residue and a His‐Tag (eIL‐5‐C‐His) was produced in Escherichia coli and purified by affinity chromatography, refolded, and polished by size‐exclusion chromatography as described in Ref. 9. The eIL‐5‐C‐His homodimers were then chemically coupled to VLPs derived from the cucumber mosaic virus (CuMVTT) Ref. 9 via the heterobifunctional cross‐linker SMPH. Derivatization of the VLP shows the typical “VLP‐ladder” with CuMV monomeric and multimeric subunits (Figure S2A, lane 2) caused by cross‐linking of VLP‐internal Cys and Lys Ref. 9. Coupling of CuMV subunits with dimeric eIL‐5‐C‐His molecules (Figure S2A, lane 1) is shown on a reducing SDS‐PAGE gel by the presence of additional coupling bands that correspond to the molecular mass of monomeric or dimeric eIL‐5 plus monomeric or multimeric CuMV subunits (Figure S2A, lane 3). Successful covalent attachment of eIL‐5‐C‐His to CuMVTT was confirmed by Coomassie staining (Figure S2A) and Western blot using an anti‐His antibody, thus staining only His‐tagged eIL‐5 (Figure S2B). Coupling efficiency for all batches was between 40% and 70% (ie, between 70 and 120 IL‐5 molecules were displayed per VLP). A further vaccine polishing step by size‐exclusion chromatography was performed to remove free uncoupled eIL‐5 from the vaccine (Figure S2A and B, lane 4 and Figure S2A and B, lane 5).
3.2. Antibody titer responses upon vaccination
Antibody titers in serum of horses were evaluated once per month. Thirteen previously placebo‐treated horses (Group 1) received a basic vaccination consisting of three immunization, and 17 previously immunized horses (Group 2) received a single boost at the beginning of the season. Three horses of Group 2 had received an additional booster in June. One vaccine dose contained 300 μg of eIL‐5‐CuMVTT (based on CuMV protein, corresponding to about 150 μg of displayed eIL‐5) formulated in sodium phosphate buffer without adjuvants. Anti‐CuMVTT antibody titers were used as surrogate marker for successful vaccination as the immune system is expected to readily induce antibodies against the foreign CuMVTT particles. In total, 30 out of 30 vaccinated horses developed antibodies against both IL‐5 and CuMVTT. However, the Group 1 horses which were de novo immunized with three doses (Figure 1A and C) showed higher but less stable anti‐IL5 titers than the horses receiving a single booster immunization (Group 2) (Figure 2A and C). Antibody titer against eIL‐5 and CuMVTT can be observed in all naive horses upon eIL‐5‐CuMVTT vaccination already after the second vaccine injection (Figure 2). All de novo immunized Group 1 horses developed anti‐IL‐5 antibody titers, suggesting that the vaccination regimen of two injections prior to IBH season plus one booster in the middle of the season is sufficient for auto‐antibody induction and maintenance in first season horses. After having reached high anti‐IL‐5 titers upon the two initial vaccinations, antibody titers continuously dropped. The booster injection efficiently increased the antibody titer in mid‐season (Figure 1A and B). Anti‐CuMVTT titers followed the same pattern; however, peak levels were lower when compared to anti‐IL‐5 levels, indicating that the immune response was dominated by anti‐IL‐5 (Figure 1C and D).
Figure 1.
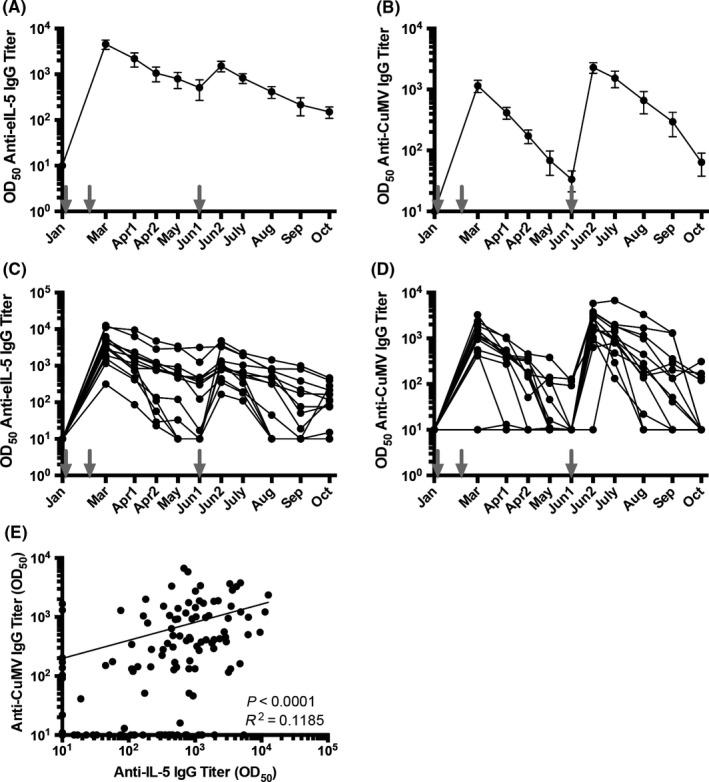
Antibody titer of Group 1 horses against eIL‐5 and CuMVTT‐VLP. Vaccinations are indicated by gray arrows. A, Mean antibody titer of anti‐eIL‐5 IgG. B, Mean antibody titer of anti‐CuMV IgG. C, Antibody titer of anti‐eIL‐5 IgG of single horses. D, Antibody titer of anti‐CuMV IgG of single horses. All antibody titers are calculated with naive serum subtracted on logarithmic scales, limit of detection is titers ≤10. E, Correlation of anti‐CuMV IgG and anti‐IL‐5 IgG antibody titer of single horses, 13 horses and 10 time points each (from January until October). VLP, virus‐like particle
Figure 2.
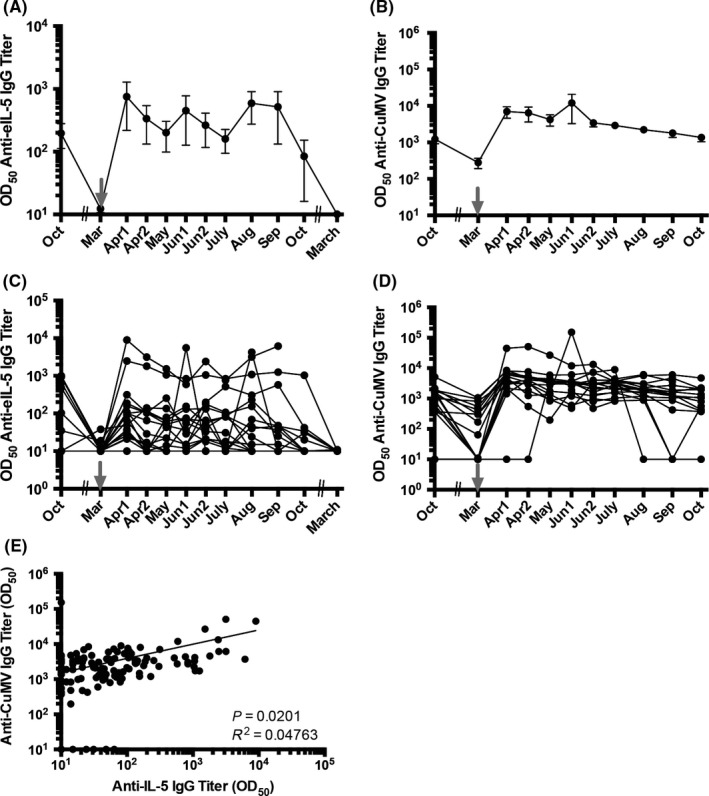
Antibody titer of Group 2 horses against eIL‐5 and CuMVTT‐VLP. Vaccinations are indicated by gray arrows. A, Mean antibody titer of anti‐eIL‐5 IgG. B, Mean antibody titer of anti‐CuMV IgG. C, Antibody titer of anti‐eIL‐5 IgG of single horses. D, Antibody titer of anti‐CuMV IgG of single horses. All antibody titers are calculated with naive serum subtracted on logarithmic scales, limit of detection is titers ≤10. E, Correlation of anti‐CuMV IgG and anti‐IL‐5 IgG antibody titer of single horses, 17 horses and eight time points each (from April until October). VLP, virus‐like particle
In the boosted Group 2 horses, all horses developed antibodies against IL‐5 and CuMVTT. Importantly, the single booster at season start for Group 2 was sufficient to induce long‐lasting anti‐IL5 antibodies throughout the season in almost all of the horses (Figure 2A and C). Interestingly, after induction of anti‐IL‐5 antibodies, titers were decreasing only slightly to reach a steady‐state during IBH season and only dropped toward season's end. Anti‐CuMVTT antibody titers showed an overall similar pattern and increased upon vaccination before they slowly but continuously declined (Figure 2B and D).
Although anti‐IL‐5 antibody titers between single horses were slightly more variable than antibody titers against the carrier VLP, both antibody responses correlated well overall (Figures 1E and 2E), demonstrating that anti‐CuMVTT titers do not interfere with the induction of anti‐IL‐5 antibodies.
3.3. Antibody specificity assessed by linear epitope mapping
Linear epitope mapping by PEPperPRINT (PEPperCHIP Custom Peptide Microarray, Heidelberg, Germany)29 from serum of three eIL‐5‐VLP vaccinated horses showed three epitopes, whereof one major epitope was recognized by all three horses tested. Each of the two minor epitopes was recognized by two horse sera (Figures 3A and S3). Structural models of dimeric eIL‐5 based on dimeric human IL‐5 (huIL‐5) crystal structure place the major epitope in a loop, and both minor epitopes in two different alpha helices (Figure 3B, magenta: loop—GNLMI, blue: α‐helices—EEVFQGIDT, cyan: α‐helices—MNRLVAETLT). As expected, the surface structural model of dimeric eIL‐5 shows that all epitopes are exposed on the surface of the molecule and thus accessible to antibodies (Figure 3C). Mapping of the dimeric eIL‐5 model onto the human IL‐5 receptor 5 alpha (huIL‐5Rα) with the recognized epitopes marked is shown in Figure 3D. In parallel, the dimeric huIL‐5 was mapped onto the huIL‐5Rα (Figure 3E). Furthermore, the huIL‐5 epitope, which is recognized by the registered anti‐huIL‐5 neutralizing antibody reslizumab (Ref. 28), is displayed on the dimeric huIL‐5. Interestingly, the epitopes on the huIL‐5 recognized by reslizumab were similar regions compared to the epitopes recognized by the sera of vaccinated horses. The data presented rely on in silico interaction between eIL‐5 and the huIL‐5‐IL‐5Rα receptor (modeled by the hu IL‐5‐IL‐5Rα interaction) and their predictive value may therefore be limited. However, the model reveals a comparable binding site found in the human analogous, which indeed strongly suggests induction of neutralization antibodies upon eIL‐5‐CuMVTT vaccination (Figure 3D and E: magenta—major equine epitope, yellow—reslizumab epitope).
Figure 3.
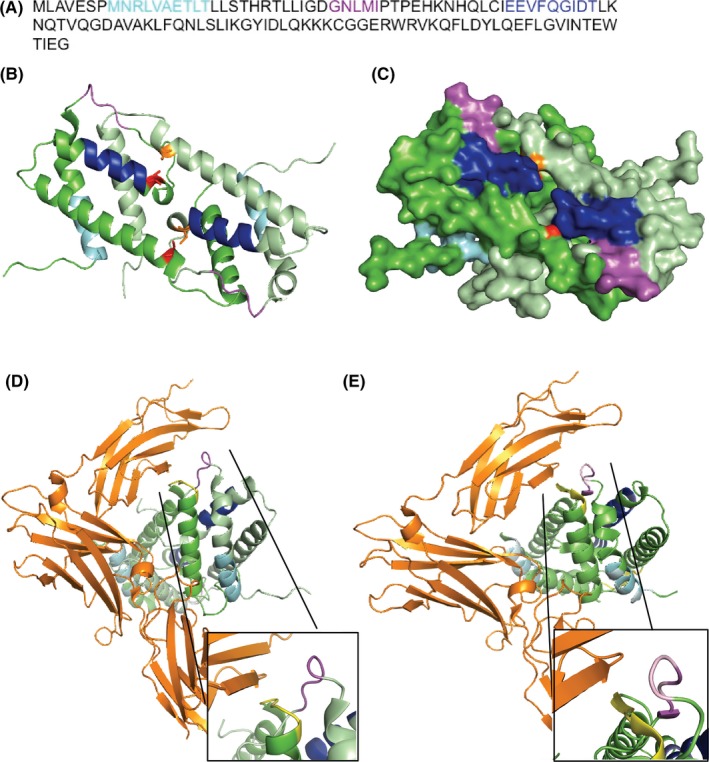
Antibody specificity by linear epitope mapping. A‐D, Colored epitopes recognized by vaccine‐induced self‐antibodies, major epitope: magenta (loop—GNLMI), minor epitope 1: blue (α‐helices—EEVFQGIDT), minor epitope 2: cyan (α‐helices—MNRLVAETLT); eIL‐5 monomers in green, disulfide bridges in red and orange. A, Linear amino acid sequence of eIL‐5 with colored linear epitopes. B, Structure model of dimeric eIL‐5 based on calculated monomeric eIL‐5 plotted on the human dimeric crystal structure. C, Surface model of dimeric eIL‐5 based on calculated monomeric eIL‐5 plotted on the human dimeric crystal structure. D, Mapping of the dimeric eIL‐5 model onto the human IL‐5 receptor 5 alpha (huIL‐5Rα) with marked epitopes of reslizumab (yellow) and corresponding equine epitopes. E, Mapping of the dimeric huIL‐5 onto the huIL‐5Rα with marked epitopes of reslizumab (yellow) and corresponding equine epitopes
3.4. Blood eosinophilia
Eosinophil levels were measured once per month in all horses. The data were analyzed according to intention to treat (ITT), thus including all horses, without exclusion of animals. The de novo immunized Group 1 horses that were vaccinated twice in January and February followed by a booster vaccination in June showed statistically significant reduction of eosinophil levels throughout the treatment season (Figure 4A, black circles) compared to the placebo‐treated first season (Figure 4A, gray circles). Group 2 horses had received five immunizations in the previous “1st year” season and a single booster vaccination in the follow‐up “2nd year” in March just before the season started. However, three horses of Group 2 with low anti‐IL‐5 titers received an additional boost in June. As expected, Group 2 horses showed comparable blood eosinophil levels throughout both vaccinated seasons (Figure 4B). However, there was an unexpected spike of eosinophils early in the first treatment year (Figure 4B, “1st year,” filled circles), which remains unexplained but perhaps was due to free eIL‐5 present in the earlier vaccine preparation. Most importantly, the course of eosinophil levels negatively correlated with antibody levels, indicating that anti‐IL‐5 antibodies likely caused the reduction of eosinophil levels (Figures 1 and 4A,C).
Figure 4.
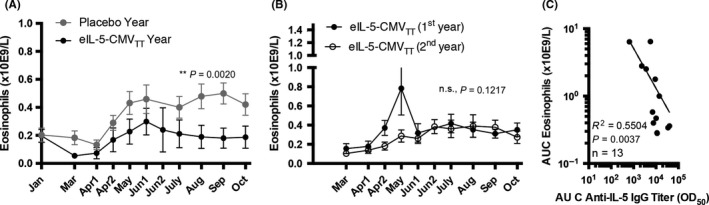
Course of eosinophil levels in blood. A, Course of seasonal blood eosinophil levels in Group 1 horses of placebo‐treated season (gray circles, n = 13) and 1st year vaccination season in “2nd year” (black circles, n = 13). B, Course of seasonal blood eosinophil levels in Group 2 horses of 1st year vaccination season (filled circles, n = 17) and 2nd year vaccination season (empty circles, n = 17). C, Correlation of eosinophil levels in blood vs anti‐IL‐5 antibody levels shown by area under the curve (AUC) from Group 1 horses
3.5. Reduced IBH lesion scores in eIL‐5‐CuMVTT vaccinated horses
Lesion scores for all horses were recorded at least once per month from March until October. The data were analyzed according to ITT, thus including all horses that participated during all three seasons: ie, (a) Pre‐treatment; (b) first year treatment/placebo; (c) second year treatment/first year treatment. Seasonal progression of lesion scores of Group 1 horses was comparable to “Pre‐season” and placebo treatment during “1st year.” However, upon vaccination during “2nd year,” lesion scores over the season clinically improved highly significantly (Figure 5A). Comparably, Group 2 horses had been vaccinated using eIL‐5‐CuMVTT in both seasons “1st year” and “2nd year” and showed a statistically significant improved course of lesion scores in both vaccinated years when comparing to their “Pre‐season” or placebo‐treated horses. Moreover, lesion course of Group 2 horses even further improved in the second year treatment when compared to first year treatment (Figure 5B). Area under the curve (AUC) of mean average lesions scores per year of both groups (Figure 5C), average lesion scores for single horses (Figure 5D), and mean average lesion score per year (Figure 5E Group 1, Figure 5F Group 2) also showed improvement of lesions scores upon vaccination with even enhanced improvement during the second treatment year.
Figure 5.
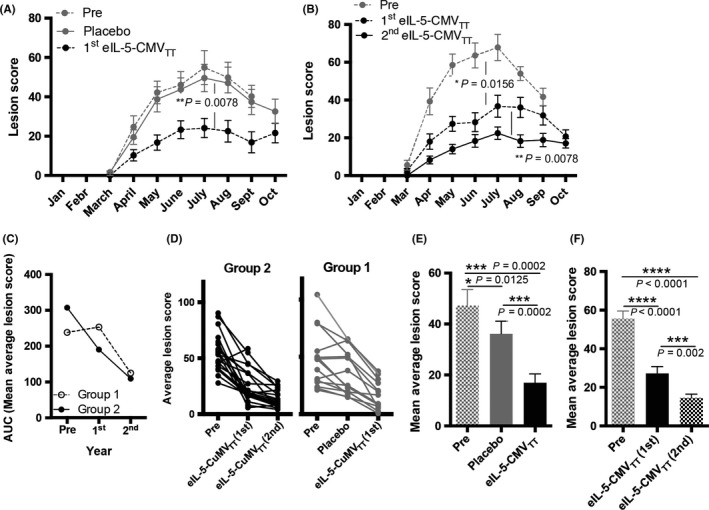
Improving IBH lesion scores by vaccination with eIL‐5‐CuMVTT vaccine. A & B, Mean monthly lesion score from March until October in “Pre‐evaluation” year (n = 34), “1st year” (placebo‐controlled double‐blind randomized study, n = 34), and “2nd year” (half crossover follow‐up vaccination, n = 30). Months with more than one measurement show mean values. A, Group 1 horses in “Pre‐season” (dotted gray line, n = 15), blinded placebo treatment “1st year” (continuous gray line, n = 15), and first season with vaccine treatment “2nd year” (dotted black line, n = 13). B, Group 2 horses in “Pre‐season” (dotted gray line, n = 19), blinded vaccine treatment “1st year” (dotted black line, n = 19), and follow‐up second year vaccine treatment (continuous black line, n = 17). C, Area under the curve of mean average lesion score per year of Group 1 (empty circles) and Group 2 (filled circles). D, Average (of monthly mean) lesion score per year of single horses in Group 2 (black lines) and Group 1 (gray lines) during “Pre‐season” (pre), placebo treatment or eIL‐5‐CuMVTT vaccination “1st year,” and eIL‐5‐CuMVTT vaccination “2nd year”. E, Mean average lesion score of all Group 1 horses during “Pre‐season” year (pre, gray pattern, n = 15), blinded placebo treatment “1st year” (filled gray, n = 15), and eIL‐5‐CuMVTT vaccination “2nd year” (filled black, n = 13). F, Mean average lesion score of all Group 2 horses during “Pre‐season” (pre, gray pattern, n = 19), blinded first year eIL‐5‐CuMVTT vaccination “1st year” (filled black, n = 19), and follow‐up eIL‐5‐CuMVTT vaccination “2nd year” (black pattern, n = 17). All graphs include all horses, n = 34 (“Pre‐season”, “1st year”), n = 30 (“2nd year”), independent of antibody titer: ITT, intention to treat; IBH, insect‐bite hypersensitivity
Analogous to the Psoriasis Area and Severity Index (PASI) used in human psoriasis patients, the IBH Severity Index (ISI) 50 and ISI 75 score describes the percentage of horses that improved their IBH symptoms ≥50% or ≥75%, respectively. The ISI score data only include horses, which were present in the current follow‐up “2nd year” study (n = 30) and compared scores between all 3 years. In the first treatment year, 53% of vaccinated horses reached an ISI 50, whereas only 8% in the placebo group reached the ISI 50. An ISI 75 was reached by 23% of vaccinated horses. No placebo‐treated horse reached ISI 75 (Figure 6A). In the second treatment year, 69% of de novo immunized Group 1 horses and 88% of Group 2 horses reached an ISI 50. An ISI 75 was reached by 31% of Group 1 horses and by 47% of Group 2 horses (Figure 6B).
Figure 6.
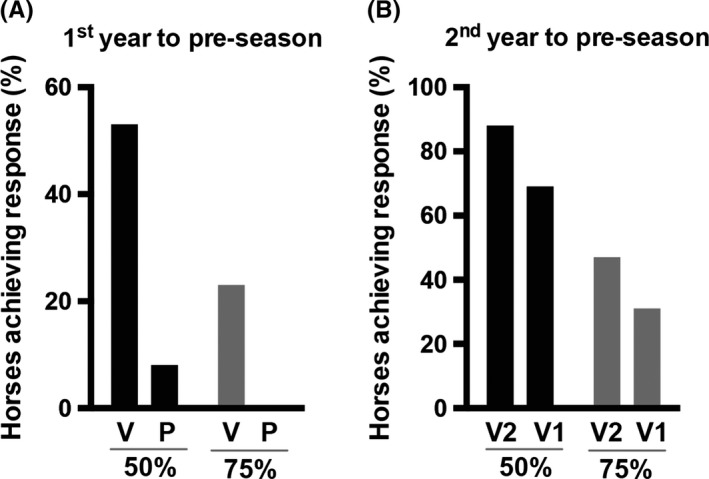
Improvement of IBH severity score (ISI) by vaccination with eIL‐5‐CuMVTT vaccine. A & B, All graphs include all horses, n = 30 (“Pre‐season”, “1st year”, “2nd year”), independent of antibody titer: ITT, intention to treat. ISI score with ISI 50 and ISI 75. ISI 50 includes % of horses that improved clinical lesion score by 50% and more, ISI 75 includes % of horses that improve clinical lesion score by 75% and more. A, ISI 50 (black) and ISI 75 (gray) score of “1st year” in the blinded study comparing vaccinated (V) and placebo‐treated (P) horses to “Pre‐season.” B, ISI 50 (black) and ISI 75 (gray) score of Group 1 (V1) and Group 2 (V2) horses of “2nd year” to “Pre‐season.” IBH, insect‐bite hypersensitivity
3.6. No increase in parasite presence after eIL‐5‐CuMVTT vaccination and no other side effects
Due to the known protective role of eosinophils during helminth infections, parasite presence in horse excrement was recorded before and after the season for all animals in both treatment years. No difference of parasite presence was detected between prior to (pre) and post (post) vaccination in 1st year and 2nd year (Figure S4A). Delta helminth presence of post‐ and pre‐vaccination is comparable between Group 1 and Group 2 in “1st year” and “2nd year,” with a tendency toward lower helminthes in 2nd year vaccinated horses of Group 2 (Figure S4B). In general, however, helminth presence in autumn was higher than in spring in both groups probably due to higher exposure on the pasture during the summer months. Of note, the only helminth parasite type found in any of the horses was the strongyle nematode, which is the most common one for horses. No other parasites (nematode and cestode) were detected. With regards to other side effects, we did not observe health issues possibly related to the vaccine. In particular, no indication for immune complex disease was found, neither on the skin nor by blood chemistry kidney parameters (blood urea, serum creatinine, albumin, total protein, sodium, potassium, sodium/potassium ratio, calcium, phosphate) before and after each IBH season (data not shown).
4. DISCUSSION
In the present study, we demonstrate that targeting eosinophils by vaccinating against IL‐5 may be a long‐term therapy for IBH in horses. We have previously shown a therapeutic effect of this VLP‐based vaccine in a one‐year placebo‐controlled clinical study.9 Here, we report that vaccination can be successfully performed over a second season and that antibody responses are more long‐lived in the second treatment year. Thus, the benefit of cytokine‐blocking antibodies normally used in humans by means of mAbs can be brought to large companion animals, such as horses, by way of active vaccination. Indeed, the size and weight of a horse precludes usage of mAbs due to excessive costs of such a therapy based on passive vaccination, which would require frequent injection of very high amounts of antibodies. In contrast to mAbs, our therapeutic vaccine is injected at low doses and after the initial first year treatment consisting of a basic vaccination regimen with three injections, the present study shows that one yearly booster is sufficient to maintain the therapeutic effect during the whole season. Antibody titers dropped toward end of the IBH season in both first and second treatment year supporting the reversibility and safety of the approach.
In naive horses, two initial vaccine injections using eIL‐5‐CuMVTT at weeks 0 and 4 prior to IBH season successfully induced anti‐self antibodies against eIL‐5. The vaccine booster in the middle of the season at week 19 increased antibody titers that had been dropping by that time. Thus, the third injection was necessary for prolonging antibody titers in the first season. Interestingly, mean anti‐IL‐5 antibody titers were one log higher than mean anti‐CuMVTT antibody titers most likely due to the efficient coupling of IL‐5 to the VLPs.30 In contrast to the horses in the previous study that received five immunizations in the first treatment year (Ref. 9), peak levels were higher but had a more pronounced mid‐season drop. Nevertheless, the clinical efficacy measured by ISI score showed stronger protection using the three injection regimen over the five injection regimen, although the vaccine preparation was slightly different in the five injection year. For follow‐up 2nd year vaccination, one vaccine booster using eIL‐5‐CuMVTT successfully induced long‐lasting anti‐self antibodies against IL‐5. Anti‐IL‐5 antibody titers correlated well to CuMVTT‐titers, which is consistent with observations in humans immunized against IL‐1β and may indicate that T cell help rather than B cells were the limiting factor to drive the IgG responses against both eIL‐5 and VLP.31 In addition, these data demonstrate that high anti‐CuMVTT titers do not inhibit induction of IL‐5‐specific antibodies. Anti‐IL‐5 antibody titers were slightly more variable than anti‐CuMVTT titers. This may be explained by the notion that anti‐IL‐5 antibodies may bind to host‐IL‐5 in the serum and that such antibodies in antigen‐antibody complexes may no longer be detectable by ELISA. The natural variations in levels of IL‐5 between subjects will perhaps lead to different rates of depletion of anti‐IL‐5 antibodies and hence give a perception of variability in anti‐IL‐5 antibody responses, as compared to CuMVTT. This is further supported by a tendency of increasing antibody titers against IL‐5 toward the end of the season in the 2nd year vaccinated horses in the absence of further vaccinations.
Based on in silico prediction analysis, the antibodies induced by eIL‐5‐CuMVTT vaccination are suggested to recognize comparable epitopes as the IL‐5 neutralizing human monoclonal antibody reslizumab. Thus, the induced antibodies are expected to interfere with binding of IL‐5 to the IL‐5‐receptor.
In the normal physiological state, enhanced numbers of eosinophils are only found in blood when increased numbers of eosinophils are required in tissues. The eosinophil has three life phases, the bone marrow, blood, and final tissue phase. The blood‐to‐tissue ratio in humans is about 1:100.32 The blood phase is considered merely to be a “passing‐through” or “waiting for extravasation” phase in circulation until eosinophils finally reach the tissues or die in the majority of cases. The life span of an eosinophil in blood ranges between 8 to 18 hours. Tissue life span, however, is considered to range from 2 to 5 day, and cytokines such as IL‐5 or chemokines such as eotaxin can further increase their life span to weeks. Locally produced IL‐5 is known to increase eosinophil half‐life in tissues (reviewed in Ref. 33). Vaccination using three injections of eIL‐5‐CuMVTT vaccine in weeks 0, 4, and 19 statistically significantly reduced blood eosinophil levels throughout the IBH season when comparing to the previous placebo treatment season in the same horses. In addition, the course of eosinophil levels negatively correlated with antibody titers, indicating a causative relation between high antibody titers and low eosinophil levels.
Clinical scores of eIL‐5‐CuMVTT vaccinated horses were found to be strongly decreased when compared to pre‐treatment season. Group 1 de novo vaccinated horses showed statistically significantly reduced clinical signs compared to previous placebo‐treated season. Depending on the vaccination regimen and vaccine formulation in “1st year” therapy, between 50% and 70% of horses reached an ISI 50, a 50% and higher improvement of symptoms. “2nd year” therapy was able to further increase the 50% response to almost 90%. A 75% and higher improvement of symptoms was achieved in 20% to 30% in “1st year” therapy, and almost in 50% of horses in “2nd year” therapy. Thus, we confirmed a clinical effect during the first year vaccination (basic vaccination) and a continuous and even stronger clinical benefit in the follow‐up year, which can be explained by more stable and long‐lasting antibody titers and perhaps increased affinity.
Vaccination with eIL‐5‐CuMVTT was safe and well tolerated. There was no difference in helminth presence found during the study period when comparing first year and second year vaccinated horses, indicating that eosinophil effector function against parasites is not dramatically impaired. Furthermore, it has been shown that eosinophil recruitment to healthy, non‐inflamed tissues and function of eosinophils within tissues may be IL‐5‐independent, indicating that homeostatic activities of eosinophils in tissues may not be impaired. This further supports the safety of the vaccination approach (Ref. 33,34). In addition, no other side effects were noticed during the study.
Taken together, eIL‐5‐CuMVTT successfully induced anti‐IL‐5 antibodies and mediated statistically significant reduction of eosinophil levels in blood upon two initial and one mid‐season booster vaccination. As a consequence, lesion scores were reduced in vaccinated horses when compared to the previous year with placebo treatment in the same horses. Symptoms were significantly reduced in the first treatment season and even further reduced in the second treatment season upon a single booster vaccination. Enhanced efficacy in the second treatment season was paralleled by more sustained antibody titers. This study suggests a basic vaccination regimen consisting of three vaccinations in the first year and an annual booster for the following years. Thus, eIL‐5‐CuMVTT may be a safe and effective way to treat IBH in horses, a finding that may be extended to other species, in particular humans for the treatment of eosinophilic asthma.
CONFLICT OF INTEREST
AFG, VF, FT, KB, MB, MK, TMK, and MFB are involved in the development of active immunotherapies. The authors FO and AZ have no conflict of interests to disclose.
AUTHOR CONTRIBUTIONS
AFG planned, performed and analyzed the experiments and clinical studies, interpreted the data and wrote the manuscript. VF planned, performed and analyzed experiments and the in silico structure model analysis. FO, KB, MB, MK and AZ performed experiments. FT contributed to discussions. TMK and MFB interpreted the data and contributed to writing the manuscript and discussions.
Supporting information
ACKNOWLEDGMENTS
We thank all horse owners who participated with their horse(s) in our clinical studies. We thank Christoph Giese (ETH Zurich) for helpful discussions.
Fettelschoss‐Gabriel A, Fettelschoss V, Olomski F, et al. Active vaccination against interleukin‐5 as long‐term treatment for insect‐bite hypersensitivity in horses. Allergy. 2019;74:572–582. 10.1111/all.13659
Funding informationThis project was supported by funding of the Swiss National Science Foundation (SNF Grant CRSII3_154490), the Commission for Technology and Innovation (CTI Grant 25758.1 PFLS‐LS), and Benchmark Vaccines Limited, UK.
REFERENCES
- 1. Baker KP, Quinn PJ. A report on clinical aspects and histopathology of sweet itch. Equine Vet J. 1978;10:243‐248. [DOI] [PubMed] [Google Scholar]
- 2. Braverman Y, Ungar‐Waron H, Frith K, et al. Epidemiological and immunological studies of sweet itch in horses in Israel. Vet Rec. 1983;112:521‐524. [DOI] [PubMed] [Google Scholar]
- 3. Anderson GS, Belton P, Jahren E, Lange H, Kleider N. Immunotherapy trial for horses in British Columbia with Culicoides (Diptera: Ceratopogonidae) hypersensitivity. J Med Entomol. 1996;33:458‐466. [DOI] [PubMed] [Google Scholar]
- 4. Kurotaki T, Narayama K, Oyamada T, Yoshikawa H, Yoshikawa T. Immunopathological study on equine insect hypersensitivity (“kasen”) in Japan. J Comp Pathol. 1994;110:145‐152. [DOI] [PubMed] [Google Scholar]
- 5. Anderson GS, Belton P, Kleider N. Culicoides obsoletus (Diptera: Ceratopogonidae) as a causal agent of Culicoides hypersensitivity (sweet itch) in British Columbia. J Med Entomol. 1991;28:685‐693. [DOI] [PubMed] [Google Scholar]
- 6. Quinn PJ, Baker KP, Morrow AN. Sweet itch: responses of clinically normal and affected horses to intradermal challenge with extracts of biting insects. Equine Vet J. 1983;15:266‐272. [DOI] [PubMed] [Google Scholar]
- 7. Fadok VA, Greiner EC. Equine insect hypersensitivity: skin test and biopsy results correlated with clinical data. Equine Vet J. 1990;22:236‐240. [DOI] [PubMed] [Google Scholar]
- 8. Greiner EC, Fadok VA, Rabin EB. Equine Culicoides hypersensitivity in Florida: biting midges aspirated from horses. Med Vet Entomol. 1990;4:375‐381. [DOI] [PubMed] [Google Scholar]
- 9. Fettelschoss‐Gabriel A, Fettelschoss V, Thoms F, et al. Treating insect‐bite hypersensitivity in horses with active vaccination against IL‐5. J Allergy Clin Immunol. 2018;142:1194‐1205. [DOI] [PubMed] [Google Scholar]
- 10. Bachmann MF, Dyer MR. Therapeutic vaccination for chronic diseases: a new class of drugs in sight. Nat Rev Drug Discov. 2004;3:81‐88. [DOI] [PubMed] [Google Scholar]
- 11. Dunshea FR, Colantoni C, Howard K, et al. Vaccination of boars with a GnRH vaccine (Improvac) eliminates boar taint and increases growth performance. J Anim Sci. 2001;79:2524‐2535. [DOI] [PubMed] [Google Scholar]
- 12. Baker DL, Powers JG, Ransom JI, et al. Reimmunization increases contraceptive effectiveness of gonadotropin‐releasing hormone vaccine (GonaCon‐Equine) in free‐ranging horses (Equus caballus): limitations and side effects. PLoS One. 2018;13:e0201570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chackerian B, Frietze KM. Moving towards a new class of vaccines for non‐infectious chronic diseases. Expert Rev Vaccines. 2016;15:561‐563. [DOI] [PubMed] [Google Scholar]
- 14. El Turabi A, Bachmann MF. Noninfectious Disease Vaccines (41). Philadelphia, PA: Elsevier; 2018. [Google Scholar]
- 15. Schaffartzik A, Hamza E, Janda J, Crameri R, Marti E, Rhyner C. Equine insect bite hypersensitivity: what do we know? Vet Immunol Immunopathol. 2012;147:113‐126. [DOI] [PubMed] [Google Scholar]
- 16. Foster AP, McKelvie J, Cunningham FM. Inhibition of antigen‐induced cutaneous responses of ponies with insect hypersensitivity by the histamine‐1 receptor antagonist chlorpheniramine. Vet Rec. 1998;143:189‐193. [DOI] [PubMed] [Google Scholar]
- 17. Benarafa C, Collins ME, Hamblin AS, Cunningham FM. Role of the chemokine eotaxin in the pathogenesis of equine sweet itch. Vet Rec. 2002;151:691‐693. [PubMed] [Google Scholar]
- 18. Collins PD, Marleau S, Griffiths‐Johnson DA, Jose PJ, Williams TJ. Cooperation between interleukin‐5 and the chemokine eotaxin to induce eosinophil accumulation in vivo. J Exp Med. 1995;182:1169‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Palframan RT, Collins PD, Williams TJ, Rankin SM. Eotaxin induces a rapid release of eosinophils and their progenitors from the bone marrow. Blood. 1998;91:2240‐2248. [PubMed] [Google Scholar]
- 20. Benarafa C, Collins ME, Hamblin AS, Sabroe I, Cunningham FM. Characterisation of the biological activity of recombinant equine eotaxin in vitro. Cytokine. 2002;19:27‐30. [DOI] [PubMed] [Google Scholar]
- 21. Strath M, Dent L, Sanderson C. Infection of IL5 transgenic mice with Mesocestoides corti induces very high levels of IL5 but depressed production of eosinophils. Exp Hematol. 1992;20:229‐234. [PubMed] [Google Scholar]
- 22. Kopf M, Brombacher F, Hodgkin PD, et al. IL‐5‐deficient mice have a developmental defect in CD5 + B‐1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity. 1996;4:15‐24. [DOI] [PubMed] [Google Scholar]
- 23. Legrand F, Klion AD. Biologic therapies targeting eosinophils: current status and future prospects. J Allergy Clin Immunol Pract. 2015;3:167‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roufosse F. Targeting the interleukin‐5 pathway for treatment of eosinophilic conditions other than asthma. Front Med (Lausanne). 2018;5:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zeltins A, West J, Zabel F, et al. Incorporation of tetanus‐epitope into virus‐like particles achieves vaccine responses even in older recipients in models of psoriasis, Alzheimer's and cat allergy. Vaccines. 2017;2:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kallberg M, Wang H, Wang S, et al. Template‐based protein structure modeling using the RaptorX web server. Nat Protoc. 2012;7:1511‐1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kusano S, Kukimoto‐Niino M, Hino N, et al. Structural basis of interleukin‐5 dimer recognition by its alpha receptor. Protein Sci. 2012;21:850‐864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang J, Kuvelkar R, Murgolo NJ, et al. Mapping and characterization of the epitope(s) of Sch 55700, a humanized mAb, that inhibits human IL‐5. Int Immunol. 1999;11:1935‐1944. [DOI] [PubMed] [Google Scholar]
- 29. Khairy WOA, Qian K, Shao H, Ye J, Qin A. Identification of two conserved B‐cell epitopes in the gp90 of reticuloendothelial virus using peptide microarray. Vet Microbiol. 2017;211:107‐111. [DOI] [PubMed] [Google Scholar]
- 30. Jegerlehner A, Storni T, Lipowsky G, Schmid M, Pumpens P, Bachmann MF. Regulation of IgG antibody responses by epitope density and CD21‐mediated costimulation. Eur J Immunol. 2002;32:3305‐3314. [DOI] [PubMed] [Google Scholar]
- 31. Cavelti‐Weder C, Timper K, Seelig E, et al. Development of an interleukin‐1β vaccine in patients with type 2 diabetes. Mol Ther. 2016;24:1003‐1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kita HAC, Gleich GJ, editors. Biology of Eosinophils. St Louis, MO: Mosby; 1998. [Google Scholar]
- 33. Park YM, Bochner BS. Eosinophil survival and apoptosis in health and disease. Allergy Asthma Immunol Res. 2010;2:87‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mesnil C, Raulier S, Paulissen G, et al. Lung‐resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest. 2016;126:3279‐3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


