Abstract
BACKGROUND
Inflammation is causally related to atherothrombosis. Treatment with canakinumab, a monoclonal antibody that inhibits inflammation by neutralizing interleukin-1β, resulted in a lower rate of cardiovascular events than placebo in a previous randomized trial. We sought to determine whether an alternative approach to inflammation inhibition with low-dose methotrexate might provide similar benefit.
METHODS
We conducted a randomized, double-blind trial of low-dose methotrexate (at a target dose of 15 to 20 mg weekly) or matching placebo in 4786 patients with previous myocardial infarction or multivessel coronary disease who additionally had either type 2 diabetes or the metabolic syndrome. All participants received 1 mg of folate daily. The primary end point at the onset of the trial was a composite of nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death. Near the conclusion of the trial, but before unblinding, hospitalization for unstable angina that led to urgent revascularization was added to the primary end point.
RESULTS
The trial was stopped after a median follow-up of 2.3 years. Methotrexate did not result in lower interleukin-1β, interleukin-6, or C-reactive protein levels than placebo. The final primary end point occurred in 201 patients in the methotrexate group and in 207 in the placebo group (incidence rate, 4.13 vs. 4.31 per 100 person-years; hazard ratio, 0.96; 95% confidence interval [CI], 0.79 to 1.16). The original primary end point occurred in 170 patients in the methotrexate group and in 167 in the placebo group (incidence rate, 3.46 vs. 3.43 per 100 person-years; hazard ratio, 1.01; 95% CI, 0.82 to 1.25). Methotrexate was associated with elevations in liver-enzyme levels, reductions in leukocyte counts and hematocrit levels, and a higher incidence of non–basal-cell skin cancers than placebo.
CONCLUSIONS
Among patients with stable atherosclerosis, low-dose methotrexate did not reduce levels of interleukin-1β, interleukin-6, or C-reactive protein and did not result in fewer cardiovascular events than placebo. (Funded by the National Heart, Lung, and Blood Institute; CIRT ClinicalTrials.gov number, NCT01594333.)
INFLAMMATION PLAYS A CRITICAL ROLE IN atherothrombosis.1,2 In the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS), treatment with canakinumab, a monoclonal antibody that selectively neutralizes interleukin-1β, resulted in fewer cardiovascular events than placebo, without lowering lipid levels or blood pressure.3 In that trial, the magnitude of risk reduction for major cardiovascular events, cardiovascular death, and death from any cause with canakinumab was greatest among patients with the largest reductions in levels of interleukin-6 and high-sensitivity C-reactive protein,4,5 which suggests that the benefit was related to the targeting of the interleukin-1β–interleukin-6–C-reactive protein pathway of innate immunity. These results thus provide proof of principle that inhibiting inflammation can prevent atherosclerotic events.6–8
We hypothesized that an alternative approach to inhibition of inflammation that involved the use of low-dose methotrexate might also result in lower cardiovascular event rates. Low-dose methotrexate is an inexpensive, effective, and widely used treatment for inflammatory conditions, including rheumatoid arthritis, psoriatic arthritis, and juvenile idiopathic arthritis.9,10 Furthermore, in observational studies, patients with rheumatoid arthritis and psoriatic arthritis who received low-dose methotrexate had fewer cardiovascular events than patients who received other therapies or placebo.11–13 We report here the results of the Cardiovascular Inflammation Reduction Trial (CIRT), which was conducted in collaboration with the National Heart, Lung, and Blood Institute (NHLBI) and was planned in parallel with CANTOS. In CIRT, we assessed low-dose methotrexate in the secondary prevention of atherothrombotic events among patients with a history of myocardial infarction or multivessel coronary artery disease who additionally had either type 2 diabetes or the metabolic syndrome.14
Methods
TRIAL DESIGN AND OVERSIGHT
This randomized, double-blind, placebo-controlled, investigator-initiated trial was funded by the NHLBI. The trial protocol, available with the full text of this article at NEJM.org, was designed by the trial executive committee with input from NHLBI staff and an NHLBI-appointed protocol-review committee, the members of which subsequently served as the trial data and safety monitoring board. The protocol was approved by the institutional review board at each of the 417 centers in North America that participated in the trial (Section A in the Supplementary Appendix, available at NEJM.org).
All trial functions — including data collection, adjustment of doses of methotrexate and placebo, site monitoring, end-point adjudication, and statistical support — were performed at the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital in Boston, with the use of a single, multipurpose central electronic data-capture system (e-SOCDAT, SOCAR Research). The trial drug (Trexall in 5-mg tablets) and matching placebo were purchased from Teva Pharmaceuticals, which was responsible for the manufacturing, packaging, and distribution; Teva Pharmaceuticals donated the costs for packaging and shipping but had no role in the design or conduct of the trial. The first and last authors prepared the first draft of the manuscript, had full access to the trial databases, generated trial analyses, made the decision to submit the manuscript for publication, and assume responsibility for the accuracy and completeness of the data and analyses and for the fidelity of the trial to the protocol.
TRIAL POPULATION
Patients 18 years of age or older were eligible if they had a history of myocardial infarction or multivessel coronary disease and either type 2 diabetes or the metabolic syndrome. Eligible patients were in a medically stable condition and had completed any planned revascularization procedures. The trial inclusion and exclusion criteria were similar to or were more restrictive than those in the guidelines for use of methotrexate in patients with rheumatoid arthritis published by the American College of Rheumatology.15 Patients with a history of chronic infection, tuberculosis, interstitial pneumonitis, pulmonary fibrosis, alcohol abuse, hepatic or renal dysfunction, or New York Heart Association class IV heart failure were excluded, as were women of childbearing potential and patients who were receiving treatment with oral glucocorticoids or other immunosuppressive agents. Details of the enrollment criteria are provided in Section B in the Supplementary Appendix.
RUN-IN PHASE, RANDOMIZATION, TRIAL DOSING, AND MONITORING
Medical records for all potential participants were reviewed centrally before enrollment to ensure eligibility. Once this process was completed, participants entered an open-label run-in phase lasting 5 to 8 weeks during which they received 1 mg of oral folic acid daily, along with oral methotrexate once weekly in doses increasing sequentially from 5 mg to 10 mg to 15 mg. Participants who had adverse effects or laboratory abnormalities during the run-in phase were excluded from further participation (Section B and Fig. S1 in the Supplementary Appendix).
Participants who successfully completed the trial run-in — with success defined as their having taken 15 mg of methotrexate once weekly for at least 2 consecutive weeks without adverse effects or laboratory abnormalities — were then randomly assigned in a 1:1 ratio, by means of a computer algorithm, to continue methotrexate, initially at a dose of 15 mg, or to receive placebo (Section C in the Supplementary Appendix). All participants continued taking folate daily. Randomization was stratified according to site, type of index event (multivessel coronary disease alone or myocardial infarction), time since the index event (≥6 months or <6 months), and status with respect to risk factors (the metabolic syndrome alone or diabetes). At 4 months, the dose was increased, per protocol, to 20 mg of methotrexate (or matching placebo). A computerized algorithm (based on levels of centrally measured laboratory variables and reported symptoms, assessed every 2 months, with treatment assignments concealed) was used to adjust the dose of methotrexate or placebo in a standardized manner at all sites (Section D and Figs. S2 and S3 in the Supplementary Appendix).
END POINTS
At trial initiation on April 4, 2013, the primary end point was the first occurrence of a major adverse cardiovascular event (a composite of non-fatal myocardial infarction, nonfatal stroke, or cardiovascular death). On January 24, 2018, after review by the NHLBI and an independent external panel, the trial end point was expanded to include hospitalization for unstable angina that led to urgent coronary revascularization; this end point would provide greater power and allow for a smaller overall sample size. Other than the members of the data coordinating center, who had access to unblinded data and had no role in this decision, no members of the investigative team or the independent external panel were aware of any unblinded trial data at the time of this decision.
Secondary end points included death from any cause; a composite of major adverse cardiovascular events plus any coronary revascularization; hospitalization for congestive heart failure; and a composite of major adverse cardiovascular events plus coronary revascularization, hospitalization for congestive heart failure, or death from any cause. Tertiary end points included the components of the final primary end point as well as coronary revascularization and arterial revascularization.
STATISTICAL ANALYSIS
The initial sample-size calculation, which was determined on the basis of the original primary end point of major adverse cardiovascular events, was revised after the addition of hospitalization for unstable angina that led to urgent revascularization. The anticipated trial sample size was reduced from 7000 to 5500, and the targeted number of primary end-point events (including the expanded definition) was increased from 530 to 634 to give the trial 90% power to detect a 23% lower rate of the primary end point in the methotrexate group than the placebo group. However, before these changes could be fully implemented across trial operations, the data and safety monitoring board, at a meeting on March 13, 2018, recommended early termination of the trial because it had crossed a pre-specified boundary for futility for both the original and final end points and because of the lack of evidence of a reduction in C-reactive protein level with methotrexate treatment. The data and safety monitoring board further requested that all participants return for a safety follow-up visit after an additional 6 months of follow-up. These recommendations were accepted by the NHLBI on April 2, 2018, at which time both enrollment and efficacy follow-up ceased. In this article, we report the original and final primary end points.
The trial protocol provides details of the pre-specified statistical analysis plan. In brief, the distributions of the change from enrollment to the end of active run-in, and then to 8 months after randomization, in interleukin-1β, interleukin-6, high-sensitivity C-reactive protein, lipid levels, markers of hepatic function, and hematologic measures were compared between the placebo group and the methotrexate group with the use of Wilcoxon rank-sum tests. Log-rank tests and Cox proportional-hazards models, stratified according to type of index event, time of index event, and status with respect to the metabolic syndrome alone or diabetes at enrollment, were used to analyze both the original and final primary end points, according to the intention-to-treat principle. For secondary and tertiary end points, results are reported as point estimates and 95% confidence intervals. The 95% confidence intervals have not been adjusted for multiplicity, and therefore inferences drawn from these intervals may not be reproducible.
RESULTS
PATIENTS
Of 9321 potential participants who provided informed consent and attended a screening visit, 6158 were eligible and were enrolled in the open-label run-in phase. At the time the trial was terminated, 4786 of the 6158 eligible patients had completed the run-in phase and had been randomly assigned to either low-dose methotrexate (2391 patients) or placebo (2395 patients) (Fig. S4 in the Supplementary Appendix). The median age of the patients was 66 years, 19% were women, and 22% identified themselves as nonwhite or Hispanic (Table 1). The qualifying event for trial participation was a previous myocardial infarction in 61% of the patients and multivessel coronary disease without previous infarction in 39%. Qualifying coexisting conditions were diabetes in 68% and the metabolic syndrome without diabetes in 32%. Data from a small cluster of sites with major Good Clinical Practice violations are not included in the analyses reported here, a decision that was made before the data were unblinded (Section E in the Supplementary Appendix).
Table 1.
Baseline Characteristics of the Trial Participants.*
| Characteristic | Low-Dose Methotrexate (N = 2391) | Placebo (N = 2395) |
|---|---|---|
| Median age (IQR) — yr | 65.6 (59.7–71.8) | 66.0 (59.8–71.7) |
| Female sex — no. (%) | 461 (19.3) | 437 (18.2) |
| Country — no. (%) | ||
| Canada | 407 (17.0) | 404 (16.9) |
| United States | 1984 (83.0) | 1991 (83.1) |
| Nonwhite race or Hispanic ethnic group — no./total no. (%)† | 545/2346 (23.2) | 485/2322 (20.9) |
| Current smoker — no. (%) | 267 (11.2) | 270 (11.3) |
| Alcohol use — no. (%) | ||
| Rarely or never | 1487 (62.2) | 1473 (61.5) |
| ≤1 drink/wk | 514 (21.5) | 520 (21.7) |
| >1 drink/wk | 390 (16.3) | 402 (16.8) |
| Median body-mass index (IQR)‡ | 31.6 (28.2–35.5) | 31.3 (28.1–35.6) |
| Hypertension — no. (%) | 2153 (90.0) | 2169 (90.6) |
| Qualifying event — no. (%) | ||
| Myocardial infarction | 1451 (60.7) | 1458 (60.9) |
| Multivessel coronary disease | 940 (39.3) | 937 (39.1) |
| Qualifying coexisting condition — no. (%) | ||
| Diabetes | 788 (33.0) | 823 (34.4) |
| Metabolic syndrome | 771 (32.2) | 780 (32.6) |
| Diabetes and metabolic syndrome | 832 (34.8) | 792 (33.1) |
| History of congestive heart failure | 288 (12.0) | 332 (13.9) |
| History of percutaneous coronary intervention | 1396 (58.4) | 1420 (59.3) |
| History of coronaryartery bypass grafting | 1010 (42.2) | 1032 (43.1) |
| Family history of premature myocardial infarction — no./total no. (%)§ | 550/2118 (26.0) | 536/2116 (25.3) |
| Family history of premature stroke — no./total no. (%)§ | 142/2144 (6.6) | 136/2160 (6.3) |
| Use of ACE inhibitor or ARB — no. (%) | 1736 (72.6) | 1724 (72.0) |
| Use of statin — no. (%) | 2058 (86.1) | 2052 (85.7) |
| Use of beta-blocker — no. (%) | 1870 (78.2) | 1905 (79.5) |
| Use of antiplatelet or antithrombotic agent — no. (%) | 2082 (87.1) | 2054 (85.8) |
| Median lipid levels (IQR) — mg/dl | ||
| Total cholesterol | 141.0 (122.0–168.0) | 140.9 (122.0–164.0) |
| LDL cholesterol | 68.0 (54.0–87.0) | 68.0 (53.3–86.0) |
| HDL cholesterol | 41.0 (34.7–49.0) | 41.0 (35.0–48.0) |
| Triglycerides | 135.4 (98.0–191.2) | 136.0 (98.2–191.6) |
| Median high-sensitivity Creactive protein level (IQR) — mg/liter | 1.53 (0.78–3.59) | 1.50 (0.70–3.29) |
| Median interleukin-1β level (IQR) — pg/ml | 1.59 (0.49–3.17) | 1.46 (0.53–3.11) |
| Median interleukin-6 level (IQR) — pg/ml | 2.37 (1.53–3.76) | 2.30 (1.58–3.51) |
| Median glycated hemoglobin level (IQR) — % | 6.6 (6.0–7.5) | 6.5 (5.9–7.5) |
There were no significant differences between the groups in the characteristics at baseline (P<0.05). Data for lipid and inflammatory biomarkers as well as glycated hemoglobin are the values at enrollment, before the active-treatment run-in phase. Effects of the run-in phase on these and other variables are described in Table S1 in the Supplementary Appendix. To convert the values for cholesterol to millimoles per liter, multiply by 0.02586. To convert the values for triglycerides to millimoles per liter, multiply by 0.01129. ACE denotes angiotensin-converting enzyme, ARB angiotensin-receptor blocker, HDL high-density lipoprotein, IQR interquartile range, and LDL low-density lipoprotein.
Race or ethnic group was reported by the patient.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
A family history of premature myocardial infarction or premature stroke was considered to be an event occurring in the mother before the age of 65 or in the father before the age of 55.
LABORATORY FINDINGS
During the open-label run-in phase, initiation of low-dose methotrexate was associated with increases in serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) and decreases in white-cell counts. Methotrexate did not reduce high-sensitivity C-reactive protein levels during the run-in phase (Table S1 in the Supplementary Appendix). At randomization, the median level of high-sensitivity C-reactive protein was 1.6 mg per liter, and the median low-density lipoprotein cholesterol level was 67 mg per deciliter (1.73 mmol per liter).
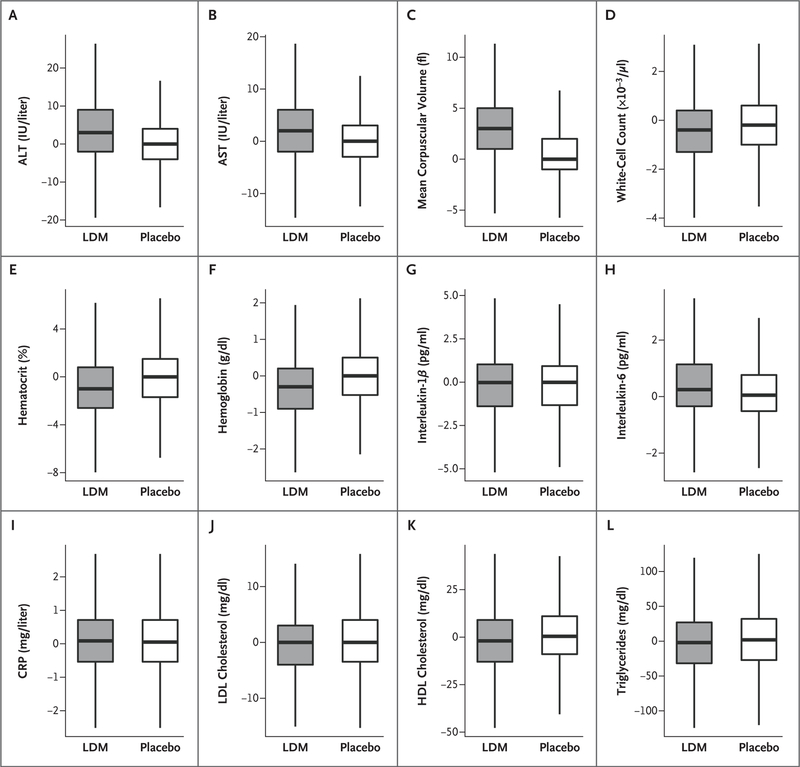
At 8 months after randomization, patients assigned to receive methotrexate had significantly greater increases from baseline in median levels of ALT and AST and in mean corpuscular volume than patients assigned to placebo and significantly greater reductions from baseline in the total white-cell count, hematocrit level, and hemoglobin level (P<0.001 for all comparisons between the methotrexate group and the placebo group). At 8 months, treatment with low-dose methotrexate did not result in greater reductions from baseline than placebo in levels of C-reactive protein, interleukin-1β, or interleukin-6. Long-term methotrexate treatment did not alter C-reactive protein levels, which were measured annually throughout the trial (Fig. 1, and Table S2 and Fig. S5 in the Supplementary Appendix).
Figure 1. Laboratory Findings.
Shown are the effects of low-dose methotrexate (LDM) and placebo on hepatic enzyme levels (alanine aminotransferase [ALT] and aspartate aminotransferase [AST]), hematologic measures (mean corpuscular volume, white-cell count, hematocrit level, and hemoglobin level), inflammatory mediators (interleukin-1β, interleukin-6, and high-sensitivity Creactive protein [CRP] levels), and lipid levels (low-density lipoprotein [LDL] cholesterol, high-density lipoprotein [HDL] cholesterol, and triglycerides). Data shown are the changes from enrollment to 8 months after randomization. The horizontal line in each box represents the median, the top and bottom of the boxes the interquartile range, and the whiskers 1.5 times the interquartile range.
FOLLOW-UP AND EFFECTS ON CLINICAL END POINTS
During the trial, the computerized dosing algorithm directed adjustments to doses at 21% of the trial visits of patients in the methotrexate group and at 18% of the visits of patients in the placebo group. Among patients who were still taking the assigned methotrexate or placebo at their last visit before trial completion, the mean weekly dose was 18.8 mg in the methotrexate group and 19.0 mg in the placebo group. At the final visit before the trial was stopped, 21% of the patients in the methotrexate group and 22% in the placebo group had permanently discontinued the trial regimen.
After a median follow-up of 2.3 years (maximum, 5 years), a first occurrence after randomization of a final primary end-point event (non-fatal myocardial infarction, nonfatal stroke, cardiovascular death, or hospitalization for unstable angina that led to urgent revascularization) was reported in 201 patients in the methotrexate group and in 207 in the placebo group (incidence rate, 4.13 vs. 4.31 per 100 person-years; hazard ratio, 0.96; 95% confidence interval [CI], 0.79 to 1.16; P = 0.67) (Table 2 and Fig. 2). For the original primary end point (nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death), the corresponding numbers were 170 in the methotrexate group and 167 in the placebo group (incidence rate, 3.46 vs. 3.43 per 100 person-years; hazard ratio, 1.01; 95% CI, 0.82 to 1.25; P = 0.91). There were no significant between-group differences in any of the pre-specified composite secondary cardiovascular end points or in any individual component of these end points.
Table 2.
Cardiovascular Clinical End Points.
| End Point | Low-Dose Methotrexate (N = 2391) | Placebo (N = 2395) | Hazard Ratio (95% CI)* | P Value | ||
|---|---|---|---|---|---|---|
| no. of patients | incidence rate/100 person-yr | no. of patients | incidence rate/100 person-yr | |||
| Primary end points† | ||||||
| Final primary end point: major adverse cardio- vascular event or hospitalization for unstable angina that led to urgent revascularization | 201 | 4.13 | 207 | 4.31 | 0.96 (0.79–1.16) | 0.67 |
| Original primary end point: major adverse cardiovascular event | 170 | 3.46 | 167 | 3.43 | 1.01 (0.82–1.25) | 0.91 |
| Secondary end points† | ||||||
| Death from any cause | 96 | 1.80 | 83 | 1.55 | 1.16 (0.87–1.56) | |
| Major adverse cardiovascular event or any coronary revascularization | 278 | 5.86 | 288 | 6.15 | 0.95 (0.81–1.12) | |
| Hospitalization for congestive heart failure | 48 | 0.95 | 53 | 1.06 | 0.89 (0.60–1.31) | |
| Major adverse cardiovascular event, coronary revascularization, hospitalization for congestive heart failure, or death from any cause | 344 | 7.30 | 345 | 7.42 | 0.98 (0.84–1.14) | |
| Tertiary end points | ||||||
| Nonfatal myocardial infarction | 113 | 2.29 | 114 | 2.32 | 0.99 (0.76–1.29) | |
| Nonfatal stroke | 28 | 0.55 | 30 | 0.60 | 0.91 (0.54–1.52) | |
| Cardiovascular death | 49 | 0.92 | 43 | 0.80 | 1.14 (0.76–1.72) | |
| Hospitalization for unstable angina that led to urgent revascularization | 41 | 0.81 | 50 | 1.01 | 0.81 (0.53–1.22) | |
| Coronary revascularization | 190 | 3.95 | 205 | 4.30 | 0.92 (0.75–1.12) | |
| Arterial revascularization | 195 | 4.05 | 185 | 3.84 | 1.05 (0.86–1.28) | |
Confidence intervals have not been adjusted for multiplicity, and therefore inferences drawn from these intervals may not be reproducible.
Major adverse cardiovascular events included nonfatal myocardial infarction, nonfatal stroke, and cardiovascular death.
Figure 2. Cumulative Incidence of the Final Primary End Point and the Original Primary End Point.
Shown is the cumulative incidence of the final primary end point of major adverse cardiovascular events (nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death) or hospitalization for unstable angina that led to urgent revascularization (Panel A) and the cumulative incidence of the original primary end point of nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death (Panel B). The inset in each panel shows the same data on an enlarged y axis.
Cardiovascular death was confirmed in 49 patients in the methotrexate group and in 43 in the placebo group (incidence rate, 0.92 vs. 0.80 per 100 person-years; hazard ratio, 1.14; 95% CI, 0.76 to 1.72). A total of 96 deaths from any cause occurred in the methotrexate group, and 83 occurred in the placebo group (incidence rate, 1.80 vs. 1.55 per 100 person-years; hazard ratio, 1.16; 95% CI, 0.87 to 1.56). We observed no effect modification in subgroup analyses stratified according to type of index event, time since index event, status with respect to diabetes or the metabolic syndrome at the time of enrollment, time spent enrolled in the trial, or levels of base-line high-sensitivity C-reactive protein above or below the trial median (data not shown).
ADVERSE EVENTS
Rates of serious adverse events, including bleeding and infection, were similar in the two groups (Table 3). Mouth sores and oral pain were more prevalent in the methotrexate group than in the placebo group, as was unintended weight loss. Modest leukopenia and elevations of ALT and AST levels were also more common in the methotrexate group. Cancers developed in more patients in the methotrexate group than in the placebo group (52 vs. 30; rate ratio, 1.72; P= 0.02), an effect that was due almost entirely to a larger number of patients with non–basal-cell skin cancer in the methotrexate group (31 vs. 10; rate ratio, 3.08; P = 0.002).
Table 3.
Adverse Events and Selected Laboratory Data.*
| Adverse Event or Laboratory Variable | Low-Dose Methotrexate (N = 2391) | Placebo (N = 2395) | P Value | ||
|---|---|---|---|---|---|
| no. of patients | incidence rate/100 person-yr | no. of patients | incidence rate/100 person-yr | ||
| Adverse event | |||||
| Any event | 1488 | 62.4 | 1399 | 56.0 | 0.004 |
| Serious event | 569 | 13.5 | 549 | 13.0 | 0.52 |
| Infection or infestation | |||||
| Any event | 659 | 16.5 | 584 | 14.4 | 0.02 |
| Serious event | 111 | 2.24 | 121 | 2.47 | 0.50 |
| Gastrointestinal disorder | |||||
| Any event | 350 | 7.79 | 284 | 6.23 | 0.006 |
| Serious event | 60 | 1.20 | 46 | 0.92 | 0.22 |
| Neurologic disorder | |||||
| Any event | 213 | 4.53 | 195 | 4.12 | 0.37 |
| Serious event | 53 | 1.06 | 55 | 1.11 | 0.89 |
| Hemorrhage† | |||||
| Any event | 132 | 2.71 | 111 | 2.28 | 0.20 |
| Serious event | 32 | 0.63 | 25 | 0.50 | 0.44 |
| Cancer | |||||
| Reported in case-report forms from visits | |||||
| Any cancer | 52 | 1.03 | 30 | 0.60 | 0.02 |
| Non–basal-cell skin cancer | 31 | 0.61 | 10 | 0.20 | 0.002 |
| Determined on the basis of a MedDRA query, case-report forms from visits, and adverse-event reports | |||||
| Any cancer | 106 | 2.15 | 95 | 1.93 | 0.51 |
| Non–basal-cell skin cancer | 33 | 0.65 | 12 | 0.24 | 0.003 |
| Mouth sores or oral pain‡ | 96 | 1.95 | 56 | 1.13 | 0.001 |
| Unintended weight loss‡ | 104 | 2.10 | 73 | 1.47 | 0.02 |
| Alanine aminotransferase >3x normal range | 49 | 0.97 | 17 | 0.34 | <0.001 |
| Aspartate aminotransferase >3x normal range | 39 | 0.77 | 21 | 0.42 | 0.03 |
| Leukopenia§ | 241 | 5.14 | 172 | 3.63 | <0.001 |
Serious adverse events were those that resulted in death, were life-threatening, led to hospitalization or prolongation of hospitalization, caused clinically significant incapacity, or were deemed to be an important medical event as judged by the investigator.
Data are determined on the basis of a standardized Medical Dictionary for Regulatory Activities (MedDRA) query.
Data are from an explicit question on case-report forms from visits and from adverse-event reports.
Data are from results from laboratory assays and from adverse-event reports.
Discussion
In this randomized, double-blind, placebo-controlled trial, low-dose methotrexate did not reduce levels of interleukin-1β, interleukin-6, or high-sensitivity C-reactive protein and was not associated with fewer cardiovascular events than placebo among patients with atherosclerosis whose condition was stable but who were at high cardiovascular risk. Methotrexate was associated with modest elevations in liver enzyme levels and reductions in leukocyte counts and hematocrit levels, as well as a higher incidence of non–basal-cell skin cancers than placebo. There was no difference between the groups in all-cause mortality.
Because the findings for low-dose methotrexate in CIRT contrast with those for canakinumab in CANTOS,3 a comparison of these two contemporary trials, which were designed and conducted in parallel, is informative. Both CIRT and CANTOS enrolled patients with atherosclerosis who were in stable condition but at high risk and who received aggressive treatment with lipid-lowering therapies. CANTOS, however, by design, included patients with residual inflammatory risk16 and thus limited enrollment to patients with persistently elevated high-sensitivity C-reactive protein levels; this trial design resulted in a median baseline C-reactive protein level among participants of 4.2 mg per liter (the approximate 90th percentile of the normal distribution). By contrast, CIRT did not screen for C-reactive protein level but instead required participants to have either diabetes or the metabolic syndrome. This trial design resulted in a median high-sensitivity C-reactive protein level of only 1.6 mg per liter at randomization.
In addition, in CANTOS, the reduction from baseline in interleukin-6 and C-reactive protein levels was 35 to 40 percentage points greater in the group assigned to receive canakinumab than in the group assigned to receive placebo,3–5 where-as in CIRT, methotrexate did not lower either of these inflammatory biomarkers or interleukin-1β. Thus, although abundant data indicate that inflammation contributes critically to atherothrombosis,1–3 an important provisional hypothesis deriving from these two contemporary trials is that reducing the risk of cardiovascular events may depend on the pathway targeted. Inhibition of interleukin-1β–interleukin-6 signaling, a process initiated at the level of the NLRP3 inflammasome,17 effectively reduced cardiovascular events in CANTOS, and human genetic data implicate this pathway as causal in atherothrombosis. Studies with neutral outcomes involving other anti-inflammatory agents (e.g., the phospholipase inhibitor darapladib and the p38 MAP kinase blocker losmapimod)18,19 that also did not lower levels of interleukin-1β, interleukin-6, or C-reactive protein over the long term point to pathways that appear to be less critical in atherothrombosis.
The observations in CANTOS, CIRT, and other trials highlight the importance of considering the mechanistic diversity of inflammatory pathways in atherosclerosis and of exploring approaches to their inhibition. Understanding these differences is likely to be crucial for future studies targeting inflammation in atherosclerosis. The mechanism involved in the efficacy of methotrexate in rheumatoid arthritis remains poorly understood despite the wide clinical use of that agent, but it probably reflects adenosine-mediated antiinflammatory effects.20,21 In contrast, canakinumab has only modest additional benefit in rheumatoid arthritis among patients already receiving treatment with methotrexate.22 Thus far, only targeting the interleukin-1β–interleukin-6 pathway with canakinumab has proven effective in reducing cardiovascular event rates. Agents such as colchicine and oral NLRP3 inhibitors that may also intersect with this pathway are currently under investigation or in development.23
The data reported here raise several additional issues. Before the initiation of CIRT, observational data had repeatedly shown an association of low-dose methotrexate use with reduced vascular event rates in patients with rheumatoid arthritis or psoriatic arthritis.11–13 The reported benefits in these observational studies may apply only to patients with existing systemic inflammatory conditions, but it is also possible that unmeasured confounding arising from the selective use of methotrexate led to bias in the estimates. Similarly, although there is evidence that methotrexate administered to treat rheumatoid arthritis lowers the C-reactive protein level, data showing this effect derive almost exclusively from studies that enrolled individuals with inflammatory disease flares. It is thus possible that the ability of methotrexate to reduce the C-reactive protein level is limited to clinical situations in which inflammation levels are high. Some of the reported reduction in the C-reactive protein level in patients with rheumatoid arthritis or resistant juvenile arthritis might have resulted from regression to the mean. Finally, in this trial, methotrexate resulted in more cases of non– basal-cell skin cancers than placebo, a finding that was unexpected and that merits further exploration.24–26
In summary, in this randomized, placebo-controlled trial involving patients with stable atherosclerosis, low-dose methotrexate did not reduce levels of interleukin-1β, interleukin-6, or C-reactive protein and did not result in fewer cardiovascular events than placebo.
Supplementary Material
Acknowledgments
Supported by grants (UO1 HL101422 and UO1 HL101389) from the National Heart, Lung, and Blood Institute. The trial drug and matching placebo were purchased from Teva Pharmaceuticals, which donated costs for packaging and shipping.
Footnotes
A complete list of the CIRT Investigators is provided in the Supplementary Appendix, available at NEJM.org.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Contributor Information
Paul M Ridker, Center for Cardiovascular Disease Prevention, Division of Preventive Medicine, Divisions of Cardiovascular Medicine, Brigham and Women’s Hospital, Boston
Brendan M. Everett, Center for Cardiovascular Disease Prevention, Division of Preventive Medicine, Divisions of Cardiovascular Medicine, Brigham and Women’s Hospital, Boston
Aruna Pradhan, Center for Cardiovascular Disease Prevention, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston
Jean G. MacFadyen, Center for Cardiovascular Disease Prevention, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston
Daniel H. Solomon, Rheumatology, Brigham and Women’s Hospital, Boston
Elaine Zaharris, Center for Cardiovascular Disease Prevention, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston
Virak Mam, Center for Cardiovascular Disease Prevention, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston
Ahmed Hasan, National Heart, Lung, and Blood Institute, Bethesda, MD
Yves Rosenberg, National Heart, Lung, and Blood Institute, Bethesda, MD
Erin Iturriaga, National Heart, Lung, and Blood Institute, Bethesda, MD
Milan Gupta, McMaster University, Hamilton, ON, Canada
Michelle Tsigoulis, Canadian Collaborative Research Network, Brampton, ON, Canada.
Subodh Verma, St. Michael’s Hospital, Toronto, ON, Canada
Michael Clearfield, Touro University, Vallejo, CA
Peter Libby, Divisions of Cardiovascular Medicine, Brigham and Women’s Hospital, Boston
Samuel Z. Goldhaber, Divisions of Cardiovascular Medicine, Brigham and Women’s Hospital, Boston
Roger Seagle, Cardiology Associates Carolina, Morganton, NC
Cyril Ofori, Wooster Community Hospital, Wooster, Ohio
Mohammad Saklayen, Dayton Veteran Affairs Medical Center, Dayton, Ohio
Samuel Butman, Verde Valley Medical Center, Cottonwood, AZ
Narendra Singh, Atlanta Heart Specialists, Atlanta
Michel Le May, University of Ottawa Heart Institute, Ottawa, ON, Canada
Olivier Bertrand, Laval University, Quebec City, QB, Canada
James Johnston, KMH Cardiology, Diagnostic and Research Centres, Mississauga, ON, Canada
Nina P. Paynter, Center for Cardiovascular Disease Prevention, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston
Robert J. Glynn, Center for Cardiovascular Disease Prevention, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston
REFERENCES
- 1.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685–95. [DOI] [PubMed] [Google Scholar]
- 2.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol 2009;54:2129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–31. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomized controlled trial. Lancet 2018;391:319–28. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Libby P, MacFadyen JG, et al. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Eur Heart J 2018;39: 3499–507. [DOI] [PubMed] [Google Scholar]
- 6.Baylis RA, Gomez D, Mallat Z, Pasterkamp G, Owens GK. The CANTOS trial: one important step for clinical cardiology but a giant leap for vascular biology. Arterioscler Thromb Vasc Biol 2017;37(11): e174–e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ibañez B, Fuster V. CANTOS: a gigantic proof-of-concept trial. Circ Res 2017;121: 1320–2. [DOI] [PubMed] [Google Scholar]
- 8.Weber C, von Hundelshausen P. CANTOS trial validates the inflammatory pathogenesis of atherosclerosis: setting the stage for a new chapter in therapeutic targeting. Circ Res 2017;121:1119–21. [DOI] [PubMed] [Google Scholar]
- 9.Weinblatt ME, Coblyn JS, Fox DA, et al. Efficacy of low-dose methotrexate in rheumatoid arthritis. N Engl J Med 1985; 312:818–22. [DOI] [PubMed] [Google Scholar]
- 10.Giannini EH, Brewer EJ, Kuzmina N, et al. Methotrexate in resistant juvenile rheumatoid arthritis: results of the U.S.A.– U.S.S.R. double-blind, placebo-controlled trial. N Engl J Med 1992;326:1043–9. [DOI] [PubMed] [Google Scholar]
- 11.Choi HK, Hernán MA, Seeger JD, Robins JM, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet 2002; 359:1173–7. [DOI] [PubMed] [Google Scholar]
- 12.Westlake SL, Colebatch AN, Baird J, et al. The effect of methotrexate on cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology (Oxford) 2010;49: 295–307. [DOI] [PubMed] [Google Scholar]
- 13.Micha R, Imamura F, Wyler von Ballmoos M, et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol 2011; 108:1362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Everett BM, Pradhan AD, Solomon DH, et al. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J 2013;166(2):199–207.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum 2008;59:762–84. [DOI] [PubMed] [Google Scholar]
- 16.Ridker PM. Residual inflammatory risk: addressing the obverse side of the atherosclerosis prevention coin. Eur Heart J 2016;37:1720–2. [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM. From C-reactive protein to interleukin-6 to interleukin-1: moving upstream to identify novel targets for atheroprotection. Circ Res 2016;118:145–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Donoghue ML, Braunwald E, White HD, et al. Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID-TIMI 52 randomized clinical trial. JAMA 2014;312:1006–15. [DOI] [PubMed] [Google Scholar]
- 19.O’Donoghue ML, Glaser R, Cavender MA, et al. Effect of losmapimod on cardiovascular outcomes in patients hospitalized with acute myocardial infarction: a randomized clinical trial. JAMA 2016; 315:1591–9. [DOI] [PubMed] [Google Scholar]
- 20.Cronstein BN, Naime D, Ostad E. The antiinflammatory mechanism of methotrexate: increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. J Clin Invest 1993;92:2675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan ES, Cronstein BN. Methotrexate — how does it really work? Nat Rev Rheumatol 2010;6:175–8. [DOI] [PubMed] [Google Scholar]
- 22.Alten R, Gomez-Reino J, Durez P, et al. Efficacy and safety of the human anti-IL-1β monoclonal antibody canakinumab in rheumatoid arthritis: results of a 12-week, phase II, dose-finding study. BMC Musculoskelet Disord 2011;12:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toldo S, Abbate A. The NLRP3 inflammasome in acute myocardial infarction. Nat Rev Cardiol 2018;15:203–14. [DOI] [PubMed] [Google Scholar]
- 24.Krathen MS, Gottlieb AB, Mease PJ. Pharmacologic immunomodulation and cutaneous malignancy in rheumatoid arthritis, psoriasis, and psoriatic arthritis. J Rheumatol 2010;37:2205–15. [DOI] [PubMed] [Google Scholar]
- 25.Lange E, Blizzard L, Venn A, Francis H, Jones G. Disease-modifying anti-rheumatic drugs and non-melanoma skin cancer in inflammatory arthritis patients: a retrospective cohort study. Rheumatology (Oxford) 2016;55:1594–600. [DOI] [PubMed] [Google Scholar]
- 26.Solomon DH, Kremer JM, Fisher M, et al. Comparative cancer risk associated with methotrexate, other non-biologic and biologic disease-modifying anti-rheumatic drugs. Semin Arthritis Rheum 2014;43: 489–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.