Abstract
The aim of this study was to investigate the dose optimization strategy for the sacrum to reduce the risk of pelvic insufficiency fracture (PIF).
Using a retrospective study design, we analyzed data from 28 patients with cervical cancer who underwent postoperative adjuvant radiotherapy in our department from June 2017 to January 2018. Among these patients, 20 (71.4%) underwent external beam radiation therapy in the pelvic lymphatic drainage area (node-negative patients). Overall, 8 patients (28.6%) underwent radiotherapy in the pelvic lymphatic drainage area with a simultaneous integrated boost (node-positive patients). Furthermore, 20 patients were assigned to 2 groups of plans according to the prescribed doses of 5000 and 4500 cGy/25. Each group had 3 plans according to 3 different dose limit conditions: “pelvic bones and sacrum unlimited,” “pelvic bones limited,” and “pelvic bones + sacrum limited.” The irradiation dose of the sacrum and pelvis was analyzed in three limited optimization models.
The planning target volume conformity index and homogeneity index, based on different optimization modes in the 4500 and 5000 cGy plans, showed no significant differences. The D50% and Dmean of the pelvis + sacrum limited mode were significantly lower than those of the pelvic limited mode (P < .001). The dose of the sacrum and pelvis in the 4500 cGy plan in the lymphatic drainage area was significantly lower than that of the 5000 cGy plan (P < .001). In the lymph node boost group, the irradiation dose of the sacrum and pelvis was significantly increased (P ≤ .001).
Increasing the limitation of the sacrum, on the basis of pelvic bone limitation, in cervical cancer intensity-modulated radiation therapy can significantly reduce the dose to the sacrum. Compared with the dose of 5000 cGy to the lymphatic drainage area, the dose of 4500 cGy was the largest influencing factor to reduce the dose to the sacrum.
Keywords: cervical cancer, IMRT (intensity modulation radiation therapy), sacrum limitation
1. Introduction
In recent years, the efficacy of cervical cancer treatment has gradually increased, with many patients experiencing prolonged survival. Therefore, the reduction in side effects would improve the quality of life of cancer patients. The intensity-modulated radiation therapy (IMRT) technique can significantly reduce the incidence of acute toxicity compared with conventional radiotherapy for cervical carcinoma. However, pelvic insufficiency fractures (PIFs) are rarely noticed. Recent studies have shown that the incidence of PIF is increasing.[1–4] PIF can lead to intractable pain and limited mobility, which can seriously impact quality of life. On the contrary, PIF is easily misdiagnosed as metastasis or recurrence, which leads to incorrect treatment. Studies have shown that most PIFs occur in the sacrum. D50% of the sacrum is an important predictor of PIF.[1] Therefore, reducing the dose of sacrum is an important way to reduce the risk of PIF. The aim of this study was to investigate the dose optimization strategy for the sacrum to provide a dosimetric basis for further study of PIF in patients with cervical cancer.
2. Methods and materials
2.1. Patients
We analyzed data from 28 patients with cervical cancer who underwent postoperative adjuvant radiotherapy in our department from June 2017 to January 2018. Patient characteristics are summarized in Table 1. Among them, 20 patients (71.4%) underwent external beam radiation therapy in the pelvic lymphatic drainage area (node-negative patients) and 8 patients (28.6%) underwent radiotherapy in the pelvic lymphatic drainage area with a sequential boost (node-positive patients). All patients underwent treatment with IMRT. The International Federation of Gynecology and Obstetrics stage distribution was 3 IB, 5 IIA, 8 IIB, 6 IIIA, 5 IIIB, and 1 IIIC. The study was approved by the Ethics Committee of The Second Affiliated Hospital of Soochow University. Due to the retrospective nature of the study, the need for informed consent was waived.
Table 1.
Patient characteristics.
2.2. Position fixation and CT scan
All patients were in a supine position and fixed with a carbon fiber frame and thermoplastic film from Orfit, Belgium. The GE's CT590 (General Electric, Waukesha, WI) large aperture analog positioning machine was used for the planned computed tomography (CT) scan. Scan layer thickness and layer spacing selection was 2.5 mm. The scan ranged from the 3rd lumbar vertebrae to 2 cm below the level of the anus. Positioning images were sent to the Pinnacle v9.0 (Philips Medical Systems, Eindhoven, The Netherlands) treatment planning system for target delineation and planning design. After the plan was confirmed, the patient was treated at the Medical Synergy Accelerator (Elekta Synergy; Elekta Oncology Systems, Crawley, UK).
2.3. Delineation of target area and organ at risk (OAR)
According to the Radiation Therapy Oncolgy Group (RTOG) guidelines,[5] clinical target volume (CTV) includes the vaginal stump and pelvic lymphatic drainage areas. The CTV was expanded by 5 mm to create the planning target volume (PTV). In the 8 patients with lymph node boost, the metastatic lymph nodes were located in front of the sacrum. The PTV of lymph nodes (PGTVnd) was obtained by expanding the corresponding metastatic nodes with a margin of 5 mm. OAR included the intestine (including the small intestine and colon), rectum, bladder, femoral head, pelvis (including the hip and tibia), and the tibia (including the ankle). The contours of the pelvis and sacrum were artificially modified based on the treatment planning system automatic delineation. The sketches of CTV, PTV, and the sacrum and pelvis are shown in Fig. 1.
Figure 1.
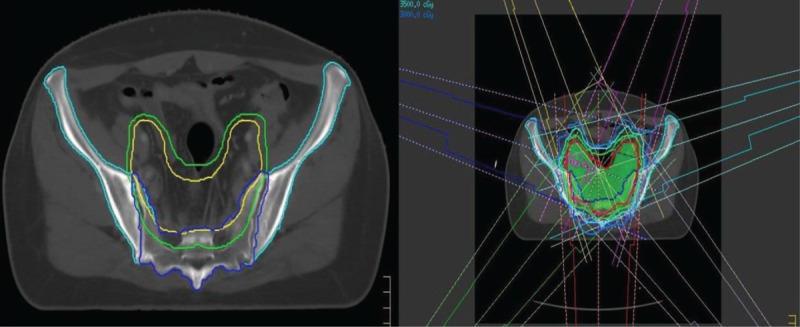
Radiotherapy target area, sacrum, pelvic delineation, and design of the radiation therapy plan.
2.4. Treatment plan design
The drainage area irradiation group uses 7-field IMRT technology (Fig. 1). Twenty patients were assigned 2 sets of plans according to the prescribed doses of 5000 and 4500 cGy/25. Each group had 3 plans according to 3 limited optimization models: “pelvic bones and sacrum unlimited,” “pelvic bones limited,” and “pelvic bones + sacrum limited.” The lymph node addition group was performed on the basis of 5000 cGy in the drainage area by the 7-field IMRT technique, and the sequential lymph node boost was 600 cGy/3 times or 1600 cGy/8 times. Treatment included 4 to 5 irradiation fields. According to whether or not there was a boost and dosage, the composition was divided into 3 subgroups: “5000 cGy group,” “5600 cGy group,” and “6600 cGy group.” The “pelvic bones + sacrum limited” mode was used in the 5600 and 6600 cGy groups. According to QUANTEC recommended dose limits,[6] 5000 cGy < 5% is the dose limit for the femoral head and V15 and V45 serve as dose limits for the intestinal tract. The rectum and bladder do not have a limited recommendation below 5000 cGy. Therefore, the ALARA method (the dose is reduced as reasonably as possible) was used to reduce the dose. The dose limit for the pelvis is V10 <90% and V20 <80%.[7–9] The limit of sacrum irradiation is based on the pelvic irradiation dose limit, and the sacrum was added as an independent dose limit optimization condition; the ALARA method was used to minimize its dose.
2.5. Dose assessment of target area and OAR
-
(1)
The percentage of PTV covered by the prescribed dose;
-
(2)
Homogeneity index (HI), HI = DRmaxR/DRp, where DRmaxR is the maximum dose, DRpR is the prescribed dose, and the closer the HI value is to one, the better the uniformity of the target region[10];
-
(3)
Conformity Index (CI), CI = VRD99%R/VRPTV, where VRD99% is the volume included in the 99% prescription dose and VRPTV is the volume of the PTV, and the closer the CI is to one, the better the target conformity[10];
-
(4)
Mean dose (Dmean), Dmean was used to assess the dose of the bladder, rectum, pelvis, and sacrum;
-
(5)
Maximum dose (Dmax), using Dmax to evaluate the pelvis and sacrum;
-
(6)
The sacrum dose was assessed using D50% (dose at 50% volume). The volume value, HI, and CI of the overall patient were expressed as the mean value, and the remaining parameters were expressed as the median value.
2.6. Statistical methods
Differences between the 2 sets of data were compared using the paired t test using SPSS 19.0 statistical software (SPSS Inc., Chicago, IL). The Pearson method was used to analyze the correlation between the 2 sets of data. A P < .05 was considered statistically significant.
3. Results
3.1. Volume of target area, pelvis, and sacrum
The volumes of PTV, PGTVnd (8 cases), pelvis, and sacrum were 1297.6 ± 413.5, 102.5 ± 192.2, 845.5 ± 93.0, and 246.8 ± 40.0 cc, respectively. The pelvic bone had a volume of 107.6 ± 38.33 cc (13.08%) overlapping in the PTV, and the sacrum had a volume of 55.13 ± 22.51 cc (22.52%) overlapping in the PTV.
3.2. Target dose
All planned PTV prescription doses (4500 or 5000 cGy) covered between 95.05% and 98.89%. The PTV, CI, and HI based on different optimization modes in the 4500 and 5000 cGy plans for the lymphatic drainage area are summarized in Tables 2 to 5. For the irradiation plan of 4500 and 5000 cGy, there was no significant difference in CI and HI between the pelvic bone limit and the pelvic + sacrum limited mode.
Table 2.
Planning target volume (PTV) and organ at risk (OAR) doses in the radiotherapy group of 5000 and 4500 cGy lymphatic drainage areas (pelvic bones and sacrum were not limited).
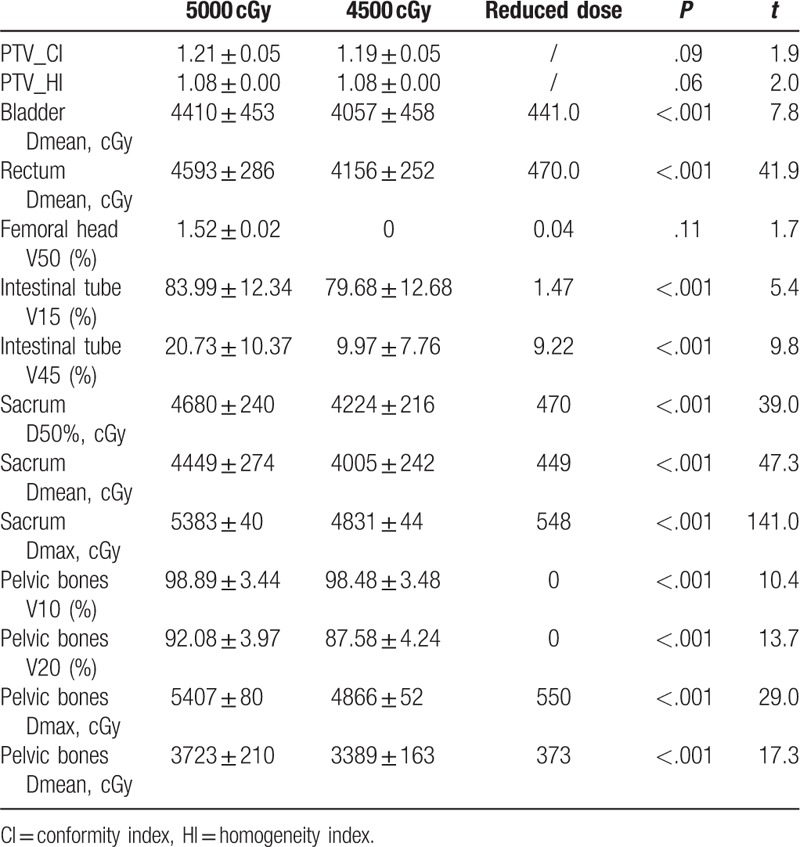
Table 5.
Planning target volume (PTV) and organ at risk (OAR) doses in 2 limited modes: “Pelvic bones Limited” and “Pelvic bones + Sacrum Limited.”.
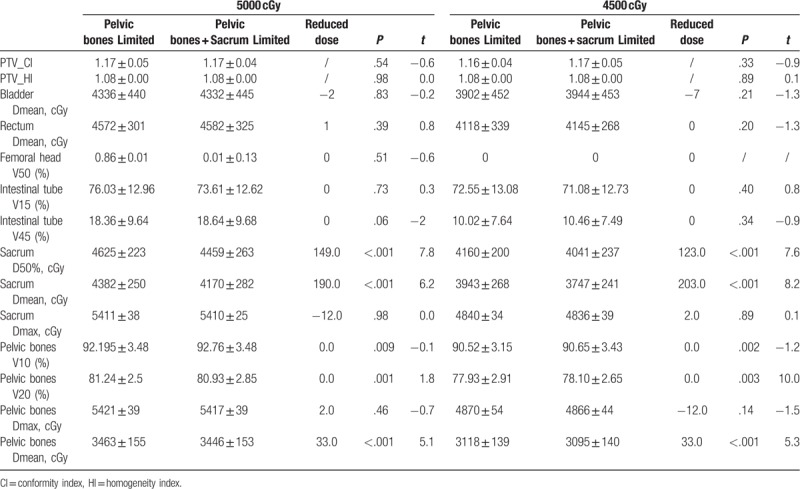
Table 3.
Planning target volume (PTV) and organ at risk (OAR) doses in the radiotherapy group of 5000 and 4500 cGy lymphatic drainage areas (pelvic bones was limited).
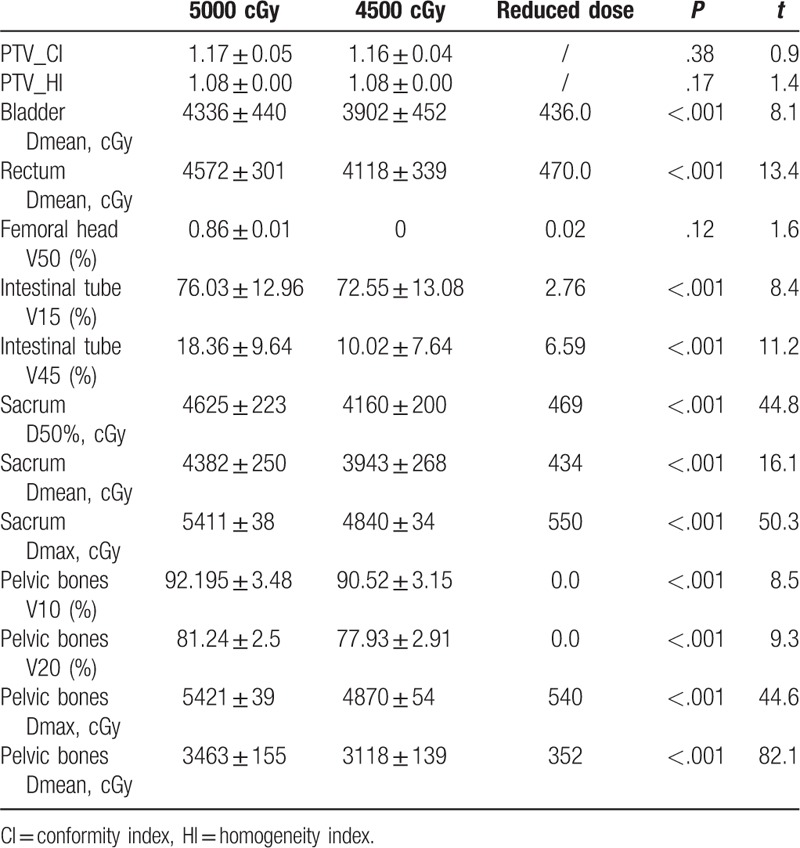
The sacrum and pelvis doses are summarized in Tables 2 to 6. In the plan to receive 5000 cGy irradiation in the lymphatic drainage area, the D50% of the 3 limited modes (infinite pelvic and sacrum, pelvic bone limit, pelvic + sacrum limit) were 4680, 4625, and 4459 cGy, respectively. The D50% and Dmean of the pelvis + sacrum limited mode were significantly lower than the pelvic limited mode (P < .001). At the same time, the pelvic V20 and Dmean also had a small decrease. Similarly, the D50% of the 3 limited modes in the 4500 cGy plan were 4224, 4160, and 4041 cGy, respectively. The pelvic + sacrum limited mode also significantly reduced the sacrum D50% and Dmean (P = .001). Among them, sacrum D50% and Dmean decreased by 123 and 203 cGy, respectively. At the same time, the Dmean of the pelvis was also significantly reduced, but the V10 and V20 increased slightly.
Table 6.
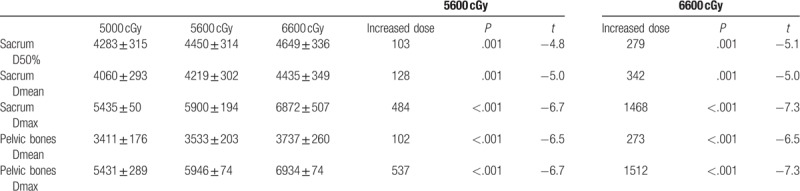
In the lymph node boost group, the radiation dose increased to 5600 and 6600 cGy, and increased the dose of the sacrum and pelvic bones (cGy).
The dose of the sacrum and pelvis of the 4500 cGy plan in the lymphatic drainage area was significantly lower than that of the 5000 cGy plan (P = .001). The sacrum and pelvic doses had reduced D50%, Dmean, and Dmax by 470, 449, and 548 cGy, respectively; The pelvic limited mode reduced by 469, 434, and 550 cGy, respectively, and the pelvic + sacrum limited mode reduced by 436, 415, and 556 cGy, respectively.
In the lymph node boost group, the irradiation dose of the sacrum and pelvis was significantly increased (P ≤ .001). In the 5600 cGy group, the sacrum D50%, Dmean, and Dmax increased by 103, 128, and 434 cGy, respectively. The pelvic Dmean and Dmax increased by 102 and 537 cGy, respectively. In the 6000 Gy group, the sacrum D50, Dmean, and Dmax increased by 279, 342, and 1468 cGy, respectively. The pelvic Dmean and Dmax increased by 273 and 1512 cGy, respectively.
The correlation between the volume of the sacrum or pelvis overlapping in the PTV and the D50% of the sacrum is shown in Fig. 2. In the 5000 cGy plan, the D50% of the 3 limited modes (both pelvic and sacrum, pelvic bone limit, pelvic + sacrum limit) were highly correlated with the volume of the sacrum overlap in the PTV (r = 0.788, 0.799, and 0.784, respectively; all P < .001). The volume overlapping with the pelvis in the PTV was moderately correlated (r = 0.387, 0.585, and 0.612, respectively; P = .09, .007, and .004, respectively). Similarly, the correlation between D50% in the 4500 cGy plan and the overlapping volume of the sacrum and pelvis was r = 0.791, 0.792, and 0.784, respectively for the sacrum (all P = .001) and r = 0.387, 0.584, and 0.596, respectively (P = .09, .007, and .006, respectively) for the pelvis.
Figure 2.
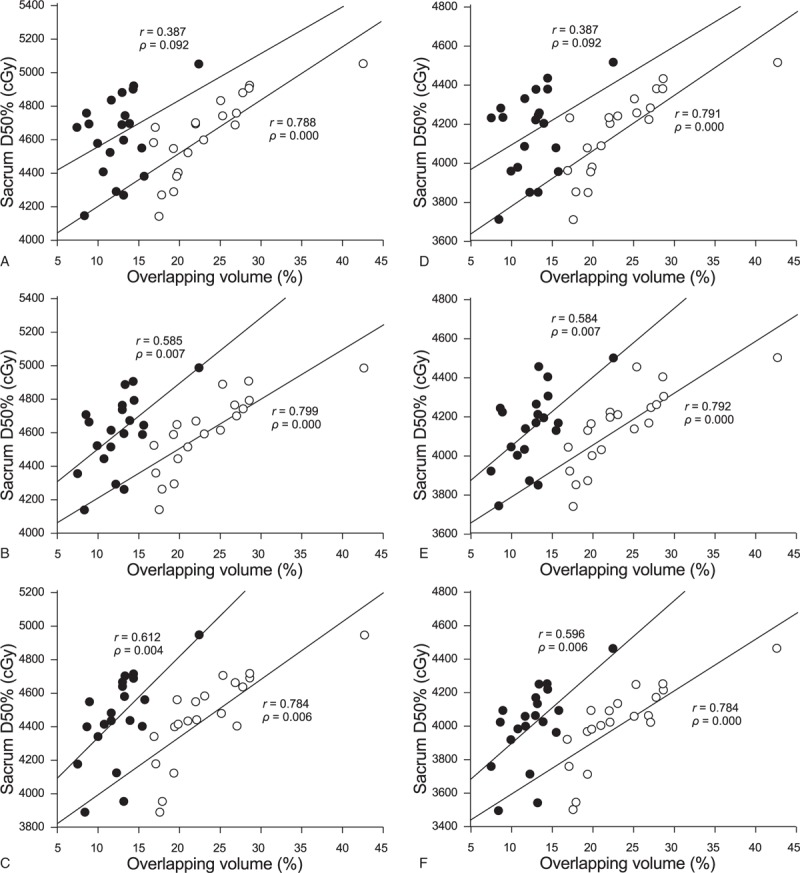
Correlation between the overlapping volume of the sacrum, pelvis, and planning target volume (PTV) and D50% of the sacrum (Fig. A, B, and C, respectively) when the lymphatic drainage area receives 5000 cGy radiation therapy, in the 3 limited modes “pelvic bones and sacrum unlimited,” “pelvic bones limited,” and “pelvic bones + sacrum limited.” Correlation between D50% and overlapping volume: Figures D, E, and F are the D50% and overlapping volume correlations for the 3 limited modes in the 4500 cGy plan, respectively. ● is the volume of PTV and pelvis overlap; ○ is the product of PTV and tibia overlap.
3.3. Other OARs
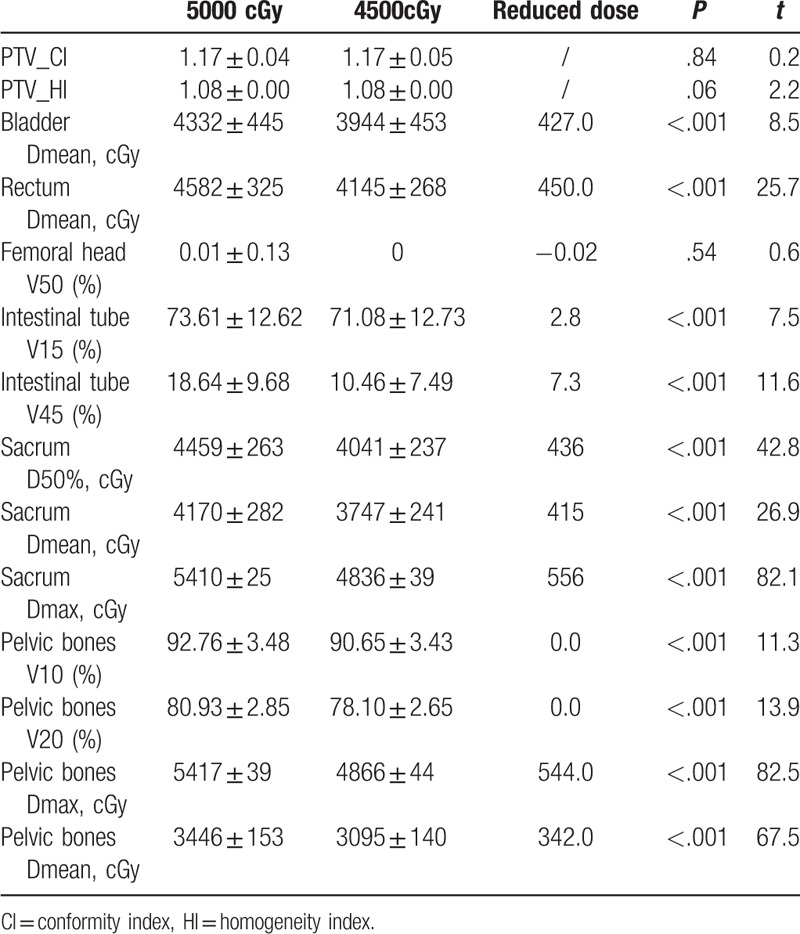
There were no significant differences in bladder, rectal, femoral head, and intestinal tube doses between the pelvic bone limitation and the pelvic + tibia limited mode, either in the 5000 or 4500 cGy lymphatic drainage group (see Table 5). The bladder, rectal, and intestinal tube doses in the 4500 cGy group were significantly lower than the 5000 cGy group (P < .001) (see Tables 2–4).
Table 4.
Planning target volume (PTV) and organ at risk (OAR) doses in the radiotherapy group of 5000 and 4500 cGy lymphatic drainage areas (pelvic bones and sacrum were limited).
4. Discussion
PIF frequently occurs in patients with locally advanced cervical cancer who receive chemoradiotherapy. In a previous study, all patients presented with more than 1 fracture of the sacrum.[1] Osteoporosis is the most important risk factor for PIF after radiotherapy.[11,12] Irradiation dose is closely related to decreased bone density, bone cell damage and necrosis, and humeral or pubic pain.[13–15] Ramlov et al[1] reported that there was a steep dose--response relationship between PIF and sacrum dose in cervical cancer patients older than 50 years. When D50% increased from 35 to 40 Gy, PIF risk increased from 22% to 45%. Therefore, reducing the dose to the sacrum (including the ankle joint) is an important method to reduce PIF. We aimed to explore the feasibility of reducing the dose to the sacrum by selecting the optimal optimization strategy in the IMRT plan for cervical cancer patients.
The volume of pelvic bone receiving low-dose irradiation is closely related to the blood toxicity of patients with cervical cancer radiotherapy.[7] Therefore, V10 and V20 are the main limiting methods for reducing blood toxicity.[7–9] However, a dose limit for radiation therapy to the sacrum, which is part of the pelvis, has not been studied. Therefore, in this paper, all 28 patients were independently delineated sacrum (including ankle joint). The limitation of the sacrum was increased, on the basis of the pelvic bone limitation in the lymphatic drainage area, including V20, V30, V40, and V45 by using the ALARA method without compromising the dose of the target area and other OAR doses. In the case of the target dose (target coverage, CI, and HI) and other OAR (including bladder, rectum, femoral head, and intestine) doses without a significant change, the pelvic and sacrum limited mode can significantly reduce the sacrum D50 and Dmean, in either the 5000 or 4500 cGy group. The dose reduction range was from 123 to 203 cGy. This shows that the dose modulation function of IMRT has slightly reset the dose in the irradiation area. Unlimited tissues, such as gluteal muscles around the pelvis, may be subject to increased doses.
The National Comprehensive Cancer Network (NCCN) guidelines recommend a dose of 4500 cGy (4000–5000 cGy) for primary tumor and lymphatic drainage in patients with untreated cervical cancer.[16] The recommended dose for external irradiation of the lymphatic drainage area for adjuvant radiotherapy after hysterectomy is recommended to be 45 to 50 Gy. For visible unresected lymph nodes, a 10 to 15-Gy dose can be added with a simultaneous integrated boost. Radiation doses of 45 and 50 Gy for the drainage areas are commonly used in most domestic and international radiotherapy centers. The tumor radiotherapy physician selects the irradiation dose according to the actual patient situation. However, there is no clear report on the difference in the dose to the sacrum, due to the 2 doses in the drainage area. The sacrum dose based on the 4500 cGy plan in this study was significantly lower than 5000 cGy. D50, Dmean, and Dmax were reduced by 436 to 470, 415 to 449, and 548–556 cGy, respectively, in 3 limited modes (unlimited doses of sacrum and pelvis, limited pelvic bone, and pelvis and sacrum). The dose of sacrum reduced by dose irradiation at 4500 cGy, instead of 5000 cGy, was significantly greater than that reduced by the limited mode optimization.
Three plans were designed in the lymph node boost group: the 5000 cGy plan in the drainage area, and the PGTVnd sequential addition to 5600 and 6600 cGy on the basis of 5000 cGy. The results showed that the sacrum and pelvis doses were significantly increased based on the 5600 and 6600-cGy boost modes, and the dose increase was more obvious in the 6600-cGy boost mode. D50% of the sacrum increased by 103 and 279 cGy, Dmean increased by 128 and 342 cGy, and Dmax increased by 434 and 1468 cGy. Among these, the increase in sacrum Dmax was the largest.
Ramlov et al[1] reported that there was a steep dose--effect relationship between PIF and sacrum dose in patients older than 50 years. The D50% of the sacrum is an important predictor of PIF. When the D50% of the sacrum increased from 35 to 40 Gy, the risk of PIF increased from 22% to 45%. An increase of D50% by 5 Gy may multiply the risk of PIF. However, the dose--response curve produced by Ramlov et al[1] is not necessarily suitable for our study. For example, the D50% of patients included in their study (including 31 cases with 4500 cGy divided into 25 drainage areas, 65 cases with drainage area 5000 cGy, and lymph node 6000 cGy divided into 30 simultaneous doses, 5 patients with a 4500 cGy drainage area based on sequential lymph node addition from 5600 to 6600 cGy) was 3780 cGy (2970–4670 cGy), which is lower than the 4041 cGy (3498–4460 cGy) of the pelvic and metatarsal limited mode (lowest) in our 4500 cGy drainage area (see Fig. 3). Therefore, further research is needed.
Figure 3.
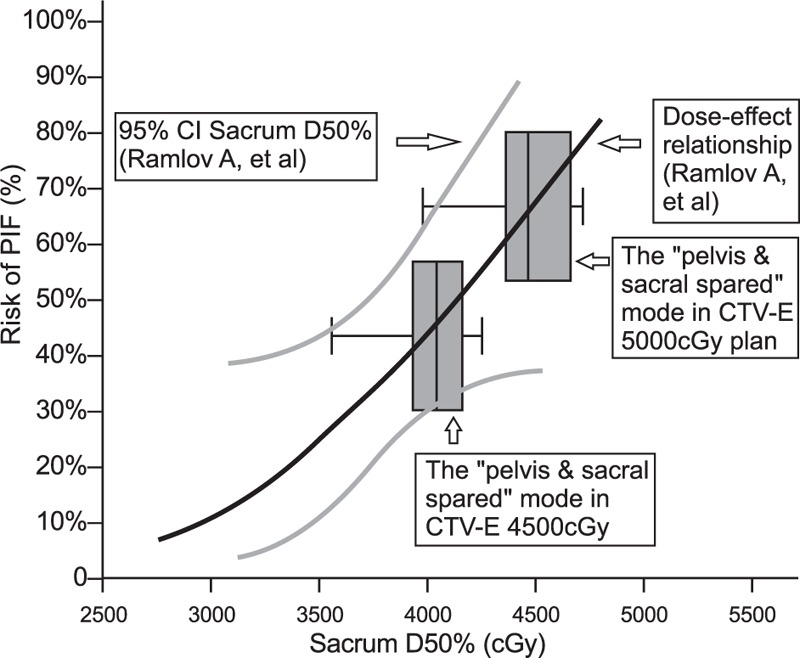
PIF risk levels in the 5000 and 4500 cGy drainage irradiation group with a “pelvic + sacral limited” pattern of sacral D50% in Ramlov et al.[1] CI = conformity index, CTV = clinical target volume, PIF = pelvic insufficiency fractures.
The sacrum D50% is positively correlated with the volume of the sacrum or pelvis overlap in the PTV, especially the overlapping volume of the sacrum, as shown in Fig. 2. The large volume of the CTV at the pelvic level results in a large overlapping volume with the pelvis and sacrum. On the contrary, the size of the PTV margin added on the basis of CTV will directly affect the overlap volume, and ultimately affect the dose of the sacrum. Improving the placement method and increasing the use of IGRT frequency can reduce the PTV margin, especially the backward margin. Relative to the pelvis, the D50% of the sacrum has a higher and more pronounced correlation with the overlapping volume of the sacrum. This also demonstrates the feasibility of reducing the D50% of the sacrum by adding the limit of the sacrum at the base of the pelvic limit.
In our study, when the PGTVnd was increased to 5600 and 6600 cGy in the lymph node boost group, the D50% of the sacrum increased by 103 and 279 cGy, respectively. This indicates that the addition of lymph nodes will increase the dose to the sacrum. However, the number of patients (8 cases) in this group was small, and the location and size of the anterior sacrum lymph nodes will directly affect the increased dose. In addition, the Dmax of the sacrum had the largest increase (537 and 1512 cGy). However, the Dmax may not be the main cause of PIF. Ramlov et al[1] reported that only 23% of the fractures overlapped with high-dose areas above 5500 cGy. Therefore, it is considered that PIF does not result from irradiation of a high dose to a small volume but by an increase in the dose to the entire bone.
There are few studies on the relationship between PIF occurrence and irradiation technology after pelvic radiotherapy. A study by Oh et al[17] showed that the 4-field box technique significantly reduced the incidence of PIF compared with the front-to-back technique (5-year cumulative incidence 35.8% vs 17.1%, P = .001). Another retrospective study from the Memorial Sloan-Kettering Cancer Center showed that the incidence of PIF using IMRT was almost identical to the incidence of PIF using 4-field cassette technology.[18] However, both of the above retrospective studies lacked dosimetric information for the sacrum. When using high-dose intracavitary brachytherapy (HDR-ICBT), some doses are scattered to the pubis. This leads to a further increase in the dose to the pelvic bone on the basis of external irradiation. Therefore, HDR-ICBT is a risk factor for PIF.[3]
There are limitations in the current study. Although the preliminary test results appear to have addressed the question of dose optimization strategy of sacrum limitation in cervical cancer IMRT planning, the efficacy of the study was influenced by its retrospective design and small sample size, due to the inevitable potential selection bias and inability to adjust for outcome related confounders. We plan to focus on population-based morbidity and its relationship with sacrum dose in future studies.
In summary, increasing the limitation of the sacrum, on the basis of pelvic bone limitation, in cervical cancer IMRT can significantly reduce the sacrum dose. Compared with the dose of 5000 cGy to the lymphatic drainage area, a dose of 4500 cGy was the most important factor to reduce the dose to the sacrum.
Author contributions
Conceptualization: Jianjun Qian.
Data curation: Qi Guo.
Project administration: Qi Guo.
Software: Shang Cai.
Visualization: Shang Cai.
Writing – original draft: Qi Guo.
Writing – review & editing: Ye Tian.
Footnotes
Abbreviations: CI = conformity index, CTV = clinical target volume, HI = homogeneity index, IMRT = intensity-modulated radiation therapy, OAR = organ at risk, PGTVnd = PTV of lymph nodes, PIF = pelvic insufficiency fracture, PTV = planning target volume.
This work was supported in part by the Jiangsu Medical Innovation Team under Grant [number CXDT-37] and Jiangsu Provincial Key Project [number BL2018657].
The authors have no conflict of interest.
References
- [1].Ramlov A, Pedersen EM, Røhl L, et al. Risk factors for pelvic insufficiency fractures in locally advanced cervical cancer following intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys 2017;97:1032–9. [DOI] [PubMed] [Google Scholar]
- [2].Lustberg MB, Reinbolt RE, Shapiro CL. Bone health in adult cancer survivorship. J Clin Oncol 2012;30:3665–74. [DOI] [PubMed] [Google Scholar]
- [3].Yamamoto K, Nagao S, Suzuki K, et al. Pelvic fractures after definitive and postoperative radiotherapy for cervical cancer: a retrospective analysis of risk factors. Gynecol Oncol 2017;147:585–8. [DOI] [PubMed] [Google Scholar]
- [4].Meixel AJ, Hauswald H, Delorme S, et al. From radiation osteitis to osteoradionecrosis: incidence and MR morphology of radiation-induced sacral pathologies following pelvic radiotherapy. Eur Radiol 2018;28:3550–9. [DOI] [PubMed] [Google Scholar]
- [5].Small W, Jr, Mell LK, Anderson P, et al. Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy in postoperative treatment of endometrial and cervical cancer. Int J Radiat Oncol Biol Phys 2008;71:428–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Haken RKT, Marks LB, Bentzen SM, et al. Quantitative analyses of normal tissue effects in the clinic (QUANTEC): clinical use. Radiother Oncol 2010;98suppl 2:S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mell LK, Kochanski JD, Roeske JC, et al. Dosimetric predictors of acute hematologic toxicity in cervical cancer patients treated with concurrent cisplatin and intensity-modulated pelvic radiotherapy. Int J Radiat Oncol Biol Phys 2006;66:1356–65. [DOI] [PubMed] [Google Scholar]
- [8].Daoming Z, Hui G, Qi Z, et al. Dosimetric analysis of postoperative radiotherapy for cervical cancer with defined pelvic IMRT. Chin J Radiat Oncol 2017;26:1303–7. [Google Scholar]
- [9].Rose BS, Aydogan B, Liang Y, et al. Normal tissue complication probability modeling of acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys 2011;79:800–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Stieler F, Wolff D, Schmid H, et al. A comparison of several modulated radiotherapy techniques for head and neck cancer and dosimetric validation of VMAT. Radiother Oncol 2011;101:388–93. [DOI] [PubMed] [Google Scholar]
- [11].Ogino I, Okamoto N, Ono Y, et al. Pelvic insufficiency fractures in postmenopausal woman with advanced cervical cancer treated by radiotherapy. Radiother Oncol 2003;68:61–7. [DOI] [PubMed] [Google Scholar]
- [12].Kim HJ, Boland PJ, Meredith DS, et al. Fractures of the sacrum after chemoradiation for rectal carcinoma: incidence, risk factors, and radiographic evaluation. Int J Radiat Oncol Biol Phys 2012;84:694–9. [DOI] [PubMed] [Google Scholar]
- [13].Williams HJ, Davies AM. The effect of X-rays on bone: a pictorial review. Eur Radiol 2006;16:619–33. [DOI] [PubMed] [Google Scholar]
- [14].Waldenström AC, Olsson C, Wilderäng U, et al. Pain and mean absorbed dose to the pubic bone after radiotherapy among gynecological cancer survivors. Int J Radiat Oncol Biol Phys 2011;80:1171–80. [DOI] [PubMed] [Google Scholar]
- [15].Waldenström AC, Olsson C, Wilderäng U, et al. Relative importance of hip and sacral pain among long-term gynecological cancer survivors treated with pelvic radiotherapy and their relationships to mean absorbed doses. Int J Radiat Oricol Biol Phys 2012;84:428–36. [DOI] [PubMed] [Google Scholar]
- [16].National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Cervical Cancer, V.4. 2019. Available at: https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf. [Google Scholar]
- [17].Oh D, Huh SJ, Nam H, et al. Pelvic insufficiency fracture after pelvic radiotherapy for cervical cancer: analysis of risk factors. Int J Radiat Oncol Biol Phys 2007;69:1183–8. [DOI] [PubMed] [Google Scholar]
- [18].Shih KK, Folkert MR, Kollmeier MA, et al. Pelvic insufficiency fractures in patients with cervical and endometrial cancer treated with postoperative pelvic radiation. Gynecol Oncol 2013;128:540–3. [DOI] [PubMed] [Google Scholar]


