Abstract
Rationale:
Sarcoidosis is an idiopathic granulomatous disease. Although the lungs are most commonly involved, any organ may be affected. To assist with future diagnoses, we describe a rare case of peritoneal sarcoidosis in a young female patient, and present a literature review.
Patient concerns:
A 32-year-old female patient presented to our institution with abdominal discomfort. She was evaluated with contrast-enhanced abdominal computed tomography (CT), and multiple enlarged lymph nodes were detected at the hepatic artery and left gastric artery nodal stations. The patient was lost during follow-up, but returned after 7 months and again underwent abdominal CT. This revealed diffuse nodular thickening of the peritoneum and the appearance of omental cake in her abdomen.
Diagnosis:
Excisional biopsy of a lymph node was performed and extrapulmonary sarcoidosis was confirmed.
Interventions:
The patient was treated with corticosteroid.
Outcomes:
A follow-up abdominal CT scan after two weeks revealed decreases in the numbers and sizes of the previously enlarged lymph nodes, and improvement in the ascites and peritoneal thickening.
Lessions:
Peritoneal sarcoidosis should be considered as an additional differential diagnosis when peritoneal carcinomatosis or tuberculous peritonitis are suspected. In this regard, serum levels of angiotensin-converting enzyme (ACE) may be a valuable diagnostic indicator of unusual sarcoidosis presentations.
Keywords: computed tomography, peritoneal sarcoidosis
1. Introduction
Sarcoidosis is an idiopathic granulomatous disease. Although the lungs are most commonly involved, any organ may be affected.[1] Peritoneal sarcoidosis is rare, and accurate discrimination between this disease and carcinomatosis or tuberculosis is important because therapeutic approaches differ completely.[2] We describe a rare case of peritoneal sarcoidosis in a young female patient, and accompany this with a review of the relevant literature.
2. Case presentation
A 32-year-old female patient presented at our outpatient clinic with abdominal discomfort. Her medical record revealed a cholecystectomy performed a year prior to her admission, but no other medical history or current medication. She underwent abdominal computed tomography (CT) that showed multiple enlarged lymph nodes along the hepatic artery and the left gastric artery. The largest node was approximately 2 cm in diameter and showed central low density on a contrast enhanced scan (Fig. 1A). There were no signs of malignant lesions in other abdominal solid organs, the gastrointestinal tract, or the genitourinary system. She was transferred to the oncology department and underwent whole body positron emission tomography-computed tomography (PET-CT). The enlarged lymph nodes seen on the abdominal CT scan showed strong 18F-fluorodeoxyglucose (FDG) uptake (Fig. 1B). Liver function and total bilirubin level were within normal limits. Tumor markers and viral markers were unremarkable. On the basis of CT and PET-CT findings, we suspected a lymphoproliferative disorder such as lymphoma or Castleman disease. However, the patient was lost during follow-up.
Figure 1.
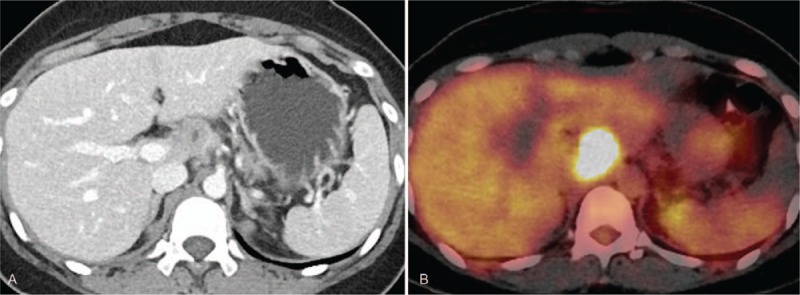
(A) A lymph node along the hepatic artery is approximately 2 cm in diameter and shows central low density on a contrast enhanced CT scan. (B) The lymph node shows strong 18F-FDG uptake on a PET-CT.
After 7 months she visited our gastroenterology department reporting weight loss of 8 kg over the preceding 2 weeks. A repeat abdominal CT scan showed significant aggravation of the lymphadenopathy of the porta hepatis and mesentery, development of diffuse nodular thickening of the peritoneum and omental cake appearance (Fig. 2). Chest CT scan revealed enlarged lymph nodes in the right upper paratracheal, subcarinal and anterior diaphragmatic areas, and left unilateral pleural effusion. Laboratory analyses indicated that serum angiotensin-converting enzyme (ACE) concentrations were elevated to 119 U/L (reference range: 8–52 U/L).
Figure 2.

(A) On axial and (B) coronal reformatted CT images of a contrast enhanced CT scan performed 7 months later shows significant aggravation of the lymphadenopathy of the porta hepatis, development of diffuse nodular thickening of the peritoneum and ascites.
The possibility of malignancy was assessed with excisional biopsy of a lymph node involving removal of a round encapsulated mass approximately 2 cm in diameter from adjacent to the celiac trunk. On pathologic examination, the lymph node showed capsular fibrosis and numerous granulomas comprising epithelioid histiocytes surrounded by an outer layer of lymphocytes, which is consistent with sarcoidosis (Fig. 3). Pathogen-specific staining revealed no acid-fast bacilli or fungal organisms (Ziehl-Neelsen and Gomori methenamine silver and periodic acid-Schiff stains, respectively). We diagnosed extrapulmonary sarcoidosis.
Figure 3.

Lymph node shows capsular fibrosis and numerous granulomas. Granulomas are composed of epithelioid histiocytes and surrounded by a rim of lymphocytes (A. H&E, ×100). In some granulomas, central foci of fibrinoid necrosis is observed (B. H&E, ×200).
Corticosteroid therapy was started, whereupon the patient's condition improved dramatically. A follow-up abdominal CT scan after 2 weeks revealed decreases in the numbers and sizes of the previously enlarged lymph nodes, and improvement in the ascites and peritoneal thickening. The patient has been treated continuously since discharge.
3. Discussion
Sarcoidosis is a multisystemic granulomatous disease of unknown cause. It may affect any organ in the body, but pulmonary involvement is most common, followed by involvement of the skin, eyes, lymph nodes and other organs.[3] Extrapulmonary involvement is reported in 30% of all cases, with the abdomen the most frequent site and typical presentation of homogenous hepatosplenomegaly. Approximately 5% to 15% of extrapulmonary cases show multiple hypodense nodules scattered in the liver and spleen.[4]
Lymph node involvements are noted in approximately 30% of all sarcoidosis cases. The celiac axis, porta hepatis, paraaortic and paracaval nodal stations are frequently affected.[5] Lymph nodes affected by sarcoidosis are significantly smaller and more discrete than those affected by lymphoma, which are conglomerated.[5]
Peritoneal sarcoidosis is rare and follows the pattern of generalized sarcoidosis. It is presented between the second and fourth decade of life, predominantly among females.[6] The most common presentations of peritoneal sarcoidosis are ascites or granulomatous peritoneal nodules. Pathologic examination through peritonea l biopsy is necessary to rule out peritoneal carcinomatosis or tuberculous peritonitis.[7]
Our patient fit the demographic profile for peritoneal sarcoidosis. She initially presented with multi-stational lymphadenopathy that progressed over 7 months to peritoneal involvement without hepatomegaly or splenomegaly. Ascites, diffuse thickening of the peritoneum, peritoneal nodules, and an omental cake appearance were observed, all of which may be consistent with peritoneal carcinomatosis or tuberculous peritonitis. Since tuberculosis is endemic in South Korea, we usually consider tuberculous peritonitis as a differential diagnosis, especially in young patients with no evidence of primary malignancies.
The patient's elevated serum ACE level was noteworthy. ACE has particular diagnostic value for sarcoidosis.[8] Epithelioid cells within sarcoid granulomas are thought to produce ACE, resulting in elevated serum ACE levels in approximately 60% of patients with sarcoidosis. ACE levels are thought to be proportional to the total granuloma volume, hence indicative of sarcoidosis activity.[5] In one population-based cohort, the sensitivity and specificity of high ACE for sarcoidosis diagnosis were 41.4% and 89.9%, respectively.[9] Our patient showed an elevated ACE level on her second visit, but we cannot evaluate whether or not ACE level was changed by the aggravation of peritoneal sarcoidosis, because ACE levels were not measured on her first visit.
Corticosteroid medications are considered the first line of treatment for sarcoidosis requiring therapy, and can reduce systemic inflammation and organ damage. Steroid-sparing agents such as methotrexate, azathioprine, and anti-TNF-α are also used. Our patient received corticosteroid therapy and showed notable improvement on follow-up abdominal CT scan performed two weeks later.
In conclusion, peritoneal sarcoidosis should be considered as an additional differential diagnosis when peritoneal carcinomatosis and tuberculous peritonitis are suspected. Serum ACE levels have considerable utility in this regard.
Author contributions
Conceptualization: Seung Wook Lee, Min Hee Lee.
Data curation: Seung Wook Lee, Jung Min Jung.
Formal analysis: Jung Min Jung.
Investigation: Seung Wook Lee, Seo Youn Choi, Jung Min Jung.
Methodology: Jung Min Jung.
Resources: Ji Eun Lee, Boem Ha Yi.
Supervision: Min Hee Lee.
Validation: Min Hee Lee, Boem Ha Yi.
Visualization: Ji Eun Lee.
Writing - Original Draft: Seung Wook Lee.
Writing - Review & Editing: Min Hee Lee, Seo Youn Choi.
Footnotes
Abbreviations: ACE = angiotensin-converting enzyme, CT = computed tomography, FDG = 18F-fluorodeoxyglucose, PET-CT = positron emission tomography-computed tomography.
This study was funded by Soonchunhyang University Research Fund.
Min Hee Lee received grants from Soonchunhyang University Research Fund. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Seung Wook Lee, Ji Eun Lee, Seo Youn Choi, Boem Ha Yi, and Jung Min Jung declare that they have no conflict of interest.
This study was approved by the institutional review boards of Soonchunhyang University Bucheon Hospital where the patient data were collected. Informed written consent was obtained from the patient for publication of this case report and accompanying images.
References
- [1].Baughman RP, Lower EE, Gibson K. Pulmonary manifestations of sarcoidosis. Presse Med 2012;41(6 Pt 2):e289–302. [DOI] [PubMed] [Google Scholar]
- [2].Gorkem U, Gungor T, Bas Y, et al. Abdominal sarcoidosis may mimic peritoneal carcinomatosis. Case Rep Obstet Gynecol 2015;2015:263945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. New Engl J Med 2007;357:2153–65. [DOI] [PubMed] [Google Scholar]
- [4].Bernaciak J, Spina JC, Curros ML, et al. Case report: peritoneal sarcoidosis in an unusual location. Semin Respir Crit Care Med 2002;23:597–600. [DOI] [PubMed] [Google Scholar]
- [5].Warshauer DM, Lee JK. Imaging manifestations of abdominal sarcoidosis. Am J Roentgenol 2004;182:15–28. [DOI] [PubMed] [Google Scholar]
- [6].Iyer S, Afshar K, Sharma OP. Peritoneal and pleural sarcoidosis: an unusual association - review and clinical report. Curr Opin Pulm Med 2008;14:481–7. [DOI] [PubMed] [Google Scholar]
- [7].Gezer NS, Basara I, Altay C, et al. Abdominal sarcoidosis: cross-sectional imaging findings. Diagn Interv Radiol 2015;21:111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kwon CI, Park PW, Kang H, et al. The usefulness of angiotensin converting enzyme in the differential diagnosis of Crohn's disease and intestinal tuberculosis. Korean J Intern Med 2007;22:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ungprasert P, Carmona EM, Crowson CS, et al. Diagnostic utility of angiotensin-converting enzyme in sarcoidosis: a population-based study. Lung 2016;194:91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


