Supplemental Digital Content is available in the text
Keywords: endoscopic submucosal dissection, meta-analysis, proton pump inhibitors, systematic review, vonoprazan
Abstract
Background:
Vonoprazan, a novel potassium-competitive acid blocking agent, has been used in the management of endoscopic submucosal dissection (ESD)-induced artificial ulcers. This study aimed to perform a systematic review and meta-analysis for the comparison of the effects of vonoprazan and proton pump inhibitors (PPIs) in treating ESD-induced artificial ulcers and preventing delayed bleeding in randomized controlled trial and cohort studies.
Methods:
We searched OVID-MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), Google Scholar, and clinical trial registries in April 2018 to identify all studies that assess and compare the effects of vonoprazan and PPI in treating ESD-induced artificial ulcers and preventing delayed bleeding. Primary outcome of ulcer healing rate and secondary outcomes of shrinkage rate, ulcer size, and delayed bleeding were studied.
Results:
A total of 1265 patients from 12 studies were included in the final analysis. Healing rate at 4 weeks post-ESD was significantly higher in the vonoprazan group than in the PPI group (relative ratio [RR] 1.20 [1.03–1.40]). However, healing rate at 8 weeks post-ESD was significantly higher in the PPI group than in the vonoprazan group (RR 0.68 [0.48–0.97]).
There was no evidence of significant difference between groups in shrinkage rate at 4 weeks post-ESD, shrinkage rate at 8 weeks post-ESD, delayed bleeding, ulcer size at 0 weeks post-ESD, and ulcer size at 8 weeks post-ESD.
Conclusions:
There was no substantial difference in ulcer healing and post-ESD bleeding between vonoprazan and PPIs. However, vonoprazan more rapidly and effectively treated artificial ulcers after ESD than did PPIs.
1. Introduction
Endoscopic submucosal dissection (ESD), an advanced endoscopic procedure, has become an alternative to surgical resection for early gastric cancer.[1,2] However, this technique causes artificial ulceration, which can be linked to delayed bleeding from the ulcer. This is a well-known complication of ESD.[1] It is generally recommended to administer a proton pump inhibitor (PPI) after ESD, because the inhibition of gastric acid secretion can improve the healing of ESD-induced ulcers.[2–4] However, PPIs have several limitations, such as short plasma half-life, slow onset of effects, and problems related to cytochrome P450 (CYP) 2C19 polymorphism.[5–7]
Recently, a novel potassium-competitive acid-blocking agent (P-CAB) called vonoprazan (TAKECAB; Takeda Pharmaceutical Co. Ltd., Tokyo, Japan), has been developed that is stronger, faster, and exhibits longer-lasting acid suppression than conventional PPIs.[8] The acid-inhibitory effect of vonoprazan has been reported to be more potent than that of PPIs, with greater impact against acid-related diseases such as gastroesophageal reflux disease or Helicobacter pylori (H pylori) infection.[9]
Vonoprazan may have an efficacy comparable to or better than that of PPIs in the treatment of artificial ulcers resulting from ESD. Therefore, vonoprazan has been preferred in the management of ESD-induced artificial ulcers.[10] There is a growing number of reports comparing the effectiveness of vonoprazan with that of PPIs in treating ESD-induced ulcers. However, the findings have been variable, and reported outcomes are conflicting. Furthermore, no previous systematic review and meta-analysis has been published regarding this issue. Therefore, we have performed a systematic review and meta-analysis to assess and compare the effects of vonoprazan and PPI in the treatment of ESD-induced artificial ulcers and prevention of delayed bleeding in randomized clinical trials (RCT) and cohort studies.
2. Methods
We developed the protocol for this review and registered it in the international prospective register of systematic reviews PROSPERO network (registration number: CRD42018091656; www.crd.york.ac.uk/PROSPERO).
The described systematic review and meta-analysis was conducted in accordance with the protocol recommended by the Cochrane Collaboration[11] and with the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines.[12] It was reported according to the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines.[13]
2.1. Ethical issues
This systematic review does not require ethical approval or informed consent because there was no direct contact with individual patients, and only previously published data were included in the review.
2.2. Search strategy and inclusion criteria
Two authors (KBJ and CGJ) independently searched OVID-MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), and Google Scholar in April 2018. We also searched registered trials described in clinical trial registries, which are listed in the Appendix (see Supplemental Digital Content, which shows worldwide clinical trial registries that we searched for).
The search terms included vonoprazan, Takecab, P-CAB, TAK-438, potassium-competitive, endoscopic submucosal dissection, and ESD. The reference lists of the identified studies and eligible articles were also searched manually in order to identify additional relevant studies.
Studies included in our analysis were selected based on the following inclusion criteria: study design (SD), randomized clinical trial or cohort study; patients (P), patients who underwent ESD for gastric adenoma or a possible node-negative early gastric cancer; intervention (I), anti-ulcer medication with potassium-competitive acid blocker including vonoprazan; comparator (C), anti-ulcer medication with PPI including lansoprazole, esomeprazole, or rabeprazole; and outcome (O), primary outcomes including ulcer healing rate at 4 weeks and 8 weeks after ESD, and secondary outcomes including shrinkage rate and ulcer size at 4 weeks and 8 weeks after ESD, and delayed bleeding event. No language or date restrictions were applied.
Review articles, case reports, case-series, letters to editor, commentaries, laboratory science studies, and any non-relevant studies were excluded from analysis.
2.3. Study identification and data extraction
Inclusion and exclusion criteria for the selection of studies were determined before systematic search. In the first stage, 2 authors (KBJ and KH) independently excluded irrelevant articles by examining the titles and abstracts of the articles identified by the variety of search strategies described above. If the report was determined eligible based on the title or abstract, the full article was retrieved. Full-text versions of potentially relevant studies, as determined by at least 1 author, were retrieved and evaluated in the second stage of study selection. The articles that met the inclusion criteria were assessed separately by 2 authors (KBJ and KH), and any discrepancies were resolved through discussion. If consensus could not be reached, the dispute was resolved with the help of the third investigator (KJG). When the study samples overlapped in ≥2 articles, we selected the article with the most comprehensive population. For studies with insufficient or missing data, we attempted to contact the authors. If this was unsuccessful, we extrapolated data from the text or tables, or when possible, made calculations using relevant data given in the study.
2.4. Data extraction
Using a data extraction form that had been developed in advance, 2 reviewers (CGJ and KBJ) independently extracted the following information: first author, year of publication, study design, country, study period, publication language, anti-ulcer medication, endoscopic measurement, ulcer healing rate, shrinkage rate, ulcer size at 4 weeks and 8 weeks after ESD, and delayed bleeding event.
2.5. Study quality assessment
The quality of the studies was independently assessed by the same 2 reviewers (KBJ and KH) using the “risk of bias” tool in case of RCTs[11] and the Newcastle-Ottawa scale (NOS) in case of cohort studies.[14] Quality assessment was performed only for peer-reviewed articles.
Risk of bias (ROB) was evaluated by considering the following 7 potential sources of bias: random sequence generation, allocation concealment, blinding of the participants, blinding of the outcome assessors, incomplete outcome data, selective reporting, and other bias. The methodology of each trial was graded as “high,” “low," or “unclear," to reflect a high ROB, low ROB, and uncertain bias, respectively.[11]
NOS evaluation involves 3 quality parameters: selection, comparability, and exposure assessment.[14] This evaluation method assigns a maximum score of 4 for selection, 2 for comparability, and 3 for exposure.
2.6. Statistical analysis
We computed the pooled relative ratio (RR) with 95% confidence intervals (CIs) for dichotomous data, and standardized mean difference (SMD) with 95% CI for continuous data. We used the χ2 test for evaluation of homogeneity and the I2 test for evaluation of heterogeneity. We regarded a level of 10% significance (P < .10) in the χ2 statistic or an I2 >50% as indicating considerable heterogeneity, and used the Mantel-Haenszel random-effect model for these cases. In all other cases, we applied the Mantel–Haenszel fixed-effect model.[11,15]
If the number of studies with substantial heterogeneity was <10, the t-statistic (Hartung-Knapp-Sidik-Jonkman method) was used instead of the z-test in all random effects analysis to decrease the error rate.[16] We carried out subgroup analysis based on study design if necessary. We also conducted sensitivity analyses to evaluate the influence of a single study on the overall estimate by excluding one study at a time in case of substantial heterogeneity. We calculated the number needed to treat (NNT) based on absolute risk reduction as an estimate of the overall clinical impact of the intervention.[17]
Publication bias was assessed using Begg funnel plot and Egger linear regression test. P < .1 was used to identify the presence of a publication bias, or funnel plots for each data set were visually assessed for asymmetry.[11] If fewer than 10 studies were included, publication bias was not assessed.[11] If data were reported as a median (P25 to P75), median (range), or mean (standard error of mean), we calculated mean and standard deviation from these values.[18] We performed all analyses using Review Manager software (version 5.3, The Cochrane Collaboration, Oxford, UK) and Comprehensive meta-analysis software (version 2.0, Biostat, Englewood, NJ).
2.7. Evidence synthesis
All synthetic results were based on both peer-reviewed articles and correspondences. As quality assessment was performed only for peer-reviewed articles, the evidence grade was not determined.
3. Results
3.1. Literature search and study characteristics
The search of OVID-MEDLINE, EMBASE, CENTRAL, and Google Scholar produced 356 studies, and 8 additional studies were identified in manual research. Of the 98 reports identified by searching clinical trial registries, 9 were completed studies. After adjusting for duplicates (n = 8), 365 studies remained. A total of 438 studies were eliminated because they appeared to be outside the scope of interest after reviewing the title and abstracts. In the first stage of study selection, kappa value between 2 reviewers was 0.774. The full texts of the remaining 15 studies were reviewed in more detail. As the study samples overlapped between certain pairs of studies,[10,19–23] we selected the one with the most comprehensive population.[20,22,24] In the second stage of study selection, kappa value between 2 reviewers was 0.824. Thus, 12 studies comprising a total of 1265 patients were included in the final analysis. These studies met the inclusion criteria and were included in this systematic review and meta-analysis (Fig. 1).
Figure 1.
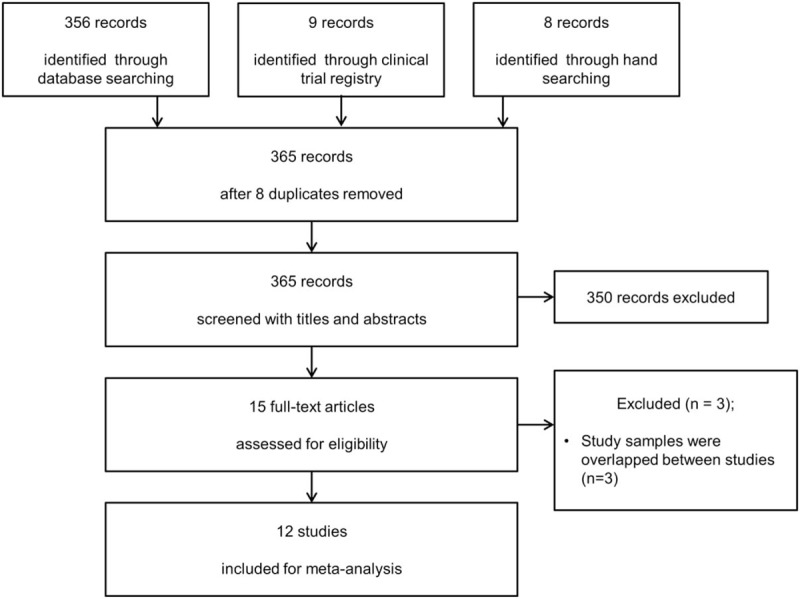
Flow diagram showing the number of abstracts and articles identified and evaluated during the review.
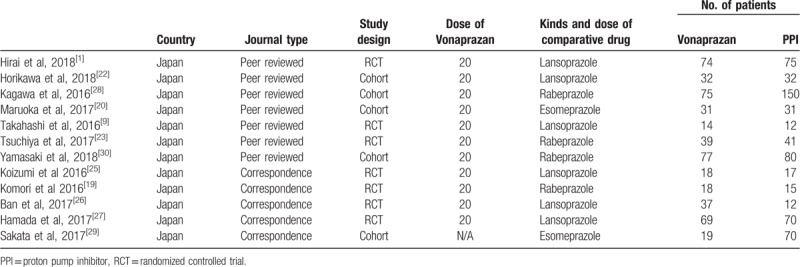
The main characteristics of studies included in this systematic review and meta-analysis are summarized in Table 1. The study included a total of 7 RCTs[1,9,23–27] and 5 cohort studies.[20,22,28–30] Of these, 7 were peer-reviewed articles[1,9,20,22,23,28] and 5 were correspondences.[24–27,29] Comparator drugs used were lansoprazole,[1,9,22,25–27] rabeprazole,[23,24,28,30] and esomeprazole.[20,29] IV-PPI administration was started before ESD in some studies[24,27–29] and after ESD in others.[1,9,20,22,23,25,26,30] All the studies were performed in Japan and the reports were written in English.
Table 1.
Summary of randomized, controlled trials included in the meta-analysis.
3.2. Study quality assessment
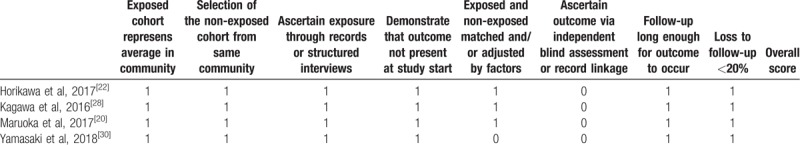
Quality assessment for peer-reviewed articles was performed using the “risk of bias” tool for RCTs[11] and the NOS for cohort studies.[14] Of the RCTs, one study mentioned the use of random sequence generation and none adequately described allocation concealment. Random sequence generation was adequately described in one study.[9] Allocation concealment, blinding of participants, and blinding of outcome assessors were not performed in any of the studies. All studies were listed in a clinical trial registry (Table 2).[1,9,23] Of the cohort studies, almost all the parameters for NOS were adequately described. Methods matched and/or adjusted by factors between exposed and nonexposed were well described in 3 studies.[20,22,28] Methods to ascertain outcome via independent blind assessment or record linkage were not described in any of the studies (Table 3).
Table 2.
Quality assessment of included randomized clinical trials based on Cochrane risk of bias tool.

Table 3.
Quality assessment of included cohort studies using the Newcastle-Ottawa quality assessment scale.
3.3. Healing rate
3.3.1. Healing rate at 4 weeks post-ESD
In all, 5 studies, including 3 RCTs[1,23,24] and 2 cohort studies,[20,30] reported healing rate at 4 weeks after ESD. Of these, 4 studies were peer-reviewed,[1,23,24,30] and 1 was correspondence.[24] Healing rate at 4 weeks post-ESD was significantly higher in the vonoprazan group than in the PPI group (RR 1.20 [1.03–1.40], I2 = 46.05, Pχ2 = .103) (Fig. 2). The number needed to treat harm (NNTH) was 14.9, with a 95% CI of 6.6 to ∞. The number needed to treat benefit (NNTB) was 59.4. In subgroup analyses, healing rate at 4 weeks post-ESD was significantly higher in the vonoprazan group than in the PPI group in cohort studies (RR 1.80 (1.11–2.92), I2 = 0.0, Pχ2 = .490, NNTH 7.1, 95% CI NNTH 4.0 to NNTH 32.6). However, there was no evidence for significant difference in healing rate in RCTs (RR 1.14 [0.97–1.35], I2 = 52.01, Pχ2 = .124, NNTB 131.0 95% CI NNTH 8.9 to ∞ to NNTB 7.8).
Figure 2.
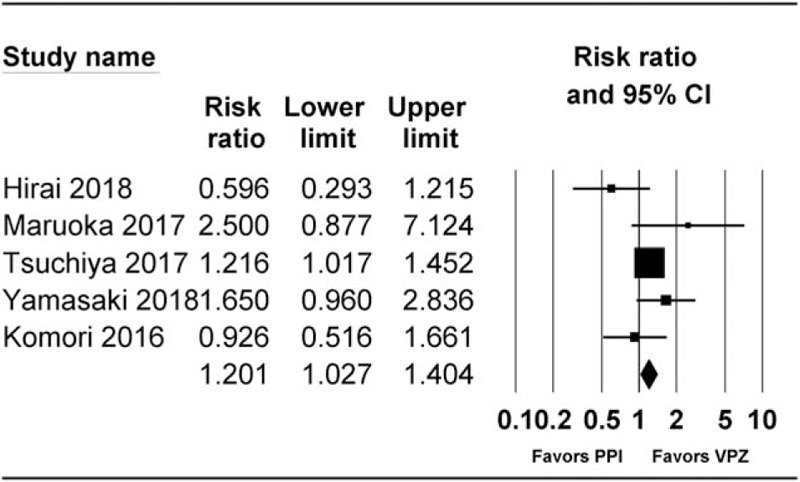
Forest plot of the studies comparing healing rate at 4 weeks post-ESD between vonoprazan-treated and proton pump inhibitor (PPI)-treated groups. The figure depicts individual trials as filled squares with relative sample size and the 95% confidence interval (CI) of the difference as a solid line. The diamond shape indicates the pooled estimate and uncertainty for the combined effect. CI = confidence intervals, PPI = proton pump inhibitor.
3.3.2. Healing rate at 8 weeks post-ESD
A total of 4 studies, including 3 RCTs[1,23,25] and 1 cohort study,[28] reported healing rates at 4 weeks after ESD. Of these, 3 studies were peer-reviewed[1,23,28] and 1 was correspondence.[25] Healing rate at 8 weeks post-ESD was significantly higher in the PPI group than in the vonoprazan group (RR 0.68 [0.48–0.97], I2 = 86.59, Pχ2 < .001, NNTB 5.3 95% CI NNTB 3.7 to NNTB 9.6) (Fig. 3). In subgroup analysis, healing rate at 8 weeks post-ESD was significantly higher in the PPI group than in the vonoprazan group in RCT (RR 0.54 [0.29–0.99], I2 = 0.0, Pχ2 = 0.375, NNTB 9.8 95% CI NNTB 5.1 to NNTB 134.0). When performing sensitivity analysis by excluding 1 study at a time, we found that the significance of results did not change with decreasing heterogeneity.
Figure 3.
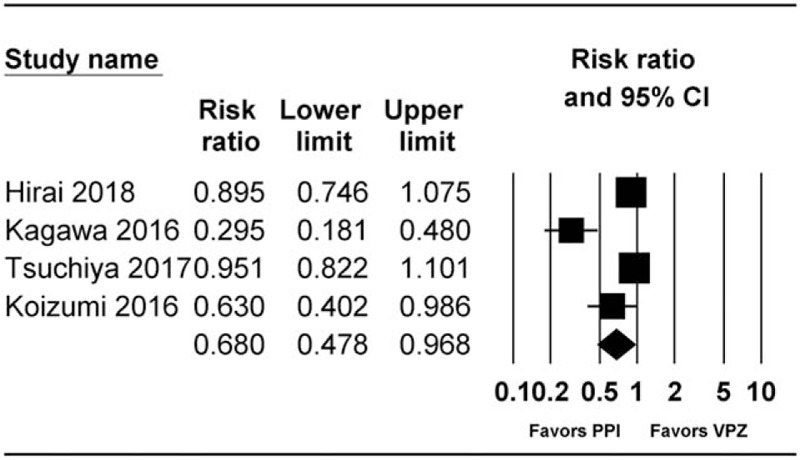
Forest plot of studies comparing healing rate at 8 weeks post-ESD between vonoprazan-treated and PPI-treated groups. The figure depicts individual trials as filled squares with relative sample size and the 95% CI of the difference as a solid line. The diamond shape indicates the pooled estimate and uncertainty for the combined effect. CI = confidence intervals, PPI = proton pump inhibitor.
3.4. Shrinkage rate
3.4.1. Shrinkage rate at 4 weeks post-ESD
Two studies, including 1 RCT[1] and 1 cohort study[30] reported shrinkage rate at 4 weeks post-ESD. All the studies were peer-reviewed.[1,30] There was no evidence of significant difference in shrinkage rate at 4 weeks post-ESD between the groups (RR 1.032 [0.930–1.146], I2 = 0.812, Pχ2 = 0.315, NNTB 29.6 95% CI NNTH 13.2 to ∞ to NNTB 7.0).
3.4.2. Shrinkage rate at 8 weeks post-ESD
Three RCTs[1,23,26] reported shrinkage rate at 8 weeks post-ESD. Of these, 2 studies were peer-reviewed[1,23] and 1 was correspondence.[26] There was no evidence of significant difference in shrinkage rate at 8 weeks post-ESD (RR 1.186 [0.866–1.625], I2 = 75.939, Pχ2 = 0.016, NNTH 11.7 95% CI NNTH 5.4 to CI NNTB 79.8). Sensitivity analysis, which involved excluding one study at a time, showed no change in significance of the results as the heterogeneity decreased.
3.5. Delayed bleeding
A total of 11 studies, including 6 RCTs[1,9,23–25,27] and 5 cohort studies,[20,22,28–30] reported healing rate at 4 weeks post-ESD. Of these, 7 studies were peer-reviewed[1,9,20,22,23,28,30] and 4 studies were correspondence (Fig. 4).[24,25,27,29]
Figure 4.
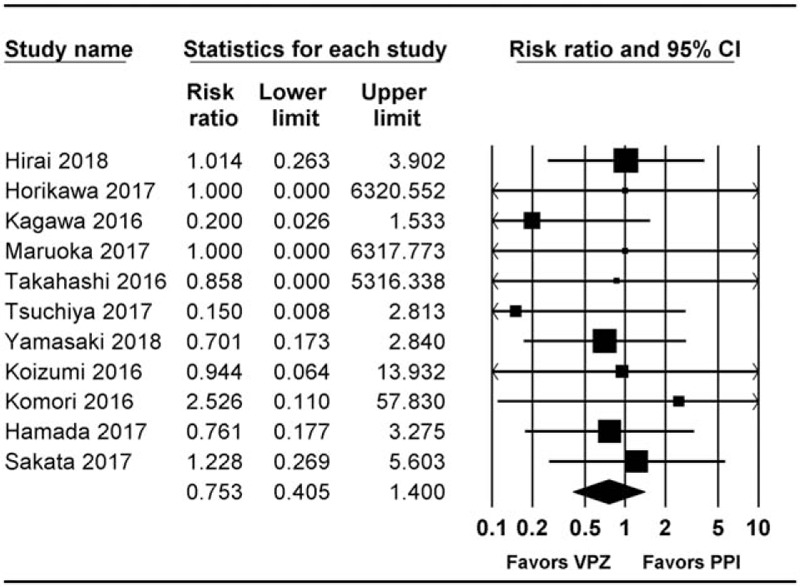
Forest plot of studies comparing rate of delayed bleeding between vonoprazan-treated and PPI-treated groups. The figure depicts individual trials as filled squares with relative sample size and the 95% CI of the difference as a solid line. The diamond shape indicates the pooled estimate and uncertainty for the combined effect. CI = confidence intervals, PPI = proton pump inhibitor.
There was no evidence of significant difference in delayed bleeding between groups (RR 0.753 [0.405–1.400], I2 = 0.0, Pχ2 = .947, NNTB 44.4 95% CI NNTH 596.0 to ∞ to NNTB 21.4).
In subgroup analysis, there was no evidence of significant differences in both RCTs (RR 0.82 [0.34–2.00], I2 = 0.0, Pχ2 = .856, NNTB 74.7 95% CI NNTH 40.6 to ∞ to NNTB 19.5) and cohort studies (RR 0.64 [0.24–1.88], I2 = 0.0, Pχ2 = .742, NNTB 32.6 95% CI NNTH 3546.3 to .3I NNTB 16.2).
3.6. Ulcer size
3.6.1. Ulcer size at 0 weeks post-ESD
A total of 9 studies, including 5 RCTs[1,9,23–25] and 4 cohort studies,[20,22,28,30] reported ulcer size at 0 weeks after ESD. Of these, 7 studies were peer-reviewed[1,9,20,22,23,28,30] and 2 were correspondence.[24,25] There was no evidence for significant difference between the 2 groups in ulcer size at 0 weeks post-ESD (standardized mean difference [SMD] 0.2 [−0.1 to 0.5], I2 = 77.7, Pχ2 < .001). In subgroup analysis, there was no evidence for significant difference in ulcer size at 0 weeks post-ESD for both RCT and cohort studies (SMD 0.3 [−0.2 to 0.9], I2 = 80.2, Pχ2 < .001 and SMD 0.0 (−0.1 to 0.2), I2 = 22.1, Pχ2 = .278, respectively).
3.6.2. Ulcer size at 4 weeks post-ESD
In total, 4 RCTs[1,9,24,25] reported ulcer size at 4 weeks after ESD. Of these, 2 studies were peer-reviewed,[1,9] and 2 were correspondence.[24,25] Ulcer size at 4 weeks post-ESD was significantly higher in the vonoprazan group than in the PPI group (SMD) 0.28 (0.0–0.5), I2 = 32.7, Pχ2 = .216).
3.6.3. Ulcer size at 8 weeks post-ESD
A total of 3 studies, including 2 RCTs[1,25] and 1 cohort study,[28] reported ulcer size at 8 weeks after ESD. Of these, 2 studies were peer-reviewed[1,28] and 1 was correspondence.[25] There was no evidence of significant difference between the 2 groups in ulcer size at 8 weeks post-ESD (SMD 0.0 [−0.0 to 0.0] I2 = 0.0, Pχ2 = .569). In subgroup analysis for RCT, there was no evidence of significant differences in ulcer size at 4 weeks post-ESD (SMD 0.0 [−0.0 to 0.0] I2 = 0.3, Pμ2 = .317).
3.7. Publication bias
Publication bias was assessed only for delayed bleeding because the number of studies included was <10 in the assessment of other outcomes. No evidence of publication bias was detected by either Egger regression test (P = .786) or funnel plot (Fig. 5).
Figure 5.
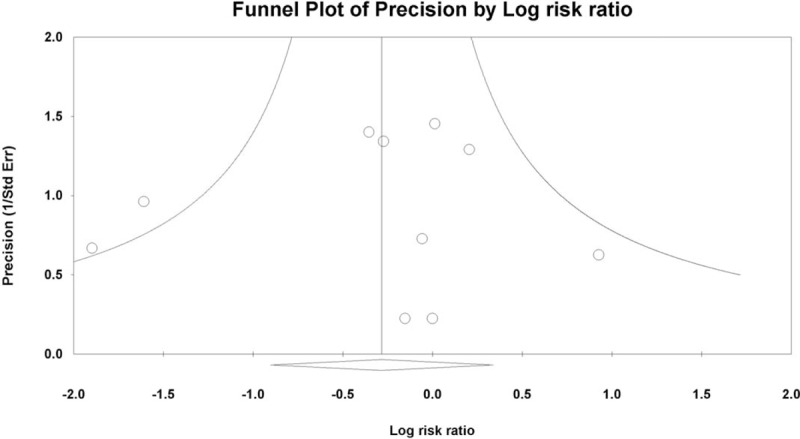
Funnel plot of studies comparing healing rate at 4 weeks post-ESD between vonoprazan-treated and PPI-treated groups. White circles: comparisons included. White diamond: pooled observed log risk ratio.
4. Discussion
We conducted a systematic review and meta-analysis of RCT and cohort studies, directly comparing vonoprazan and PPIs in terms of their effects on artificial ulcer healing and post-ESD bleeding. Our findings can be summarized as 3 main results. First, vonoprazan enhanced the healing rate of artificial ulcers to a greater extent than PPIs until 4 weeks after ESD, but PPIs had enhanced the healing rate to a greater extent than vonoprazan at 8 weeks post-ESD. Second, no significant differences in ulcer shrinkage rates between PPI-treated groups and vonoprazan-treated groups were observed at 8 weeks post-ESD. Lastly, no significant differences in post-ESD bleeding were observed between vonoprazan-treated groups and PPI-treated groups.
To accelerate ulcer healing, clot stabilization through elevation of intragastric pH is required.[4,31,32] Inhibition of gastric acid production may contribute toward a neutral pH, which can stabilize blood clots and prevent recurrent bleeding,[33,34] as blood coagulation and platelet aggregation are pH-dependent.[4] In particular, the healing speed of ESD-induced ulcers is greater than that of peptic ulcers as a result of differences in their histology.[34,35] The proper muscle layer under peptic ulcers is partially replaced by fibrosis resulting from chronic inflammation. However, the proper muscle layer under the endoscopic resection area is not damaged, so marginal blood flow can facilitate healing of artificial ulcers.[35] This healing process must function effectively to minimize the occurrence of post-ESD complications.[23] Despite these differences, artificial ulcers induced by gastric ESD are typically treated with PPI for 4 to 8 weeks, just as in case of peptic ulcers.[22]
Vonoprazan, a novel and orally active P-CAB, is considered a potential alternative to PPIs in the treatment of acid-related diseases such as reflux esophagitis and gastroduodenal ulcers as well as in the eradication of H pylori.[36–38] It has gained popularity owing to its superior characteristics compared to conventional PPIs, such as rapid onset of action, longer duration of action, and consistent acid suppression.[8,39] Moreover, it is not affected by acid secretion state, mealtimes, or CYP2C19 polymorphism.[40,41]
Most studies found that vonoprazan was superior to PPIs in promoting healing or preventing delayed bleeding. However, we observed no significant difference between the 2 drugs in terms of rate of induced ulcer shrinkage or delayed bleeding, which may be a result of the differences in study design and characteristics. Our study showed that ulcer healing rates for the first 4 weeks were superior in patients taking vonoprazan compared with those taking PPIs, although shrinkage rates were not significantly different. This might be because vonoprazan acts faster than PPIs in ensuring that the optimal gastric pH is achieved. Previous studies demonstrated that PPIs showed a delay in the sustained reduction of acid,[42] whereas vonoprazan was reported to achieve steady-state acid levels as early as 1 day after ESD.[6]
This study compared the efficacy of vonoprazan in healing post-ESD artificial ulcers with PPIs based on studies that investigated different PPIs and used different durations of administration. As a result, it showed that the healing rate of vonoprazan was superior to that of PPI at 4 weeks post-ESD. On the contrary, the healing rate of PPI was superior to that of vonoprazan at 8 weeks post-ESD. However, there was no significant difference in the ulcer shrinkage rate at 4 or 8 weeks after ESD between the vonoprazan group and the PPI group. In most studies, PPI was administered intravenously during 2 days post-ESD, which may be associated with an increase in gastric pH and partly contribute to the observed rapidity in the healing process. However, in early healing of post-ESD ulcers, vonoprazan was more beneficial than PPI.
In our study, although healing rate at 4 weeks post-ESD was significantly higher in the vonoprazan than in the PPI group in cohort studies, the healing rate exhibited by the former group did not reach clinical significance, which may be a result of lower power from a small number of patients and a small number of available studies. Thus, additional well-designed, large studies may clarify the issues presented here.
Our secondary aim was the evaluation of the preventive effect of vonoprazan on delayed bleeding compared to PPIs. Clinically, the prevention of delayed bleeding is crucial after ESD. The frequency of delayed bleeding after ESD has been reported to be approximately 5%,[43–46] and most delayed bleeding events develop within the first 2 weeks after ESD.[35,47] Therefore, vonoprazan is excellent at preventing delayed bleeding in theory, owing to its persistent, fast, and highly potent suppression of acid production.[6,36,40] In fact, there was no significant difference in the incidence of delayed bleeding after ESD between the vonoprazan and PPI groups. There may be 2 reasons for this result. First, acid suppression by both vonoprazan and PPIs was potent enough to prevent delayed bleeding. Second, meticulous coagulation in thick blood vessels with potential bleeding afterward was performed during ESD procedure or second look endoscopy.
The present systematic review and meta-analysis has several limitations. First, large heterogeneity was observed between included studies for some variables. The outcomes of healing rate and shrinkage rate at 8 weeks after ESD and ulcer size at 0 weeks after ESD showed substantial heterogeneity. This may have resulted from differences in the type of study, the different kinds of PPIs investigated, and diverse dosage regimens for PPI administration. In response to this limitation, we tried to conduct the subgroup analysis for study design. In addition, we conducted sensitivity analyses to evaluate the influence of a single study on the overall estimate by excluding one study at a time.
Secondly, some of the evidence available is from retrospective cohort studies. As random sequence generation and allocation concealment were not performed in cohort studies, their inclusion could have led to selection bias. However, cohort studies included in this meta-analysis adequately described the selection parameters. Thus, we assumed that our analysis may not be influenced by selection bias.
Lastly, all the trials included in this analysis were from Japan, without any appropriate published data from Western countries. Thus, the results may not be generalizable to other races.
Nevertheless, our study has demonstrated strength through the application of rigorous methodologies to provide the first systematic review investigating the effects of vonoprazan and PPI in the treatment of ESD-induced artificial ulcers and prevention of delayed bleeding.
Based on current data, there seemed to be no substantial difference in the ulcer healing rate and frequency of post-ESD bleeding between the vonoprazan-treated and PPI-treated groups. However, our data conclusively demonstrated that the efficacy of vonoprazan was superior to that of PPI for post-ESD ulcer healing in the early phase of the healing process. In this study, vonoprazan and PPI exhibited equal effectiveness in diminishing the incidence of post-ESD bleeding, suggesting that conventional PPIs administered by initial intravenous infusion might affect the prevention of postoperative bleeding following gastric ESD.
In conclusion, in the present study, vonoprazan more rapidly and effectively treated artificial ulcers after ESD than did PPIs. Further studies with larger numbers of patients are warranted to clarify the efficacy of vonoprazan compared with PPI.
Author contributions
The guarantor for this article is Beom Jin Kim, M.D., Ph.D.
Beom Jin Kim conceived and designed the study. Geunjoo Choi and Jae Gyu Kim collected the data. Hyun Kang analysed the data. Hyun Kang wrote the manuscript. All authors have approved the final version of this article and its list of authors.
Conceptualization: Beom Jin Kim.
Data curation: Beom Jin Kim, Geunjoo Choi.
Formal analysis: Hyun Kang.
Funding acquisition: Hyun Kang.
Investigation: Jae Gyu Kim.
Methodology: Beom Jin Kim.
Supervision: Beom Jin Kim.
Writing – original draft: Hyun Kang.
Writing – review & editing: Jae Gyu Kim.
Beom Jin Kim orcid: 0000-0002-0938-6697.
Supplementary Material
Footnotes
Abbreviations: CI = confidence intervals, CYP = cytochrome P450, ESD = Endoscopic submucosal dissection, H pylori = Helicobacter pylori, MOOSE = Meta-analysis of Observational Studies in Epidemiology, NNT = number needed to treat, NNTB = number needed to treat benefit, NNTH = number needed to treat harm, NOS = Newcastle-Ottawa scale, P-CAB = potassium-competitive acid blocking agent, PPI = proton pump inhibitor, PRISMA = Preferred Reporting Items for Systematic reviews and Meta-Analysis, RCT = randomized clinical trial, RR = relative ratio, SMD = standardized mean difference.
Funding: This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2018R1A2A2A05021467).
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Supplemental Digital Content is available for this article.
References
- [1].Hirai A, Takeuchi T, Takahashi Y, et al. Comparison of the effects of vonoprazan and lansoprazole for treating endoscopic submucosal dissection-induced artificial ulcers. Dig Dis Sci 2018;63:974–81. [DOI] [PubMed] [Google Scholar]
- [2].Ye BD, Cheon JH, Choi KD, et al. Omeprazole may be superior to famotidine in the management of iatrogenic ulcer after endoscopic mucosal resection: a prospective randomized controlled trial. Aliment Pharmacol Ther 2006;24:837–43. [DOI] [PubMed] [Google Scholar]
- [3].Uedo N, Takeuchi Y, Yamada T, et al. Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am J Gastroenterol 2007;102:1610–6. [DOI] [PubMed] [Google Scholar]
- [4].Yang Z, Wu Q, Liu Z, et al. Proton pump inhibitors versus histamine-2-receptor antagonists for the management of iatrogenic gastric ulcer after endoscopic mucosal resection or endoscopic submucosal dissection: a meta-analysis of randomized trials. Digestion 2011;84:315–20. [DOI] [PubMed] [Google Scholar]
- [5].Shin JM, Inatomi N, Munson K, et al. Characterization of a novel potassium-competitive acid blocker of the gastric H,K-ATPase, 1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamin e monofumarate (TAK-438). J Pharmacol Exp Ther 2011;339:412–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sakurai Y, Mori Y, Okamoto H, et al. Acid-inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects—a randomised open-label cross-over study. Aliment Pharmacol Ther 2015;42:719–30. [DOI] [PubMed] [Google Scholar]
- [7].Furuta T, Shirai N, Sugimoto M, et al. Influence of CYP2C19 pharmacogenetic polymorphism on proton pump inhibitor-based therapies. Drug Metab Pharmacokinet 2005;20:153–67. [DOI] [PubMed] [Google Scholar]
- [8].Sugano K. Vonoprazan fumarate, a novel potassium-competitive acid blocker, in the management of gastroesophageal reflux disease: safety and clinical evidence to date. Therap Adv Gastroenterol 2018;11: 1756283X17745776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Takahashi K, Sato Y, Kohisa J, et al. Vonoprazan 20 mg vs lansoprazole 30 mg for endoscopic submucosal dissection-induced gastric ulcers. World J Gastrointest Endosc 2016;8:716–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Maruoka D, Arai M, Kasamatsu S, et al. Superior healing efficacy of a new potassium-competitive acid blocker vonoprazan (TK-438) than that of proton pump inhibitors for post gastric endoscopic submucosal dissection artificial ulcers. Gastroenterology 2016;150:S680. [Google Scholar]
- [11].Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. UK: The Cochrane Collaboration; 2011. [Google Scholar]
- [12].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [13].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:23. [DOI] [PubMed] [Google Scholar]
- [14].Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses 2009. [Google Scholar]
- [15].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol 2014;14:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Naing C, Aung K, Mak JW. Reporting ’number needed to treat’ in meta-analyses: a cross-sectional study. J Evid Based Med 2012;5:232–7. [DOI] [PubMed] [Google Scholar]
- [18].Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Komori H, Ueyama H, Nagahara A, et al. Vonoprazan versus rabeprazole for the healing effect of gastric ulcers after endoscopic submucosal dissection: A prospective randomized controlled trial. J Gastroenterol Hepatol 2016;31:291. [Google Scholar]
- [20].Maruoka D, Arai M, Kasamatsu S, et al. Vonoprazan is superior to proton pump inhibitors in healing artificial ulcers of the stomach post-endoscopic submucosal dissection: a propensity score-matching analysis. Dig Endosc 2017;29:57–64. [DOI] [PubMed] [Google Scholar]
- [21].Horikawa Y, Mizutamari H, Mimori N, et al. Effect of vonoprazan in artificial ulcer healing after gastric endoscopic submucosal dissection. United Eur Gastroenterol J 2016;4:A321. [Google Scholar]
- [22].Horikawa Y, Mizutamari H, Mimori N, et al. Short-term efficacy of potassium-competitive acid blocker following gastric endoscopic submucosal dissection: a propensity score analysis. Scand J Gastroenterol 2018;53:243–51. [DOI] [PubMed] [Google Scholar]
- [23].Tsuchiya I, Kato Y, Tanida E, et al. Effect of vonoprazan on the treatment of artificial gastric ulcers after endoscopic submucosal dissection: Prospective randomized controlled trial. Dig Endosc 2017;29:576–83. [DOI] [PubMed] [Google Scholar]
- [24].Komori H, Ueyama H, Nagahara A, et al. A Prospective randomized controlled trial of vonoprazan vs rabeprazole for gastric ulcers after endoscopic submucosal dissection. United Eur Gastroenterol J 2016;4:A391. [Google Scholar]
- [25].Koizumi A, Yamashita H, Okada A. Comparison of lansoprazole with vonoprazan for treating post-endoscopic submucosal dissection ulcers. United Eur Gastroenterol J 2016;4:A387. [Google Scholar]
- [26].Ban H, Sugimoto M, Otsuka T, et al. Advantage of potassium-competitive acid blocker to healing of artificial ulcer after endoscopic submucosal dissection: prospective randomized trial. Gastroenterology 2017;152:S254. [Google Scholar]
- [27].Hamada K, Uedo N, Tonai Y, et al. Effectiveness of a vonoprazan on prevention of bleeding from endoscopic submucosal dissection-induced gastric ulcers: a prospective randomized phase II study. Gastroenterology 2017;152:S257. [DOI] [PubMed] [Google Scholar]
- [28].Kagawa T, Iwamuro M, Ishikawa S, et al. Vonoprazan prevents bleeding from endoscopic submucosal dissection-induced gastric ulcers. Aliment Pharmacol Ther 2016;44:583–91. [DOI] [PubMed] [Google Scholar]
- [29].Sakata Y, Yamazaki T, Yasui Y, et al. A Comparative study of therapeutic effect by vonoprazan and esomeprazole on bleeding after gastric endoscopic submucosal dissection. United Eur Gastroenterol J 2017;5:A342. [Google Scholar]
- [30].Yamasaki A, Yoshio T, Muramatsu Y, et al. Vonoprazan is superior to rabeprazole for healing endoscopic submucosal dissection: induced ulcers. Digestion 2018;97:170–6. [DOI] [PubMed] [Google Scholar]
- [31].Tarnawski AS. Cellular and molecular mechanisms of gastrointestinal ulcer healing. Dig Dis Sci 2005;50suppl 1:S24–33. [DOI] [PubMed] [Google Scholar]
- [32].Suzuki S, Gotoda T, Kusano C, et al. The efficacy and tolerability of a triple therapy containing a potassium-competitive acid blocker compared with a 7-day PPI-based low-dose clarithromycin triple therapy. Am J Gastroenterol 2016;111:949–56. [DOI] [PubMed] [Google Scholar]
- [33].Oh JH, Choi MG, Dong MS, et al. Low-dose intravenous pantoprazole for optimal inhibition of gastric acid in Korean patients. J Gastroenterol Hepatol 2007;22:1429–34. [DOI] [PubMed] [Google Scholar]
- [34].Lee SY, Kim JJ, Lee JH, et al. Healing rate of EMR-induced ulcer in relation to the duration of treatment with omeprazole. Gastrointest Endosc 2004;60:213–7. [DOI] [PubMed] [Google Scholar]
- [35].Kakushima N, Fujishiro M, Kodashima S, et al. Histopathologic characteristics of gastric ulcers created by endoscopic submucosal dissection. Endoscopy 2006;38:412–5. [DOI] [PubMed] [Google Scholar]
- [36].Jenkins H, Sakurai Y, Nishimura A, et al. Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther 2015;41:636–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Martinucci I, Blandizzi C, Bodini G, et al. Vonoprazan fumarate for the management of acid-related diseases. Expert Opin Pharmacother 2017;18:1145–52. [DOI] [PubMed] [Google Scholar]
- [38].Inatomi N, Matsukawa J, Sakurai Y, et al. Potassium-competitive acid blockers: advanced therapeutic option for acid-related diseases. Pharmacol Ther 2016;168:12–22. [DOI] [PubMed] [Google Scholar]
- [39].Yang X, Li Y, Sun Y, et al. Vonoprazan: a novel and potent alternative in the treatment of acid-related diseases. Dig Dis Sci 2018;63:302–11. [DOI] [PubMed] [Google Scholar]
- [40].Garnock-Jones KP. Vonoprazan: first global approval. Drugs 2015;75:439–43. [DOI] [PubMed] [Google Scholar]
- [41].Ashida K, Sakurai Y, Nishimura A, et al. Randomised clinical trial: a dose-ranging study of vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the treatment of erosive oesophagitis. Aliment Pharmacol Ther 2015;42:685–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Shimatani T, Inoue M, Kuroiwa T, et al. Acid-suppressive effects of rabeprazole, omeprazole, and lansoprazole at reduced and standard doses: a crossover comparative study in homozygous extensive metabolizers of cytochrome P450 2C19. Clin Pharmacol Ther 2006;79:144–52. [DOI] [PubMed] [Google Scholar]
- [43].Kim SJ, Choi CW, Kang DH, et al. Second-look endoscopy and factors associated with delayed bleeding after endoscopic submucosal dissection. World J Gastrointest Endosc 2016;8:173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Toyokawa T, Inaba T, Omote S, et al. Risk factors for perforation and delayed bleeding associated with endoscopic submucosal dissection for early gastric neoplasms: analysis of 1123 lesions. J Gastroenterol Hepatol 2012;27:907–12. [DOI] [PubMed] [Google Scholar]
- [45].Choi CW, Kim HW, Kang DH, et al. Clinical outcomes of second-look endoscopy after gastric endoscopic submucosal dissection: predictive factors with high risks of bleeding. Surg Endosc 2014;28:2213–20. [DOI] [PubMed] [Google Scholar]
- [46].Mannen K, Tsunada S, Hara M, et al. Risk factors for complications of endoscopic submucosal dissection in gastric tumors: analysis of 478 lesions. J Gastroenterol 2010;45:30–6. [DOI] [PubMed] [Google Scholar]
- [47].Lee SH, Lee CK, Chung IK, et al. Optimal duration of proton pump inhibitor in the treatment of endoscopic submucosal dissection-induced ulcers: a retrospective analysis and prospective validation study. Dig Dis Sci 2012;57:429–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


