Abstract
Background:
Nasopharyngeal carcinoma (NPC) is a prevalent malignancy in Asia particularly southern China. Comparisons of outcomes of conformal radiotherapy (CRT) and intensity-modulated radiotherapy (IMRT) have been still debated. This meta-analysis was carried out to compare oncologic outcomes of CRT and IMRT in the treatment of NPC.
Methods:
A literature search was performed through PubMed, Embase, and the Cochrane library databases from their inceptions to November 10, 2018. Two authors assessed the included studies and extracted data independently. Relative studies that compared oncologic outcomes between CRT and IMRT for NPC were included.
Results:
A total of 13 eligible studies were included, which contained 1 RCT, 1 prospective study, and 11 retrospective studies. Our meta-analysis showed that IMRT has increased overall survival (OR = 0.51, 95% CI = 0.41–0.65, P < .00001), locoregional control rate (OR = 0.59, 95% CI = 0.52–0.67, P < .00001), disease-free survival (OR = 0.77, 95% CI = 0.65–0.91, P = .002), and metastasis-free survival (OR = 0.71, 95% CI = 0.54–0.94, P = .01) in comparison with CRT.
Conclusion:
The results of this meta-analysis indicate IMRT should be a better option for the treatment of NPC because patients who underwent IMRT may benefit from increased overall survival, locoregional control rate, disease-free survival, and metastasis-free survival compared with CRT.
Keywords: conformal radiotherapy, CRT, IMRT, intensity-modulated radiotherapy, meta-analysis, nasopharyngeal carcinoma
1. Introduction
Nasopharyngeal carcinoma (NPC), a tumor originated from the epithelial cells of the nasopharynx, is a prevalent malignancy in Asia particularly Southern China.[1] Because of the anatomic location of nasopharynx and its high radiosensitivity, radiotherapy-based treatment for NPC is the standard treatment. Conformal radiotherapy (CRT) is constantly developing and proved in the late 1990s as a recommended treatment for cancer for its better target coverage and decreased toxicity to normal organs. More recently, the application of intensity-modulated radiotherapy (IMRT) has reached a breakthrough in treatment outcomes of NPC. IMRT produces more accurate dose homogeneity around targets and less toxicity to normal organs than CRT.[2] The advantages of IMRT to spare the parotid gland have been demonstrated repeatedly, and its use for NPC was more prevailed than CRT.[2,3] However, results showing that IMRT in terms of oncologic outcomes had limited success compared with CRT, and most results were only come from China or other Asian countries.[4–6] For the time being, there is no undisputed evidence that IMRT is superior to CRT regarding overall survival, locoregional control, disease-free survival, and metastasis-free survival.[4–6]
Thus, the treatment of CRT and IMRT for NPC is still hard for doctors and patients to choose. In this paper, the meta-analysis aims to guide doctors and patients for the treatment of NPC.
2. Methods
2.1. Literature search strategy
All included studies published up to November 10, 2018 were acquired by searching PubMed, EMBASE, and Cochrane Library, which were performed by 2 authors (G-JH and M-SL) independently, with the following key words: intensity modulation radiation therapy, IMRT, conformal radiotherapy, CRT, nasopharyngeal carcinoma, nasopharyngeal cancer, nasopharyngeal neoplasm, and NPC.
2.2. Eligibility criteria
The inclusion criteria were: only original studies were included; comparing CRT and IMRT on oncologic outcomes, which contain overall survival, locoregional control, disease-free survival, and metastasis-free survival; written in the English language.
2.3. Data extraction
The data of oncologic outcomes were extracted from included studies by 2 authors (M-SL and H-BL) and then checked by another author (G-JH). The information included study name, publication year, study design, number of patients, sex, mean follow-up time.
2.4. Study quality assessment
Because included studies were 1 RCT, 1 prospective study, and 11 retrospective studies, so study quality was all assessed by the Newcastle-Ottawa quality assessment scale. Studies with scores of 6 or higher considered high quality are eligible for this paper.
2.5. Statistical analysis
Review Manager Version 5.3 (Cochrane Collaboration, Oxford, UK) was used for this meta-analysis. The heterogeneity of included studies was evaluated by calculating the I2 statistic. The I2 test was used to assess the extent of inconsistency among the results. A fixed-effects model was applied when the I2 statistic < 50%, and a random-effects model was applied when the I2 statistic>50%. Outcomes were compared by odds ratio (OR) and 95% CIs. Dichotomous variables were compared by weighted odds ratio and 95% CIs. The z statistic was involved to test for overall pooled effect, which significance was set at P < .05.
3. Results
3.1. Eligible studies and characteristics of studies
In the meta-analysis, 13 studies were included, which contains 1 RCT, 1 prospective study, and 11 retrospective studies (Fig. 1). And a total of 14,745 patients of NPC were included, of whom 10,033 (68.1%) underwent CRT and 4712 (31.9%) underwent IMRT. Table 1 showed the characteristics of all included studies, while detailed data were shown in Table 2.[6–18]
Figure 1.
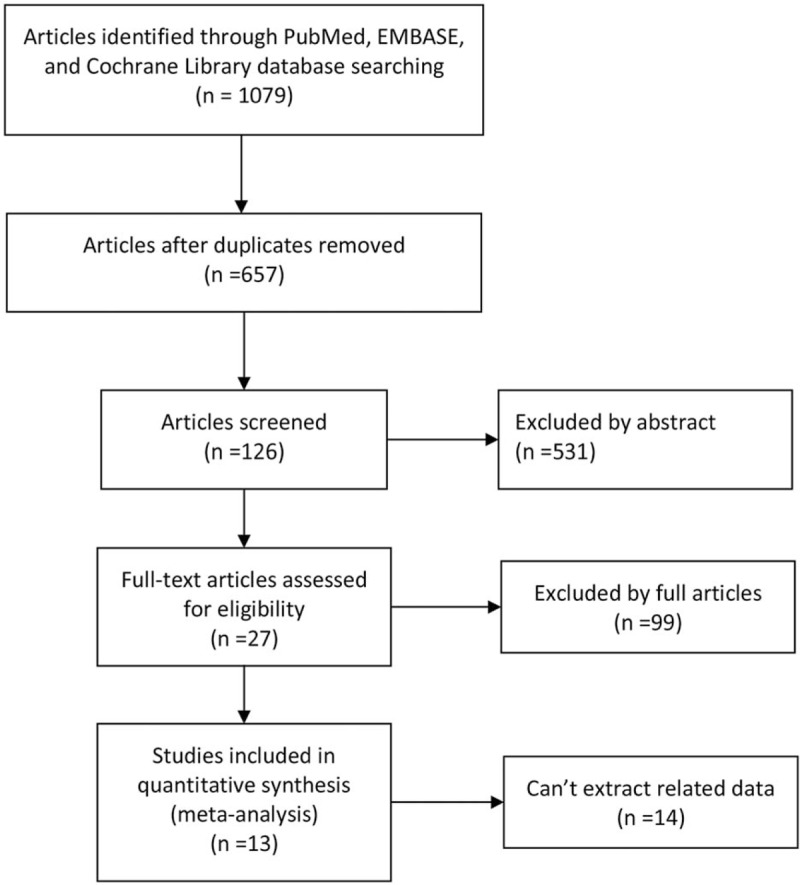
The PRISMA flow chart to show the process of searching and screening citations.
Table 1.
Characteristics and demographics of included studies.
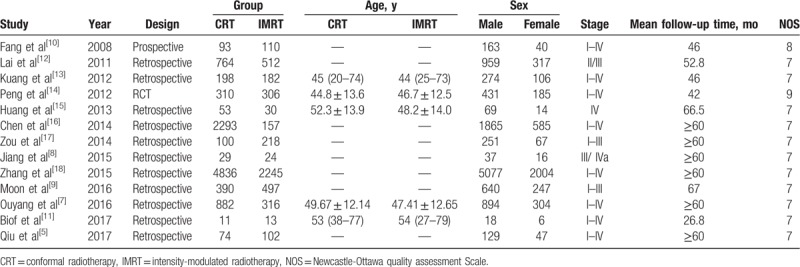
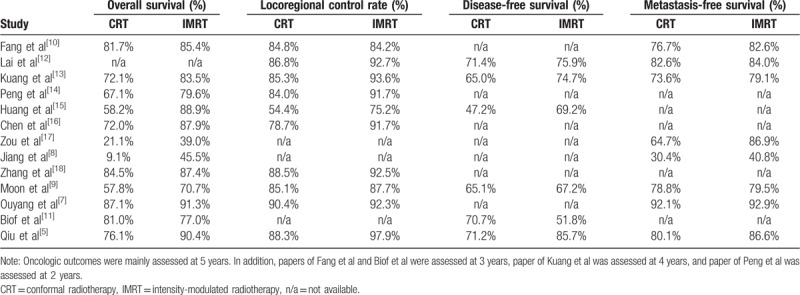
Table 2.
Detailed date of oncologic outcomes.
3.2. Quality assessment, heterogeneity analysis, and publication bias
The study considered as high quality is eligible for this research. In the studies, 1 study scored 9 points, 1 study scored 8 points, and others scored 7; it indicated that included studies were considered high quality (Table 1).
There was no significant heterogeneity in the analysis of locoregional control and disease-free survival. So a fixed-effects model was used. Others were performed by a random-effects model because of the I2 statistic>50%. The publication bias for the current meta-analysis was estimated by the funnel plot.
3.3. Meta-analysis of oncologic outcomes
3.3.1. Overall survival
Twelve studies provided detailed data on overall survival with 8818 patients in the CRT group and 4200 patients in the IMRT group. And heterogeneity was evaluated between the studies (χ2 = 30.04, P = .002, I2 = 63%), so a random effect model was performed to calculate the pooled effect. Results of the pooled effect demonstrated that the difference between the CRT group and the IMRT group on overall survival was statistically of significance (The total pooled effect: OR = 0.51, 95% CI = 0.41–0.65, P < .00001; Fig. 2A), which distinctly favored IMRT. The funnel plot (Fig. 3A) of the included studies on overall survival showed an inverted symmetric funnel.
Figure 2.
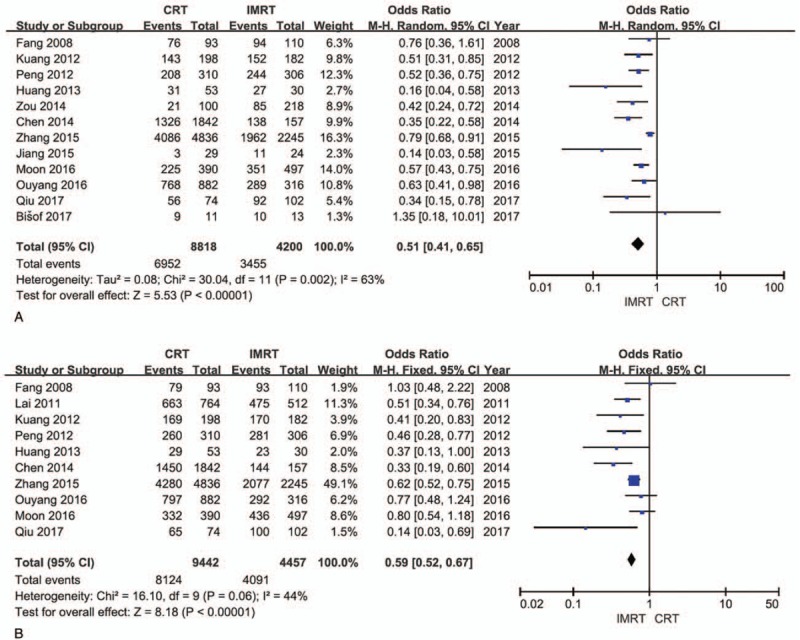
Forest plots. A, Overall survival. B, Locoregional control. CI = confidence interval, CRT = conformal radiotherapy, IMRT = intensity-modulated radiotherapy, OR = odds ratio.
Figure 3.
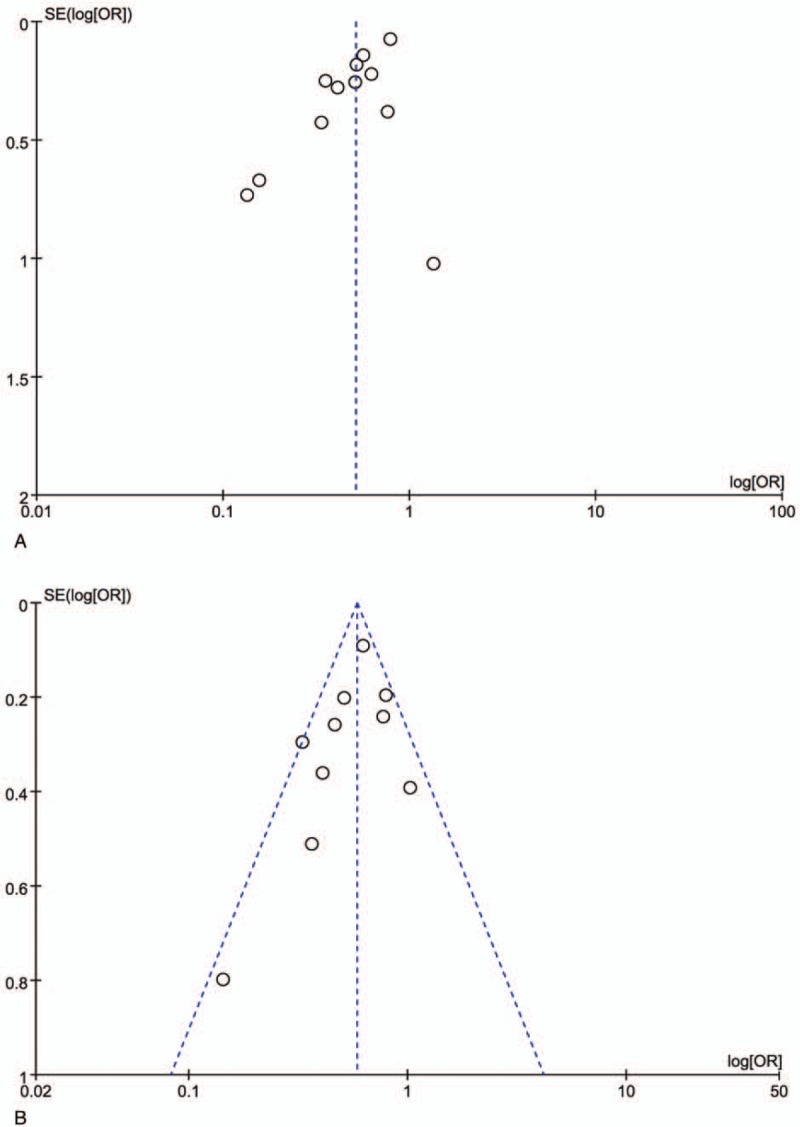
The funnel plot. A, Overall survival. B, Locoregional control. OR = odds ratio, SE = standard Error.
3.3.2. Locoregional control rate
Ten studies provided detailed data on locoregional control with 9442 patients in the CRT group and 4457 patients in the IMRT group. And heterogeneity was evaluated among the included studies (χ2 = 16.10, P = .06, I2 = 44%), so a fixed effect model was performed to calculate the pooled effect. Results of the pooled effect demonstrated that the difference between the CRT group and the IMRT group on recurrence rate was statistically of significance (The total pooled effect: OR = 0.59, 95% CI = 0.52–0.67, P < .00001; Fig. 2B). The funnel plot (Fig. 3B) of the included studies on locoregional control rate showed an inverted symmetric funnel.
3.3.3. Disease-free survival
Six studies provided detailed data on disease-free survival with 1490 patients in the CRT group and 974 patients in the IMRT group. And heterogeneity was evaluated between the studies (χ2 = 8.06, P = .15, I2 = 38%), so a fixed effect model was performed to calculate the pooled effect. Results of the pooled effect demonstrated that the difference between the CRT group and the IMRT group on disease-free survival was statistically of significance (The total pooled effect: OR = 0.77, 95% CI = 0.65–0.91, P = .002; Fig. 4A).
Figure 4.
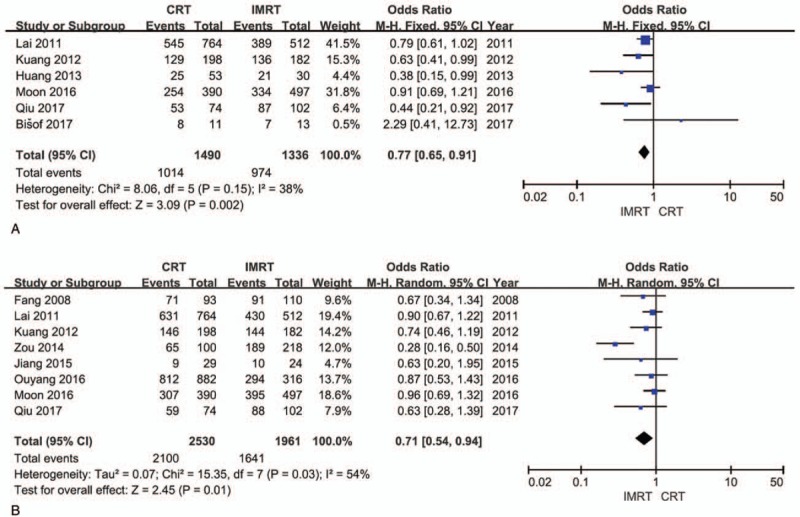
Forest plots. A, Disease-free survival. B, Metastasis-free survival. CI = confidence interval, CRT = conformal radiotherapy, IMRT = intensity-modulated radiotherapy, OR = odds ratio.
3.3.4. Metastasis-free survival
Eight studies provided detailed data on metastasis-free survival with 2530 patients in the CRT group and 1641 patients in the IMRT group. And heterogeneity was evaluated among the included studies (χ2 = 15.35, P = .03, I2 = 54%), so a random effect model was performed to calculate the pooled effect. Results of the pooled effect demonstrated that the difference between the CRT group and the IMRT group on metastasis-free survival was statistically of significance (The total pooled effect: OR = 0.71, 95% CI = 0.54–0.94, P = .01; Fig. 4B).
4. Discussion
Before technology application of IMRT, CRT was successfully applied to the treatment of NPC as a standardized technique.[7,8] After the application of IMRT in the treatment of NPC, results showed that IMRT can improve oncologic outcomes. Comparisons of outcomes of CRT and IMRT have been still debated. Moon et al[9] compared outcomes of CRT and IMRT in patients with NPC, 390 and 497 patients were treated with CRT and IMRT respectively. Results displayed that IMRT was associated with a better local progression-free survival and overall survival than CRT for the treatment of NPC. IMRT was significantly superior in terms of overall survival for advanced primary tumors.[1] Fang et al[10] reported a study to investigate survival outcomes for patients with NPC treated by CRT and IMRT. Two hundred three NPC patients, who were treated by CRT (n = 93) or IMRT (n = 110) between March 2002 and July 2004, were analyzed. Results showed that the 3-year locoregional control, metastasis-free survival, and overall survival rates were 84.8%, 76.7%, and 81.7% for the CRT group, respectively, compared with 84.2%, 82.6%, and 85.4% for the IMRT group. Lee et al[19] also suggested that IMRT treatment showed no further improvement compared with CRT. The CRT group achieved a significant improvement in local failure-free rate. Neurological damage and bone/soft tissue necrosis were reduced significantly. However, 5-year disease-specific survival rates were 81% in the CRT group and 85% in the IMRT group, while the corresponding neurological toxicity rates were 3.5% and 1.8%. However, Bisof et al[11] conducted a retrospective study to compare IMRT and CRT in the treatment of NPC with regard to outcomes and dose distribution to the planning target volumes and to the organs at risk. Results showed that the mean dose and dose to 50% parotid glands volume as well as the maximal dose to the spinal cord were significantly lower in the IMRT group than in the CRT group. IMRT was also superior in coverage of the planning target volumes. The 3-year overall survival rates and disease-free survival rates of patients in the IMRT group and the CRT group were 77% and 81%, 51.9% and 70.7%, respectively.
With respect to overall survival, the results of the pooled effect vividly demonstrated that the difference between the CRT group and the IMRT group was statistically of significance (The total pooled effect: OR = 0.51, 95% CI = 0.41–0.65, P < .00001), which distinctly favored IMRT. With respect to locoregional control rate, the result of the pooled effect vividly demonstrated that the difference between the CRT group and the IMRT group was statistically of significance (The total pooled effect: OR = 0.59, 95% CI = 0.52–0.67, P < .00001), which distinctly favored IMRT. With respect to disease-free survival, the result of the pooled effect vividly demonstrated that the difference between the CRT group and the IMRT group was statistically of significance (The total pooled effect: OR = 0.77, 95% CI = 0.65–0.91, P = .002), which distinctly favored IMRT. With respect to metastasis-free survival, the result of the pooled effect vividly demonstrated that the difference between the CRT group and the IMRT group was statistically of significance (The total pooled effect: OR = 0.71, 95% CI = 0.54–0.94, P = .01), which distinctly favored IMRT.
IMRT has increased overall survival, locoregional control rate, disease-free survival, and metastasis-free survival compare with CRT, which indicated the truly fact that IMRT can deliver irradiation dose more accurate to target organs and reduce toxicity to normal organs. Moreover, the use of IMRT technology and doctors’ skills for the treatment of NPC are becoming increasingly advanced and mature. Therefore, IMRT should be superior to CRT for the treatment of NPC.
In a study of Wu et al,[3] IMRT had been applied in the treatment of NPC for nearly 20 years, while it is known about the 10-year survival outcomes. They aimed to evaluate the 10-year survival outcomes for patients with NPC receiving IMRT. Data on 614 patients with newly diagnosed NPC treated by IMRT between 2004 and 2008 were reviewed retrospectively. Survival outcomes stratified by tumor stage were compared. The 10-year local relapse-free survival rates for T1, T2, and T3 were 94.2%, 92.5%, and 91.4%, respectively, while significantly higher than that of T4 (79.3%). Furthermore, the 10-year overall survival rates were respectively 100%, 87.1%, 75.5%, and 55.6% for stages I, II, III, and IV. Late toxicities were assessable for 495 (80.6%) patients, and results showed that most were Grade I/II damages. Xerostomia and hearing impairment remained the most troublesome. So, IMRT could achieve satisfactory survival outcomes for NPC patients with acceptable late toxicities. Tian et al[6] investigated the feasibility of reirradiation with IMRT for recurrent T1 to T2 NPC by assessing long-term survival rates and late complication rates. Sixty patients who had been irradiated were diagnosed with locally recurrent T1 to T2 NPC and underwent reirradiation with IMRT. Severe radiation toxicities were assessed. The 5-year local failure-free survival, distant failure-free survival, and overall survival rates were 85.7%, 96.1%, and 67.2%, respectively. The most common severe complications were headache, mucosal necrosis, cranial neuropathy, and temporal lobe necrosis. Thirty-nine patients developed at least 1 severe complication and 18 patients died. The conclusion indicated that excellent disease control could be achieved by reirradiation with IMRT for recurrent T1 to T2 NPC. However, the main challenge remained severe late complications. Chan et al[1] conducted a study to assess the efficacy and toxicities of reirradiation by using IMRT in patients with locally advanced recurrent NPC. Thirty-eight patients with consecutive recurrent T3 to T4 NPC treated between 2005 and 2013 were analyzed retrospectively. The 3-year overall survival rate, progression-free survival rate, and local control rate were 47.2%, 17.5%, and 44.3%, respectively. 73.7% of patients experienced ≥1 grade 3 late toxicities, and 3 patients died of massive epistaxis. Temporal lobe necrosis developed sooner with a higher total biological equivalent dose. Therefore, adequate tumor dose coverage was important for treating recurrent T3 to T4 NPC.
Several limitations of this meta-analysis could be resulted from various types of biases. First, the meta-analysis was based on 1 RCT, 1 prospective study, and 11 retrospective studies. Second, the number of patients analyzed was small. Third, the tumor stage of patients, the length of follow-up time, and radiological dose were inconsistent. So, results may have been influenced by these biases.
Our meta-analysis showed that IMRT has increased overall survival, locoregional control rate, disease-free survival, and metastasis-free survival in comparison with CRT. Therefore, to minimize the risk of bias, more large, multicenter, and randomized-controlled trials should be needed to be performed.
5. Conclusion
IMRT has increased overall survival, locoregional control rate, disease-free survival, and metastasis-free survival in comparison with CRT. In conclusion, IMRT should be a better option for the treatment of NPC because patients who underwent IMRT may benefit from better oncologic outcomes compared with CRT.
Acknowledgment
The authors are grateful to Dr Huizi Li for his statistical support for the study.
Author contributions
All authors have read and approved the final manuscript.
Conceptualization: Meng-Si Luo, Hong-Bing Liu.
Data curation: Meng-Si Luo, Guan-Jiang Huang.
Formal analysis: Meng-Si Luo, Guan-Jiang Huang.
Investigation: Guan-Jiang Huang, Hong-Bing Liu.
Methodology: Meng-Si Luo, Guan-Jiang Huang, Hong-Bing Liu.
Resources: Meng-Si Luo.
Supervision: Hong-Bing Liu.
Validation: Hong-Bing Liu.
Writing – original draft: Meng-Si Luo, Guan-Jiang Huang.
Writing – review & editing: Meng-Si Luo, Hong-Bing Liu.
Footnotes
Abbreviations: CI = confidence interval, CRT = conformal radiotherapy, IMRT = intensity-modulated radiotherapy, NPC = nasopharyngeal carcinoma, OR = odds ratio.
M-SL and G-JH have contributed equally to this work as joint first authors.
Ethics approval and patient written informed consent were not required because all analyses in our study were performed based on data from published studies.
The authors are responsible for the content and writing of the paper.
The authors have no conflicts of interest to disclose.
References
- [1].Chan OS, Sze HC, Lee MC, et al. Reirradiation with intensity-modulated radiotherapy for locally recurrent T3 to T4 nasopharyngeal carcinoma. Head Neck 2017;39:533–40. [DOI] [PubMed] [Google Scholar]
- [2].Li JG, Venigalla P, Leeman JE, et al. Patterns of nodal failure after intensity modulated radiotherapy for nasopharyngeal carcinoma. Laryngoscope 2017;127:377–82. [DOI] [PubMed] [Google Scholar]
- [3].Wu LR, Liu YT, Jiang N, et al. Ten-year survival outcomes for patients with nasopharyngeal carcinoma receiving intensity-modulated radiotherapy: an analysis of 614 patients from a single center. Oral Oncol 2017;69:26–32. [DOI] [PubMed] [Google Scholar]
- [4].Veldeman L, Madani I, Hulstaert F, et al. Evidence behind use of intensity-modulated radiotherapy: a systematic review of comparative clinical studies. Lancet Oncol 2008;9:367–75. [DOI] [PubMed] [Google Scholar]
- [5].Qiu WZ, Peng XS, Xia HQ, et al. A retrospective study comparing the outcomes and toxicities of intensity-modulated radiotherapy versus two-dimensional conventional radiotherapy for the treatment of children and adolescent nasopharyngeal carcinoma. J Cancer Res Clin Oncol 2017;143:1563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tian YM, Guan Y, Xiao WW, et al. Long-term survival and late complications in intensity-modulated radiotherapy of locally recurrent T1 to T2 nasopharyngeal carcinoma. Head Neck 2016;38:225–31. [DOI] [PubMed] [Google Scholar]
- [7].Ouyang PY, Shi D, Sun R, et al. Effect of intensity-modulated radiotherapy versus two-dimensional conventional radiotherapy alone in nasopharyngeal carcinoma. Oncotarget 2016;7:33408–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jiang H, Wang G, Song H, et al. Analysis of the efficacy of intensity-modulated radiotherapy and two-dimensional conventional radiotherapy in nasopharyngeal carcinoma with involvement of the cervical spine. Oncol Lett 2015;10:2731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Moon SH, Cho KH, Lee CG, et al. IMRT vs. 2D-radiotherapy or 3D-conformal radiotherapy of nasopharyngeal carcinoma: survival outcome in a Korean multi-institutional retrospective study (KROG 11-06). Strahlenther Onkol 2016;192:377–85. [DOI] [PubMed] [Google Scholar]
- [10].Fang FM, Chien CY, Tsai WL, et al. Quality of life and survival outcome for patients with nasopharyngeal carcinoma receiving three-dimensional conformal radiotherapy vs. intensity-modulated radiotherapy: a longitudinal study. Int J Radiat Oncol Biol Phys 2008;72:356–64. [DOI] [PubMed] [Google Scholar]
- [11].Bisof V, Rakusic Z, Bibic J, et al. Comparison of intensity modulated radiotherapy with simultaneous integrated boost (IMRT-SIB) and a 3-dimensional conformal parotid gland-sparing radiotherapy (ConPas 3D-CRT) in treatment of nasopharyngeal carcinoma: a mono-institutional experience. Radiol Med 2018;123:217–26. [DOI] [PubMed] [Google Scholar]
- [12].Lai SZ, Li WF, Chen L, et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys 2011;80:661–8. [DOI] [PubMed] [Google Scholar]
- [13].Kuang WL, Zhou Q, Shen LF. Outcomes and prognostic factors of conformal radiotherapy versus intensity-modulated radiotherapy for nasopharyngeal carcinoma. Clin Transl Oncol 2012;14:783–90. [DOI] [PubMed] [Google Scholar]
- [14].Peng G, Wang T, Yang KY, et al. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol 2012;104:286–93. [DOI] [PubMed] [Google Scholar]
- [15].Huang HI, Chan KT, Shu CH, et al. T4-locally advanced nasopharyngeal carcinoma: prognostic influence of cranial nerve involvement in different radiotherapy techniques. ScientificWorldJournal 2013;2013:439073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chen C, Yi W, Gao J, et al. Alternative endpoints to the 5-year overall survival and locoregional control for nasopharyngeal carcinoma: a retrospective analysis of 2,450 patients. Mol Clin Oncol 2014;2:385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zou X, Han F, Ma WJ, et al. Salvage endoscopic nasopharyngectomy and intensity-modulated radiotherapy versus conventional radiotherapy in treating locally recurrent nasopharyngeal carcinoma. Head Neck 2015;37:1108–15. [DOI] [PubMed] [Google Scholar]
- [18].Zhang MX, Li J, Shen GP, et al. Intensity-modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two-dimensional radiotherapy: a 10-year experience with a large cohort and long follow-up. Eur J Cancer 2015;51:2587–95. [DOI] [PubMed] [Google Scholar]
- [19].Lee AW, Ng WT, Chan LL, et al. Evolution of treatment for nasopharyngeal cancer—success and setback in the intensity-modulated radiotherapy era. Radiother Oncol 2014;110:377–84. [DOI] [PubMed] [Google Scholar]


