Abstract
Patient: Female, 3
Final Diagnosis: Recurrent sarcoma botryoides
Symptoms: Vaginal mass
Medication: —
Clinical Procedure: Surgical resection • adjuvant chemotherapy
Specialty: Obstetrics and Gynecology
Objective:
Unusual setting of medical care
Background:
Sarcoma botryoides, known as embryonal rhabdomyosarcoma (ERMS), is a malignant tumor which arises from embryonic muscle cells. The incidence of ERMS in the uterine cervix rarely occurs at a very young age. With sufficient resources, management of this disease is not difficult. However, in limited resources settings, such as in Indonesia, the situation is more challenging. This case report aims to highlight the difficulties encountered in diagnosing and treating patients with sarcoma botryoides.
Case Report:
A 3-year-old female patient came the outpatient clinic of our hospital with a protruding mass from her vagina resembling a bunch of grapes which easily bled. She underwent surgery to remove the mass. After the procedure, she did not return to the hospital for the recommended adjuvant chemotherapy treatment due to limited funds. Three months later, she came to the outpatient clinic with the same complaint, despite smaller size. Due to limited resources, we only evaluated the metastasis using chest x-ray and did not perform intra-operative biopsy. In the second surgery, a wide excision with 1–2 cm margin was performed, followed by adjuvant chemotherapy for 6 series. We achieved a satisfactory outcome in this case, and 18 months after the surgery, the patient was still in remission.
Conclusions:
Sarcoma botryoides is a rare malignancy. The effective treatment for sarcoma botryoides is wide excision with safe margin of 1–2 cm, followed by 6–12 cycles of vincristine, actinomycin D, and cyclophosphamide (VAC) regiment as an adjuvant chemotherapy. A family’s understanding of the treatment plan is important to achieve desired outcomes. Even with limited resources, this malignancy can still be properly treated.
MeSH Keywords: Health Resources; Indonesia; Infant; Rhabdomyosarcoma, Embryonal; Uterine Cervical Neoplasms
Background
Sarcoma botryoides, also known as embryonal rhabdomyosarcoma (ERMS), is a subgroup of rhabdomyosarcoma (RMS), a malignant tumor arising from embryonic muscle cells. It is the most common soft tissue sarcoma in childhood and young adulthood, accounting for 4% to 6% of all malignancies in this age group [1]. This tumor typically presents as a “grape-like” tumor. It is usually reported as a vaginal tumor in the female reproductive tract of infants and rarely in the uterine cervix [2].
ERMS of the uterine cervix usually occurs in women in their late teens and early 20s [3]. In this case, the malignancy occurred at a very young age, presenting with a protruding mass out of the vagina. This case report aimed to highlight the difficulties encountered in diagnosing and treating patients with sarcoma botryoides in a limited resources setting in Indonesia.
Case Report
A 3-year-old female patient was brought to the outpatient clinic of our hospital with the chief complaint of a protruding mass in her vagina for 7 months. The mass was initially small, and the patient was brought to the hospital only after realizing its rapid growth. From history taking, the mass resembled a bunch of grapes, with a tendency to bleed. Gynecologic examination showed a 10×10 cm multinodular solid mass with smooth surface protruding through the vaginal introitus (Figure 1). Chest x-ray revealed no metastases in the lungs. A tumor excision with biopsy was performed afterwards. Pathological analysis of the biopsy revealed a malignant spindle mesenchymal tumor, suspecting leiomyosarcoma with differential diagnosis of rhabdomyosarcoma. After the surgery, the patient was scheduled for follow-up monitoring. However, the patient did not come back to the hospital due to insufficient fund and no health insurance.
Figure 1.
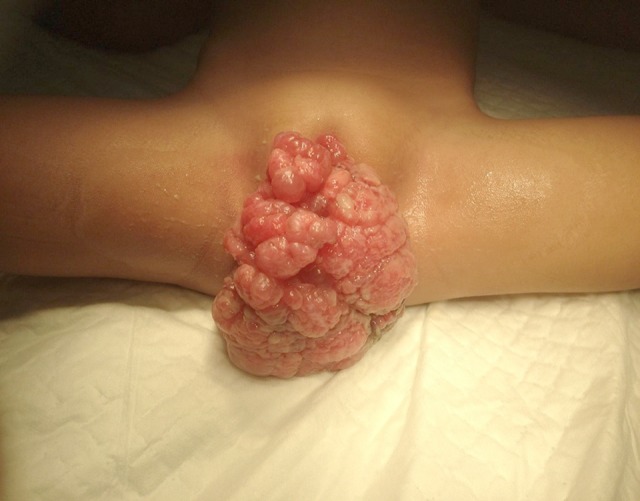
Protruding vaginal mass during the first visit.
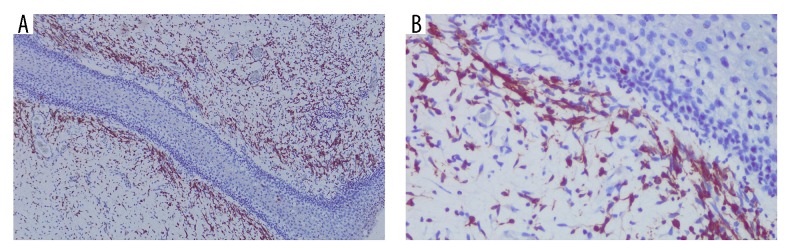
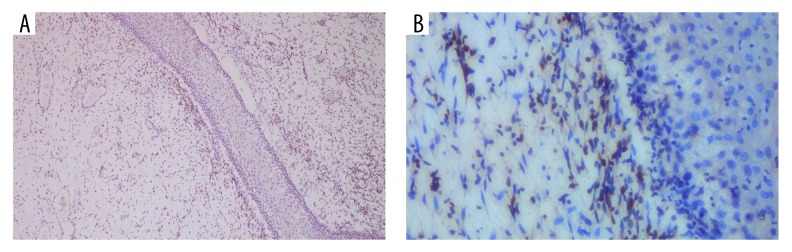
Three months after the first surgery, the patient came back with recurrent vaginal mass. This time, the patient was covered by national health insurance. Gynecologic examination showed identical characteristics with the previous tumor at the same location, only smaller in size (6×5 cm) (Figure 2). A second chest x-ray was performed and revealed no metastases. In the second surgery, a wide excision with 2 cm of margin of healthy tissue was performed without intraoperative biopsy due to limited resources. Post-operative pathological examination showed malignant spindle mesenchymal tumor, suggesting a sarcoma botryoides finding. Further immunohistochemistry (IHC) examination revealed positive anti-desmin antibody at the tumor cell cytoplasm and positive anti-myogenin antibody at the tumor cell nuclei. Both stains also showed the typical sign of Nicholson cambium layer, supporting the diagnosis of ERMS (Figures 3, 4).
Figure 2.
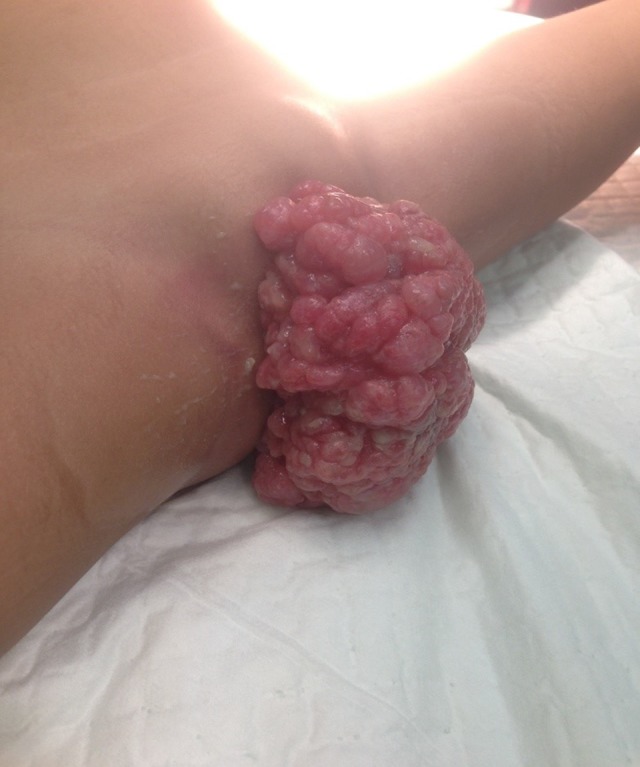
Recurrent mass 3 months after the first surgery.
Figure 3.
Anti-desmin antibody showed polypoid-shape tissue covered in squamous epithelial. There was mesenchymal tumor growth with tightly and loosely packed arrangements of alternating zones. Beneath the epithelial layer, there was hypercellular area of rounded-nuclear hyperchromatic spindle-shaped cell, arranged eccentrically with eosinophilic cytoplasm forming the cambium layer. There were also spread of inflammatory cells. (A) 100× magnification. (B) 400× magnification.
Figure 4.
Anti-myogenin antibody gave the positive result in tumor cell nuclei and showed the typical sign of Nicholson cambium layer. (A) 100× magnification. (B) 400× magnification.
Due to the recurrence of the tumor, adjuvant chemotherapy was given to the patient. A combination set of vincristine, actinomycin D, and cyclophosphamide (VAC) was given for 6 cycles with the duration of 5 days in each cycle, and 20 days of break between each cycle. Vincristine with the dose of 1.5 mg/m2 was given only on the first day of every cycle, while actinomycin D with the dose of 0.3 mg/m2 and cyclophosphamide with the dose of 150 mg/m2 given from the first day until the fifth day of every cycle. Follow-up of 18 months post chemotherapy showed that the patient was still in remission.
Discussion
Sarcoma botryoides may appear as abnormal vaginal bleeding, prolapsing mass through vagina, or abdominal-pelvic mass [4]. The major physical finding is the presence of a mass in the vagina. RMS should be suspected in women with an abnormal mass in the vagina that has great tendency to bleed [5]. A study of sarcoma patients in Norway showed the common symptoms of RMS were post-menopausal bleeding (31–46%), premenopausal abnormal uterine bleeding (27–34%), abdominal pain (4–13%), abdominal distension (8–17%), voiding problems (1–2%), and asymptomatic (1–2%) [6].
Risk factors of sarcoma botryoides have been unclear, due to the low quantity of cervical sarcoma botryoides cases reported in the literature. Some literature reports mention the following risk factors: aging, certain race (African-American women have incidence of twice that as white American), more than 5 years usage of tamoxifen, and history of radioactive exposure. While the number of parity, menarche age, and menopause have not been found to affect RMS [7]. One study found that exposure to chemical agents, maternal age more than 30 years, low socio-economy status, and environment factors contributed to the incidence of RMS [8].
Diagnosis of sarcoma botryoides is based on histopathology and post-surgery immunohistochemistry, although in some cases it is done by preoperative histopathology or intraoperative frozen section [9]. In our case, IHC evaluation revealed positive anti-desmin antibody and positive anti-myogenin antibody. This finding was similar to a previous case report of sarcoma botryoides in a 17-month old infant, where IHC evaluation showed focal positivity for desmin and myogenin [2].
The Intergroup Rhabdomyosarcoma Study Group (IRSG) protocol recommends staging and grouping of the tumor. Staging is determined by primary tumor location, tumor size, regional lymph node involvement, and the presence of metastasis. Grouping categorizes patients according to the extent of disease remaining after the initial surgical procedure(s) but before beginning chemotherapy and radiation therapy. The staging and grouping systems and the tumor histologic subtype are all used to make decisions about treatment [10].
The choice of treatment for sarcoma botryoides includes radical surgery, fertility-sparing surgery, chemoradiation, and multiple approaches. Optimal treatment of the tumor is not yet established due to the scarce number of cases. However, multiple approaches to treatment result in better prognosis [11]. Tumors in the RMS group have a greater tendency to have early recurrence, which makes adjuvant chemotherapy considered as a post-surgical treatment. Until now, the combination of both has been preferred [12]. Adjuvant chemotherapy was given to our patient after the second surgery due to recurrence of the tumor, indicated by growth of the tumor at the same location with identical characteristic, but smaller in size, at 3 months after the first surgery.
The regimen of the chemotherapy among teenagers and young adults with sarcoma botryoides is vincristine, actinomycin D, and cyclophosphamide (VAC), which are given for between 6 to 12 cycles [1]. When resected appropriately and embryonal cells shown in histopathology analysis, sarcoma botryoides provides remarkably better prognosis with multi-agent adjuvant chemotherapy as compared to other RMS tumors [13]. In our case, we gave vincristine of 1.5 mg/m2 on the first day plus actinomycin D of 0.3 mg/m2 and cyclophosphamide of 150 mg/m2 from the first day until the fifth day in each cycle. This dose of VAC was used in a previous study, and that patient remained alive and well with no evidence of disease 44 months after treatment [14].
Overall, sarcomas usually have a poor prognosis with high recurrence risk for all stages, ranging from 45–73% (40% recurrence in the lung, 13% in the pelvic area). Moreover, the majority of patients experiencing recurrence do so within 2 years after the primary therapy [15]. Another study stated that the survival rate in patients with RMS ranged between 20–63% with a mean value of 47%. The metastatic pathway of tumor is through the myometrium, pelvic blood vessels and lymphatic, surrounding pelvic and abdomen structures, and further metastasis to the lung [5]. However, the prognosis of cervical sarcoma botryoides is much better than other genital RMS, especially when the tumor appears as a single polypoid lesion and the lesion is removed [16].
Since 1959, 7 cases of cervical ERMS in infants have been reported, including our patients [2,17–20]. From 6 previous reported cases, 2 of which were cases of recurrent ERMS, the earlier recurrent case was treated with chemotherapy and brachytherapy, while the later one was treated with surgery and chemotherapy [2,19]. However, the information written in the previous reported cases was incomplete. There was only 1 case report that stated the IHC evaluation result. For the cases that used chemotherapy, none reported the dose of the chemotherapy regiments used (Table 1).
Table 1.
Sarcoma botryoides of the uterine cervix in infant (reported cases since 1959).
| Author | Patient age | Chief complaints | IHC | Treatment | Relapse | Therapy for Relapse | Outcome |
|---|---|---|---|---|---|---|---|
| Crawford (1959) [18] | 11-month old | Growth in the perineal region in the past 3 months | Not stated | Surgery (total abdominal hysterectomy and colpectomy) | No | A&W*, 18 months | |
| Kobi et al. (2009) [19] | 1-year old | Not stated | Not stated | Surgery (no further explanation) | No | A&W*, 29 months | |
| Kobi et al. (2009) [19] | 2-year old | Not stated | Not stated | Surgery (no further explanation) | No | A&W*, 8 months | |
| van Sambeeck et al. (2014) [2] | 17-month old | Abnormal vaginal bleeding and vaginal tissue loss with a “grape bunch” appearance | Focal positivity for desmin and myogenin | Chemotherapy (VAI*** for 9 cycles) | Yes (6 months after chemotherapy) | Chemotherapy and brachytherapy (no further explanation) | A&W*, 12 months |
| Yasmin et al. (2015) [17] | 7-month old | Protruding mass in the vaginal area for 7 days | Not stated | Surgery (subtotal hysterectomy) and chemotherapy (5 cycles, no explanation about the regiment) | Yes (2 months after chemotherapy) | Surgery and chemotherapy (no further explanation) | Not stated |
| ALSaleh et al. (2017) [20] | 18-month old | Spontaneous intermittent painless vaginal bleeding in the past 10 months | Not stated | Neoadjuvant chemotherapy (VAC** for 10 cycles) → Surgery (total abdominal hysterectomy and bilateral salpingectomy with upper vaginectomy) → Chemotherapy (VAC** for 5 cycles) | No | A&W*, 12 months | |
| Current study | 3-year old | protruding mass from vagina for 7 months | Positive anti-desmin and anti-myogenin antibody | Tumor excision | Yes (3 months after tumor excision) | Surgery (Wide excision with safe margin of 1–2 cm) → Chemotherapy (VAC** for 6 cycles) | A&W*, 18 months |
IHC – immunohistochemistry;
A&W – alive and well;
VAC – vincristine, actinomycin D, cyclophosphamide;
VAI – vincristine, actinomycin D, ifosfamid.
In our case, there were several difficulties encountered in diagnosing and treating the patient. Firstly, if only the parents had brought their child to the hospital earlier, the mass would not have become that large. Secondly, evaluation of the metastasis should be done with computed tomography (CT) scan and/or magnetic resonance imaging (MRI) instead of chest x-ray. However, due to the limited resources in the area, no CT scan or MRI was available. Thirdly, because of the lack of understanding from the patient’s parents, the patient underwent surgery 2 times. They thought that if the mass was already removed, there was no need for further treatment. Fourthly, intraoperative biopsy to evaluate the safe margin for the wide excision was not done due to the lack of pathological analysis resources in the hospital.
Conclusions
Sarcoma botryoides is a rare malignancy. The effective treatment for sarcoma botryoides is wide excision with safe margin of 1 cm to 2 cm, followed by 6–12 cycles of VAC regiment as an adjuvant chemotherapy. A family’s understanding of the treatment plan is important for complete resolution. Even with limited resources, this malignancy can still be properly treated.
Footnotes
Department and Institution where work was done
Department of Obstetrics and Gynecology Ulin General Hospital, Banjarmasin, Indonesia
Conflict of interest
None.
References:
- 1.Behtash N, Mousavi A, Tehranian A, et al. Embryonal rhabdomyosarcoma of the uterine cervix: Case report and review of the literature. Gynecol Oncol. 2003;91(2):452–55. doi: 10.1016/s0090-8258(03)00539-0. [DOI] [PubMed] [Google Scholar]
- 2.van Sambeeck SJ, Mavinkurve-Groothuis AM, Flucke U, Dors N. Sarcoma botryoides in an infant. BMJ Case Rep. 2014;2014 doi: 10.1136/bcr-2013-202080. pii: bcr2013202080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Houghton JP, McCluggage WG. Embryonal rhabdomyosarcoma of the cervix with focal pleomorphic areas. J Clin Pathol. 2007;60(1):88–89. doi: 10.1136/jcp.2005.034769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jayi S, Bouguern H, Fdili FZ, et al. Embryonal rhabdomyosarcoma of the cervix presenting as a cervical polyp in a 16-year-old adolescent: A case report. J Med Case Rep. 2014;8:241. doi: 10.1186/1752-1947-8-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berek JS. Berek & Novak’s gynecology. 14 ed. Philadelphia: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 6.Nordal RR, Thoresen SO. Uterine sarcomas in Norway 1956–1992: Incidence, survival and mortality. Eur J Cancer. 1997;33(6):907–11. doi: 10.1016/s0959-8049(97)00040-3. [DOI] [PubMed] [Google Scholar]
- 7.Koivisto-Korander R, Butzow R, Koivisto AM, Leminen A. Clinical outcome and prognostic factors in 100 cases of uterine sarcoma: Experience in Helsinki University Central Hospital 1990–2001. Gynecol Oncol. 2008;111(1):74–81. doi: 10.1016/j.ygyno.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Grufferman S, Wang HH, DeLong ER, et al. Environmental factors in the etiology of rhabdomyosarcoma in childhood. J Natl Cancer Inst. 1982;68(1):107–13. [PubMed] [Google Scholar]
- 9.Giuntoli RL, 2nd, Metzinger DS, DiMarco CS, et al. Retrospective review of 208 patients with leiomyosarcoma of the uterus: Prognostic indicators, surgical management, and adjuvant therapy. Gynecol Oncol. 2003;89(3):460–69. doi: 10.1016/s0090-8258(03)00137-9. [DOI] [PubMed] [Google Scholar]
- 10.Raney RB, Maurer HM, Anderson JR, et al. The Intergroup Rhabdomyosarcoma Study Group (IRSG): Major lessons from the IRS-I through IRS-IV studies as background for the current IRS-V treatment protocols. Sarcoma. 2001;5(1):9–15. doi: 10.1080/13577140120048890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khosla D, Gupta R, Srinivasan R, et al. Sarcomas of uterine cervix: Clinicopathological features, treatment, and outcome. Int J Gynecol Cancer. 2012;22(6):1026–30. doi: 10.1097/IGC.0b013e31825a97f6. [DOI] [PubMed] [Google Scholar]
- 12.Arndt CA, Donaldson SS, Anderson JR, et al. What constitutes optimal therapy for patients with rhabdomyosarcoma of the female genital tract? Cancer. 2001;91(12):2454–68. [PubMed] [Google Scholar]
- 13.McDowell HP. Update on childhood rhabdomyosarcoma. Arch Dis Child. 2003;88(4):354–57. doi: 10.1136/adc.88.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeisler H, Mayerhofer K, Joura EA, et al. Embryonal rhabdomyosarcoma of the uterine cervix: Case report and review of the literature. Gynecol Oncol. 1998;69(1):78–83. doi: 10.1006/gyno.1998.4962. [DOI] [PubMed] [Google Scholar]
- 15.Walterhouse D, Watson A. Optimal management strategies for rhabdomyosarcoma in children. Paediatr Drugs. 2007;9(6):391–400. doi: 10.2165/00148581-200709060-00006. [DOI] [PubMed] [Google Scholar]
- 16.Abdeljalil K, Asma B, Kouira M, et al. Embryonal rhabdomyosarcoma of the uterine cervix: Two cases report and literature review. Open J Obstet Gynecol. 2014;4:868–73. [Google Scholar]
- 17.Crawford EJ. Sarcoma botryoides: A case report. Am J Obstet Gynecol. 1959;78:618–20. doi: 10.1016/0002-9378(59)90536-8. [DOI] [PubMed] [Google Scholar]
- 18.Kobi M, Khatri G, Edelman M, Hines J. Sarcoma botryoides: MRI findings in two patients. J Magn Reson Imaging. 2009;29(3):708–12. doi: 10.1002/jmri.21670. [DOI] [PubMed] [Google Scholar]
- 19.Yasmin F, Ahmed MA, Begum T, et al. A case report of rhabdomyosarcoma of uterine cervix in a 7-month-old child. BIRDEM Med J. 2017;7(3):242–44. [Google Scholar]
- 20.ALSaleh N, ALwadie H, Gari A. Rhabdomyosarcoma of the genital tract in an 18-month-old girl. J Surg Case Rep. 2017;2017(4):rjx080. doi: 10.1093/jscr/rjx080. [DOI] [PMC free article] [PubMed] [Google Scholar]