Abstract
Background
This study aimed to investigate the factors associated with sarcopenia in elderly residents in three nursing homes in Suzhou City, East China including the association with nutrition and physical exercise.
Material/Methods
Elderly residents (n=316) from three nursing homes included 112 men and 204 women. The appendicular skeletal muscle index (ASMI), grip strength, and movements were measured to diagnose sarcopenia. The correlation between sarcopenia with age, sex, body mass index (BMI), ASMI, upper arm circumference, calf circumference, muscle content, grip strength, dietary intake, degree and duration of movement were also assessed.
Results
The prevalence of sarcopenia was 28.8% (30.4% for men and 27.9% for women). Patients with sarcopenia were older compared with controls. Height, BMI, upper arm circumference, calf circumference and arm muscle mass, lower limb muscle mass, limb skeletal muscle index and ASMI, grip strength, and pace of movement were lower than controls. The prevalence of sarcopenia correlated with the intake of meat, fish, eggs, and milk, and duration of weekly aerobic and resistance exercise. Logistic regression analysis showed a positive correlation between the prevalence of sarcopenia and age, and a negative correlation between BMI and consumption of meat, eggs, and milk.
Conclusions
The prevalence of sarcopenia in elderly residents in three nursing homes in Suzhou City was 28.8%. Increasing age was a risk factor for sarcopenia. Increased BMI and a diet containing meat, eggs, and milk were protective factors. The findings from this study provide support that adequate dietary protein can prevent sarcopenia in the elderly.
MeSH Keywords: Exercise, Nutrition Assessment, Risk Factors, Sarcopenia, Secondary Prevention
Background
Changes in the musculoskeletal system associated with aging are a serious concern in the elderly population. Sarcopenia was first described in 1989 by Rosenberg to describe reduced muscle mass and decreased muscle strength associated with aging [1]. With the increased understanding of sarcopenia, in 2011 an international sarcopenia working group defined sarcopenia as an ‘age-associated loss of skeletal muscle mass and function’ [2]. Previous studies have shown that muscle loss after the age of 30 years occurs at a rate of between 1–5% per year [3,4]. Muscle loss of 30% can affect normal muscle function, and muscle loss of 40% can threaten life, and rapid skeletal muscle loss in the limbs is an independent risk factor that predicts all-cause mortality [5–7].
Age-related loss of muscle is often accompanied by an increase in intramuscular and visceral fat content [8,9], but reduced subcutaneous fat. Increased intramuscular fat content induces a decline in physiological functions of muscle and movement disorders, including unsteady gait and falls [10]. Therefore, the early identification and prevention of sarcopenia and its complications are essential to improve the quality of life of the elderly population. Previous studies on sarcopenia have mainly focused on elderly residents in the community or hospital inpatients. However, in the developed eastern regions of China, a large number of elderly people choose to spend their later years in nursing homes. The effects of nutritional habits and physical exercise on the prevalence of sarcopenia in older adults in nursing homes remain poorly understood.
Therefore, this study aimed to investigate the factors associated with sarcopenia in elderly residents in three nursing homes in Suzhou City, East China including the association with nutrition and physical exercise.
Material and Methods
Participants
This case-control study included elderly residents of three retirement homes in Suzhou City, East China. The study was conducted between September 2016 to April 2017. The study included elderly people aged more than 60 years of age who voluntarily participated in the study and signed informed consent. The exclusion criteria included elderly individuals with cognitive impairment, speech impairment, or hearing loss, individuals with mental illness, severe metabolic disease, organ failure, individuals who had a pacemaker or artificial joint, serious disability, incomplete clinical information, or who refused to participate in the study (Figure 1). This study was approved by the Ethics Committee of Suzhou Municipal Hospital, and the written consent was obtained from all participants.
Figure 1.
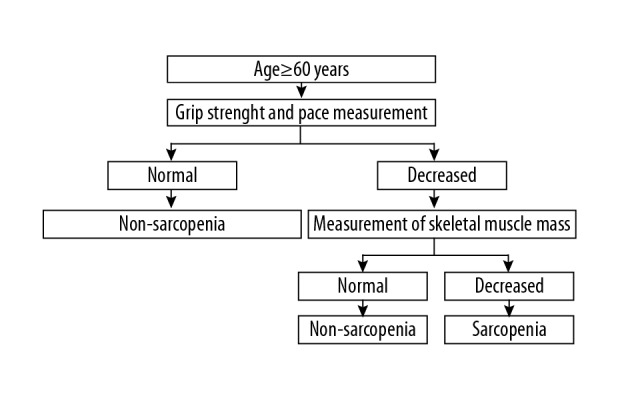
Diagnostic flow chart of sarcopenia.
Evaluation using an electronic questionnaire
The data of elderly people in three nursing homes, the Jinshi Community, the Chunhui Nursing Home, and Yiyang Elderly Apartments in Suzhou City were studied using cluster sampling. Study investigators received training to conduct face-to-face interviews and evaluations using an electronic questionnaire before physical examinations were performed by the professional medical staff.
The questionnaire recorded data on gender, age, marital status, education level, height, weight, upper arm circumference, calf circumference, contents of upper and lower limb muscles, grip strength, pace of movement, and frequency of eating protein from meat, fish, eggs, soy, and tofu (>50 g/day), dairy products including milk and yogurt (>200 g/day), calcium supplements (>300 mg/day), vitamin D supplements (>400 IU/day), duration of weekly aerobic exercise that included jogging, cycling, swimming, and tai chi, and duration of weekly resistance training, including climbing stairs, and weight training.
Measurement of muscle mass
Muscle mass was measured by bioelectrical impedance analysis using a DBA-210 human component analyzer (Beijing Donghuayuan Medical Equipment Co., Ltd., China). Before measurement of muscle mass, the study participants went without food and drink for two hours and measurements were performed with the participants with clothing removed from their arms and legs, and barefoot. Study participants stood still on the test platform of the foot electrode and held the handle electrode with arms straight about 30° away from the trunk while the measurements of muscle mass were recorded.
Measurement of grip strength
After adjusting the grip distance, a Jamar dynamometer (Sammons Preston, Bolingbrook, IL, USA) was used to measure the grip strength of the arm when the participants held the grip in a sitting position, with the upper arm and forearm at a 90° angle and the testing arm slightly abducted at 30°. The maximum value in the two tests was taken as the grip strength and used for statistical analysis.
Measurement of the pace of movement
The start and end points of a 4-meter straight line were tagged with a colored ribbon. The time when the first foot crossed the ribbon was taken as the start and, the pace of movement was calculated with the distance divided by the difference between start and end times measured using a stopwatch. The fastest pace of movement in two tests was used in the final statistical analysis.
Measurement of the upper arm and calf circumference
The upper arm circumference was measured around the arm at the midpoint between the acromion and the olecranon of the ulna, with the upper limb in a naturally hanging state. The calf circumference was measured with the tape placed horizontally around the thickest part of the calf while the legs were separated by a distance equal to the shoulder width.
Diagnostic criteria for sarcopenia
Sarcopenia was diagnosed according to a consensus report of the Asian Working Group for Sarcopenia (Figure 1) [11]. Reduced muscle mass was defined using the appendicular skeletal muscle mass index (ASMI) calculated as the appendicular skeletal muscle mass/height2 (men <7.0 kg/m2 and women <5.7 kg/m2) according to measurement using bioelectrical impedance analysis. Reduced pace of movement was defined as a step speed <0.8 m/s. Low muscle strength was defined as grip strength <26 kg for men and <18 kg for women. The elderly people who met the diagnostic criteria were included in the sarcopenia group. The control group included the non-sarcopenia group.
Statistical analysis
Data were statistically analyzed using SPSS version 17.0 software. Data for the measurements were expressed as the mean ± standard deviation (SD). The t-test was used for comparison between two groups, and analysis of variance (ANOVA) was used for comparison between multiple groups. Measurement data were represented by the percentage or rate and a chi-squared (χ2) test was performed to compare the groups. Pearson’s correlation analysis was used for continuous variables, Spearman’s correlation analysis was used for classification variables, and logistic regression analysis was used to analyze sarcopenia-associated factors. P<0.05 indicated a statistically significant difference.
Results
Comparison of demographic data between study participants with or without sarcopenia
There were initially 370 participants in the study, and 316 met the study inclusion criteria (112 men and 204 women). The prevalence of sarcopenia was 28.8% (30.4% for men and 27.9% for women). No significant differences were found between men and women. Reduced height, body mass, body mass index (BMI), upper arm circumference, calf circumference, arm muscle mass, lower limb muscle mass, limb skeletal muscle mass, the appendicular skeletal muscle mass index (ASMI), grip strength, and pace of movement, and increased age were found in the sarcopenia group compared with the control group (Table 1).
Table 1.
Comparison of demographic data between control and sarcopenia group.
| Control (n=225) | Sarcopenia (n=91) | Test value | P value | |
|---|---|---|---|---|
| Age | 74.99±9.55 | 81.70±8.95 | −5.77 | 0.00 |
| Sex (Male),n(%) | 78 (34.67) | 34 (37.36) | 0.21 | 0.65 |
| Height (cm) | 159.19±8.21 | 154.20±11.29 | 3.83 | 0.00 |
| Body mass (Kg) | 61.53±10.56 | 53.69±9.66 | 6.12 | 0.00 |
| BMI (kg/m2) | 24.28±3.72 | 22.23±3.48 | 4.51 | 0.00 |
| Biceps (cm) | 28.88±5.73 | 27.16±3.57 | 2.67 | 0.01 |
| Calf (cm) | 33.92±3.83 | 31.67±3.74 | 4.76 | 0.00 |
| Upper limb muscle mass (Kg) | 4.57±1.27 | 3.20±0.79 | 11.57 | 0.00 |
| Lower limb muscle mass (Kg) | 13.30±1.17 | 10.13±2.55 | 8.47 | 0.00 |
| Limbs’ skeletal muscle mass (Kg) | 17.86±4.29 | 13.33±3.17 | 9.12 | 0.00 |
| ASMI (kg/m2) | 6.97±1.11 | 5.44±0.78 | 12.01 | 0.00 |
| Grip strength (Kg) | 25.37±7.48 | 15.23±5.91 | 12.75 | 0.00 |
| Pace (m/s) | 0.91±0.28 | 0.58±0.36 | 7.67 | 0.00 |
Compared with men,
P<0.05; compared with control group,
P<0.05.
Pearson’s correlation analysis of sarcopenia-associated indicators in the elderly population
Pearson’s correlation analysis showed that regardless of gender, ASMI was positively correlated with body mass index (BMI), upper arm circumference, calf circumference, upper limb and lower limb muscle mass, grip strength, and pace of movement in the elderly population. Pace of movement and grip strength were negatively correlated with age. A positive correlation was also found between upper arm circumference and arm muscle content, between calf circumference and lower limb muscle content, between upper limb muscle content and grip strength, and between lower limb muscle content and pace of movement. ASMI, calf circumference, and muscle content of the upper and lower limbs were negatively correlated with age in elderly women (Table 2).
Table 2.
Pearson correlation analysis of sarcopenia with related indicators in the elderly.
| Age | BMI | ASMI | Biceps circumference | Calf circumference | Upper limb muscle mass | Lower limb muscle mass | Grip strength | Pace | |
|---|---|---|---|---|---|---|---|---|---|
| Age | −0.015 | −0.122 | −0.147 | −0.229* | −0.174 | −0.099 | −0.512** | −0.427** | |
| BMI | 0.047 | 0.381** | 0.511** | 0.622** | 0.411** | 0.166 | 0.183 | 0.029 | |
| ASMI | −0.418** | 0.325** | 0.288** | 0.438** | 0.895** | 0.921** | 0.462** | 0.264** | |
| Biceps circumference | 0.005 | 0.362** | 0.243** | 0.557** | 0.320** | 0.272** | 0.128 | −0.001 | |
| Calf circumference | −0.225** | 0.455** | 0.409** | 0.287** | 0.457** | 0.333** | 0.383** | 0.229* | |
| Upper limb muscle mass | −0.408** | 0.344** | 0.828** | 0.219** | 0.362** | 0.810** | 0.494** | 0.286** | |
| Lower limb muscle mass | −0.458** | 0.049 | 0.859** | 0.212** | 0.369** | 0.766** | 0.433** | 0.244* | |
| Grip strength | −0.585** | 0.160* | 0.613** | 0.078 | 0.285** | 0.575** | 0.639** | 0.583** | |
| Pace | −0.593** | −0.026 | 0.411** | 0.027 | 0.223** | 0.343** | 0.432** | 0.554** |
The boldface is the correlation coefficient between indicators for men, while the non-boldface is the correlation coefficient between indicators for women.
P<0.05;
P<0.01.
Dietary intake and the prevalence of sarcopenia
Significant differences were found in the prevalence of sarcopenia between the groups with different dietary intake of meat, fish, eggs, and milk. However, no statistically significant difference was found for the prevalence of sarcopenia between the groups with different frequencies of intake of soy products, calcium supplements, and vitamin D supplements (Table 3).
Table 3.
Effect s of dietary and different intake frequencies on the prevalence of sarcopenia.
| Meat | Fish | Eggs | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Sarcopenia | Prevalence (%) | Control | Sarcopenia | Prevalence (%) | Control | Sarcopenia | Prevalence (%) | ||||
| 4~7 days/week | 126 | 27 | 17.65 | 70 | 17 | 19.54 | 156 | 45 | 22.39 | |||
| 1~3 day(s)/week | 79 | 51 | 39.23 | 121 | 50 | 29.24 | 22 | 9 | 29.03 | |||
| Never eating | 20 | 13 | 39.39 | 34 | 24 | 41.38 | 47 | 37 | 44.05 | |||
| χ2 | 17.99 | 8.13 | 13.56 | |||||||||
| P value | <0.01 | <0.05 | <0.01 | |||||||||
| Bean products | Dairy | Calcium tablet | Vitamin D | |||||||||
| Control | Sarcopenia | Prevalence (%) | Control | Sarcopenia | Prevalence (%) | Control | Sarcopenia | Prevalence (%) | Control | Sarcopenia | Prevalence (%) | |
| 4~7 days/week | 82 | 25 | 23.36 | 139 | 37 | 21.02 | 50 | 17 | 25.37 | 35 | 12 | 25.53 |
| 1~3 day(s)/week | 99 | 50 | 33.56 | 22 | 12 | 35.29 | 7 | 4 | 36.36 | 7 | 3 | 30.00 |
| Never eating | 44 | 16 | 26.67 | 64 | 42 | 39.62 | 168 | 70 | 29.41 | 183 | 76 | 29.34 |
| χ2 | 3.32 | 11.95 | 0.73 | 0.29 | ||||||||
| P value | 0.19 | <0.01 | 0.69 | 0.87 | ||||||||
Pairwise comparison of the effect of dietary intake on the prevalence of sarcopenia
Pairwise comparison showed that the frequencies of intake of meat, fish, eggs, and milk during 4–7 days per week were associated with the lowest prevalence of sarcopenia in the elderly population. No difference was found in the prevalence of sarcopenia for dietary intake of protein between the 1–3 days/week group and the ‘never eat’ group. Different prevalence of sarcopenia was found for intake frequencies of meat or milk, but not for fish or eggs, between the 4–7 days/week group and the 1–3 days/week group (Table 4).
Table 4.
Pairwise comparison of effect of intake frequencies on prevalence of sarcopenia.
| Groups | Meat | Fish | Eggs | Dairy | ||||
|---|---|---|---|---|---|---|---|---|
| χ2 | P value | χ2 | P value | χ2 | P value | χ2 | P value | |
| G1 vs. G2 | 16.40 | <0.01 | 2.82 | >0.05 | 0.66 | >0.05 | 3.24 | <0.05 |
| G1 vs. G3 | 7.61 | <0.05 | 8.18 | <0.05 | 13.56 | <0.01 | 11.35 | <0.01 |
| G2 vs. G3 | 0.00 | >0.05 | 2.92 | >0.05 | 2.13 | >0.05 | 0.20 | >0.05 |
G1 – 4~7 days/week; G2 – 1~3 day(s)/week; G3 – never eating.
Duration of weekly aerobic exercise and the prevalence of sarcopenia
A significant difference was found in the prevalence of sarcopenia between the groups with different hours per week (<150 min, 150–300 min, 300–450 min, and >450 min) of aerobic exercise, such as walking, cycling, swimming, and tai chi (χ2=16.28; P=0.001) (Table 5).
Table 5.
Effect of weekly aerobic exercise duration on the prevalence of sarcopenia.
| Control | Sarcopenia | Prevalence (%) | χ2 | P value | |
|---|---|---|---|---|---|
| <150 min | 91 | 58 | 38.93 | 16.28 | 0.001 |
| 150–300 min | 70 | 22 | 23.91 | ||
| 300–450 min | 26 | 6 | 18.75 | ||
| >450 min | 38 | 5 | 11.63 |
Duration of weekly resistance training and the prevalence of sarcopenia
Significant differences were found in the prevalence of sarcopenia between the groups for different duration of weekly resistance training (<40–90 min, 90–120 min, 120–180 min, and >180 min) that included climbing stairs, standing from sitting, weight training, and standing on tiptoe (Table 6).
Table 6.
Effect of weekly resistance training duration on the prevalence of sarcopenia.
| Control | Sarcopenia | Prevalence (%) | χ2 | P value | |
|---|---|---|---|---|---|
| <150 min | 152 | 81 | 34.76 | 15.86 | 0.001 |
| 150–300 min | 47 | 7 | 12.96 | ||
| 300–450 min | 20 | 3 | 13.04 | ||
| >450 min | 6 | 0 | 0.00 |
Spearman’s correlation analysis of factors associated with sarcopenia
Spearman’s correlation analysis of sarcopenia with age, gender, BMI, upper arm circumference, calf circumference, different dietary intake frequencies (meat, fish, eggs, soy, milk, calcium tablets, and vitamin D), and duration of different ways of movement (aerobic exercise and resistance training) showed that age was positively correlated with the prevalence of sarcopenia, but BMI, upper arm circumference, calf circumference, increased intake frequency of dietary meat, fish, eggs, and milk, and long duration of aerobic exercise and resistance training were negatively correlated with sarcopenia (Table 7).
Table 7.
Spearman correlation analysis of senile sarcopenia with related factors.
| Spearman correlation coefficient r | P value | |
|---|---|---|
| Age | 0.307 | 0.000 |
| Sex | 0.026 | 0.651 |
| BMI | −0.253 | 0.000 |
| Upper arm circumference | −0.192 | 0.001 |
| Calf circumference | −0.280 | 0.000 |
| Meat | −0.229 | 0.000 |
| Fish | −0.159 | 0.005 |
| Eggs | −0.201 | 0.000 |
| Bean products | −0.051 | 0.369 |
| Dairy | −0.193 | 0.001 |
| Calcium tablets | −0.028 | 0.624 |
| VitaminD | −0.027 | 0.635 |
| Aerobic exercise | −0.226 | 0.000 |
| Resistance training | −0.221 | 0.000 |
Logistic regression analysis of factors associated with sarcopenia
Logistic regression analysis of sarcopenia with other related factors showed that age was positively correlated with the risk of sarcopenia, but increased BMI and intake of meat, eggs, and milk were negatively correlated with the risk of sarcopenia (Table 8).
Table 8.
Logistic regression analysis of sarcopenia with related factors.
| B | Sig. | OR | 95%CI | |
|---|---|---|---|---|
| Age | 0.079 | 0.000 | 1.082 | (1.043, 1.122) |
| BMI | −0.201 | 0.000 | 0.818 | (0.730, 0.916) |
| Upper arm circumference | −0.026 | 0.641 | 0.974 | (0.873, 1.087) |
| Calf circumference | −0.038 | 0.409 | 0.962 | (0.879, 1.054) |
| Meat | ||||
| Never eating* | 0.024 | |||
| 1~3 day(s)/week | −0.441 | 0.409 | 0.643 | (0.107, 0.850) |
| 4~7 days/week | −1.198 | 0.023 | 0.302 | (0.226, 1.832) |
| Fish | ||||
| Never eating* | 0.908 | |||
| 1~3 day(s)/week | −0.106 | 0.804 | 0.899 | (0.403, 2.821) |
| 4~7 days/week | 0.064 | 0.897 | 1.066 | (0.389, 2.079) |
| Eggs | ||||
| Never eating* | 0.014 | |||
| 1~3 day(s)/week | −1.200 | 0.038 | 0.301 | (0.177, 0.756) |
| 4~7 days/week | −1.005 | 0.007 | 0.366 | (0.097, 0.937) |
| Dairy | ||||
| Never eating* | 0.032 | |||
| 1~3 day(s)/week | −0.666 | 0.213 | 0.514 | (0.198, 0.794) |
| 4~7 days/week | −0.924 | 0.009 | 0.397 | (0.180, 1.467) |
| Aerobic exercise | ||||
| <150 mina | 0.352 | |||
| 150–300 min | −0.149 | 0.692 | 0.861 | (0.100, 1.110) |
| 300–450 min | 0.010 | 0.987 | 1.010 | (0.315, 3.237) |
| >450 min | −1.098 | 0.073 | 0.333 | (0.412, 1.802) |
| Resistance training | ||||
| 40–90 mina | 0.168 | |||
| 90–120 min | −1.101 | 0.036 | 0.333 | |
| 120–180 min | −0.769 | 0.291 | 0.463 | (0.111, 1.929) |
| >180 min | −19.994 | 0.999 | 0.000 | (0.119, 0.931) |
| Constant | 1.997 | 0.390 | 7.370 | |
Reference category.
Discussion
The prevalence of sarcopenia was 28.8% (30.4% in elderly men and 27.9% in elderly women) at three retirement homes in Suzhou City, with no significant difference between men and women. This finding was higher than the prevalence of sarcopenia in Taiwan or Japan reported by Wu et al. and Tanimoto et al. [12,13]. Fielding et al. reported that the prevalence of sarcopenia in European and American populations was 7–27% in men, 10–23% in women, and was between 30–50% in the population aged 80 years and older [2]. The prevalence of sarcopenia has previously been reported to increase with age and was reported to be higher in men than in women [14]. However, Coin et al. reported no gender difference in the prevalence of sarcopenia in the elderly population [15]. Therefore, the prevalence of sarcopenia in the present study was higher than that found in previous studies. These differences might have been caused by differences in race, study inclusion criteria, and diagnostic criteria of sarcopenia [16].
Grip strength, walking speed, and limb muscle index are the three common indicators for diagnosing sarcopenia, as recommended by the Asian sarcopenia working group. Two indicators of physical function, grip strength and walking speed, significantly correlate with poor prognosis, disability, and mortality due to sarcopenia [17,18]. The results of the present study showed that the age of the population with sarcopenia was significantly higher, whereas BMI, grip strength and walking speed were significantly lower, compared with a normal population. Pearson’s correlation analysis indicated that the appendicular skeletal muscle mass index (ASMI) of both men and women in the elderly population positively correlated with BMI, grip strength, and pace of movement, which has been previously reported [19]. Also, a positive correlation was found between upper arm circumference and arm muscle content, calf circumference and lower limb muscle content, the content of the upper limb muscles and grip strength, and lower extremity muscle content and pace of movement, but pace of movement and grip strength were negatively correlated with age. These results indicated that physical function, including grip strength and movement, had the tendency to decline gradually with the increasing age. The muscle content of the limbs affects their function, and with the decline in physical function, the prevalence of sarcopenia increases [20]. Preserving limb muscle content can delay the decline of grip strength and pace of movement, postpone the occurrence and development of sarcopenia, and reduce the risk of the poor prognosis associated with sarcopenia [17,18].
An adequate intake of nutritional supplements and protein and energy balance as part of a program of therapy in the elderly can help to prevent or reverse sarcopenia [21–23]. This study found that a regular and frequent weekly intake of meat, fish, eggs, or milk was correlated with a low risk of sarcopenia in the elderly population, but the intake frequency of bean products, calcium supplements, or vitamin D supplements had no influence on the prevalence of sarcopenia. This finding was consistent with the findings reported by Beasley et al. [24]. The effect of animal proteins on increasing muscle mass is greater than that of plant proteins, which may be related to the fact that animal proteins can provide more energy and improve muscle function more efficiently. Animal-based proteins, such as milk protein, are rich in casein and whey protein and can better promote muscle protein synthesis, increase muscle mass, improve muscle strength and pace of movement, and improve movement when compared with soybean protein [25].
In the present study, further analysis showed that the frequency of intake of meat, fish, eggs, and dairy products in the 4–7 days/week group was associated with the lowest prevalence of sarcopenia. No difference was observed in the prevalence of sarcopenia between the 1–3 days/week group and the never eat protein group. These results suggested that high protein intake might help to reduce the risk of sarcopenia. The current dietary protein recommendation of 0.8 g/kg/day may not be sufficient to maintain the normal functioning of skeletal muscles in the elderly population [24]. Previous studies have shown that supplementing the diet with more than the recommended amount of protein can improve muscle function in the frail elderly population [26]. Muscle synthesis is reduced in patients with protein deficiency, and it may be more appropriate for elderly people to increase their total protein intake to 1.2–1.5 g/kg/day [27]. Studies have shown that vitamin D supplementation is beneficial for preventing sarcopenia [28]. In 2014, the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis recommended that vitamin D should be used at a dose of >800 IU/day to prevent sarcopenia [29]. However, in the present study, the findings did not show any impact of different frequencies of intake of oral vitamin D supplements on the prevalence of sarcopenia, possibly because fewer people took vitamin D supplements, or because the dose was insufficient.
This study showed that aerobic exercise and resistance training could reduce the risk of sarcopenia, which was consistent with previous studies that showed that aerobic exercise, endurance exercise, and resistance exercise could increase muscle mass and strength in patients with sarcopenia [30–32]. Extending the duration of aerobic exercise leads to increased numbers of mitochondria, enhanced enzyme activity, increased muscle protein synthesis, and increased muscle mass [31,33]. Resistance training increases muscle mass, strength, and muscle fiber volume and reduces myostatin synthesis [34,35]. These mechanisms may explain why aerobic exercise and resistance training reduce the prevalence of sarcopenia [36].
Univariate analysis in this study showed that sarcopenia was positively correlated with age and negatively correlated with increased BMI, upper arm circumference, calf circumference, increased frequency of intake of dietary meat, fish, eggs, and milk, and duration of aerobic exercise and resistance training. Regression analysis showed that age was a risk factor, but increased BMI and intake of meat, eggs, and dairy products were protective factors for sarcopenia. These findings are supported by the findings from previous studies that reported that increased age was a risk factor for sarcopenia and increased BMI was a protective factor for sarcopenia in the elderly population [24,37]. These findings indicate that the appropriate supplementation of high-quality animal proteins, such as meat, eggs, and milk, could help delay the occurrence and development of sarcopenia.
Anthropometric methods, such as measurement of upper arm circumference and calf circumference, can be used to indirectly determine muscle mass in a risk prediction model for sarcopenia, but are affected by the increase in body fat content and the decrease in skin elasticity during aging, and their correlations with sarcopenia may not be significant [38]. However, in the present study, univariate analysis showed that the duration of aerobic exercise and resistance training were negatively correlated with the risk of sarcopenia, but regression analysis did not find a reduction in the prevalence of sarcopenia with increased duration of aerobic exercise and resistance training. This finding was similar to the findings of previous studies that the improvement in the muscle function of high-intensity resistance training had similar results to low-intensity resistance training [39]. This finding might be due to the significant increase in muscle mass and improvement in function needed for the longer duration of exercise [39]. The relationship between duration or intensity of exercise intervention and the prevention of sarcopenia deserves further investigation.
This study had several limitations. First, in the elderly population studied, the presence of chronic diseases and therapeutic drugs not identified in the study might have affected indicators such as muscle strength and walking speed, leading to bias in the results [40]. Second, the last revised European consensus on the definition and diagnosis of sarcopenia recommended that dual-energy x-ray absorptiometry (DXA) is the method commonly used for analyzing body composition and muscle mass [41]. However, in this study, muscle mass was measured by bioelectrical impedance analysis, rather than using imaging methods such as computed tomography (CT) or magnetic resonance imaging (MRI) [42]. Although the method of bioelectrical impedance analysis was more practical, convenient, and economical, may not be the most accurate method [14]. Third, this study enrolled elderly people from urban communities or retirement institutions but not from rural regions, and was not fully representative of the Chinese population. Finally, this study was a cross-sectional survey, and it was difficult to determine the causal relationship between sarcopenia and other influencing factors. These limitations support the need for further validation of the results in future large-scale controlled studies.
Conclusions
This study reported a prevalence of sarcopenia of 28.8%, 30.4% for men and 27.9% for women, in three elderly retirement homes in East China. These study findings showed that increased age was associated with an increased risk of sarcopenia, while a high-quality protein diet reduced the risk of developing sarcopenia. The findings indicate that there are feasible measures that can be used to prevent sarcopenia and improve the quality of life of the elderly.
Footnotes
Source of support: Departmental sources
References
- 1.Rosenberg IH. Sarcopenia: Origins and clinical relevance. Clin Geriatr Med. 2011;27(3):337–39. doi: 10.1016/j.cger.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12(4):249–56. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bortz WM., 2nd A conceptual framework of frailty: A review. J Gerontol A Biol Sci Med Sci. 2002;57(5):M283–88. doi: 10.1093/gerona/57.5.m283. [DOI] [PubMed] [Google Scholar]
- 4.Avers D, Brown M. White paper: Strength training for the older adult. J Geriatr Phys Ther. 2009;32(4):148–52. [PubMed] [Google Scholar]
- 5.Szulc P, Munoz F, Marchand F, et al. Rapid loss of appendicular skeletal muscle mass is associated with higher all-cause mortality in older men: The prospective MINOS study. Am J Clin Nutr. 2010;91(5):1227–36. doi: 10.3945/ajcn.2009.28256. [DOI] [PubMed] [Google Scholar]
- 6.Locquet M, Beaudart C, Hajaoui M, et al. Three-year adverse health consequences of sarcopenia in community-dwelling older adults according to 5 diagnosis definitions. J Am Med Dir Assoc. 2019;20(1):43–46.e2. doi: 10.1016/j.jamda.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Anker MS, von Haehling S, Springer J, et al. Highlights of mechanistic and therapeutic cachexia and sarcopenia research 2010 to 2012 and their relevance for cardiology. Arch Med Sci. 2013;9(1):166–71. doi: 10.5114/aoms.2013.33356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stenholm S, Harris TB, Rantanen T, et al. Sarcopenic obesity: Definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11(6):693–700. doi: 10.1097/MCO.0b013e328312c37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bai H, Sun J, Du G, Jiao F. Association of moderate aerobic exercise and rho-associated kinase 2 concentration in subjects with dyslipidemia. Arch Med Sci. 2017;13(4):807–12. doi: 10.5114/aoms.2017.68142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zamboni M, Mazzali G, Fantin F, et al. Sarcopenic obesity: aA new category of obesity in the elderly. Nutr Metab Cardiovasc Dis. 2008;18(5):388–95. doi: 10.1016/j.numecd.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15(2):95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 12.Wu IC, Lin CC, Hsiung CA, et al. Epidemiology of sarcopenia among community-dwelling older adults in Taiwan: A pooled analysis for a broader adoption of sarcopenia assessments. Geriatr Gerontol Int. 2014;14(Suppl 1):52–60. doi: 10.1111/ggi.12193. [DOI] [PubMed] [Google Scholar]
- 13.Tanimoto Y, Watanabe M, Sun W, et al. Association of sarcopenia with functional decline in community-dwelling elderly subjects in Japan. Geriatr Gerontol Int. 2013;13(4):958–63. doi: 10.1111/ggi.12037. [DOI] [PubMed] [Google Scholar]
- 14.Kim JS, Kim WY, Park HK, et al. Simple age specific cutoff value for sarcopenia evaluated by computed tomography. Ann Nutr Metab. 2017;71(3–4):157–63. doi: 10.1159/000480407. [DOI] [PubMed] [Google Scholar]
- 15.Coin A, Perissinotto E, Enzi G, et al. Predictors of low bone mineral density in the elderly: The role of dietary intake, nutritional status and sarcopenia. Eur J Clin Nutr. 2008;62(6):802–9. doi: 10.1038/sj.ejcn.1602779. [DOI] [PubMed] [Google Scholar]
- 16.Zeng Y, Hu X, Xie L, et al. The prevalence of sarcopenia in chinese elderly nursing home residents: A comparison of 4 diagnostic criteria. J Am Med Dir Assoc. 2018;19(8):690–95. doi: 10.1016/j.jamda.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 17.Gale CR, Martyn CN, Cooper C, Sayer AA. Grip strength, body composition, and mortality. Int J Epidemiol. 2007;36(1):228–35. doi: 10.1093/ije/dyl224. [DOI] [PubMed] [Google Scholar]
- 18.Taekema DG, Gussekloo J, Westendorp RG, et al. Predicting survival in oldest old people. Am J Med. 2012;125(12):1188–94.e1. doi: 10.1016/j.amjmed.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 19.Wang QQ, Jing XM, Bi YZ, et al. Human umbilical cord Wharton’s jelly derived mesenchymal stromal cells may attenuate sarcopenia in aged mice induced by hindlimb suspension. Med Sci Monit. 2018;24:9272–81. doi: 10.12659/MSM.913362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maeda K, Koga T, Nasu T, et al. Predictive accuracy of calf circumference measurements to detect decreased skeletal muscle mass and european society for clinical nutrition and metabolism-defined malnutrition in hospitalized older patients. Ann Nutr Metab. 2017;71(1–2):10–15. doi: 10.1159/000478707. [DOI] [PubMed] [Google Scholar]
- 21.Wu GH, Kong FZ, Dong XF, et al. Association between hyperhomocysteinemia and stroke with atherosclerosis and small artery occlusion depends on homocysteine metabolism-related vitamin levels in Chinese patients with normal renal function. Metab Brain Dis. 2017;32(3):859–65. doi: 10.1007/s11011-017-9978-3. [DOI] [PubMed] [Google Scholar]
- 22.Cheng Y, Kong FZ, Dong XF, et al. Influence of renal function on the association between homocysteine level and risk of ischemic stroke. Am J Transl Res. 2017;9(10):4553–63. [PMC free article] [PubMed] [Google Scholar]
- 23.Chung JH, Hwang HJ, Shin HY, Han CH. Association between sarcopenic obesity and bone mineral density in middle-aged and elderly Korean. Ann Nutr Metab. 2016;68(2):77–84. doi: 10.1159/000442004. [DOI] [PubMed] [Google Scholar]
- 24.Beasley JM, Shikany JM, Thomson CA. The role of dietary protein intake in the prevention of sarcopenia of aging. Nutr Clin Pract. 2013;28(6):684–90. doi: 10.1177/0884533613507607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips SM, Tang JE, Moore DR. The role of milk- and soy-based protein in support of muscle protein synthesis and muscle protein accretion in young and elderly persons. J Am Coll Nutr. 2009;28(4):343–54. doi: 10.1080/07315724.2009.10718096. [DOI] [PubMed] [Google Scholar]
- 26.Tieland M, van de Rest O, Dirks ML, et al. Protein supplementation improves physical performance in frail elderly people: A randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. 2012;13(8):720–26. doi: 10.1016/j.jamda.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Morley JE, Argiles JM, Evans WJ, et al. Society for sarcopenia, cachexia, and wasting disease. Nutritional recommendations for the management of sarcopenia. J Am Med Dir Assoc. 2010;11(6):391–96. doi: 10.1016/j.jamda.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tieland M, Brouwer-Brolsma EM, Nienaber-Rousseau C, et al. Low vitamin D status is associated with reduced muscle mass and impaired physical performance in frail elderly people. Eur J Clin Nutr. 2013;67(10):1050–55. doi: 10.1038/ejcn.2013.144. [DOI] [PubMed] [Google Scholar]
- 29.Rizzoli R, Stevenson JC, Bauer JM, et al. The role of dietary protein and vitamin D in maintaining musculoskeletal health in postmenopausal women: A consensus statement from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) Maturitas. 2014;79(1):122–32. doi: 10.1016/j.maturitas.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Cornish SM, Chilibeck PD. Alpha-linolenic acid supplementation and resistance training in older adults. Appl Physiol Nutr Metab. 2009;34(1):49–59. doi: 10.1139/H08-136. [DOI] [PubMed] [Google Scholar]
- 31.Harber MP, Konopka AR, Douglass MD, et al. Aerobic exercise training improves whole muscle and single myofiber size and function in older women. Am J Physiol Regul Integr Comp Physiol. 2009;297(5):R1452–59. doi: 10.1152/ajpregu.00354.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wasenius NS, Simonen M, Penttinen L, et al. Effect of maternal weight during pregnancy on offspring muscle strength response to resistance training in late adulthood. Adv Med Sci. 2018;63(2):353–58. doi: 10.1016/j.advms.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Short KR, Vittone JL, Bigelow ML, et al. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab. 2004;286(1):E92–101. doi: 10.1152/ajpendo.00366.2003. [DOI] [PubMed] [Google Scholar]
- 34.Brotto M, Abreu EL. Sarcopenia: Pharmacology of today and tomorrow. J Pharmacol Exp Ther. 2012;343(3):540–46. doi: 10.1124/jpet.112.191759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J, Al-Mallah M, Juraschek SP, et al. The association of clinical indication for exercise stress testing with all-cause mortality: The FIT Project. Arch Med Sci. 2016;12(2):303–9. doi: 10.5114/aoms.2016.59255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whittemore LA, Song K, Li X, et al. Inhibition of myostatin in adult mice increases skeletal muscle mass and strength. Biochem Biophys Res Commun. 2003;300(4):965–71. doi: 10.1016/s0006-291x(02)02953-4. [DOI] [PubMed] [Google Scholar]
- 37.Yu R, Wong M, Leung J, et al. Incidence, reversibility, risk factors and the protective effect of high body mass index against sarcopenia in community-dwelling older Chinese adults. Geriatr Gerontol Int. 2014;14(Suppl 1):15–28. doi: 10.1111/ggi.12220. [DOI] [PubMed] [Google Scholar]
- 38.Ishii S, Tanaka T, Shibasaki K, et al. Development of a simple screening test for sarcopenia in older adults. Geriatr Gerontol Int. 2014;14(Suppl 1):93–101. doi: 10.1111/ggi.12197. [DOI] [PubMed] [Google Scholar]
- 39.Reid KF, Martin KI, Doros G, et al. Comparative effects of light or heavy resistance power training for improving lower extremity power and physical performance in mobility-limited older adults. J Gerontol A Biol Sci Med Sci. 2015;70(3):374–80. doi: 10.1093/gerona/glu156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishikawa S, Naito S, Iimori S, et al. Loop diuretics are associated with greater risk of sarcopenia in patients with non-dialysis-dependent chronic kidney disease. PloS One. 2018;13(2):e0192990. doi: 10.1371/journal.pone.0192990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edmunds K, Gislason M, Sigurethsson S, et al. Advanced quantitative methods in correlating sarcopenic muscle degeneration with lower extremity function biometrics and comorbidities. PloS One. 2018;13(3):e0193241. doi: 10.1371/journal.pone.0193241. [DOI] [PMC free article] [PubMed] [Google Scholar]


