Abstract
Higher trait optimism and/or lower cynical hostility are associated with healthier behaviors and lower risk of morbidity and mortality, yet their association with health care utilization has been understudied. Whether these psychological attitudes are associated with breast cancer screening behavior is unknown. To assess the association of optimism and cynical hostility with screening mammography in older women and whether sociodemographic factors acted as mediators of these relationships, we used Women's Health Initiative (WHI) observational cohort survey data linked to Medicare claims. The sample includes WHI participants without history of breast cancer who were enrolled in Medicare Parts A and B for ≥2 years from 2005–2010, and who completed WHI baseline attitudinal questionnaires (n = 48,291). We used survival modeling to examine whether screening frequency varied by psychological attitudes (measured at study baseline) after adjusting for sociodemographic characteristics, health conditions, and healthcare-related variables. Psychological attitudes included trait optimism (Life Orientation Test-Revised) and cynical hostility (Cook Medley subscale), which were self-reported at study baseline. Sociodemographic, health conditions, and healthcare variables were self-reported at baseline and updated through 2005 as available. Contrary to our hypotheses, repeated events survival models showed that women with the lowest optimism scores (i.e., more pessimistic tendencies) received 5% more frequent screenings after complete covariate adjustment (p < .01) compared to the most optimistic group, and showed no association between cynical hostility and frequency of screening mammograms. Sociodemographic factors did not appear to mediate the relationship between optimism and screenings. However, higher levels of education and higher levels of income were associated with more frequent screenings (both p < .01). We also found that results for optimism were primarily driven by women who were aged 75 or older after January 2009, when changes to clinical guidelines lead to uncertainty about risks and benefits of screening in this age group. The study demonstrated that lower optimism, higher education, and higher income were all associated with more frequent screening mammograms in this sample after repeated events survival modeling and covariate adjustment.
Keywords: breast cancer, cynical hostility, optimism, psychological attitudes, screening mammograms
1. Introduction
While regular screenings have clear positive implications for early detection of disease, screening recommendations increasingly weigh the health risks of over-screening as well. Excess detection of early-stage breast cancers, for example, could expose women to treatment-related physical and emotional harms without a clear mortality benefit.[1] Evolving breast cancer screening guidelines therefore attempt to balance the potential risks of over-screening with potential benefits, especially in older women.[2–5] Where evidence is insufficient, guidelines now urge physicians to weigh their patient's “values regarding specific benefits and harms.”[6] These individualized considerations are shaped in part by complex interrelated characteristics including health and socioeconomic status (SES), access to care, race/ethnicity, health risk perceptions, and psychological traits.
White women typically report 12% to 15% higher screening rates than Black and Hispanic women,[9] and women earning ≤125% of the federal poverty level are half as likely as higher-income peers to undergo screenings.[10] Health care access and insurance coverage also impact screening rates[10] independent of race/ethnicity.[5] Yet even as physicians are now required to incorporate patients’ values around screenings, there is a gap in understanding how psychological attitudes influence screening behavior.
Women with high dispositional optimism (positive future expectation[11]) or low cynical hostility (mistrust of others[12]) have lower rates of coronary heart disease, cancer-related mortality, and overall mortality,[8] in part driven by healthier behaviors.[13–19] Higher optimism has also been associated with lower risk of re-hospitalization after coronary artery bypass surgery.[18,20] These psychological attitudes also vary by SES[7] and race/ethnicity,[8] likely due to differential exposures to stress and discrimination. High levels of psychological attitudes of pessimism and cynical hostility, even if influenced by past events, may negatively influence the decision to screen,[7,21] particularly if a woman is more likely to perceive that the test may not make a difference (e.g., pessimistic outlook) or that the healthcare personnel involved in the testing will be disrespectful or unhelpful (e.g., cynical hostile outlook). Yet to our knowledge, no data exist about the role of optimism and cynical hostility with respect to screening mammography in aging postmenopausal women.
The current analysis seeks to extend existing knowledge about optimism, cynical hostility, and screening mammography by linking demographic, psychosocial, and health-related factors from WHI participants to Medicare claims data. We examine whether optimism and cynical hostility are independently correlated with mammogram screening frequency, hypothesizing that
-
(1)
more optimistic and less cynically hostile women would have more frequent screenings, and that
-
(2)
sociodemographic factors may mediate this effect.
2. Methods
2.1. Overview
We examined the association between psychological attitudes (optimism and cynical hostility, measured at study baseline) and mammogram screening frequency using repeated events survival analyses, adjusted first for age only, then additional health and healthcare related factors, and then additionally for sociodemographic covariates. Survival models are appropriate for analyzing data where the outcome is the time to a specific event of interest (i.e., screening mammography), and in this case, the event can happen repeatedly. These models account for multiple screenings per person, and therefore for variation both within and between subjects.
2.2. Study population
Between 1994 and 1998, the WHI,[22] the largest longitudinal study of post-menopausal U.S. women, recruited 161,808 women from diverse racial/ethnic and socioeconomic backgrounds, ages 50 to 79, from 24 states and the District of Columbia. Eligible women participated in either the clinical trial (CT; n = 68,132) or the observational study (OS; n = 93,676).[22] Exclusion criteria relevant to the current study include: substance abuse (except smoking or alcohol), mental illness (severe depression, dementia), life expectancy under 3 years, other randomized trial participation, and plans to move within 3 years.[22] Our analysis includes CT or OS participants still enrolled in the WHI on January 1, 2005 (n = 115,399) who had linked Medicare claims (n = 102,855), had baseline scores for optimism and cynical hostility, were continuously enrolled in fee-for-service (FFS) Medicare Parts A and B for at least 2 years during 2005 to 2010, and were free from breast cancer before 2005 (final n = 48,291, see Fig. 1). Medicare Parts A and B provide coverage for inpatient and outpatient care, respectively, while Medicare Part C includes individuals in managed care plans (whose claims we could not observe), and Part D claims are those for prescription drugs. For the Women's Health Initiative study, institutional review board approval was obtained at each clinical center and all participants were provided written informed consent.
Figure 1.
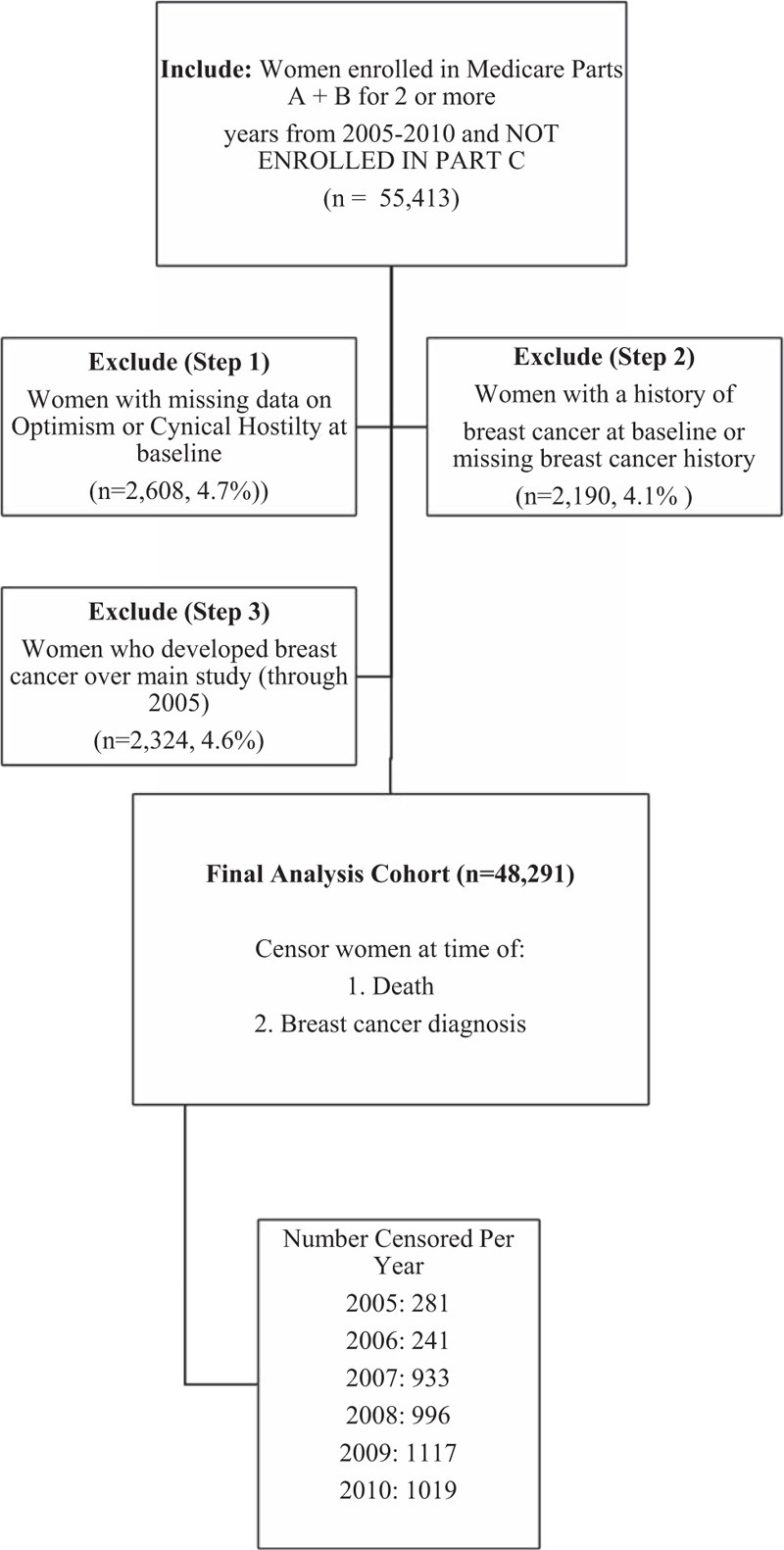
Flowchart of cohort creation. Notes: Censorship rates in 2005 and 2006 are lower because women were required to have at least 2 years of Medicare claims starting in 2005, so censorship in these first 2 years was due to breast cancer diagnosis alone.
2.3. Outcome variable: screening mammograms background
Screening mammograms were recommended every 1 to 2 years by the USPSTF during the study period (2005–2010) for women aged 40 and older (although revised guidelines at the end of 2009 increased the regular screening age from 40 to 50 and concluded that there was insufficient evidence around screenings for women over 75).[6] Screening mammograms were included in Medicare preventive service coverage, and were subject to co-insurance (20% in absence of supplementary policy), but not to deductibles.
2.4. Measuring screenings with claims data
We measured screening mammogram frequency using repeated events survival analysis. Medicare Physician/Supplier Part B Carrier claims (including outpatient physician services) contained screening mammogram records (HCPCS/CPT Codes 76092, 77057, G0202). This objective method of measuring receipt of screening mammograms reduces or eliminates recall bias, which may otherwise be influenced by psychological attitudes. We used a modified published algorithm[23] to recode screening mammograms as diagnostic if they occurred within 9 months of a previous mammogram, or after diagnosis of breast cancer. The WHI-Medicare link has been successfully used to measure utilization in prior work.[24]
2.5. Predictor variables
Baseline optimism was assessed by the 6-item Life Orientation Test-Revised (LOT-R),[11] with scores ranging from 6 to 30; higher scores indicate greater optimism and lower scores indicate greater pessimism. Women were classified by quartile of score in the analysis sample, consistent with prior studies in the WHI:[8] Least (<22), Mid-low (22 to <24), Mid-high (24 to <26), Most (26+).
Baseline cynical hostility was assessed using the 13-item cynicism subscale of the Cook Medley hostility questionnaire. Scores range from 0 to 13, with higher scores indicating greater cynicism.[25] Women were classified by quartile of score in the analysis sample, consistent with prior studies:[8] Least (<1), Mid-low (1 to <3), Mid-high (3 to <5), Most (5+).
2.6. Covariates and potential confounders
All survival models adjust for age on Jan 1, 2005 (continuous). Subsequent models also adjust for the following covariates: OS or CT (active hormone therapy, placebo arm, or not randomized into hormone therapy, including Dietary Modification trial participants), and original Medicare eligibility (65+, disability, end-stage renal disease). Factors capturing potential screening barriers included lack of insurance at WHI baseline (which could have preceded Medicare enrollment)[26] and having a regular medical provider.[27] Depressive symptoms[28] and social support[29] were included for their association with cancer screenings. Depressive symptoms were measured using the Burnam Screening Algorithm,[30] a questionnaire that includes 6 items from the Center for Epidemiologic Studies Depression Scale (CES-D) and 2 items from the Diagnostic Interview Scale (DIS), with a cutoff of ≥0.06 indicating depression.[30] The WHI social support construct is a continuous measure using 9 items selected from the Medical Outcomes Study).[31] Baseline health behaviors associated with breast cancer screening or risk included alcohol consumption,[32] exercise[33] (MET-hours/week <2.5, 2.5 to <18.5, and 18.5 or greater), high cholesterol requiring pills ever,[34] and family history of first-degree female relatives with breast cancer (none, or ≥1). Additional health factors associated with screenings or cancer risk were updated through 2005 when available: smoking status (current, past, or never smoker),[32] obesity (BMI ≥ 30[35]), hypertension ever,[36] diabetes ever,[37] and breast biopsies (0, 1, or ≥2). Family or personal history of breast cancer or benign breast disease is associated with higher mammography utilization.[32]
Models including sociodemographic variables additionally adjust for race/ethnicity, education, and income as self-reported at WHI baseline: race/ethnicity (White, Black, Hispanic/Latina, Asian or Pacific Islander, American Indian or Alaskan Native, and Other which could include mixed race); education (less than high school, high school or GED, some school past high school, college or some post-graduate/post-professional training, and graduate degree or higher); gross total family annual income (reported incomes collapsed into 4 categories: <$20,000; $20,000-$49,999; $50,000 to $74,999; and ≥$75,000).
2.7. Statistical methods
2.7.1. Descriptive methods
We described baseline sample characteristics according to race/ethnicity, income, education, mean optimism and cynical hostility scores, and covariates noted above (n = 48,291). We also described the distribution of mean optimism and cynical hostility at various race/ethnicity, education, and income levels, and tested for significant associations using chi-squared tests.
2.7.2. Longitudinal methods
Precise mammography screening frequency was examined using multivariate repeated events survival analyses, adjusted for relevant covariates, in 3 sets of models using Cox proportional hazards models for conditional risk set recurrent event data with a time scale measured in years (PROC PHREG, SAS Version 9.3).[38] Variance of the coefficients was adjusted using the robust sandwich estimator to address within-person correlation of events.
Survival analyses model the “hazard” of a specific event occurring over time (e.g., any number of days, weeks, years, etc). Rather than specifying an interval over which an event may have occurred (e.g., whether a woman received a mammogram in 2 years), these models account for the fact that the time intervals until an event occurs will vary, and that this variation (1 year vs 5 years until an event occurs, for example) yields additional important data. Cox proportional hazards regression models are a class of regression models for this type of data which also allow for covariate adjustment.
Survival models better characterize the frequency of screenings over time and account for events which might alter normal screening timelines, such as receipt of a diagnostic mammogram without diagnosis of breast cancer (effectively extending the time a woman could wait before additional screening). In our analysis, each woman enters the risk set at a valid starting event, and is assumed not to be at risk of a second event until her first event is complete, thus “resetting the clock.” Valid starting events included: study start, screening mammogram, or a diagnostic mammogram without breast cancer diagnosis. Screenings and/or diagnoses that occurred within 8 weeks of each other were considered part of the same event; therefore, if a woman had a screening mammogram followed by diagnosis of breast cancer within 8 weeks this would count as a screening “start point” and a diagnosis “end point” (censoring following data) and the days in between were excluded from analyses. Women were removed from the risk set when the study period ended, or due to breast cancer diagnosis or death (for examples of screening timelines, see Fig. 2).
Figure 2.
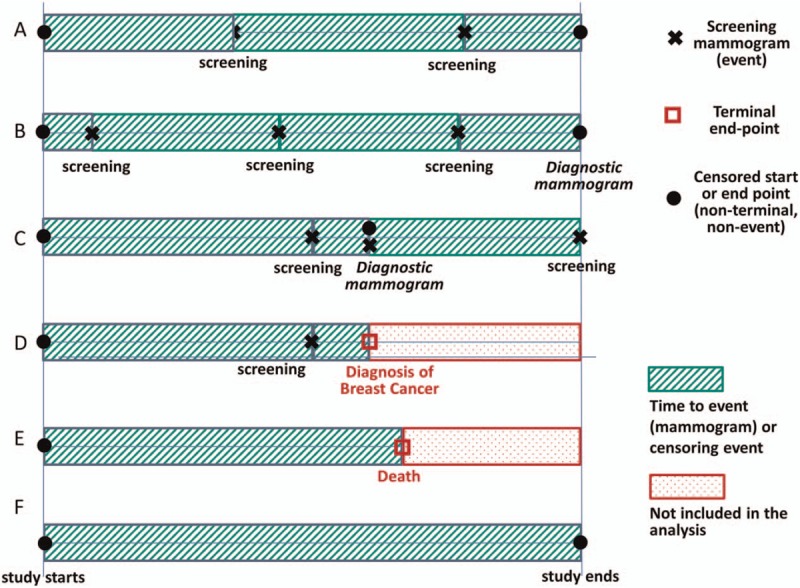
Screening mammography timelines in survival models. Notes: the figure above provides examples of potential individual participant timelines and how these timelines are interpreted for survival models. Intervals with diagonal lines are included in the analyses; those with dots are excluded from analyses because they follow either death or a breast cancer diagnosis. Screening mammograms (designated with a black “X”) are considered events, and survival models assess how the hazard for the event (screening mammogram) differs for women with, for example, least vs most optimism scores. A diagnosis of breast cancer or death (both designated with a square) are terminal end points, meaning that the individual is no longer eligible for the event. “Censored” start and end points indicate that the exact date of the previous or subsequent screening mammogram (the event) is unknown, for example, at the beginning of the observation period, and at the end of the study follow-up period. Diagnostic mammograms are considered definite start points (they re-set a woman's clock for a potential new screening at that exact time); however, they are considered censored end-points (they are not the screening event of interest, so they function similar to ending an eligible follow-up window). In the figure above, Timeline A represents a woman with 2 screening mammograms during her follow up, and ends at the study end (censored end point). Timelines B and C include diagnostic mammograms as well as screening mammograms. Timelines D and E show a case where follow-up is ended due to breast cancer diagnosis (D) or death (E). Finally, Timeline F shows a case where a woman has no events during her entire follow-up period and until the study ends.
Model 1 examined the main effects of optimism and cynical hostility as predictors for screenings (both attitudes modeled together in a single model) after adjustment for age. Model 2 includes Model 1, and also adjusts for health- and healthcare variables. In Model 3, variables for race/ethnicity, income, and education were added to Model 2 to observe the extent to which these variables mediated the association between attitudes and screening frequency. We considered sociodemographic variables to have a mediation-like effect if, when added, the attitude effects became non-significant. Finally, a fourth model (Model 4) included all variables in Model 3, plus the interaction between quartiles of each attitude and whether women were age 75 or older during an eligible screening interval that also occurred after January 1, 2009. This model was added to account for the possibility that the USPSTF guideline revisions at the end of 2009, which introduced more uncertainty when screening women age 75 or older, may drive differential patterns in mammography by age.
We considered P values less than .05 to be statistically significant.
3. Results
3.1. Descriptive characteristics
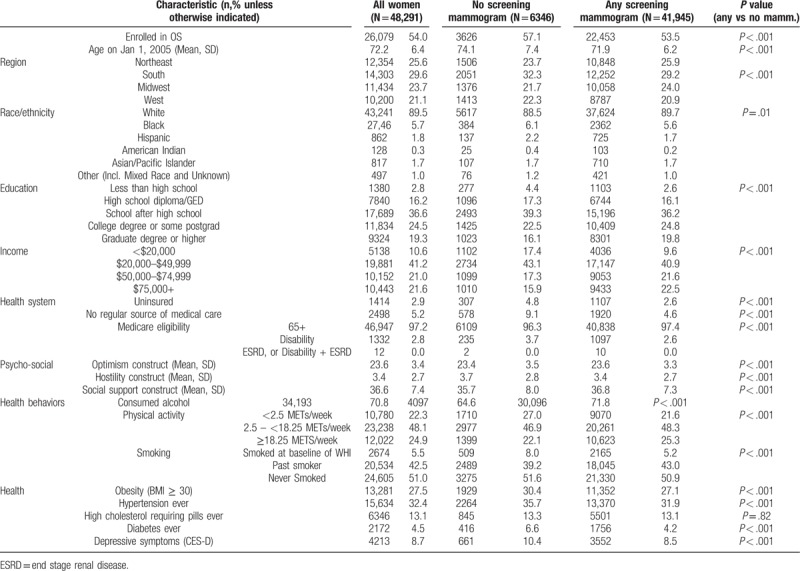
Overall, women had an average age of 72.2 (SD 6.4), were primarily white (89.5%) and were relatively well-educated with high-income: only 2.8% did not complete high school and 10.6% reported household incomes under $20,000 annually (Table 1). Women with no screenings vs any screening mammograms were significantly more likely to be older, racial/ethnic minorities, lower SES, disabled, uninsured, and without a regular medical provider. These women with no screenings were also more likely to be current or past smokers, reported less exercise, and were less likely to report any alcohol consumption. Women with no (vs any) screenings also had slightly lower mean optimism scores (23.4 vs 23.6, P < .001, which matched the mean scores for the original enrollment cohort) and slightly higher mean cynical hostility scores (3.7 vs 3.4, P < .001, which also matched means for the original enrollment cohort). By contrast, women with any screening mammograms (vs no screenings) reported higher social support. Optimism quartile distributions by score were as follows: Least (23%), Mid-Low (25%), Mid-High (24%), Most (28%). Hostility quartile distributions by score were as follows: Least (15%), Mid-Low (26%), Mid-High (27%), Most (31%). We did not find evidence that our Exclusion Steps or Censoring criteria impacted the distribution of attitudes, compared to the enrollment cohort.
Table 1.
Baseline characteristics and comparison of women with none vs any screening mammograms.
Mean optimism was generally lower for minority women (except for black women, who resembled whites, Fig. 3). Mean cynical hostility was higher for minority groups, with the exception of Asian/Pacific islander women. Optimism generally increased with increased education and income, while the reverse was true for cynical hostility. Differences were statistically significant by quartiles of optimism and cynical hostility (chi-square tests, all P < .01).
Figure 3.
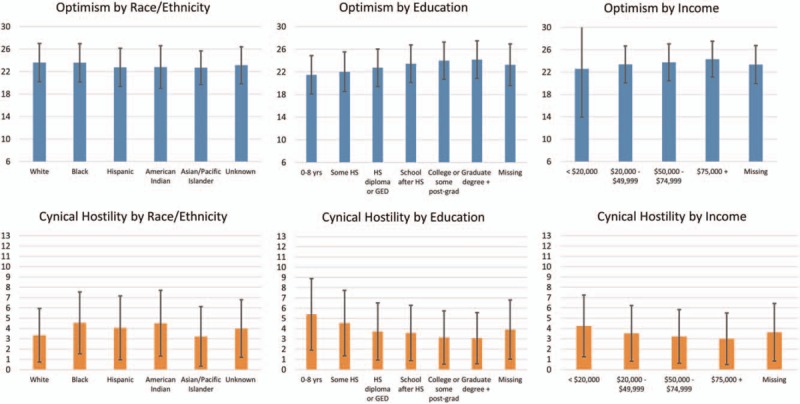
Distribution of attitudes by race/ethnicity and SES variables.
3.2. Frequency of screening mammograms
Proportional hazards assumptions were satisfied for all survival models (modeling until first screening event), meaning that the hazard of screening for different levels of each independent variable is proportional across the screening interval (e.g., level 1 vs 2 of a variable confers twice the risk of screening across the entire screening interval)
In Model 1, which included each attitude plus adjustment for age, optimism was not significant (P = .10), while most (vs least) cynically hostile women had 5% lower hazard of screening (P < .01, Table 2).
Table 2.
Repeated events survival analysis of optimism, cynical hostility, race/ethnicity and socioeconomic status with screening mammograms (robust variance estimator).
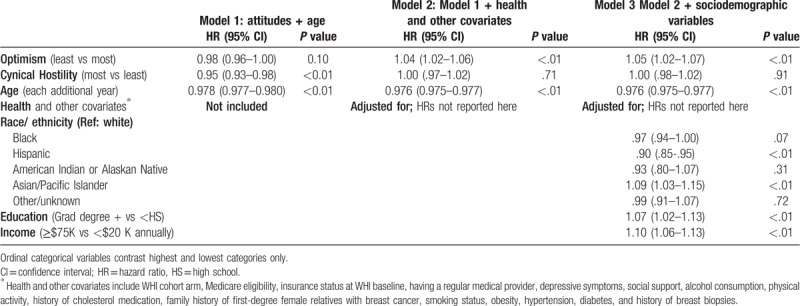
In Model 2, which additionally adjusted for a host of health and other covariates, least optimistic women had 4% more frequent screenings compared to the most optimistic quartile of women (P < .01, Table 2). Cynical hostility was not significantly associated with obtaining screening mammograms after these additional covariate adjustments.
In Model 3, least vs most optimistic women had 5% more frequent screening mammograms (P < .01), while cynical hostility remained non-significant. Sociodemographic variables did not appear to mediate the association between attitudes and screening mammogram frequency, after adjusting for health and healthcare related variables. The results for optimism and cynical hostility, after adjustment for the health and other covariates included in Model 2, remained robust to other model specifications including: modeling attitudes as continuous variables, modeling each attitude independently, and assessing the constructs of optimism and pessimism individually via the optimism and pessimism subscales.
In Model 3, several sociodemographic variables were significant independent predictors of mammogram screening frequency, even after adjusting for attitudes and for health and healthcare related variables. The most educated women in the sample had 7% more frequent screenings than those without a high school degree (P = <.01). Those women in the highest income bracket (whose household earnings were at least $75,000 annually) had 10% more frequent screenings than those women with household incomes under $25,000 annually (P < .01). Models indicated significant differences in screenings across racial/ethnic groups; however, due to smaller sample sizes the individual group estimates should be interpreted with caution. Compared to white women (reference group), Hispanic women had 10% less frequent screenings (P < .01), while Asian and Pacific Islander women had 9% more frequent screenings (P < .01).
In Model 4 (not shown), we found a significant interaction (overall interaction P value <.01) between optimism and whether a woman was age 75 or older during an eligible screening interval that also occurred after January 1, 2009 (the year of the USPSTF policy change). This interaction showed that overall model results were driven primarily by women who were 75 or older during eligible screening intervals (HR: 1.05, 95% CI 1.03–1.08), whereas women who had not turned 75 during their eligible screening intervals did not show a significant effect of optimism (HR 0.99, 95% CI 0.95–1.03). We did not find a significant interaction between cynical hostility and this age cutoff (P = .71).
4. Discussion
Contrary to our hypotheses, repeated events survival models showed that after adjusting for age as well as health and other covariates, women with the lowest optimism scores (i.e., women with more pessimistic tendencies) received 5% more frequent screenings than those in the most optimistic group. Also contrary to our hypotheses, there was no association between cynical hostility and frequency of screening mammograms after adjusting for age as well as health and other covariates. However, consistent with expectations and with past literature,[10] higher income, education, and identifying as an Asian/Pacific Islander was associated with higher screening frequency, while identifying as Hispanic/Latina was associated with lower screening frequency. Finally, we found that results for optimism were primarily driven by women who were 75 or older during an eligible screening interval that also occurred after January 1, 2009.
Our results for least optimistic women receiving more frequent screenings were surprising, based on a large body of literature demonstrating that more optimistic women tend to be healthier and adhere to medical advice more readily than less-optimistic peers,[16,18] and would therefore be expected to undergo screening at higher rates. One explanation for the fact that we see differences between the simplest models and those adjusted for other covariates beyond age is that we have adjusted for factors which may operate to increase the likelihood of screening in the presence of higher trait optimism, including patterns of healthier behaviors (less smoking, more physical activity) and overall better physical health (lower average BMI, less hypertension, less diabetes) and mental health (fewer depressive symptoms). With greater consideration, these findings make sense based on a literature demonstrating that people with pessimistic tendencies use more primary care physician visits, specialty care, and hospital treatment.[39] Having more exposure to the health system may increase the opportunity for screening to be recommended or at least discussed by health care providers. Our work also adds directly to existing literature by rigorously measuring preventive mammography utilization over time using repeated events survival analysis, rather than simply assessing whether or not any screenings occurred, and by using claims data instead of self-report, thus reducing potential validity threats based on differential recall.
We found significant associations between screenings and income, education, and race/ethnicity. These factors may capture barriers to receipt of screenings, but may also capture cultural differences in perceived risks and benefits of screenings: for example, some studies document lower perceived benefit of mammography among Hispanic/Latina women who do not have any symptoms of breast cancer.[40] The current study is not designed to capture such differences in perceptions of risks and benefits, which may vary by race/ethnicity, cultural factors, and medical practice patterns in one's community or country of origin. However, these factors could supersede health insurance, income, and other factors in driving screening behaviors. There may be additional factors not captured in this analysis that could disproportionately hinder Hispanic/Latina women, or enable Asian/Pacific Islander women, when it comes to screening mammography.
We found that older optimists (i.e., those facing uncertainty about risks and benefits of screening after the change to the USPSTF recommendations) were less likely to receive screening mammograms. Changes to screening recommendations likely increase uncertainty and anxiety around the risks and benefits of screenings for both patients and providers, and patients’ dispositional attitudes about the future are likely to influence how they manage this uncertainty. This underscores the importance of comprehensive shared decision making conversations with providers, especially given that studies have shown providers most often discuss benefits of screenings, but do not routinely address screening risk or ask patients about their screening preferences.[41] This may be in part because those rare clinical practice guidelines that do advise shared decision-making often “provide no guidance about how to do this and communicate the evidence in a way patients will understand”.[42]
5. Limitations
Despite the strengths of this analysis, it has several important limitations. WHI women were typically healthier than post-menopausal woman on average in the U.S. Only baseline measurements of optimism and cynical hostility were used, and although they are considered relatively stable traits, they can change over time[43] or in the presence of targeted interventions.[44,45] This analysis could not observe women's utilization in Medicare part C (and these women were thus excluded), or the presence of supplemental coverage (Medigap) plans which are more likely to be purchased by higher SES women and which could lower costs for individual screenings. Our identification of women eligible for screening mammograms based on available health and demographic characteristics may under or over-estimate eligibility especially if health conditions are miscoded. This study was not designed to observe the content of patient-provider interactions which preceded screening mammograms. The WHI also does not measure individuals’ perception regarding breast or other cancer risk, which may be inaccurate. For example, underestimation of true objective risk is termed the “optimistic bias”, which is distinct from, but often confused with, dispositional optimism.[46,47] In studies that have measured both dispositional optimism and optimistic bias (unrealistic optimism), dispositional optimists tend to have accurate perceptions of their health risks and benefits (i.e., they do not have a so-called optimistic bias).[48] Therefore, underestimation of breast cancer risk is unlikely to explain the observed lower rates of mammography screening among women with higher levels of dispositional optimism. Additionally, it would be difficult to generalize these results to other screening behaviors in men or to other types of screenings.
6. Conclusions and policy implications
Lower optimism, higher education, and higher income all predicted more frequent screening mammograms in this sample after repeated events survival modeling and covariate adjustment. Guidelines for screening mammography which place increased decision-making burden on an individual's perception of risk may result in women with certain attitudinal traits being more or less likely to receive screenings. Physicians should proceed cautiously when applying new and evolving guidelines which place increased decision-making burden on a woman's individual perceptions of risk and belief that good or bad things may happen to her. More work is needed to determine whether these differences may influence over- or under-screening for certain groups of women. Comprehensive shared decision-making for screening mammograms in the face of uncertainty about risks and benefits should help ensure that women are making fully informed decisions, regardless of dispositional attitude or prior perceptions of risk.
Author contributions
Conceptualization: Ana M. Progovac, Julie M. Donohue, Chung-Chou H. (Joyce) Chang, Karen A. Matthews, Elizabeth B. Habermann, Lewis H. Kuller, Hilary A. Tindle.
Formal analysis: Ana M. Progovac, Mary Pettinger, Chung-Chou H. (Joyce) Chang, Hilary A. Tindle.
Funding acquisition: Hilary A. Tindle.
Investigation: Ana M. Progovac, Lewis H. Kuller, Milagros Rosal, Wenjun Li, Lorena Garcia, Hilary A. Tindle.
Methodology: Ana M. Progovac, Mary Pettinger, Julie M. Donohue, Chung-Chou H. (Joyce) Chang, Karen A. Matthews, Elizabeth B. Habermann, Milagros Rosal, Wenjun Li, Lorena Garcia, Hilary A. Tindle.
Resources: Lewis H. Kuller, Hilary A. Tindle.
Software: Mary Pettinger.
Supervision: Julie M. Donohue, Chung-Chou H. (Joyce) Chang, Karen A. Matthews, Elizabeth B. Habermann, Lewis H. Kuller, Hilary A. Tindle.
Writing – original draft: Ana M. Progovac, Julie M. Donohue, Karen A. Matthews, Elizabeth B. Habermann, Hilary A. Tindle.
Writing – review & editing: Ana M. Progovac, Mary Pettinger, Julie M. Donohue, Chung-Chou H. (Joyce) Chang, Karen A. Matthews, Elizabeth B. Habermann, Milagros Rosal, Wenjun Li, Lorena Garcia, Hilary A. Tindle.
Ana M. Progovac orcid: 0000-0002-8011-9305.
Ana Progovac orcid: 0000-0002-8011-9305.
Footnotes
Abbreviations: BMI = body mass index, CES-D = Center for Epidemiologic Studies Depression Scale, CT = clinical trial, FDA = Food & Drug Administration (U.S.), FFS = fee for service, HCPCS/CPT = Healthcare Common Procedure Coding System/Common Procedural Terminology System, HR = hazard ratio, LOT-R = Life Orientation Test-Revised, MET = metabolic equivalent, OS = observational study, SES = socioeconomic status, USPSTF = U.S. Preventive Services Task Force, WHI = Women's Health Initiative.
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, US Department of Health and Humans Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
The authors have no conflicts of interest to report.
This manuscript was prepared in collaboration with investigators of the WHI and has been reviewed and approved by the Women's Health Initiative (WHI). The short list of WHI investigators can be found at https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Short%20List.pdf
References
- [1].Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med 2012;367:1998–2005. [DOI] [PubMed] [Google Scholar]
- [2].Myers ER, Moorman P, Gierisch JM, et al. Benefits and harms of breast cancer screening: a systematic review. JAMA 2015;314:1615–34. [DOI] [PubMed] [Google Scholar]
- [3].Schonberg MA, McCarthy EP, Davis RB, et al. Breast cancer screening in women aged 80 and older: results from a national survey. Am Geriatr Soc 2004;52:1688–95. [DOI] [PubMed] [Google Scholar]
- [4].Siu AL, Bibbins-Domingo K, Grossman DC, et al. Convergence and divergence around breast cancer screening. Ann Intern Med 2016;164:301–2. [DOI] [PubMed] [Google Scholar]
- [5].Jiang M, Hughes DR, Duszak R., Jr Screening mammography rates in the Medicare population before and after the 2009 U.S. preventive services task force guideline change: an interrupted time series analysis. Women's Health Issues 2015;25:239–45. [DOI] [PubMed] [Google Scholar]
- [6].United States Preventive Services Task Force. Screening for breast cancer: U.S. preventive services task force recommendation statement. Ann Intern Med 2009;151:716–26. [DOI] [PubMed] [Google Scholar]
- [7].Robb KA, Simon AE, Wardle J. Socioeconomic disparities in optimism and pessimism. Int J Behav Med 2009;16:331–8. [DOI] [PubMed] [Google Scholar]
- [8].Tindle HA, Chang Y-F, Kuller LH, et al. Optimism, cynical hostility, and incident coronary heart disease and mortality in the women's health initiative. Circulation 2009;120:656–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Caplan LS, Wells BL, Haynes S. Breast cancer screening among older racial/ethnic minorities and whites: barriers to early detection. J Gerontol 1992. 101–10. 47 Spec No. [PubMed] [Google Scholar]
- [10].Sambamoorthi U, McAlpine DD. Racial, ethnic, socioeconomic, and access disparities in the use of preventive services among women. Prev Med 2003;37:475–84. [DOI] [PubMed] [Google Scholar]
- [11].Scheier M, Carver C, Bridges M. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. J Pers Soc Psychol 1994;67:1063–78. [DOI] [PubMed] [Google Scholar]
- [12].Cook WW, Medley DM. Proposed hostility and Pharisaic-virtue scales for the MMPI. J Appl Psychol 1954;38:414–8. [Google Scholar]
- [13].Tindle HA, Davis E, Kuller LH. Attitudes Cardiovasc Disease. Maturitas 2010;67:108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rasmussen HN, Scheier MF, Greenhouse JB. Optimism and physical health: a meta-analytic review. Ann Behav Med 2009;37:239–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Scherwitz LW, Perkins LL, Chesrtey MA, et al. Hostility and health behaviors in young adults: the CARDIA study. Am J Epidemiol 1992;136:136–45. [DOI] [PubMed] [Google Scholar]
- [16].Tinker LF, Rosal MC, Young AF, et al. Predictors of dietary change and maintenance in the women's health initiative dietary modification trial. J Am Diet Assoc 2007;107:1155–65. [DOI] [PubMed] [Google Scholar]
- [17].Brunner R, Dunbar-Jacob J, Leboff MS, et al. Predictors of adherence in the women's health initiative calcium and vitamin D trial. Behav Med 2009;34:145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tindle HA, Belnap BH, Houck PR, et al. Optimism, response to treatment of depression, and rehospitalization after coronary artery bypass graft surgery. Psychosom Med 2012;74:200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Progovac AM, Chang Y-F, ChangF C-CH, et al. Are optimism and cynical hostility associated with smoking cessation in older women? Ann Behav Med 2017;51:1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Halpin LS, Barnett SD. Preoperative state of mind among patients undergoing CABG: effect on length of stay and postoperative complications. J Nurs Care Qual 2005;20:73–80. [DOI] [PubMed] [Google Scholar]
- [21].Gallo LC, Matthews KA. Understanding the association between socioeconomic status and physical health: do negative emotions play a role? Psychol Bull 2003;129:10–51. [DOI] [PubMed] [Google Scholar]
- [22].Hays J, Hunt JR, Hubbell FA, et al. The women's health initiative recruitment methods and results. Ann Epidemiol 2003;139 Supplement:S18–77. [DOI] [PubMed] [Google Scholar]
- [23].Fenton JJ, Zhu W, Balch S, et al. Distinguishing screening from diagnostic mammograms using Medicare claims data. Med Care 2014;52:e44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lakshminarayan K, Larson JC, Virnig B, et al. Comparison of Medicare claims versus physician adjudication for identifying stroke outcomes in the Women's Health Initiative. Stroke 2014;45:815–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cook W, Medley D. Proposed hostility and Pharisaic-virtue scales for the MMPI. J Appl Psychol 1954;38:414–8. [Google Scholar]
- [26].Watson-Johnson LC, DeGroff A, Steele CB, et al. Mammography adherence: a qualitative study. J Womens Health 2011;20:1887–94. [DOI] [PubMed] [Google Scholar]
- [27].Azami-Aghdash S, Ghojazadeh M, Sheyklo SG, et al. Breast cancer screening barriers from the woman's perspective: a meta-synthesis. Asian Pac J Cancer Prev 2015;16:3463–71. [DOI] [PubMed] [Google Scholar]
- [28].Penninx BWJH, Guralnik JM, Havlik RJ, et al. Chronically depressed mood and cancer risk in older persons. J Natl Cancer Inst 1998;90:1888–93. [DOI] [PubMed] [Google Scholar]
- [29].Messina CR, Lane DS, Glanz K, et al. Relationship of social support and social burden to repeated breast cancer screening in the women's health initiative. Health Psychol 2004;23:582–94. [DOI] [PubMed] [Google Scholar]
- [30].Burnam MA, Wells KB, Leake B, et al. Development of a brief screening instrument for detecting depressive disorders. Med Care 1988;26:775–89. [DOI] [PubMed] [Google Scholar]
- [31].Matthews KA, Shumaker SA, Bowen DJ, et al. Women's health initiative: why now? What is it? What's new? Am Psychol 1997;52:101–16. [DOI] [PubMed] [Google Scholar]
- [32].Schueler KM, Chu PW, Smith-Bindman R. Factors associated with mammography utilization: a systematic quantitative review of the literature. J Womens Health (Larchmt) 2008;17:1477–98. [DOI] [PubMed] [Google Scholar]
- [33].Carpenter CL, Ross RK, Paganini-Hill A, et al. Lifetime exercise activity and breast cancer risk among post-menopausal women. Br J Cancer 1999;80:1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Furberg A-S, Veierød MB, Wilsgaard T, et al. Serum high-density lipoprotein cholesterol, metabolic profile, and breast cancer risk. J Nat Cancer Inst 2004;96:1152–60. [DOI] [PubMed] [Google Scholar]
- [35].Neuhouser ML, Aragaki AK, Prentice RL, et al. Overweight, obesity, and postmenopausal invasive breast cancer risk: a secondary analysis of the women's health initiative randomized clinical trials. JAMA Oncol 2015;1:611–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tournberg SA, Holm LE, Carstensen JM. Breast cancer risk in relation to serum cholesterol, serum beta-lipoprotein, height, weight, and blood pressure. Acta Oncol 1988;27:31–7. [DOI] [PubMed] [Google Scholar]
- [37].Talamini R, Franceschi S, Favero A, et al. Selected medical conditions and risk of breast cancer. Br J Cancer 1997;75:1699–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hosmer D, Lemeshow S. Applied Survival Analysis: Regression Modeling of Time to Event Data. New York: John Wiley; 1999. [Google Scholar]
- [39].Bock JO, Hajek A, Konig HH. The longitudinal association between psychological factors and health care use. Health Serv Res 2017;53:1065–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Austin LT, Ahmad F, McNally M-J, et al. Breast and cervical cancer screening in Hispanic women: a literature review using the health belief model. Women's Health Issues 2002;12:122–8. [DOI] [PubMed] [Google Scholar]
- [41].Hoffman RM, Lewis CL, Pignone MP, et al. Decision-making processes for breast, colorectal, and prostate cancer screening: the DECISIONS survey 2010;305_suppl:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hoffmann TC, Montori VM, Del Mar CJJ. The connection between evidence-based medicine and shared decision making 2014;312:1295–6. [DOI] [PubMed] [Google Scholar]
- [43].Chapman BP, Roberts B, Duberstein PR. Personality and longevity: knowns, unknowns, and implications for public health and personalized medicine. J Aging Res 2011;2011:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Borders A, Earleywine M, Jajodia A. Could mindfulness decrease anger, hostility, and aggression by decreasing rumination? Aggress Behav 2010;36:28–44. [DOI] [PubMed] [Google Scholar]
- [45].Malouff JM, Schutte NS. Can psychological interventions increase optimism? A meta-analysis. J Posit Psychol 2017;12:594–604. [Google Scholar]
- [46].Shepperd JA, Waters E, Weinstein ND, et al. A primer on unrealistic optimism. Curr Dir Psychol Sci 2015;24:232–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Radcliffe NM, Klein WMP. Dispositional, unrealistic, and comparative optimism: differential relations with the knowledge and processing of risk information and beliefs about personal risk. Personal Soc Psychol Bull 2002;28:836–46. [Google Scholar]
- [48].Jansen LA, Mahadevan D, Appelbaum PS, et al. Dispositional optimism and therapeutic expectations in early-phase oncology trials. Cancer 2016;122:1238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]


