Abstract
Prothoracicotropic hormone (PTTH) is a neuropeptide that triggers a cascade of events within the prothoracic gland (PG) cells, leading to the activation of all the crucial enzymes involved in ecdysone biosynthesis, the main insect steroid hormone. Studies concerning ecdysteroidogenesis predicted PTTH action using brain extract (BE), consisting in a complex mixture in which some components positively or negatively interfere with PTTH-stimulated ecdysteroidogenesis. Consequently, the integration of these opposing factors in steroidogenic tissues leads to a complex secretory pattern. A recombinant form of prothoracicotropic hormone (rPTTH) from the tobacco budworm Heliothis virescens (F.) (Lepidoptera: Noctuidae) was expressed and purified to perform in vitro tests in a standard and repeatable manner. A characterization of rPTTH primary and secondary structures was performed. The ability of rPTTH and H. virescens BE to stimulate ecdysteroidogenesis was investigated on the third day of fifth larval stage. rPTTH activity was compared with the BE mixture by enzyme immunoassay and western blot, revealing that they equally stimulate the production of significant amount of ecdysone, through a transduction cascade that includes the TOR pathway, by the phosphorylation of 4E binding protein (4E-BP) and S6 kinase (S6K), the main targets of TOR protein. The results of these experiments suggest the importance of obtaining a functional pure hormone to perform further studies, not depending on the crude brain extract, composed by different elements and susceptible to different uncontrollable variables.
Keywords: ecdysteroidogenesis, prothoracic glands, recombinant prothoracicotropic hormone, brain extract, Heliothis virescens
In insects, ecdysteroids biosynthesis and secretion by prothoracic glands (PGs) or analogous organs play an essential role in molting and metamorphosis regulation (Iga and Kataoka 2012, Hentze et al. 2013). Following the neuropeptidal stimuli of prothoracicotropic hormone (PTTH), PGs secrete ecdysone (McBrayer et al. 2007, Iga and Kataoka 2012). PTTH is produced by the neurosecretory cells of the brain, the pars intercerebralis, and it is stored in the corpora cardiaca, a pair of neuroglandular organs located behind the brain (Agui et al. 1979, Dai et al. 1994, Gullan and Cranston 2005). Once released by corpora cardiaca, PTTH acts on PGs, binding the specific tyrosine kinase receptor, Torso, on the cellular membrane, in order to activate multiple signal transduction cascades (Rewitz et al. 2009). To date, the entire process involving PTTH stimulation and protein biosynthesis in PGs is not completely elucidated, but it is clear that PTTH positively modulates cAMP increase and intracellular Ca2+ release, as observed in Manduca sexta (Linnaeus, 1763) (Lepidoptera: Sphingidae) (Smith et al. 1985, 1986; Fellner et al. 2005) and Bombyx mori (Linnaeus, 1758) (Lepidoptera: Bombycidae) (Gu et al. 1996, 1998). Moreover, following the binding with its specific receptor, PTTH leads to the activation of two specific signal transduction cascades: MAPK and PI3K/Akt/TOR. These two pathways, differently activated depending on lepidopteran species, promote protein translation of various enzymes resulting in ecdysone biosynthesis from cholesterol. It was demonstrated that in B. mori (Lin and Gu 2007, Gu et al. 2012) and Heliothis virescens (Fabricius, 1777) (Scieuzo et al. 2018) both pathways are involved in PTTH-stimulated ecdysteroidogenesis, whereas in M. sexta, the only one MAPK pathway seems to be activated (Rybczynski et al. 2001). In M. sexta, B. mori, and H. virescens, PTTH stimulation also increases protein biosynthesis and phosphorylation, in order to support ecdysone production by PGs (Rybczynski and Gilbert 1994, Pennacchio et al. 1997, Gu et al. 2012). PTTH was firstly isolated and characterized in B. mori (Kataoka et al. 1991), in which it is synthesized as pre-pro-hormone and subsequently processed in the brain, to obtain a glycosylated homodimer with active subunits of 109 amino acids, with three intra- and one interchain disulfide bonds (Ishibashi et al. 1994). Heliothis virescens PTTH shows high similarity with the entire Helicoverpa zea (Boddie, 1850) (Lepidoptera: Noctuidae) PTTH sequence (Wei et al. 2005) and with the active portion of B. mori, M. sexta, Antheraea pernyi (Guerin Meneville, 1855) (Lepidoptera: Saturniidae), Samia cynthia ricini (Drury, 1773) (Lepidoptera: Saturniidae), and Hyalophora cecropia (Linnaeus, 1758) (Lepidoptera: Saturniidae) PTTH sequence (Xu et al. 2003). In this work, a recombinant form of H. virescens PTTH (rPTTH), suitable to perform in vitro tests in a standard and repeatable manner, was expressed and purified. Moreover, the functionality of increasing concentration of rPTTH on PGs was evaluated by in vitro tests (enzyme immunoassay and western blot), in comparison to H. virescens PTTH contained in the brain extract (BE). This is to define whether the rPTTH alone or other components of the BE are responsible for triggering the transduction cascade and modulate ecdysteroidogenesis in PGs.
Results highlight that PTTH is the active component of the BE mixture, strengthening the results obtained in previous studies performed using the BE and aimed to clarify the mechanism by which the hormone stimulates ecdysteroidogenesis.
Materials and Methods
Insect Rearing and Staging
Heliothis virescens larvae were reared according to Vinson et al. (1973) on a standard artificial diet developed by Vanderzant et al. (1962). Rearing temperature was maintained at 29 ± 1°C, under a photoperiod of 16:8 (L:D) and relative humidity 70 ± 5%. Last (fifth) instar larvae were staged according to Webb and Dahlman (1985) and synchronized as reported by Pennacchio et al. (1992).
RNA Extraction, cDNA Synthesis, and Polymerase Chain Reaction
Total RNA from an equal number of brains collected from 3- to 4-d-old last (fifth) instar larvae was extracted using TRI-Reagent (Sigma–Aldrich, St. Louis, MO), according to the manufacturer’s protocol. An additional DNase treatment (Invitrogen, Waltham, MA) was carried out before the second purification step to remove any contaminating DNA. RNA was further purified by the RNeasy MinElute Clean-up Kit (Qiagen, Venio, the Netherlands) and eluted in 20 µl of RNA Storage Solution (Ambion Inc., Austin, TX), following the manufacturer’s protocol. RNA integrity was verified on an Agilent 2100 Bioanalyzer using RNA nano chips (Agilent Technologies, Palo Alto, CA) and RNA quantity was determined by a Nanodrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA). cDNA coding for the mature active form of PTTH was obtained using 5 µg of total RNA by SuperScript III Reverse Transcriptase (Invitrogen), according to the manufacturer’s protocol. cDNA was amplified by polymerase chain reaction (PCR), using the following primers:
HvirPTTHpET32FOR: 5′-GACGACGACAAGATGGGTAACATTAAAGTCGAGAAG-3′
HvirPTTHpET32REV: 5′-GAGGAGAAGCCCGGTTATTTCTCAGTAGCGTAGTAA-3′
Primers were designed starting from the nucleotide sequence of PTTH cDNA (AY172671.1), obtained from the databases available at the National Center for Information and Biotechnology (NCBI) (www.ncbi.nlm.nih.gov/). The underlined extensions were added to allow a direct cloning of the fragment into the expression vector pET32 Ek/LIC, in frame with Trx, His, and S tags (a total of 17 kDa). PCR amplification was performed using the high-fidelity Taq, KOD DNA Polymerase (Novagen, Madison, WI), according to the manufacturer’s protocol, in a thermocycler GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA).
Cloning, Expression, and Purification of rPTTH
The amplimer, coding for the active form of PTTH, was purified using Quantum Prep Freeze N Squeeze DNA Gel Extraction Spin Columns (Bio-Rad, Hercules, CA) and ligated into pET-32 Ek/LIC vector (Novagen) for bacterial expression, according to the manufacturer’s instructions (Ek/LIC Cloning Kits, Novagen). The construct was sequenced (Macrogen Europe, the Netherlands) and transformed into E. coli BL21 (DE3) chemically competent cells (Novagen) according to the manufacturer’s protocol. The recombinant mature form of PTTH was expressed growing the transformed E. coli BL21 (DE3) cells at 37°C in Luria Bertani broth (LB). rPTTH gene expression was induced at OD600 = 0.6 by adding 0.5-mM isopropyl-β-d-thiogalactopyranoside (IPTG). After growing for additional 4 h at 37°C, rPTTH was purified under denaturing conditions using affinity chromatography on nickel resin Qiagen Ni-NTA (Qiagen Ni-TED packed columns 1000-Macherey-Nagel, Bethlehem, PA) following the manufacturer’s protocol. The purified protein was dialyzed against phosphate-buffered saline (PBS) 1×, to remove urea and allow the rPTTH refolding in an homodimeric form, essential for its biological activity (Ishibashi et al. 1994). A rate of dialyzed peptide was used to verify the correct folding by native PAGE (12.5%). Proteins were prepared in a nonreducing and nondenaturing sample buffer (neither SDS in electrophoresis gel and buffer components nor β-mercaptoethanol in sample loading buffer) to preserve both the secondary structure and the native charge density of the protein. The purified fusion protein was then digested by 0.01 units of thrombin/μg of purified protein (thrombin from bovine plasma 1,000 IU/mg, Sigma–Aldrich) for 16–18 h at room temperature (25°C) in order to remove the Trx and the His tags. Thrombin was finally inactivated by heating the sample at 60°C for 10 min. The sample was analyzed by sodium dodecyl sulphate - polyacrylamide gel electrophoresis (SDS–PAGE) (15%) in order to verify the occurred digestion . Digested rPTTH was then separated from His-tag, using affinity chromatography on nickel resin Qiagen Ni-NTA (Qiagen Ni-TED packed columns 1000-Macherey-Nagel) and imidazole gradient, following the manufacturer’s protocol. Then, in order to remove imidazole, dialysis against PBS 1× was performed for 16 h. A rate of dialyzed peptide was used to verify the integrity by SDS–PAGE (15%) in the presence and in the absence of the reducing agent β-mercaptoethanol (Kataoka et al. 1991, Ishibashi et al. 1994, Shionoya et al. 2003).
Structural Characterization of rPTTH
rPTTH was treated with 60 μl of 10-mM DTT in 50-mM NH4HCO3, pH 8.5 for 45 min at 56°C to reduce cysteine residues and then with 55-mM iodoacetamide in the dark at room temperature for 30 min for the alkylation reaction. The protein was finally digested with trypsin (1/50 w/w) in 50 mM NH4HCO3, pH 8.5 overnight at 37°C. The resulting peptide mixture was directly analyzed by both MALDI tandem mass spectrometry (MALDI-MS/MS) on a MALDI TOF-TOF 5800 plus instrument (ABI Sciex, Framingham, MA) and liquid chromatography/tandem mass spectrometry (LC-MS/MS) using an LTQ Orbitrap XL Orbitrap mass spectrometer (Thermo Fisher Scientific). CD spectra were recorded at 25°C using 1-cm quartz cells in the range between 260 and 198 nm using a Jasco J-1100 CD spectrometer (Jasco, Easton, MD). Protein concentration for CD measurement was 5 µM in 10-mM sodium phosphate buffer, pH 7.4.
Extraction of PTTH
The brain extract mixture containing PTTH (hereafter referred as BE) was prepared by homogenizing brains dissected from an equal number of H. virescens 3-d (V3) and early 4-d (V4) last (fifth) instar larvae and stored in ice-cold Grace’s insect medium (Sigma–Aldrich). The homogenate was placed in boiling water for 2 min, cooled to 4°C on ice and centrifuged at 15,000 g at 4°C for 5 min (Pennacchio et al. 1997). Before being used, BE was diluted in Grace’s insect medium to 0.1 brain equivalent/µl and used immediately for the experiments described below or stored at −80°C.
In Vitro Ecdysteroidogenesis Stimulation
For dose–response experiments, PGs from 3-d (V3) old last (fifth) instar larvae were dissected at room temperature, in PBS 1× as previously reported (Pennacchio et al. 1998). Individual glands were held in cell culture wells in 100 µl of Grace’s insect medium (Sigma–Aldrich) for 30 min (time of rest) at 25°C, in order to reduce the possibility of their activation by experimental manipulation, as reported for Manduca sexta PGs (Bollenbacher et al. 1983, Smith et al. 1986). Following the dissection of PGs, Grace’s insect medium was replaced with a fresh medium containing BE or rPTTH. In vitro ecdysone biosynthesis was evaluated for a single PG incubated under different conditions: control PG incubated in Grace’s insect medium (Sigma–Aldrich); stimulated PG with 0.1 brain equivalent/µl BE; stimulated PG with 0.1 ng/µl rPTTH; stimulated PG with 0.25 ng/µl rPTTH; stimulated PG with 0.5 ng/µl rPTTH. Each condition was maintained for 3 h at 25°C. The production of ecdysone after in vitro stimulation was determined by a competitive enzyme immunoassay (EIA), as previously described (Scieuzo et al. 2018). All experiments were performed on a single PG, in four technical replicates for each of the 10 biological replicates.
Analysis of Protein Phosphorylation
To verify the ability of the rPTTH to stimulate ecdysteroidogenesis through PI3K/Akt/TOR pathway, western blot analysis were performed on PGs previously incubated in Grace’s insect medium (Sigma–Aldrich) and stimulated with 0.1 ng/µl rPTTH for 3 h at 25°C. A pool of 20 PGs for each condition (control and PGs stimulated by rPTTH) was incubated and lysed directly in Laemmli 2× sample buffer (Laemmli 1970), allowing the inhibition of proteases and phosphatases. The extracted proteins were separated by a 10% polyacrylamide gel electrophoresis and transferred on a Whatman nitrocellulose membrane (Protran, Dassel, Germany). Specific antibodies were used to evaluate the phosphorylation of two TOR targets: anti-phospho-4E-BP (Cell Signaling, catalogue number 2855S, Danvers, MA) and anti-phospho-S6K (Millipore, catalogue number 04-393, Temecula, CA). α-Actin was used as an endogenous control using an anti-α-actin antibody (Abcam, catalogue number 75186, Cambridge, United Kingdom). All antibodies were diluted 1:1,000 in Tris-buffered saline added with 0.1% Tween-20 (TBS-T) with 5% bovine serum albumin, and the incubation was carried out for 16 h. Membranes were sequentially incubated with each of three antibodies. Goat anti-rabbit conjugated to horseradish peroxidase (Invitrogen), diluted 1:15,000 in TBS-T, was used as a secondary antibody. Enhanced chemiluminescence (ECL) (LiteAB Blot kit, Euroclone, Pavia, Italy) was used for the detection and signals were measured by ChemidocTM MP System (Bio-Rad).
Statistical Analysis
Enzyme immunoassay data were expressed as the mean ± SEM of 10 independent biological replicates and evaluated by GraphPad Prism 6 software, version for Windows (www.graphpad.com) (GraphPad Software, La Jolla, CA). For the analysis, a paired t-test was used to compare the mean amount of ecdysone produced, following the in vitro stimulation by recombinant hormone and brain extract. Differences compared with a control group and differences among increasing concentration of rPTTH were analyzed by analysis of variance, followed by Tukey’s posthoc test.
Results
Cloning, Expression, and Purification of rPTTH
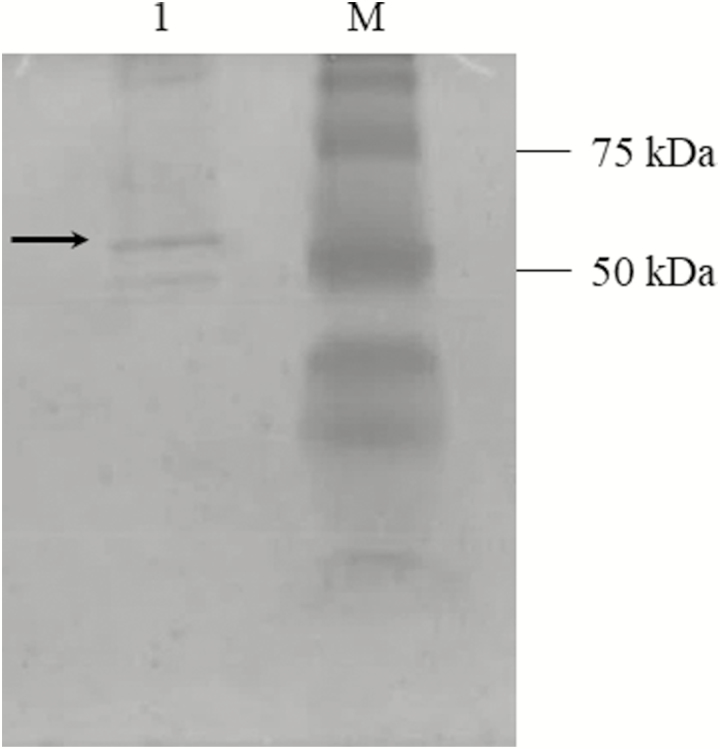
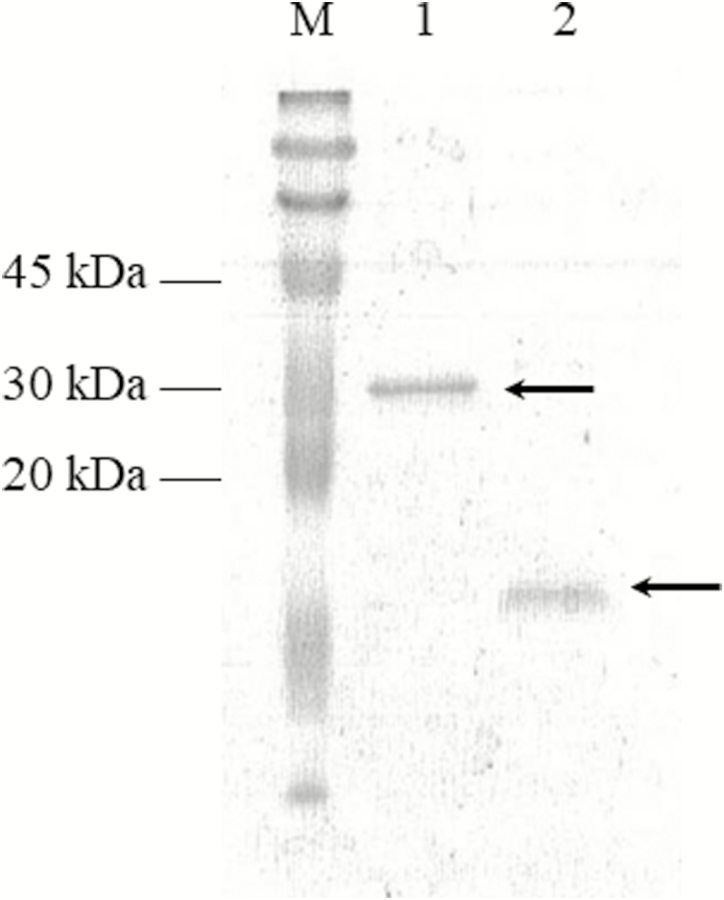
As a result of PCR amplification, a 368-bp fragment, coding for the active form of H. virescens PTTH, was purified and ligated into pET-32 Ek/LIC vector. The sequencing analysis confirmed that the amplicon was in frame under T7 promoter. rPTTH, with the expected molecular weight of 30 kDa, was detected both in supernatant and pellet, after E. coli BL21 (DE3) cells induction by IPTG (Fig. 1a, lanes 1 and 2). Although part of rPTTH was localized also in the inclusion bodies, it was mostly found in the soluble fraction, confirming its hydrophilic feature. The recombinant protein was purified using affinity chromatography on nickel resin Qiagen Ni peptide under pH gradient. rPTTH with the expected molecular weight of 30 kDa was present only in the elution fractions with Elution buffer at pH 4.5 (Fig. 1b, lanes 5–9) and not in elution fractions with Elution buffer at pH 5.9 (Fig. 1b, lanes 1–4). The correct refolding of rPTTH into the biologically active homodimeric form was analyzed by native PAGE (12.5%) that showed rPTTH with the expected molecular weight of 60 kDa (Fig. 2). Purified rPTTH was successfully digested by 0.01 units of thrombin/μg to remove the 14-kDa Trx and His tags and generate a 16-kDa peptide, corresponding to the mature rPTTH (13 kDa) with S tag (3k Da). The analysis of the sample by SDS–PAGE (15%) showed a single band corresponding to the protein of interest (Fig. 3). The correct refolding of the thrombin digested rPTTH into the biologically active homodimeric form and integrity were analyzed by SDS–PAGE (15%) in the presence and in the absence of β-mercaptoethanol (Fig. 4).
Fig. 1.
Expression and purification of rPTTH. (a) Lane M: Bio-Rad Standard Kaleidoscope Protein Molecular Weight Marker; lane 1: supernatant from E. coli BL21 (DE3) cells, induced with 0.5-mM IPTG for 4 h at 37°C and lysed under denaturing conditions; lane 2: pellet from E. coli BL21 (DE3) cells, induced with 0.5-mM IPTG for 4 h at 37°C and lysed under denaturing conditions; lane 3: supernatant from not induced and lysed E. coli BL21 (DE3) cells; and lane 4: pellet from not induced and lysed E. coli BL21 (DE3) cells. (b) Lane M: Bio-Rad Standard Kaleidoscope Protein Molecular Weight Marker (Bio-Rad); Lanes 1–4: eluate with Elution buffer at pH 5.9; lanes 5–9: eluate with Elution buffer at pH 4.5.
Fig. 2.
Native PAGE of rPTTH. Native PAGE showed the correct folding of recombinant PTTH. Lane M: Bio-Rad Standard Kaleidoscope Protein Molecular Weight Marker (Bio-Rad); lane 1: purified rPTTH (60 kDa).
Fig. 3.
SDS–PAGE of rPTTH digested with thrombin. rPTTH was digested by using 0.01 units of thrombin/μg of purified protein. The cleavage generated a 16-kDa peptide corresponding to the mature PTTH (13 kDa) and the S tag (3 kDa). Lane M: ColorBurst Electrophoresis Marker (Sigma–Aldrich); lane 1: rPTTH before thrombin digestion; lane 2: rPTTH after thrombin digestion.
Fig. 4.
SDS–PAGE of the thrombin-digested rPTTH in the presence and in the absence of β-mercaptoethanol. SDS–PAGE showed the integrity of the thrombin digested recombinant PTTH. Lane M: Bio-Rad Precision Plus Protein All Blue Standards (Bio-Rad); lane 1: unreduced, refolded rPTTH (32 kDa); lane 2: rPTTH reduced by β-mercaptoethanol (16 kDa).
Structural Characterization of rPTTH
The primary structure of purified intact rPTTH was verified by mass spectrometric procedures following essentially the mass mapping strategy. Reduced and carboxyamidomethylated rPTTH was digested with trypsin and the resulting peptide mixture directly analyzed by both MALDI MS/MS and LC-MS/MS. The mass signals recorded in the spectra were mapped onto the anticipated sequence of rPTTH leading to a 78% coverage of the protein primary structure.
The secondary structure of rPTTH was evaluated by Circular Dichroism analysis. Protein (5 μM) was dissolved in 10 mM sodium phosphate buffer, pH 7.4. The CD spectrum was recorded at 25°C and displayed a minimum at 216 nm typical of the beta-sheet structure as shown in Fig. 5. Deconvolution of the CD spectrum showed that rPTTH has 36% beta-sheet structure, whereas 14% is associated with beta-turn structures.
Fig. 5.
Analysis of rPTTH secondary structure. Circular dichroism spectrum of the PTTH sample in phosphate buffer pH 7.4 showing the minimum of absorbance at 216 nm.
Evaluation of BE and rPTTH In Vitro Activity
The evaluation of ecdysone titer produced in vitro and released by H. virescens PGs in different conditions was performed by EIA. Ecdysone in vitro secretion was enhanced by BE (0.1 brain equivalent/µl) (2,991.12 ± 945.88 pg/gland) and rPTTH (0.1 ng/µl) stimulation (4,263.96 ± 1,348.38 pg/gland), both statistically different from the control (660.84 ± 208.98 pg/gland) (F = 7.63; P < 0.05; Fig. 6a). In order to evaluate the biological activity of the recombinant hormone, PGs were also stimulated with different concentration of rPTTH (0.25 and 0.5 ng/µl). No statistically significant difference was measured between rPTTH 0.1 and 0.25 ng/µl stimulation (6,044.44 ± 1911.42 pg/gland); significant difference was observed between rPTTH 0.25 and 0.5 ng/µl stimulation (9,408.78 ± 2,975.32 pg/gland) (F = 14.54; P < 0.0001; Fig. 6b).
Fig. 6.
In vitro effects of BE and rPTTH on ecdysone synthesis by PGs. PGs explanted from 3-d (V3) last (fifth) instar larvae were stimulated with BE (0.1 brain equivalent/µl) or rPTTH (0.1 ng/µl). Control glands (CTR) were incubated in Grace’s insect medium (a). To evaluate the biological activity of rPTTH, PGs were stimulated with increasing concentration of the recombinant hormone (0.1, 0.25 and 0.5 ng/µl). (b) The ecdysone was determined by EIA after 3 h of incubation. Data are expressed as the mean concentrations of ecdysone (pg/gland) ± SEM of n = 10 experiments. Statistical analysis was performed by one-way analysis of variance followed by Tukey’s posthoc test. Different letters indicate significant differences (P < 0.05).
Phosphorylation of 4E-BP and S6K Proteins in PGs
To verify the influence of rPTTH on PI3K/Akt/TOR pathway in H. virescens ecdysteroidogenesis, western blot analysis was performed on PGs. The phosphorylation of the main targets of TOR kinase was detected using antibodies against phospho-4E-BP and phospho-S6K. The in vitro exposure of PGs explanted from 3 d (V3) old last (fifth) instar larvae to rPTTH enhanced the phosphorylation level of both 4E-BP and S6K proteins. No phosphorylation signal was detected in unstimulated PGs, incubated in Grace’s insect medium (Fig. 7).
Fig. 7.
Phosphorylation of TOR target proteins in PGs. PGs from 3-d old last (fifth) instar larvae were stimulated in vitro, in a Grace’s insect medium, added with 0.1 ng/μl of rPTTH. Unstimulated PGs were incubated in Grace’s insect medium. Incubation was maintained for 3 h at 25°C in each condition. Glands lysed in Laemmli 2× sample buffer were analyzed by western blot using antibodies against phospho-4E-BP (20 kDa) and phospho-S6K (70 kDa). Each lane represents the equivalent of 20 PGs. α-Actin (42 kDa) was used as endogenous control.
Discussion
The natural prothoracicotropic hormone (nPTTH) is a neuropeptide representing the main stimulatory factor on the PGs, capable of triggering the entire process of ecdysteroidogenesis (Huang et al. 2008). nPTTH is a brain extract component, along with other factors, such as insulin and prothoracicostatic peptide (PTSP), the natural antagonist of nPTTH (Gilbert 2012). The use of the brain extract that includes nPTTH could be deviating, as the different components effects are not easily evaluable; at certain times of the larval stage, the production of ecdysone could be also dependent on an insulin-like factor. At increasing dose of nPTTH, the effect of PTSP could be prevalent, preventing the ecdysone production. Indeed, it is known that PTSP inhibits basal (Hua et al. 1999, Dedos et al. 2001) and nPTTH-stimulated (Hua et al. 1999) ecdysteroidogenesis in vitro in a dose-dependent manner. PTSP negatively regulates the effect of the neuropeptide on target organs, in order to modulate the physiological hormone response over the time (Gilbert et al. 2000). Moreover, although the active portion of nPTTH shows a high similarity among lepidopterans, the effect of the recombinant and natural hormone could be different: in M. sexta rPTTH and nPTTH show identical effects in terms of ecdysone synthesis (in a dose-, time-, and instar-dependent manner), cAMP levels and protein phosphorylation pattern (Gilbert et al. 2000); differently, in Bombyx mori, nPTTH, and rPTTH show different ability to stimulate ecdysteroidogenesis: the ecdysone titer measured after rPTTH stimulation is significantly lower than that obtained by nPTTH stimulation and does not consistently stimulate cAMP level in last (fifth) instar larvae and in the first day of pupae (Dedos et al. 1999). In previous studies (Scieuzo et al. 2018), we observed a consistent ability of crude brain extracts to stimulate ecdysteroidogenesis in H. virescens PGs. In this work, a rPTTH from H. virescens was expressed, purified and its primary and secondary structures were characterized to obtain a hormone useful to perform in vitro tests, in a standard and repeatable manner. Our results derived from the SDS–PAGE analysis of purified rPTTH from H. virescens, in the presence and in the absence of the reducing agent β-mercaptoethanol, confirm literature data concerning the homodimeric nature of the biologically active insect PTTH (Kataoka et al. 1991, Ishibashi et al. 1994, Shionoya et al. 2003). rPTTH from H. virescens has a secondary structure composed of 36% beta-sheet and 14% of beta turn structures. This agrees with the rPTTH secondary structure observed in other insect species (Noguti et al. 1995, Zhang and Denlinger 2011). rPTTH activity on PGs was evaluated by in vitro tests in comparison to the brain extract (BE) mixture, demonstrating that rPTTH from H. virescens and nPTTH found in the BE have comparable effects. Data from experiments performed with BE stimulation at the concentration of 0.1 brain equivalent/µL (Pennacchio et al. 1997, 1998; Scieuzo et al. 2018) revealed that rPTTH has equivalent effects on in vitro PG stimulation and on the phosphorylation of the two TOR pathway targets: 4E-BP and S6K. Our results confirm literature data, indicating that nPTTH, as a component of the brain extract mixture, increases the in vitro production and secretion of ecdysone in H. virescens PGs (Pennacchio et al. 1997). Our experiments highlight that H. virescens rPTTH has a similar effect in comparison to the brain extract in the stimulation of PGs biosynthetic activity. No statistically significant differences among samples differently stimulated (nPTTH or rPTTH) were observed. Furthermore, to investigate the rPTTH quantitative biological activity, the ecdysone produced in vitro and released by H. virescens PGs was measured, using increasing concentration of the purified recombinant hormone. Overall, the in vitro activity of increasing concentration of rPTTH displays the ability to stimulate PGs to produce and secrete increasing amount of ecdysone, overcoming the potential inhibitory effect observed following the natural hormone stimulation. This allows the standardization and reproducibility of experiments aimed to study ecdysteroidogenesis. Our results show that, similarly to the natural hormone, rPTTH acts through a transduction cascade that includes multiple phosphorylations of the two main targets of TOR pathway, 4E-BP and S6K. The possibility to use the recombinant PTTH for further experiments aimed to investigate the mechanisms of ecdysteroidogenesis is of considerable importance. Comparability in the use of H. virescens rPTTH and crude brain extract strengthen data deriving from previous research, suggesting that the effect is due to a greater extent to natural PTTH, despite the presence of other stimulatory or inhibitory factors.
Acknowledgments
We would like to thank Annalisa Sileo and Donato Zaccagnino for their assistance in Heliothis virescens rearing. This work was supported by University of Basilicata (RIL funds).
References Cited
- Agui N. ,Granger N. A., Gilbert L. I., and Bollenbacher W. E.. 1979. Cellular localization of the insect prothoracicotropic hormone: in vitro assay of a single neurosecretory cell. Proc. Natl. Acad. Sci. USA. 76: 5694–5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollenbacher W. E. ,O’Brien M. A., Katahira E. J., and Gilbert L. I.. 1983. A kinetic analysis of the action of the insect prothoracicotropic hormone. Mol. Cell. Endocrinol. 32: 27–46. [DOI] [PubMed] [Google Scholar]
- Dai J. D., Mizoguchi A., and Gilbert L. I.. . 1994. Immunoreactivity of neurosecretory granules in the brain retrocerebral complex of Manduca sexta to heterologous antibodies against Bombyx prothoracicotropic hormone and bombyxin. Invertebr. Reprod. Dev. 26: 187–196. [Google Scholar]
- Dedos S. G. ,Fugo H., Nagata S., Takamiya M., and Kataoka H.. 1999. Differences between recombinant PTTH and crude brain extracts in cAMP-mediated ecdysteroid secretion from the prothoracic glands of the silkworm, Bombyx mori. J. Insect Physiol. 45: 415–422. [DOI] [PubMed] [Google Scholar]
- Dedos S. G. ,Nagata S., Ito J., and Takamiya M.. 2001. Action kinetics of a prothoracicostatic peptide from Bombyx mori and its possible signaling pathway. Gen. Comp. Endocrinol. 122: 98–108. [DOI] [PubMed] [Google Scholar]
- Fellner S. K. ,Rybczynski R., and Gilbert L. I.. 2005. Ca2+ signaling in prothoracicotropic hormone-stimulated prothoracic gland cells of Manduca sexta: evidence for mobilization and entry mechanisms. Insect Biochem. Mol. Biol. 35: 263–275. [DOI] [PubMed] [Google Scholar]
- Gilbert L. I. 2012. Insect endocrinology. Elsevier, Amsterdam, the Netherlands. [Google Scholar]
- Gilbert L. I. ,Rybczynski R., Song Q., Mizoguchi A., Morreale R., Smith W. A., Matubayashi H., Shionoya M., Nagata S., and Kataoka H.. 2000. Dynamic regulation of prothoracic gland ecdysteroidogenesis: Manduca sexta recombinant prothoracicotropic hormone and brain extracts have identical effects. Insect Biochem. Mol. Biol. 30: 1079–1089. [DOI] [PubMed] [Google Scholar]
- Gu S. H. ,Chow Y. S., Lin F. J., Wu J. L., and Ho R. J.. 1996. A deficiency in prothoracicotropic hormone transduction pathway during the early last larval instar of Bombyx mori. Mol. Cell. Endocrinol. 120: 99–105. [DOI] [PubMed] [Google Scholar]
- Gu S. H., Chow Y. S., and O’reilly D. R.. . 1998. Role of calcium in the stimulation of ecdysteroidogenesis by recombinant prothoracicotropic hormone in the prothoracic glands of the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 28: 861–867. [Google Scholar]
- Gu S. H. ,Yeh W. L., Young S. C., Lin P. L., and Li S.. 2012. TOR signaling is involved in PTTH-stimulated ecdysteroidogenesis by prothoracic glands in the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 42: 296–303. [DOI] [PubMed] [Google Scholar]
- Gullan P. J., and Cranston P. S.. . 2005. The insects: an outline of entomology. Wiley-Blackwell, Oxford, UK. [Google Scholar]
- Hentze J. L. ,Moeller M. E., Jørgensen A. F., Bengtsson M. S., Bordoy A. M., Warren J. T., Gilbert L. I., Andersen O., and Rewitz K. F.. 2013. Accessory gland as a site for prothoracicotropic hormone controlled ecdysone synthesis in adult male insects. PLoS One 8: e55131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Y. J. ,Tanaka Y., Nakamura K., Sakakibara M., Nagata S., and Kataoka H.. 1999. Identification of a prothoracicostatic peptide in the larval brain of the silkworm, Bombyx mori. J. Biol. Chem. 274: 31169–31173. [DOI] [PubMed] [Google Scholar]
- Huang X. ,Warren J. T., and Gilbert L. I.. 2008. New players in the regulation of ecdysone biosynthesis. J. Genet. Genomics 35: 1–10. [DOI] [PubMed] [Google Scholar]
- Iga M., and Kataoka H.. . 2012. Recent studies on insect hormone metabolic pathways mediated by cytochrome P450 enzymes. Biol. Pharm. Bull. 35: 838–843. [DOI] [PubMed] [Google Scholar]
- Ishibashi J. ,Kataoka H., Isogai A., Kawakami A., Saegusa H., Yagi Y., Mizoguchi A., Ishizaki H., and Suzuki A.. 1994. Assignment of disulfide bond location in prothoracicotropic hormone of the silkworm, Bombyx mori: a homodimeric peptide. Biochemistry. 33: 5912–5919. [DOI] [PubMed] [Google Scholar]
- Kataoka H. ,Nagasawa H., Isogai A., Ishizaki H., and Suzuki A.. 1991. Prothoracicotropic hormone of the silkworm, Bombyx mori: amino acid sequence and dimeric structure. Agric. Biol. Chem. 55: 73–86. [PubMed] [Google Scholar]
- Laemmli U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227: 680–685. [DOI] [PubMed] [Google Scholar]
- Lin J. L., and Gu S. H.. . 2007. In vitro and in vivo stimulation of extracellular signal-regulated kinase (ERK) by the prothoracicotropic hormone in prothoracic gland cells and its developmental regulation in the silkworm, Bombyx mori. J. Insect Physiol. 53: 622–631. [DOI] [PubMed] [Google Scholar]
- McBrayer Z. ,Ono H., Shimell M., Parvy J. P., Beckstead R. B., Warren J. T., Thummel C. S., Dauphin-Villemant C., Gilbert L. I., and O’Connor M. B.. 2007. Prothoracicotropic hormone regulates developmental timing and body size in Drosophila. Dev. Cell 13: 857–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguti T. ,Adachi-Yamada T., Katagiri T., Kawakami A., Iwami M., Ishibashi J., Kataoka H., Suzuki A., Go M., and Ishizaki H.. 1995. Insect prothoracicotropic hormone: a new member of the vertebrate growth factor superfamily. FEBS Lett. 376: 251–256. [DOI] [PubMed] [Google Scholar]
- Pennacchio F., Vinson S. B., and Tremblay E.. . 1992. Host regulation effects of Heliothis virescens (F.) larvae induced by teratocytes of Cardiochiles nigriceps (V.) (Lepidoptera, Noctuidae - Hymenoptera, Braconidae). Arch. Insect Biochem. 19: 177–192. [Google Scholar]
- Pennacchio F., Sordetti R., Falabella P., and Vinson S. B.. . 1997. Biochemical and ultrastructural alterations in prothoracic glands of Heliothis virescens (F.) (Lepidoptera: Noctuidae) last instar larvae parasitized by Cardiochiles nigriceps (V.) (Hymenoptera: Braconidae). Insect Biochem. Mol. Biol. 27: 439–450. [Google Scholar]
- Pennacchio F., Falabella P., and Vinson S. B.. . 1998. Regulation of Heliothis virescens prothoracic glands by Cardiochiles nigriceps polydnavirus. Arch. Insect Biochem. 38: 1–10. [Google Scholar]
- Rewitz K. F. ,Yamanaka N., Gilbert L. I., and O’Connor M. B.. 2009. The insect neuropeptide PTTH activates receptor tyrosine kinase torso to initiate metamorphosis. Science. 326: 1403–1405. [DOI] [PubMed] [Google Scholar]
- Rybczynski R., and Gilbert L. I.. . 1994. Changes in general and specific protein synthesis that accompany ecdysteroid synthesis in stimulated prothoracic glands of Manduca sexta. Insect Biochem. Mol. Biol. 24: 175–189. [DOI] [PubMed] [Google Scholar]
- Rybczynski R. ,Bell S. C., and Gilbert L. I.. 2001. Activation of an extracellular signal-regulated kinase (ERK) by the insect prothoracicotropic hormone. Mol. Cell. Endocrinol. 184: 1–11. [DOI] [PubMed] [Google Scholar]
- Scieuzo C. ,Nardiello M., Salvia R., Pezzi M., Chicca M., Leis M., Bufo S. A., Vinson S. B., Rao A., Vogel H., et al. 2018. Ecdysteroidogenesis and development in Heliothis virescens (Lepidoptera: Noctuidae): focus on PTTH-stimulated pathways. J. Insect Physiol. 107: 57–67. [DOI] [PubMed] [Google Scholar]
- Shionoya M. ,Matsubayashi H., Asahina M., Kuniyoshi H., Nagata S., Riddiford L. M., and Kataoka H.. 2003. Molecular cloning of the prothoracicotropic hormone from the tobacco hornworm, Manduca sexta. Insect Biochem. Mol. Biol. 33: 795–801. [DOI] [PubMed] [Google Scholar]
- Smith W. A. ,Gilbert L. I., and Bollenbacher W. E.. 1985. Calcium-cyclic AMP interactions in prothoracicotropic hormone stimulation of ecdysone synthesis. Mol. Cell. Endocrinol. 39: 71–78. [DOI] [PubMed] [Google Scholar]
- Smith W. A. ,Combest W. L., and Gilbert L. I.. 1986. Involvement of cyclic AMP-dependent protein kinase in prothoracicotropic hormone-stimulated ecdysone synthesis. Mol. Cell. Endocrinol. 47: 25–33. [DOI] [PubMed] [Google Scholar]
- Vanderzant E. S., Richardson C. D., and Fort S. W. Jr. 1962. Rearing of the bollworm on artificial diet. J. Econom. Entomol. 55: 140. [Google Scholar]
- Vinson S. B., Guillot F. S., and Hays D. B.. . 1973. Rearing of Cardiochiles nigriceps in the laboratory, with Heliothis virescens as hosts. Ann. Entomol. Soc. Am. 66: 1170–1172. [Google Scholar]
- Webb B. A., and Dahlman D. L.. . 1985. Developmental pathology of Heliothis virescens larvae parasitized by Microplitis croceipes: parasite-mediated host developmental arrest. Arch. Insect Biochem. 2: 131–139. [Google Scholar]
- Wei Z. J. ,Zhang Q. R., Kang L., Xu W. H., and Denlinger D. L.. 2005. Molecular characterization and expression of prothoracicotropic hormone during development and pupal diapause in the cotton bollworm, Helicoverpa armigera. J. Insect Physiol. 51: 691–700. [DOI] [PubMed] [Google Scholar]
- Xu W. H., and Denlinger D. L.. . 2003. Molecular characterization of prothoracicotropic hormone and diapause hormone in Heliothis virescens during diapause, and a new role for diapause hormone. Insect Mol. Biol. 12: 509–516. [DOI] [PubMed] [Google Scholar]
- Zhang Q., and Denlinger D. L.. . 2011. Molecular structure and its expression analysis of the prothoracicotropic hormone gene in the northern house mosquito, Culex pipiens, in association with diapause and blood feeding. Insect Mol. Biol. 20: 201–213. [DOI] [PMC free article] [PubMed] [Google Scholar]