Abstract
Thyroid cancer incidence varies greatly between and within high‐income countries (HICs), and overdiagnosis likely plays a major role in these differences. Yet, little is known about the situation in low‐ and middle‐income countries (LMICs). We compare up‐to‐date thyroid cancer incidence and mortality at national and subnational levels. 599,851 thyroid cancer cases in subjects aged 20–74 reported in Cancer Incidence in Five Continents volume XI from 55 countries with at least 0.5 million population, aged 20–74 years, covered by population‐based cancer registration, and 22,179 deaths from the WHO Mortality Database for 36 of the selected countries, over 2008–2012, were included. Age‐standardized rates were computed. National incidence rates varied 50‐fold. Rates were 4 times higher among women than men, with similar patterns between countries. The highest rates (>25 cases per 100,000 women) were observed in the Republic of Korea, Israel, Canada, the United States, Italy, France, and LMICs such as Turkey, Costa Rica, Brazil, and Ecuador. Incidence rates were low (<8) in a few HICs (the Netherlands, the United Kingdom, and Denmark) and lowest (3–4) in some LMICs (such as Uganda and India). Within‐country incidence rates varied up to 45‐fold, with the largest differences recorded between rural and urban areas in Canada (HIC) and Brazil, India, and China (LMICs). National mortality rates were very low (<2) in all countries and in both sexes, and highest in LMICs. The very high thyroid cancer incidence and low mortality rates in some LMICs also strongly suggest a major role of overdiagnosis in these countries.
Keywords: thyroid neoplasm, incidence, mortality, medical overuse, epidemiology
Short abstract
What's new?
The rise in thyroid cancer incidence in high‐income countries (HICs) is widely documented. By comparison, trends in low‐ and middle‐income countries (LMICs) are less well‐defined. Here, analysis of population‐based cancer registry data for thyroid cancer reveals very high incidence rates in some LMICs, comparable to those of some HICs where overdiagnosis plays an important role. The highest rates occurred in urban areas and countries where diagnostic equipment is abundant and not subject to regulatory control. The findings suggest that, similar to HICs, increased surveillance and advanced diagnostic practices in some LIMCs has produced an epidemic of thyroid cancer diagnoses.
Abbreviations
- HIC
High‐income country
- LMIC
Low‐ and middle‐income country
- TC
Thyroid cancer
Introduction
The incidence of thyroid cancer (TC) has been increasing markedly in several high‐income countries (HICs), including France, Italy, and the United States, since the 1980s, and in the Republic of Korea since the mid‐1990s.1, 2, 3 In contrast, TC mortality has remained low and stable, or has even declined.4, 5 TC incidence varies greatly between and within HICs, most likely due to differences in the detection of subclinical, indolent cancers that would never cause symptoms or death.6, 7 Yet, little is known about TC incidence in low‐ and middle‐income countries (LMICs), some of which are undergoing rapid economic transition. Here, we present the most up‐to‐date TC incidence rates for 55 countries, including several LMICs (some for the first time), and contrast these with mortality rates.
Materials and Methods
The number of new TC cases (ICD‐10 C73) in 2008–2012 and their histology were extracted from population‐based cancer registry data compiled by the International Agency for Research on Cancer into Cancer Incidence in Five Continents (CI5) Volume XI.8 Only registries that have passed a detailed assessment of comparability (adherence to international standards and guidelines), completeness (the degree to which cancers diagnosed in the catchment population are indeed registered), and validity (ascertaining that the recorded cases are accurate) checks are published in CI5, and this is an indication of their high‐quality.8 To alleviate the challenges cancer registries in LMICs may face to follow international registration standards, reference values are based on data from other cancer registries in the same region, and on the data published in the previous two volumes of CI5.9 For our study, countries with at least 0.5 million inhabitants aged 20–74 years covered by cancer registration, regardless of whether this was through a national, a regional, or a grouping of regional registries, were selected. When several regional registries were present in a country, data were pooled to obtain a proxy of the national incidence. National TC deaths from the WHO Mortality Database,10 for 2008–2012, for the selected countries, were included if vital statistics covered >70% of the population and the proportion of ill‐defined causes of death was <20%.11
Age‐standardized incidence and mortality rates (world standard population)12 were computed for adults aged 20–74 years. The analysis was restricted to patients under 75 years to enable inclusion of LMICs where the oldest age groups are often combined in population counts.
Countries were classified as LMICs or HICs according to the 2012 World Bank list of economies.
Results
Fifty‐five countries and their 333 registries (covering 15% of the world's population) met the inclusion criteria, providing incidence data for 599,851 TC cases (79% women). Australia, Austria, the Republic of Korea, the United Kingdom, and the United States provided both national and regional data. Thirty‐six of the 55 countries contributed information on 22,179 TC deaths (55% women).
During the period 2008–2012, national TC incidence rates in women aged 20–74 years varied 50‐fold (Fig. 1). The Republic of Korea showed by far the highest incidence rates (149.8 new cases per 100,000 women). Other HICs with very high incidence rates in women were Cyprus (48.7), Italy (30.3), Canada (29.5), Israel (27.6), the United States (26.8), and France (25.4). Remarkably, TC incidence rates in women were also high in several LMICs, with rates of >25 new cases per 100,000 women in Costa Rica (30.9), Turkey (30.0), and Brazil (27.5). The lowest TC incidence rates in women, in HICs, were in the Netherlands (5.5), the United Kingdom (7.3), and Denmark (7.4), and in LMICs were in Uganda (3.1), India (4.4), and Iran (4.7). The patterns in incidence rates in men and women were similar, although incidence rates in men were on average one quarter those in women (Fig. 1 b).
Figure 1.
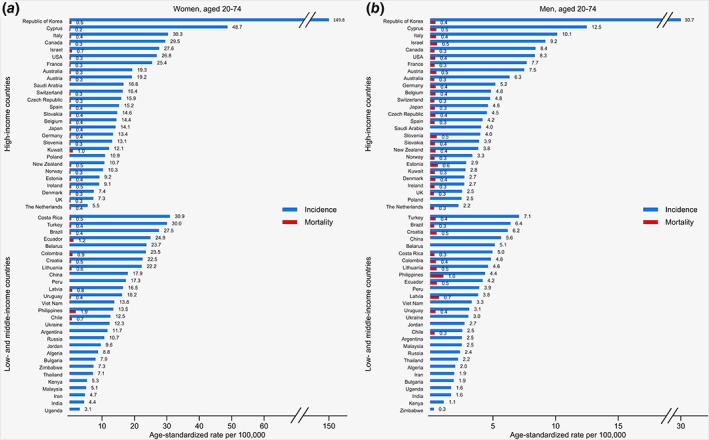
Age‐standardized incidence and mortality rates of thyroid cancer per 100,000, for 2008–2012, in women (a) and in men (b) aged 20–74 years. The incidence data presented originate from 27 national, 8 regional, and 20 combined regional registries. The data period was 2008–2012, except in Slovakia (2008–2010); Costa Rica and Iran, Golestan (2008–2011); Vietnam, Ho Chi Minh City (2009–2012); Latvia; Peru, Lima; and Zimbabwe, Harare (2010–2012). [Color figure can be viewed at wileyonlinelibrary.com]
Regional incidence rates varied up to 45‐fold, in both women and men (Fig. 2). The largest regional contrasts (≥ nine‐fold) were in countries — both HICs and LMICs — with areas of differing urbanization. For instance, in women, incidence rates in Canada ranged from 4.3 in Yukon to 39.2 in Ontario, in Brazil from 10.1 in Poços de Caldas to 110.1 in Florianópolis; in India from 1.3 in both Pune and Tripura to 15.6 in Thiruvananthapuram, and in China from 0.8 in Yanting County to 36.7 in Shanghai City. Notably, the incidence rate in Florianópolis was comparable to those in the Republic of Korea. In contrast, limited regional variation was seen in the United Kingdom (from 5.2 in Wales to 8.9 in North East England, in women).
Figure 2.
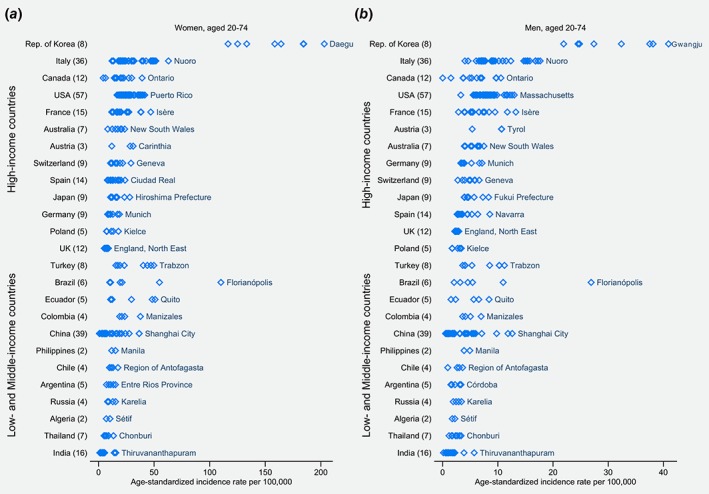
Age‐standardized incidence rates of thyroid cancer per 100,000, for 2008–2012, by regional registry, in countries with several regional registries, in women (a) and in men (b) aged 20–74 years. The number in parentheses indicates the number of regional registries in the country. Only the names of the regional registries with the highest incidence rate in each country are shown on the plot. The incidence period was 2008–2012 for the 298 regional registries, except for the after: 2008–2010: Brazil, Florianópolis; Chile, Conception and Region of Antofagasta; Ecuador, Loja; Japan, Miyagi Prefecture; Thailand, Bangkok; Italy, Caserta, Cremona, Florence and Prado, Friuli‐Venezia Giulia, Lecco, Lombardy South Pavia, Mantua, South Tyrol, Trento, and Veneto; Spain, Albacete, Asturias, Murcia, and Navarra. 2008–2011: Algeria, Sétif; Argentina, Entre Ríos Province; Brazil, Curitiba and Poços de Caldas; China, Benxi; India, Ahmedabad and Pune; Thailand, Chonburi; France, Bas‐Rhin and Manche; Italy, Barletta, Como, Ferrara, Piacenza, Sassari, Taranto, and Umbria; Spain, Canary Islands, Ciudad Real, Cuenca, and Mallorca. 2009–2012: China, Hengdong, Huaiyin District Huai'an, and Yueyanglou; India, Kamrup Urban District; Thailand, Lopburi Province; France, Limousin. 2010–2012: China, Guangzhou, Hefei, Jiangmen, Jianhu County, Wuxi, Xianju, Xiping, Yanshi, Zhongshan City, and Zhuhai; India, Tripura and Wardha; Turkey, Erzurum. [Color figure can be viewed at wileyonlinelibrary.com]
The most common histology of thyroid tumors was papillary carcinoma (Table 1). The proportion of papillary carcinoma was >80% in the countries and the regional registries with the top 5 incidence rates (with the exception of Canada, Ontario, with 70%), and ranged from 56% to 76% (and around 30% in Africa) in the registries with the bottom 5 incidence rates, among women, in both HICs and LMICs. The proportions were slightly lower among men.
Table 1.
Proportion of papillary carcinomas among new thyroid cancer cases, in subjects aged 20–74 years old, for 2008–2012, by sex and country income group, in the countries with the highest and the lowest incidence rates
| Registry | Proportion papillary carcinoma (%) | Age‐standardized rate (per 100,000) |
|---|---|---|
| Women | ||
| Highest incidence rates | ||
| High‐income countries | ||
| Republic of Korea, Daegu | 97 | 203.1 |
| Italy, Nuoro | 88 | 62.9 |
| Cyprus | 95 | 48.7 |
| Canada, Ontario | 70 | 39.2 |
| Israel | 91 | 27.6 |
| Low‐ and Middle‐income countries | ||
| Brazil, Florianópolis | 88 | 110.1 |
| Ecuador, Quito | 93 | 50.9 |
| Turkey, Trabzon | 88 | 49.8 |
| Costa Rica | 81 | 30.9 |
| Belarus | 94 | 23.7 |
| Lowest incidence rates | ||
| High‐income countries | ||
| Estonia | 70 | 9.2 |
| Ireland | 76 | 9.1 |
| Denmark | 65 | 7.4 |
| United Kingdom, South West | 66 | 6 |
| The Netherlands | 70 | 5.5 |
| Low‐ and Middle‐income countries | ||
| Kenya, Nairobi | 31 | 5.3 |
| Malaysia, Penang | 62 | 5.1 |
| Iran, Golestan Province | 65 | 4.7 |
| Uganda, Kyadondo County | 30 | 3.1 |
| India, Poona | 56 | 1.3 |
| India, Tripura | 68 | 1.3 |
| Men | ||
| Highest incidence rates | ||
| High‐income countries | ||
| Republic of Korea, Gwangju | 97 | 40.9 |
| Italy, Nuoro | 78 | 17.7 |
| Cyprus | 95 | 12.5 |
| Canada, Ontario | 66 | 10.6 |
| Israel | 85 | 9.2 |
| Low‐ and Middle‐income countries | ||
| Brazil, Florianópolis | 81 | 26.9 |
| China, Shanghai city | 87 | 12.6 |
| Turkey, Trabzon | 72 | 11.2 |
| Croatia | 75 | 6.2 |
| Belarus | 90 | 5.1 |
| Lowest incidence rates | ||
| High‐income countries | ||
| Denmark | 62 | 2.7 |
| Ireland | 62 | 2.7 |
| United Kingdom, South West | 58 | 2.3 |
| United Kingdom, England, East Midlands | 60 | 2.3 |
| The Netherlands | 65 | 2.2 |
| Poland, Lower Silesia | 63 | 1.7 |
| Poland, Lublin | 68 | 1.7 |
| Low‐ and Middle‐income countries | ||
| Bulgaria | 64 | 1.9 |
| Iran, Golestan Province | 61 | 1.9 |
| Uganda, Kyadondo County | 19 | 1.6 |
| Kenya, Nairobi | 45 | 1.1 |
| India, Tripura | 61 | 0.5 |
Only registries with at least 10 cases over 2008–2012 were considered. Countries with the top/bottom 5 incidence rates are presented. In countries with regional registries, the regional registry with the highest/lowest rate is shown. Registries are sorted by descending incidence rates. Histology data are missing for United Kingdom, Wales and Zimbabwe, Harare– the registries with the lowest incidence rates in their country, for women and men, respectively.
The large incidence rates contrasted with the low national TC mortality rates in all income settings (≤1 death per 100,000 people in almost all countries in both women and men; the highest rates <2 deaths per 100,000 women, in Ecuador and the Philippines), with similar mortality rates in both sexes (Fig. 1).
Discussion
This international comparison confirms the very high TC incidence rates in some — but not all — HICs in 2008–2012 and shows very high incidence rates in several LMICs, particularly in urban areas. Besides, the proportion of papillary carcinomas — the histological type making up the bulk of the increase in and overdiagnoses of TC in several countries2, 3, 13, 14, 15, 16, 17 — is similar in the HICs and LMICs with the highest incidence rates. Together with the uniformly low mortality rates, these findings support the existence of overdiagnosis of TC cases in LMICs.
Overdiagnosis is defined as the detection of tumors that, if left untreated, would be unlikely to progress to symptoms or death.18 It requires the existence of a reservoir of detectable cancers and activities leading to their detection.19 There is indeed a large reservoir of slow or nonprogressing TC20 (autopsy studies have revealed that around 11% of the population may unknowingly harbor a thyroid tumor),21 and several factors favor their increased detection.22
First, as the use of diagnostic imaging spreads, the chances of finding cancerous lesions after investigations for a different health problem increases. For instance, two studies in Australia and the United States showed that 11% and 15% of TC, respectively, were discovered coincidentally.23
Second, ultrasound examinations — a favored technic to investigate the thyroid gland — have become increasingly available and sensitive, encouraging opportunistic screening and thus allowing detection of smaller, asymptomatic nodules. In 2008–2009, 39% of thyroid tumors were ≤ 1 cm in the United States13 and 50% <0.8 cm in the Republic of Korea.24 In addition, the studies in the Republic of Korea demonstrated good correlation between the screening rate with ultrasound and TC incidence.17, 24
Third, the healthcare system affects access to medical examinations. Overdiagnosis is more likely to occur under privately‐oriented healthcare systems, in the absence of a gatekeeping role in primary care for referral to secondary care, and where doctors are paid by a fee‐for‐service.25 A 2012 study in 34 HICs demonstrated that the upward trend of TC incidence is closely related to healthcare systems with a low share of public spending, i.e. a system where private health insurance or patients’ direct payment are predominant.25 This type of system enables both overuse by patients and proposal of advanced medical examinations by the healthcare provider. Furthermore, where ultrasound is excluded from social health insurance benefits, there is literally no regulation on screening with ultrasound.
Finally, other elements contributing to the diagnosis of indolent tumors include the absence of guidelines regarding the management of small tumors, or conversely the recommendation to treat tumors of any size; the increase in the number of thyroid specimens delivered to pathologists after thyroidectomy for causes other than cancer; and guidelines for more thorough thyroid specimen examinations.22
In our study, the highest TC incidence rates were observed in countries and urban areas where thyroid‐gland examinations are widely available and not subject to regulatory controls, i.e., where the healthcare system is largely private and market‐oriented (e.g. the United States and Brazil) and/or doctors and diagnostic equipment are abundant (“screening pressure”) (e.g. some areas in Italy, France, the Republic of Korea, the United States, and towns in LMICs).26 For instance, in China, TC incidence rates in Shanghai City are 45 times those in the rural area of Yanting County. Previous studies in Florianópolis, Brazil16 and Thiruvananthapuram, India27 concluded that more invasive thyroid nodule management and overdiagnosis, respectively, were the most plausible explanations for their substantial TC burden — the highest in their respective countries. Where access to care is guided by strong public regulatory bodies (such as in the United Kingdom, the Netherlands, and Nordic countries), TC incidence remains low, shows little regional variability, and papillary carcinomas make up a smaller fraction of all TC diagnoses.
Differences in exposure to unknown and known risk factors (e.g., radiation exposure, obesity, and iodine intake)28 and the quality of cancer registration also contribute to the observed patterns20, 29 but are unlikely to explain more than a small proportion of the variation in TC cancer incidence.
Our study has limitations. The CI5 dataset lacks individual‐level data on risk factor exposure, on how each case was diagnosed, and on tumor size or stage at diagnosis which would give additional clues to the number of new cases which might qualify as overdiagnosis. Although TC mortality rates are highest after age 75 years, the analysis was restricted to ages <75 years to enable inclusion of LMICs. Mortality data apply to national populations, whereas some incidence data are derived from regional registries, with varying population coverage. Although we selected data from the cancer registries which had passed the thorough selection process of CI5, and mortality data from countries with medium‐ or high‐quality data, data quality still varies from one registry or from one country to another. This can lead to underestimation or overestimation of the incidence and mortality rates in some countries, yet it is unlikely to explain the extent of the differences between the countries. Finally, a regional incidence comparison requires high‐quality data and is therefore impossible in many countries.
Overdiagnosis was estimated to account for 70–90% of TC cases in HICs with high incidence rates.1 As we now also observe high incidence rates in some LMICs, we strongly suspect that overdiagnosis may account for a large proportion of cases in these settings as well. The results of our study call for a change (reduction) in thyroid‐gland examination practices in the asymptomatic general population. Such a change would contribute to avoiding harm and misuse of much‐needed healthcare resources, especially in LMICs.
Acknowledgements
We thank the population‐based cancer registries for their data, and Karen Muller and Susan Gamon from the International Agency for Research on Cancer for editing assistance.
No conflict of interest declared by any author.
References
- 1. Vaccarella S, Franceschi S, Bray F, et al. Worldwide thyroid‐cancer epidemic? The increasing impact of Overdiagnosis. N Engl J Med 2016;375:614–617. [DOI] [PubMed] [Google Scholar]
- 2. James BC, Mitchell JM, Jeon HD, et al. An update in international trends in incidence rates of thyroid cancer, 1973‐2007. Cancer Causes Control 2018;29:465–473. [DOI] [PubMed] [Google Scholar]
- 3. Dal Maso L, Panato C, Franceschi S, et al. The impact of overdiagnosis on thyroid cancer epidemic in Italy,1998–2012. Eur J Cancer 2018;94:6–15. [DOI] [PubMed] [Google Scholar]
- 4. La Vecchia C, Malvezzi M, Bosetti C, et al. Thyroid cancer mortality and incidence: a global overview. Int J Cancer 2015;136:2187–2195. [DOI] [PubMed] [Google Scholar]
- 5. Davies L, Morris L, Hankey B. Increases in thyroid cancer incidence and mortality. JAMA 2017;318:389–390. [DOI] [PubMed] [Google Scholar]
- 6. Davies L. Overdiagnosis of thyroid cancer. BMJ 2016;355:i6312. [DOI] [PubMed] [Google Scholar]
- 7. Brito JP, Morris JC, Montori VM. Thyroid cancer: zealous imaging has increased detection and treatment of low risk tumours. BMJ 2013;347:f4706. [DOI] [PubMed] [Google Scholar]
- 8. Bray F, Colombet M, Mery L, et al. Cancer incidence in five continents, vol. XI (electronic version), Lyon, France: International Agency for Research on Cancer, 2017. [Google Scholar]
- 9. Bray F, Znaor A, Cueva P, et al. Planning and developing populations‐based cancer registration in low‐ and middle‐income settings, Vol 43, Lyon, France: International Agency for Research on Cancer, 2014. [PubMed] [Google Scholar]
- 10. World Health Organization . WHO mortality database, 2017.
- 11. Mathers CD, Fat DM, Inoue M, et al. Counting the dead and what they died from: an assessment of the global status of cause of death data. Bull World Health Organ 2005;83:171–177. [PMC free article] [PubMed] [Google Scholar]
- 12. Segi M. Cancer mortality for selected sites in 24 countries (1950–1957), Sendai, Japan: Department of Public health, Tohoku University of Medicine, 1960. [Google Scholar]
- 13. Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 2014;140:317–322. [DOI] [PubMed] [Google Scholar]
- 14. O'Grady TJ, Gates MA, Boscoe FP. Thyroid cancer incidence attributable to overdiagnosis in the United States 1981‐2011. Int J Cancer 2015;137:2664–2673. [DOI] [PubMed] [Google Scholar]
- 15. Colonna M, Uhry Z, Guizard AV, et al. Recent trends in incidence, geographical distribution, and survival of papillary thyroid cancer in France. Cancer Epidemiol 2015;39:511–518. [DOI] [PubMed] [Google Scholar]
- 16. Cordioli MI, Canalli MH, Coral MH. Increase incidence of thyroid cancer in Florianopolis, Brazil: comparative study of diagnosed cases in 2000 and 2005. Arq Bras Endocrinol Metabol 2009;53:453–460. [DOI] [PubMed] [Google Scholar]
- 17. Ahn HS, Kim HJ, Kim KH, et al. Thyroid cancer screening in South Korea increases detection of papillary cancers with no impact on other subtypes or thyroid cancer mortality. Thyroid 2016;26:1535–1540. [DOI] [PubMed] [Google Scholar]
- 18. Lin JS, Bowles EJA, Williams SB, et al. Screening for thyroid cancer: updated evidence report and systematic review for the US preventive services task force. JAMA 2017;317:1888–1903. [DOI] [PubMed] [Google Scholar]
- 19. Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst 2010;102:605–613. [DOI] [PubMed] [Google Scholar]
- 20. Pacini F, Castagna MG, Brilli L, et al. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann. Oncol. 2012;23 Suppl 7:vii110‐9. [DOI] [PubMed]
- 21. Furuya‐Kanamori L, Bell KJ, Clark J, et al. Prevalence of differentiated thyroid cancer in autopsy studies over six decades: a meta‐analysis. J Clin Oncol 2016;34:3672–3679. [DOI] [PubMed] [Google Scholar]
- 22. Davies L, Morris LG, Haymart M, et al. American Association of Clinical Endocrinologists and American College of endocrinology disease state clinical review: the increasing incidence of thyroid cancer. Endocr Pract 2015;21:686–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yoo F, Chaikhoutdinov I, Mitzner R, et al. Characteristics of incidentally discovered thyroid cancer. JAMA Otolaryngol Head Neck Surg 2013;139:1181–1186. [DOI] [PubMed] [Google Scholar]
- 24. Park S, Oh CM, Cho H, et al. Association between screening and the thyroid cancer "epidemic" in South Korea: evidence from a nationwide study. BMJ 2016;355:i5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee TJ, Kim S, Cho HJ, et al. The incidence of thyroid cancer is affected by the characteristics of a healthcare system. J Korean Med Sci 2012;27:1491–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high‐income countries. JAMA 2018;319:1024–1039. [DOI] [PubMed] [Google Scholar]
- 27. Mathew IE, Mathew A. Rising thyroid cancer incidence in southern India: an epidemic of Overdiagnosis? Journal of the Endocrine Society 2017;1:480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lim H, Devesa SS, Sosa JA, et al. Trends in thyroid cancer incidence and mortality in the United States, 1974‐2013. JAMA 2017;317:1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kitahara CM, Devesa SS, Sosa JA. Increases in thyroid cancer incidence and mortality‐reply. JAMA 2017;318:390–391. [DOI] [PubMed] [Google Scholar]


