Abstract
Omadacycline is a novel aminomethylcycline approved for the treatment of community‐acquired bacterial pneumonia and acute bacterial skin and skin structure infections. This article reviews existing data pertaining to the biochemistry, mechanism of action, pharmacokinetics/pharmacodynamics, in vitro activity, and current progress with omadacycline in clinical trials. Omadacycline inhibits protein synthesis by binding to the 30S subunit of the bacterial ribosome at the tetracycline‐binding site with an affinity similar to glycylcyclines. It is able to bypass older tetracycline resistance mechanisms and demonstrates activity against bacterial strains that are tetracycline resistant. In addition, omadacycline displays broad‐spectrum activity against gram‐positive organisms (including methicillin‐resistant Staphylococcus aureus and vancomycin‐resistant enterococci), gram‐negative organisms, atypical organisms, and anaerobes. It has been evaluated against infections in adults both intravenously and orally. Dosage adjustments are not required for patients with renal impairment. Omadacycline displays a comparable efficacy and safety profile to standard‐of‐care agents, with the most common side effects observed being gastrointestinal. Currently available data for omadacycline suggest that this is a promising agent added to our antimicrobial armamentarium.
Keywords: omadacycline, Staphylococcus, Streptococcus, Enterococcus, gram‐negative, resistance
Globally, antimicrobial resistance has been an ever‐growing problem since the 1970s.1 Unfortunately, tetracyclines have not been exempt, and gram‐positive and gram‐negative bacteria have developed resistance to older tetracyclines, such as doxycycline and minocycline. This is of particular concern for infections commonly treated with tetracyclines, including community‐acquired bacterial pneumonia (CABP) and acute bacterial skin and skin structure infections (ABSSSIs). A high rate of morbidity and mortality is associated with pneumonia and skin infections, and antimicrobial resistance will only lead to worsened patient outcomes. In 2014, approximately 423,000 visits to emergency departments in the United States had pneumonia as the primary discharge diagnosis.1 According to the Centers for Disease Control and Prevention, 51,811 people died of pneumonia in the United States in 2015 (16.1 deaths per 100,000 population).2 In addition, ABSSSIs are also of growing concern. The prevalence of methicillin‐resistant Staphylococcus aureus (MRSA) infections is increasing, resulting in a significant health care burden in the United States and globally. Nearly 80% of pathogen‐positive ABSSSI cultures in a retrospective study resulted from S. aureus; 46% of these were MRSA.3 The frequency of clinically diagnosed ABSSSIs in the study population was 496/10,000 person‐years.3 This underscores the urgent need for additional antimicrobials effective against common community‐acquired pathogens.
Widespread resistance to many classes of antibiotics has led to the development of new therapeutic options. Omadacycline (previously PTK 0796; Paratek Pharmaceuticals) is a novel aminomethylcycline (synthesized from the tetracycline class) which sought United States Food and Drug Administration (FDA) approval to treat CABP and ABSSSIs.4
Omadacycline was granted Qualified Infectious Disease Product and Fast Track designations due to the need for broad‐spectrum antimicrobials with capability to combat multidrug‐resistant organisms. In April 2018, the FDA accepted the New Drug Application (NDA) and granted priority review for omadacycline to treat CABP and ABSSSIs. The NDA review included both intravenous (IV) and oral formulations. Omadacycline was granted FDA approval on October 2, 2018. This article provides a comprehensive review of omadacycline, including its chemistry, mechanism of action, microbiological spectrum of activity, resistance, pharmacokinetics, pharmacodynamics, efficacy in animal models, clinical efficacy, and safety/tolerability.
Data Sources
A literature search was performed using the PubMed electronic database with the following search terms: omadacycline and PTK 0796. A secondary search for relevant clinical trials was completed using Google Scholar and ClinicalTrials.gov. Additional information was gathered from abstracts from Infectious Diseases Week 2017, the European Congress of Clinical Microbiology and Infectious Diseases conference from 2018, and the Paratek Pharmaceuticals website.
Chemistry, Structure, and Function
Omadacycline is a semisynthetic aminomethylcycline derived from minocycline (Figure 1). It has a novel modification with an aminomethyl group present at the C9 position of the basic tetracycline structure. This modification allows omadacycline to overcome bacterial resistance mechanisms commonly used against doxycycline and minocycline, including tetracycline efflux and ribosomal protection.4 In addition, the structure modification increases antimicrobial potency, as well as limits unwanted side effects, such as nausea and emesis, commonly seen with tigecycline.5, 6
Figure 1.
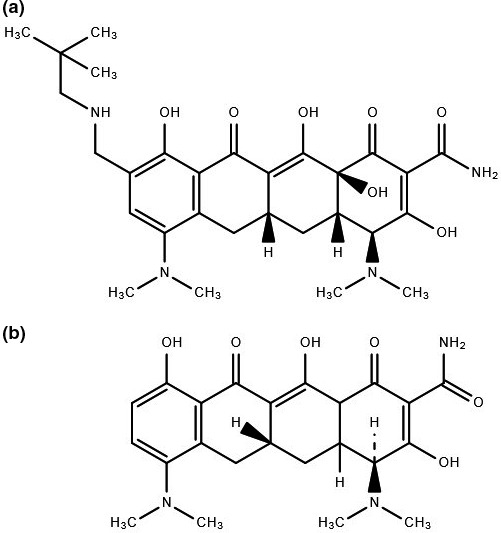
(a) Chemical structure of omadacycline. (b) Chemical structure of minocycline.
Like other tetracyclines, omadacycline inhibits protein synthesis of bacteria without having a significant impact on synthesis of DNA, RNA, or peptidoglycan. It binds to the 30S subunit of the bacterial ribosome at the tetracycline‐binding site with an affinity similar to glycylcyclines.6, 7
Microbiologic Activity
Similar to other tetracyclines, omadacycline is a broad‐spectrum antimicrobial with activity against aerobic and anaerobic gram‐positive and gram‐negative bacteria, as well as atypical bacteria (Table 1). In addition, omadacycline demonstrates activity against organisms with multidrug resistance, including tetracycline resistance. Breakpoints of ≤4, ≤0.5mg/L, ≤0.25mg/L, and ≤0.25mg/L have been defined for Enterobacteriaceae, Staphylococcus aureus, Enterococcus faecalis, and Streptococcus species, respectively.
Table 1.
In Vitro Susceptibility of Various Organisms to Omadacycline and Comparator Agents
| Organism (# of Isolates) | Omadacycline | Doxycycline | Minocycline | Tetracycline | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC50 (mg/L) | MIC90 (mg/L) | MIC Range (mg/L) | MIC50 (mg/L) | MIC90 (mg/L) | MIC Range (mg/L) | MIC50 (mg/L) | MIC90 (mg/L) | MIC Range (mg/L) | MIC50 (mg/L) | MIC90 (mg/L) | MIC Range (mg/L) | |
| Gram‐positive organisms | ||||||||||||
| MRSA (n=39)4 | 0.25 | 0.5 | 0.125–1 | 0.125 | 8 | ≤ 0.06–8 | 0.25 | 8 | ≤ 0.06–16 | 0.25 | 64 | ≤ 0.06–64 |
| MRSA (n=1438)8 | 0.12 | 0.25 | 0.03–8 | – | – | – | – | – | – | – | – | – |
| S. pneumoniae (n=41)4 | ≤ 0.06 | 0.125 | ≤ 0.06–0.25 | 2 | – | ≤ 0.06–4 | 2 | 8 | ≤ 0.06–8 | 16 | 32 | ≤ 0.06–64 |
| E. faecalis (n=31)4 | 0.25 | 0.5 | 0.125–1 | 4 | 16 | ≤ 0.06–16 | 8 | 16 | 0.125–16 | 32 | 64 | 0.125– > 64 |
| E. faecium (n=24)4 | 0.25 | 0.5 | 0.125–0.5 | 2 | 8 | ≤ 0.06–16 | 8 | 16 | 0.125–32 | 32 | 64 | 0.125– > 64 |
| E. faecium (n=390)8 | 0.06 | 0.12 | ≤ 0.015–8 | – | – | – | – | – | – | – | – | – |
| Gram‐negative organisms | ||||||||||||
| H. influenzae (n=53)4 | 1 | 2 | 0.5–8 | 0.5 | 4 | 0.125–8 | – | – | – | 2 | 32 | 0.125–64 |
| M. catarrhalis (n=408)8 | 0.25 | 0.25 | 0.06–0.5 | – | – | – | – | – | – | – | – | – |
| K. pneumoniae (n=14)4 | 2 | 4 | 1–8 | 2 | 32 | 1–64 | 2 | 64 | 2– > 64 | 2 | > 64 | 0.5– > 64 |
| K. pneumoniae (n=1771)8 | 2 | 8 | 0.25– > 32 | – | – | – | – | – | – | – | – | – |
| Enterobacter cloacae species complex (n=572)8 | 2 | 4 | 0.25– > 32 | – | – | – | – | – | – | – | – | – |
| A. baumannii (n=441)8 | 4 | 8 | 0.06– > 32 | – | – | – | – | – | – | – | – | – |
| S. maltophilia (n=315)8 | 2 | 8 | 0.25– > 32 | – | – | – | – | – | – | – | – | – |
| Atypical organisms | ||||||||||||
| Mycoplasma pneumoniae (n=20)9 | 0.125 | 0.25 | 0.125–0.25 | 0.25 | 0.5 | 0.125–0.5 | – | – | – | 0.5 | 0.5 | 0.25–0.5 |
| Ureaplasma spp. (n=20)9 | 1 | 2 | 0.25–2 | 0.25 | 4 | 0.06–4 | – | – | – | 1 | 16 | 0.25–16 |
MIC = minimum inhibitory concentration; MIC50 = minimum concentration of antibiotic that inhibits 50% of the isolates; MIC90 = minimum concentration of antibiotic that inhibits 90% of the isolates; MRSA = methicillin‐resistant Staphylococcus aureus.
Gram‐Positive Aerobic Coverage
Omadacycline provides activity against many gram‐positive organisms. In an evaluation of 339 gram‐positive isolates, omadacycline activity was compared to that of tetracycline, minocycline, doxycycline, vancomycin, and other agents with clinically relevant gram‐positive coverage.4 Against S. aureus isolates, including methicillin‐susceptible, methicillin‐resistant, and multidrug‐resistant strains, omadacycline MIC at which 90% of isolates were inhibited (MIC90) values were 0.5 mg/L. In S. aureus strains possessing tetracycline resistance, omadacycline produced MICs ranging from 0.125 to 1 mg/L. In both Enterococcus faecalis and E. faecium strains, including those resistant to vancomycin or tetracycline, omadacycline displayed activity with an MIC90 value of 0.5 mg/L. All streptococcal strains were inhibited by omadacycline at concentrations of 0.5 mg/L. Similarly, in a global surveillance study including roughly 70,000 isolates, 99.9% of all S. aureus and Enterococcus spp. were inhibited by omadacycline concentrations less than or equal to 2 mg/L.10
Gram‐Negative Aerobic Coverage
Gram‐negative coverage for omadacycline includes many Enterobacteriaceae. Against clinical pathogens, omadacycline was compared to standard‐of‐care agents. For E. coli and Klebsiella spp., respectively, MIC90 values of 2 and 4 mg/L were observed. Omadacycline also displays activity against Haemophilus influenzae and Moraxella catarrhalis.8, 11 In 3383 H. influenzae isolates, including 736 β‐lactamase positive, 99% of the organisms were inhibited by omadacycline concentrations of less than or equal to 2 mg/L. Against M. catarrhalis, 100% of the 1126 isolates were inhibited by concentrations of less than or equal to 1 mg/L. Omadacycline also demonstrates antimicrobial inhibition against multidrug‐resistant organisms. Activity against Acinetobacter baumannii‐Acinetobacter calcoaceticus spp. complex (n=2101) and other Acinetobacter spp. (n=292) was assessed, and omadacycline inhibited growth of 91.5% and 95.5% of the isolates at less than or equal to 4 mg/L, respectively.10 Against 315 Stenotrophomonas maltophilia isolates, omadacycline inhibited growth of 82.2% of the organisms evaluated.10 Against other multidrug‐resistant Enterobacteriaceae, omadacycline inhibited 85.3% of non–ceftazidime‐susceptible (n=1439) and 52.7% of non–imipenem‐susceptible isolates (n=277).8
Anaerobic, Atypical, and Other Coverage
Similar to other tetracyclines, omadacycline displays activity against a variety of other organisms. Susceptibility of omadacycline was evaluated against 186 anaerobic organisms.12 Against Bacteroides fragilis, Prevotella spp., Clostridium difficile, Clostridium perfringens, and Peptostreptococcus spp., MIC90 values were 4, 2, 0.5, 16, and 1 mg/L, respectively. These values were equivalent or within 1‐dilution difference compared to tigecycline. Omadacycline displayed comparable susceptibility to doxycycline, tetracycline, clindamycin, azithromycin, and moxifloxacin against Mycoplasma spp. and Ureaplasma spp. with MIC90 values less than or equal to 2 mg/L.9 A total of 125 dog and cat bite infection isolates were tested for omadacycline susceptibility.13 All isolates, excluding Eikenella corrodens, had omadacycline MICs less than 1 mg/L. Reduced susceptibility to all tetracyclines was observed for Eikenella spp. Omadacycline activity has also been evaluated against bioterrorism pathogens, including Bacillus anthracis and Yersinia pestis.14 The MIC90 value observed for omadacycline for B. anthracis was 0.06 mg/L compared to 0.06 mg/L for doxycycline and 0.12 mg/L for both ciprofloxacin and tetracycline. For Y. pestis, MIC90 values for omadacycline, doxycycline, tetracycline, and ciprofloxacin were 1, 1, 2, and 0.03 mg/L, respectively.
Resistance
Tetracycline resistance is common in both gram‐positive and gram‐negative organisms10, 15 The resistance mechanisms against tetracyclines can be stratified based on frequency into major and minor mechanisms.6, 7, 16 The two major mechanisms of resistance are increased number of efflux pumps and production of ribosomal protection proteins. The two minor mechanisms of resistance include modification of the ribosomal target and enzymatic inactivation.6, 7, 16 Omadacycline retains activity for organisms with these resistance genes and does not appear to be affected by resistance to other antimicrobials.7, 8, 10 No strains with induced resistance have been reported.
The primary advantage of omadacycline is its ability to bypass certain resistance mechanisms that affect older tetracycline antibiotics, including efflux pumps and ribosomal protection proteins.7, 16 When exposed to the ribosomal protection protein Tet(O), omadacycline continues to inhibit protein synthesis despite tetracycline becoming inactive in the presence of Tet(O).6 Tet(B) produces an efflux protein in gram‐negative bacteria that causes resistance in the tetracycline class, but minocycline, glycylcyclines, and omadacycline are not affected by this gene.6 In gram‐positive bacteria, the gene Tet(K) is responsible for tetracycline efflux, and omadacycline remains active despite its presence.6
In addition to evading the mechanisms of resistance above, the resistance mechanisms active against omadacycline have not been found to be clinically relevant.16 An analysis on omadacycline binding sites was performed on E. coli isolates. Omadacycline was found to have the same binding site as tetracycline and tigecycline and is susceptible to the same 16S rRNA mutations that confer binding‐site alterations. Two mutations to the 16S rRNA are required to affect the primary binding site, but when these mutations occur, tetracycline resistance results in a 4‐ to 8‐fold increase in MIC. However, this mechanism causes low level resistance and decreases the fitness of the organisms by impairing cell growth.16
Pharmacokinetics
Pharmacokinetics of both oral and IV omadacycline have been evaluated in several clinical studies. Absolute bioavailability of omadacycline is 34.5%, leading to an oral dose of 300 mg versus a 100 mg IV dose.17 Omadacycline displays linear pharmacokinetics, with higher area under the curve (AUC) and maximum observed plasma concentrations (C max) with increasing dosages.17 A mean plasma half‐life (t 1/2) of 17 hours was observed, which was independent of formulation (tablet, solution, IV) for single‐dose administration.18 In a multiple‐dose evaluation, including dosages up to 600 mg, a 13‐hour t 1/2 was observed after single dose but was comparable to the previous study on day 5 (16 hrs).17 Protein binding of omadacycline was low (20%) and nonspecific.19 Omadacycline undergoes minimal hepatic metabolism and is neither a substrate, inducer, nor inhibitor of the cytochrome P450 system. In addition, it is not a potential transport substrate or inhibitor of clinically relevant drug transporters. Omadacycline is eliminated predominately in the feces (81.1%), with some renal elimination (14.4%).19
A phase I evaluation of 16 subjects, eight with end‐stage renal disease (ESRD) and eight healthy, assessed the pharmacokinetics of omadacycline.20 The AUC values observed were comparable between ESRD and healthy subjects, irrespective of whether the omadacycline dose was administered before or after dialysis. In addition, volume of distribution and overall clearance were comparable between the two groups, suggesting that dosage adjustments are not required for omadacycline in patients with renal dysfunction.
The effect of food on omadacycline bioavailability was assessed in a phase I, randomized, four‐period, crossover study.21 The four periods consisted of overnight fasting with a standard high‐fat (nondairy) meal 3 hours after dosing, standard high‐fat (nondairy) meal 4 hours before dosing, standard high‐fat (nondairy) meal 2 hours before dosing, and standard high‐fat meal 2 hours before dosing. Substantial effects on omadacycline exposure were observed when administered in varying fed states and even greater effect when dairy was included compared to fasted states. Area under the curve reductions of 17%, 42%, and 63% occurred for nondairy meal 4 hours prior, nondairy meal 2 hours prior, and meal with dairy 2 hours prior to omadacycline receipt. These results suggest omadacycline should be taken in a fasted state with avoidance of dairy or other multivalent cations.
Finally, omadacycline penetrated well into the epithelial lining fluid (ELF), suggesting that this agent may be an option for treatment of lower respiratory tract bacterial infections. In a phase I assessment of 58 healthy adult subjects, omadacycline and tigecycline plasma, ELF, and alveolar cell concentrations were compared.22 Systemic exposure, based upon AUC0–24 and AUC0–12 values, was 3‐fold higher in the plasma, ELF, and alveolar cells for omadacycline versus tigecycline. There are no currently published data on omadacycline pharmacodynamics against gram‐negative organisms.
Pharmacodynamics
Like other tetracyclines, area under the unbound concentration‐time curve to the MIC (fAUC/MIC) is the antimicrobial activity predictor for omadacycline.23 Omadacycline pharmacodynamics were assessed in a murine pneumonia model.24 In this model, omadacycline activity was examined against four S. pneumoniae isolates. Bactericidal (≥ 3‐log kill) activity was observed in three of the four strains. Approximately 100% of the drug in plasma penetrated into the ELF. The plasma fAUC/MIC values for stasis and 2‐log10 kill were 15.79–19.83 and 18.65–56.2, respectively. Similarly, the ELF fAUC/MIC values for stasis and 2‐log10 kill were 14.18–17.80 and 17.26–47.27, respectively.
Animal Efficacy Models
Two distinct animal efficacy models of omadacycline have been described.14, 24 First, omadacycline was evaluated against four strains of S. pneumoniae (MIC 0.03–0.125 mg/L) in a murine pneumonia model.24 Omadacycline was administered subcutaneously at doses ranging from 0.1 to 25.6 mg/kg every 12 hours and was bactericidal at all doses in two strains. For the other two strains, omadacycline induced stasis at 0.92–1.28 mg/kg every 24‐hour doses and 1‐log10 kill at 1.26–18.24 mg/kg every 24‐hour dose, respectively.
In the second study, omadacycline was evaluated against B. anthracis and Y. pestis in postexposure prophylaxis (PEP) and in a delayed‐treatment model of inhalational anthrax in murine models.14 In the Bacillus PEP arm, animals were exposed to four separate doses of B. anthracis (mean 30.5 × 50% lethal dose [LD50]) by whole‐body aerosol.14 Omadacycline was administered 24 hours postexposure at doses ranging from 0.75 to 15 mg/kg IP twice/day and was compared to ciprofloxacin 30 mg/kg twice/day, doxycycline 0.75–15 mg/kg twice/day, and saline. Omadacycline‐ and doxycycline‐matched doses (2.5–15 mg/kg) demonstrated similar survival rates, but omadacycline 0.75 mg/kg‐dose survival was significantly better (p=0.0125). All 10 saline animals died (median survival 2.25 days), compared to six of ten animals in the omadacycline 0.75 mg/kg group (median survival 4.75 days), eight of ten animals in the doxycycline 0.75 mg/kg group, and two of nine animals in the ciprofloxacin group. In the delayed‐treatment anthrax model, omadacycline 15 mg/kg IP twice daily was administered 48 hours postexposure and compared to ciprofloxacin 30 mg/kg, doxycycline 15 mg/kg, and saline. No significant differences were seen between omadacycline, ciprofloxacin, and doxycycline. All 10 animals died in the saline arm (median survival 2.25 days), whereas four, two, and three animals died in the omadacycline, ciprofloxacin, and doxycycline arms, respectively.
In the Yersinia arm, animals were exposed to three separate doses of Y. pestis (mean 29.4x LD50) by whole‐body aerosol.14 Omadacycline (5–40 mg/kg IP twice/day) for 7 days was compared to doxycycline (5–40 mg/kg twice/day), ciprofloxacin (15 mg/kg twice/day), and saline. In mice treated with omadacycline 40 mg/kg or doxycycline 40 mg/kg, 90% survived (compared to 100% of ciprofloxacin‐treated mice and no (0%) saline‐treated mice). In omadacycline 40 mg/kg‐treated and ciprofloxacin‐treated mice, no viable bacteria were recovered.
Clinical Efficacy Trials
Community‐Acquired Bacterial Pneumonia
The Omadacycline for Pneumonia Treatment In the Community (OPTIC) trial was a phase III, randomized, double‐blind, multicenter study comparing the safety and efficacy of omadacycline to moxifloxacin for the treatment of adults with CABP.25 Patients were randomized to IV omadacycline 100 mg twice/day for two doses followed by 100 mg IV/day or IV moxifloxacin 400 mg/day for 3 days, with the option to switch to oral therapy or continue IV for a total of 7–14 days. Patients were included if they had clinical evidence of CABP, signs of infection or systemic inflammatory response, radiographically confirmed acute bacterial pneumonia, and CABP categorized as Pneumonia Patient Outcomes Research Team Score (PORT) risk class II, III, or IV. Patients were excluded if they received other effective antibacterial treatment within 72 hours, had hospital‐acquired or health care–associated pneumonia, empyema, lung abscess, septic shock, or end‐stage liver disease. The primary end points were early clinical response (ECR) at 72–120 hours (FDA primary end point) and clinical success at the posttreatment evaluation (PTE, occurred 5–10 days following the last treatment dose) in both the intention‐to‐treat (ITT) population and the clinically evaluable (CE) populations (European Medicines Agency [EMA] primary end point). In the ITT population at ECR evaluation, omadacycline performed similarly to moxifloxacin (81.1% vs 82.7%, treatment difference [TD] −1.6 [95% confidence interval (CI) −7.1 to 3.8]) (Table 2). At the PTE, results for omadacycline versus moxifloxacin were similar in both the ITT (87.6% vs 85.1%; TD 2.5 [−2.4 to 7.4]) and the CE (92.9% vs 90.4%; TD 2.5 [95% CI −1.7 to 6.8]) populations, respectively. By pathogen, omadacycline had similar rates of clinical success as moxifloxacin against atypical pathogens (92.4% vs 91.5%), gram‐negative bacteria (84.8% vs 80.9%), and S. pneumoniae (86% vs 91.2%), respectively. Clinical success rates for omadacycline were slightly less with S. aureus compared to moxifloxacin (72.7% vs 81.8%, respectively). Based on these results, omadacycline was non‐inferior for both the FDA and EMA end points.
Table 2.
Summary of Clinical Response in Patients Treated with Omadacycline in Phase II and III trials
| Population | Rate of Clinical Response, n (%) | % Difference (95% CI) | |
|---|---|---|---|
| Phase II cSSSI trial26 | |||
| Omadacycline | Linezolid | ||
| TOC (ITT) | 98/111 (88.3) | 82/108 (75.9) | 12.4 (1.9 to 22.9) |
| TOC (MITT) | 75/84 (89.3) | 59/78 (75.6) | 13.6 (1.4 to 25.9) |
| TOC (CE) | 98/100 (98) | 82/88 (93.2) | 4.8 (−1.7 to 11.3) |
| TOC (ME) | 75/77 (97.4) | 59/63 (93.7) | 3.8 (−4.0 to 11.5) |
| Phase III ABSSSI trial (OASIS)27 | |||
| ECR (MITT) | 268/316 (84.8) | 267/311 (85.8) | −0.7 (−6.3 to 4.9) |
| PTE (MITT) | 272/316 (86.1) | 260/311 (83.6) | 2.5 (−3.2 to 8.2) |
| PTE (CE) | 259/269 (96.3) | 243/260 (93.5) | 2.8 (−1.0 to 6.9) |
| Phase III ABSSSI trial (OASIS‐2)28 | |||
| ECR (MITT) | 315/360 (87.5) | 297/360 (82.5) | 5.0 (−0.2 to 10.3) |
| PTE (MITT) | 303/360 (84.2) | 291/360 (80.8) | 3.3 (−2.2 to 9.0) |
| PTE (CE) | 278/284 (97.9) | 279/292 (95.5) | 2.3 (−0.5 to 5.8) |
| Phase III CABP trial (OPTIC)25 | |||
| Omadacycline | Moxifloxacin | ||
| ECR (ITT) | 313/386 (81.1) | 320/388 (82.7) | −1.6 (−7.1 to 3.8) |
| PTE (ITT) | 338/386 (87.6) | 330/388 (85.1) | 2.5 (−2.4 to 7.4) |
| PTE (CE) | 316/340 (92.9) | 312/345 (90.4) | 2.5 (−1.7 to 6.8) |
ABSSSI = acute bacterial skin and skin structure infection; CABP = community‐acquired bacterial pneumonia; cSSSI = complicated skin and skin structure infection; CE = clinically evaluable; ECR = early clinical response; ITT = intent to treat; MITT = modified intent to treat; ME = microbiologically evaluable; PTE = post‐treatment evaluation; TOC = test of cure.
Skin and Skin Structure Infections (SSSI)
Phase II Trial
A phase II, randomized, controlled, investigator‐blind, multicenter study compared omadacycline monotherapy to linezolid with or without aztreonam for treatment of complicated SSSI (cSSSI).26 Included patients were ≥ 18 years old and had one of four cSSSI (wound infection, major abscess, infected ulcer in the lower extremity, or cellulitis). Any patient with an infection treatable with surgical intervention alone was excluded. Both omadacycline and linezolid were initially given as IV (100 mg IV daily vs 600 mg IV twice/day, respectively) with the option to transition to oral (200 mg orally/day vs 600 mg orally twice/day, respectively). Patients were evaluated at four specified time points: baseline, end of IV treatment, end of treatment, and 10–17 days after the last dose of treatment (test of cure [TOC] evaluation). The primary hypothesis was that safety and tolerability of omadacycline was comparable with linezolid. The secondary hypothesis was that omadacycline was noninferior to linezolid for the rate of successful clinical response at TOC. The majority of patients in the omadacycline (65.8%) and linezolid (66.7%) groups had major abscesses. The most common organism identified was S. aureus, with the majority being MRSA. Patients received an equivalent mean duration of IV treatment (4.3 days) for both drugs, and the overall mean treatment duration was similar between the omadacycline and linezolid groups (10 vs 9.6 days, respectively).
In each of the four populations assessed, omadacycline‐treated patients had higher rates of treatment success compared with patients receiving linezolid. For patients treated with omadacycline versus linezolid, clinical cure rates were 88.3% versus 75.9% (TD 12.4 [95% CI 1.9–22.9]) for the ITT population; 89.3% versus 75.6% (TD 13.6 [95% CI 1.4–25.9]) in the MITT population; 98% versus 93.2% (TD 4.8 [95% CI −1.7 to 11.3]) in the CE population; and 97.4% versus 93.7% (TD 3.8 [95% CI −4.0 to 11.5]) in the microbiologically evaluable (ME) population, respectively (Table 2). When evaluating the ME population by organism, both treatment arms achieved 100% clinical response for gram‐negative and gram‐positive bacteria other than S. aureus. For S. aureus, omadacycline had higher success rates compared with linezolid (97.2% vs 92.7%), including MRSA (97.7% vs 93.8%). A retrospective analysis of the ITT patients was performed to evaluate clinical response during the first 72 hours of starting therapy to align with the 2010 FDA guidance for the development of drugs for treatment of ABSSSI.29 Clinical response at 72 hours was similar between the omadacycline‐ and linezolid‐treated patients (96.8% vs 94.4%, respectively). These ECR data were primarily from patients transitioning from IV to oral medications in the first 72 hours of treatment.26 Based on the clinical response results for this phase II trial for cSSSI, omadacycline was deemed non‐inferior to linezolid.
Phase III Trials
The Omadacycline in Acute Skin and Skin Infections Study (OASIS) and OASIS‐2 are phase III, randomized, double‐blind, multicenter studies comparing the safety and efficacy of omadacycline to linezolid for treating adult subjects with ABSSSI.27, 28, 30, 31, 32 In the OASIS, patients were randomized 1:1 to receive IV omadacycline 100 mg twice/day for two doses followed by 100 mg IV/day or IV linezolid 600 mg twice/day with the option to continue IV or switch to oral omadacycline 300 mg/day or oral linezolid 600 mg twice/day for a total of 7–14 days.27 The primary end points were ECR at 48–72 hours (FDA primary end point) and clinical response at the PTE in the MITT population and CE population, which was 7–14 days after the last dose of treatment (EMA co‐primary end points). Included patients had a qualifying ABSSSI (≥ 75 cm2 total surface area of contiguous involved tissue) and evidence of systemic inflammatory response within 24 hours prior to randomization. Patients were excluded if ≥ 1 dose of a potentially effective antibiotic were given within 72 hours prior to the first dose of the study drug or if the patient was taking medications known to interact with linezolid, such as monoamine oxidase inhibitors (MAOIs). Unlike the phase II cSSSI study, infection type was well distributed in the OASIS population with similar percentages of patients with wound infections, cellulitis/erysipelas, and major abscesses. For ECR in the MITT population, omadacycline (84.8%) performed similarly to linezolid (85.8%; TD −0.7 [−6.3 to 4.9]) (Table 2). Omadacycline performed comparatively to linezolid at the PTE for MITT (86.1% vs 83.6%; TD 2.5 [−3.2 to 8.2]) and CE populations (96.3% vs 93.5%; TD 2.8 [−1.0 to 6.9]), respectively. Based on these results, omadacycline met noninferiority for both the FDA and EMA efficacy end points. Clinical response by pathogen at PTE in the micro‐MITT population showed comparable clinical responses for omadacycline and linezolid. Clinical success rates were high for both omadacycline and linezolid for S. aureus (83.3% vs 83.4%), including MRSA (82.6% vs 86%), respectively. Success rates were lower for both omadacycline (74.5%) and linezolid (70.3%) for Streptococcus anginosus group. On the other hand, omadacycline had lower rates of clinical success compared with linezolid for Streptococcus pyogenes (72.7% vs 88.9%, respectively). For vancomycin‐sensitive E. faecalis, clinical success rates were higher for both omadacycline and linezolid (90.0% vs 92.3%, respectively).
Multiple subgroup analyses from the OASIS trial have been performed, including patients with high body mass index (BMI), diabetes, chronic kidney disease (CKD), intravenous drug use (IVDU), and hepatitis C positive (HCV+).30, 31, 32 Patients with normal BMI compared with high BMI (i.e., overweight and obese) had similar rates of clinical success at ECR for omadacycline (84.7% vs 84.8%, respectively) and linezolid (84.9% vs 85.9%, respectively).30 Clinical success rates at PTE were similar in the MITT and CE populations regardless of treatment or BMI, and no significant differences were found between the subgroups. For patients with CKD, clinical success at ECR was similar between omadacycline and linezolid regardless of CKD staging (CKD stage 0/1 vs 2/3).31 Omadacycline performed comparatively at PTE for both the MITT and CE populations compared with linezolid. Clinical success was high in both groups across all CKD stages, and no statistical differences were found. In the IVDU subgroup analysis, no significant difference between treatment groups was found in clinical success rates at ECR for the MITT population.32 Similarly, clinical success rates were comparable between IVDU patients, IVDU/HCV+ patients, and non‐IVDU/HCV− patients at PTE in the MITT and CE populations. A significantly higher clinical success rate at PTE was found with omadacycline compared to linezolid in non‐IVDU patients in both the MITT and CE populations.
In the OASIS‐2 study, patients were randomized 1:1 to receive oral omadacycline or linezolid for a total duration of 7–14 days.28 Patients in the oral omadacycline treatment arm received 450 mg/day for the first 2 days, then 300 mg/day thereafter. Patients in the oral linezolid treatment arm received 600 mg twice/day from day 1 of treatment onwards. The same primary end points were evaluated in OASIS and OASIS‐2 (i.e., ECR at 48–72 hrs for the MITT population and PTE 7–14 days after last treatment dose for the MITT and CE populations). Primary infection type was most commonly wound infections in the omadacycline and linezolid groups (58.3% vs 59.4%), followed by cellulitis/erysipelas and major abscesses. Omadacycline achieved all primary end points for noninferiority for the FDA and EMA. For the MITT population, ECR was similar for omadacycline and linezolid (87.5% vs 82.5%; TD 5.0 [−0.2 to 10.3], respectively) (Table 2). Clinical success rates were also comparable at PTE for the MITT population (84.2% vs 80.8%; TD 3.3 [−2.2 to 9.0]) and CE population (97.9% vs 95.5%; TD 2.3 [−0.5 to 5.8]), respectively. Omadacycline maintained high rates of clinical success for all gram‐positive pathogens in the study and performed favorably over linezolid for S. aureus (82.7% vs 79.8%; n=453), MRSA (85.6% vs 79.4%; n=211), S. pyogenes (69% vs 56.3%; n=45), S. anginosus group (86% vs 73.3%; n=47), and vancomycin‐sensitive E. faecalis (100% vs 70%; n=17), respectively.
Safety and Tolerability
Omadacycline's safety and tolerability were tested in three phase III trials: OPTIC, OASIS, and OASIS‐2. In OPTIC, there were 170 (44.5%) patients in the omadacycline group and 200 (51.5%) patients in the moxifloxacin group who experienced any adverse event during the study period.25 Treatment‐emergent adverse events (TEAEs) occurred in 157 (41.1%) and 188 (48.5%) patients in the omadacycline and moxifloxacin groups, respectively (Table 3), with only 39 (10.2%) omadacycline patients and 69 (17.8%) moxifloxacin patients experiencing drug‐related TEAEs. Of those patients, only two (0.5%) patients in each group experienced a serious drug‐related TEAE. Most of these TEAEs were gastrointestinal (GI) related (vomiting and nausea) and did not cause discontinuation of the study drugs. In addition, there were no reported cases of C. difficile due to omadacycline; however, eight (2%) patients developed a C. difficile infection or complication in the moxifloxacin group. Overall, drug discontinuation due to any adverse event was low in both groups (4.4% omadacycline vs 7.2% moxifloxacin).25
Table 3.
Treatment‐Emergent Adverse Events (TEAEs) in at Least 5% of Patients Treated with Omadacycline in Phase III Study Populations25, 27, 28
| Adverse Events, n (%) | CABP | ABSSSI | ||||
|---|---|---|---|---|---|---|
| OPTIC | OASIS | OASIS‐2 | ||||
| Omadacycline IV/PO (n=382) | Moxifloxacin IV/PO (n=388) | Omadacycline IV/PO (n=323) | Linezolid IV/PO (n=322) | Omadacycline PO (n=368) | Linezolid PO (n=367) | |
| Any TEAE | 157 (41.1) | 188 (48.5) | 156 (48.3) | 147 (45.7) | 197 (53.5) | 137 (37.3) |
| Nausea | 9 (2.4) | 21 (5.4) | 10 (12.4) | 32 (9.9) | 111 (30.2) | 28 (7.6) |
| Infusion site extravasation | 4 (1.0) | 9 (2.3) | 28 (8.7) | 19 (5.9) | – | – |
| Subcutaneous abscess | – | – | 17 (5.3) | 19 (5.9) | – | – |
| Vomiting | 10 (2.6) | 6 (1.5) | 17 (5.3) | 16 (5.0) | 62 (16.8) | 11 (3) |
| Wound Infection | 1 (0.3) | – | 15 (4.6) | 15 (4.7) | 22 (6.0) | 17 (4.6) |
| ALT Increased | 14 (3.7) | 18 (4.6) | 9 (2.8) | 14 (4.3) | 19 (5.2) | 11 (3.0) |
ALT = alanine transaminase; ABSSSI = acute bacterial skin and skin structure infection; CABP = community‐acquired bacterial pneumonia; IV = intravenous; OASIS = Omadacycline in Acute Skin and Skin Infections Study; OPTIC = Omadacycline for Pneumonia Treatment In the Community; PO = oral; TEAE = treatment‐emergent adverse event.
In the OASIS trial, TEAEs occurred in 156 (48.3%) patients receiving omadacycline, which was slightly higher than those in the linezolid group (147 (45.7%) patients).27 Similar to the OPTIC trial, most TEAEs were GI related (nausea, vomiting, diarrhea) in both groups, and study drug discontinuation due to any adverse event was low (1.8% omadacycline vs 2.1% linezolid) and likely attributed to only mild or moderate nausea observed. In a subgroup analysis of patients with CKD stage 0/1, a TEAE was observed in 129 (50.6%) patients receiving omadacycline compared to 130 (48.7%) patients receiving linezolid.31 In patients with CKD stage 2/3, 26 (38.8%) patients in the omadacycline group and 16 (30.8%) in the linezolid group experienced a TEAE. Omadacycline appears to be safe in patients with both normal and impaired renal function. Another subgroup analysis examined the impact of BMI on rates of drug‐related TEAE development.30 Approximately 15 (12.5%) patients receiving omadacycline and 16 (14.8%) patients receiving linezolid experienced a drug‐related TEAE in the normal BMI group; whereas 24 (11.8%) omadacycline patients and 26 (12.1%) linezolid patients experienced a drug‐related TEAE in the high BMI group.
In the OASIS‐2 trial, 201 (54.6%) patients in the omadacycline group and 140 (38.1%) patients in the linezolid group experienced at least one adverse event.28 Of those patients who experienced an adverse event, 139 (37.8%) omadacycline patients and 52 (14.2%) linezolid patients experienced a drug‐related TEAE, with only one linezolid patient experiencing a serious drug‐related TEAE. No participants in the omadacycline group experienced a serious drug‐related TEAE. In total, six (1.6%) omadacycline patients and three (0.8%) linezolid patients discontinued the study drug due to an adverse event.
Liver function tests (LFTs) for omadacycline‐treated patients were examined during all three phase III clinical trials. In the OPTIC study, 14 (3.7%) and 8 (2.1%) patients had alanine aminotransferase (ALT) and aspartate aminotransferase (AST) elevations, respectively; in the OASIS trial, 2.8% and 2.5% of patients had ALT and AST elevations, respectively; in the OASIS‐2 trial, 5.2% and 4.6% of patients had ALT and AST elevations, respectively.25, 27, 28 Overall, few patients had LFT elevations greater than 3 times the upper limit of normal (ALT 3.2%; AST 2.8%) or total bilirubin elevations greater than 1.5 times the upper limit of normal (1.3%) in all three studies.25, 27, 28 Omadacycline's effect in patients with ESRD was also examined. Five of 16 (31.3%) patients with ESRD experienced a TEAE, with only one (6.3%) patient experiencing a TEAE that was considered to be due to the study drug.19
Further safety testing was performed to determine the effect of omadacycline on QTc prolongation. In the OPTIC trial, 42 (11.9%) patients treated with omadacycline experienced an increase in QTc greater than 450 milliseconds, with 24 (6.8%) patients experiencing an increase greater than 450 milliseconds corresponding to a greater than 30 milliseconds change from their baseline QTc interval.33 In addition, six (1.7%) patients experienced an increase in QTc greater than 500 milliseconds, with five (1.4%) patients experiencing an increase greater than 500 milliseconds corresponding to a greater than 30 milliseconds change from their baseline QTc interval. Within the OASIS trial, 23 (7.1%) patients treated with omadacycline experienced an increase in their QTc interval greater than 450 milliseconds, with only three (0.9%) patients in this group having a greater than 30 milliseconds change from baseline.33 There was one (0.3%) patient who experienced a greater than 30 milliseconds change from baseline that resulted in a QTc interval greater than 500 milliseconds. In the OASIS‐2 trial, only four (1.1%) omadacycline‐treated patients experienced an increase in QTc greater than 450 milliseconds, and no patients had a recorded QTc greater than 500 milliseconds.33
Conclusion
Omadacycline is a novel aminomethylcycline with potent broad‐spectrum activity against infectious pathogens that frequently cause community‐acquired infections, including ABSSSI and CABP, with the potential for additional coverage against organisms displaying multidrug resistance. Omadacycline displays favorable pharmacokinetic profiles allowing for once daily dosing, penetration into the ELF, and lack of renal dosing adjustments. Results from phase III studies for CABP and ABSSSI are promising, demonstrating comparable efficacy to standard‐of‐care agents. In addition, omadacycline provides a similar safety profile to comparators with GI‐related side effects representing the most common adverse drug event with mild nausea and no C. difficile–associated diarrhea observed in clinical trials. In patients with recurrent C. difficile infections or β‐lactam allergies, omadacycline is a non fluoroquinolone option that should be considered for treatment of CABP or ABSSSIs.
Conflict of interest: The authors have nothing to disclose.
References
- 1. Zaman SB, Hussain MA, Nye R, Mehta V, Mamun KT, Hossain N. A review on antibiotic resistance: alarm bells are ringing. Cureus 2017;9:e1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention . Pneumonia. https://www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed April 18, 2018.
- 3. Ray GT, Suaya JA, Baxter R. Incidence, microbiology, and patient characteristics of skin and soft‐tissue infections in a U.S. population: a retrospective population‐based study. BMC Infect Dis 2013;13:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Macone AB, Caruso BK, Leahy RG, et al. In vitro and in vivo antibacterial activities of omadacycline, a novel aminomethylcycline. Antimicrob Agent Chemother 2014;58:1127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Honeyman L, Ismail M, Nelson ML, et al. Structure‐activity relationship of the aminomethylcyclines and the discovery of omadacycline. Antimicrob Agents Chemother 2015;59:7044–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tanaka SK, Steenbergen J, Villano S. Discovery, pharmacology, and clinical profile of omadacycline, a novel aminomethylcycline antibiotic. Bioorg Med Chem 2016;24:6409–19. [DOI] [PubMed] [Google Scholar]
- 7. Draper MP, Weir S, Macone A, et al. Mechanism of action of the novel aminomethylcycline antibiotic omadacycline. Antimicrob Agent Chemother 2014;58:1279–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pfaller MA, Huband MD, Shortridge D, Flamm RK. Surveillance of omadacycline activity tested against clinical isolates from the United States and Europe as part of the 2016 SENTRY antimicrobial surveillance program. Antimicrob Agents Chemother 2018;62(4):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Waites KB, Crabb DM, Liu Y, Duffy LB. In vitro activities of omadacycline (PTK 0796) and other antimicrobial agents against human mycoplasmas and ureaplasmas. Antimicrob Agents Chemother 2016;60(12):7502–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pfaller MA, Huband MD, Rhomberg PR, Flamm RK. Surveillance of omadacycline activity against clinical isolates from a global collection (North America, Europe, Latin America, Asia‐Western Pacific), 2010–2011. Antimicrob Agents Chemother 2017;61:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pfaller MA, Rhomberg PR, Huband MD, Flamm RK. Activity of omadacycline tested against Enterobacteriaceae causing urinary tract infections from a global surveillance program. Diagn Microbiol Infect Dis 2018;91(2):179–83. [DOI] [PubMed] [Google Scholar]
- 12. Stapert L, Wolfe C, Shinabarger D, Marra A, Pillar C. In vitro activities of omadacycline and comparators against anaerobic bacteria. Antimicob Agents Chemother 2018;62(4):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goldstein EJC, Citron DM, Tyrrell KL, Leoncio E, Merriam CV. Comparative in vitro activity of omadacycline against dog and cat bite would isolates. Antimicrob Agents Chemother 2018;62(4):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Steenbergen J, Tanaka SK, Miller LL, Halasohoris SA, Hershfield JR. In vitro and in vivo activity of omadacycline against two biothreat pathogens, Bacillus anthracis and Yersinia pestis . Antimicrob Agents Chemother 2017;61(5):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Villano S, Steenbergen J, Loh E. Omadacycline: development of a novel aminomethylcycline antibiotic for treating drug‐resistant bacterial infections. Future Microbiol 2016;11(11):1421–34. [DOI] [PubMed] [Google Scholar]
- 16. Heidrich CG, Mitova S, Schedlbauer A, et al. The novel aminomethylcycline omadacycline has high specificity for the primary tetracycline‐binding site on the bacterial ribosome. Antibiotics 2016;5(4):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bundrant LA, Tzanis E, Garrity‐Ryan L, et al. Safety and pharmacokinetics of the aminomethylcycline antibiotic omadacycline administered to healthy subjects in oral multiple‐dose regimens. Antimicrob Agents Chemother 2018;62(2):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sun H, Ting L, Machineni S, et al. Randomized, open‐label study of the pharmacokinetics and safety of oral and intravenous administration of omadacycline to healthy subjects. Antimicrob Agents Chemother 2016;60(12):7431–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Flarakos J, Du Y, Gu H, et al. Clinical disposition, metabolism and in vitro drug–drug interaction properties of omadacycline. Xenobiotica 2017;47(8):682–96. [DOI] [PubMed] [Google Scholar]
- 20. Berg JK, Tzanis E, Garrity‐Ryan L, et al. Pharmacokinetics and safety of omadacycline in subjects with impaired renal function. Antimicrob Agents Chemother 2018;62(2):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tzanis E, Manley A, Villano S, Tanaka SK, Bai S, Loh E. Effect of food on the bioavailability of omadacycline in healthy participants. J Clin Pharmacol 2018;57(3):321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gotfried MH, Horn K, Garrity‐Ryan L, et al. Comparison of omadacycline and tigecycline pharmacokinetics in the plasma, epithelial lining fluid, and alveolar cells of healthy adult subjects. Antimicrob Agents Chemother 2017;61(9):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Agwuh KN, MacGowan A. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J Antimicrob Chemother 2006;58(2):256–65. [DOI] [PubMed] [Google Scholar]
- 24. Lepak AJ, Zhae M, Marchillo K, VanHecker J, Andes DR. In vivo pharmacodynamic evaluation of omadacycline (PTK 0796) against Streptococcus pneumoniae in the murine pneumonia model. Antimicrob Agents Chemother 2017;5:pii: e02368‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stets R, Popescu M, Gonong J, et al. A phase 3, randomized, double‐blind, multi‐center study to compare the safety and efficacy of moxifloxacin for the treatment of adult patients with CABP (the OPTIC study). ID Week 2017; 2017 Oct 4–8; San Diego, CA. Poster 1883. 2017.
- 26. Noel GJ, Draper MP, Hait H, Tanaka SK, Arbeit RD. A randomized, evaluator‐blind, phase 2 study comparing the safety and efficacy of omadacycline to those of linezolid for treatment of complicated skin and skin structure infections. Antimicrob Agents Chemother 2012;56(11):5650–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paratek . A phase 3 randomized, double‐blind, multi‐center study to compare the safety and efficacy of oral and IV omadacycline to linezolid for treating adult subjects with ABSSSI (The OASIS Study). 2017 Apr 24. Available from https://paratekpharma.com/media/1410/eccmid-oasis-oral-final-22apr2017-vff.pdf. Accessed May 31, 2018.
- 28. Paratek . ABSSSI‐16301 study design: top‐line data results. Omadacycline in acute skin and skin structure infections study (OASIS‐2). 2017 Jul 17. Available from http://paratekpharma.com/media/1462/final-top-line-16301-absssi-17-July-2017-v1-investor-presentation.pdf. Accessed May 31, 2018.
- 29. FDA Center for Drug Evaluation and Research (CDER) . Guidance for industry. Acute bacterial skin and skin structure infections: developing drugs for treatment. Silver Spring, MD: U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research; 2010. Available from http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071185.pdf. Accessed May 31, 2018. [Google Scholar]
- 30. Wilcox M, Cure‐Bolt N, Chitra S, Tzanis E, McGovern P. Efficacy and safety of omadacycline in patients with acute bacterial skin and skin structure infections (ABSSSI) and high body mass index or diabetes: a subgroup analysis from the OASIS trial. Poster session presented at: ID Week 2017; 2017 Oct 4–8; San Diego, CA. Available from http://paratekpharma.com/media/1489/wilcoxidweek20171838omc-oasis-1-bmi-dm.pdf. Accessed May 31, 2018.
- 31. File TM Jr, Cure‐Bolt N, Chitra S, Tzanis E, McGovern P. Efficacy and safety of omadacycline in chronic kidney disease (CKD) patients with acute bacterial skin and skin structure infections (ABSSSI): a subgroup analysis from the OASIS trial. Poster session presented at: ID Week 2017; 2017 Oct 4–8; San Diego, CA. Available from https://paratekpharma.com/media/1490/fileidweek20171834omc-oasis-1-ckd.pdf. Accessed May 31, 2018.
- 32. Ohl CA, Cure‐Bolt N, Chitra S, Tzanis E, McGovern P. Efficacy and safety of omadacycline in intravenous drug using (IVDA) and hepatitis C‐positive (HCV+) patients with acute bacterial skin and skin structure infections (ABSSSI): a subgroup analysis from the OASIS trial. Poster session presented at: ID Week 2017; 2017 Oct 4–8; San Diego, CA. Available from https://paratekpharma.com/media/1485/ohlidweek20171885omc-oasis-1-ivdu.pdf. Accessed May 31, 2018.
- 33. Darpo B, Tzanis BA, Garrity‐Ryan L, et al. Cardiac safety of omadacycline in the IV/oral phase 3 acute bacterial skin and skin structure (ABSSSI) and in the IV/oral phase 3 community‐acquired bacterial pneumonia (CABP) studies. ID Week 2017; 2017 Oct 4–8; San Diego, CA. Available from https://paratekpharma.com/media/1486/darpoidweek20171886omc-cardiac-safety-oasis-1-optic.pdf. Accessed May 31, 2018.


